Abstract
In 2011, a large multivessel survey was conducted to provide nearly synoptic sampling of Red Snapper Lutjanus campechanus throughout their reproductive season in the U.S. Gulf of Mexico. A total of 2,487 Red Snapper were caught with a female : male ratio that was approximately 1:1. The ovaries of 1,002 females were histologically examined. Females (n = 391) were found with spawning markers (postovulatory follicles and hydrated oocytes) throughout the study area, but primarily in outer shelf waters. Statistical models were developed to quantify and test the dependence of the proportion of females bearing spawning markers (spawning fraction) on female length and age, time of year, depth, gear type (vertical line or longline), or region (east or west of the Mississippi River). Most of the variance in spawning fraction was explained by the time of year; spawning fractions were generally low in spring, peaked in midsummer, and declined by fall. There was also strong statistical evidence of a positive relationship between spawning fraction and either age or length. The effects of region and gear type were not significant once time of year and size or age were accounted for. These results demonstrate the need to account for differences in the time of year and age structure of the population when the productivity of populations of Red Snapper are compared. For example, productivity has been hypothesized to be greater in the western Gulf than in the eastern Gulf, as evidenced by regional patterns of egg and larval abundance. Our results suggest that this regional difference is not due to any intrinsic difference in the biology of the fish, but simply a consequence of there being more large, old Red Snapper in the western Gulf. Recent stock assessments have indicated that Red Snapper are increasing in abundance and there is a need to continue monitoring to detect any possible compensation in reproduction.
Received November 25, 2014; accepted April 2, 2015
Many tropical and subtropical fishes exhibit indeterminate fecundity and spawn multiple times over an extended period that may last much of the year. Several studies have demonstrated that the number of eggs produced during a spawning event (batch fecundity) increases with age or body length at a faster rate than does body weight (Cooper et al. Citation2013; Hixon et al. Citation2014; He et al. Citation2015). Moreover, evidence is emerging that spawning frequency also increases with age and body size (LaPlante and Schultz Citation2007; Mehault et al. Citation2010; Fitzhugh et al. Citation2011; Cooper et al. Citation2013; Klibansky and Scharf Citation2013). As a result, egg production in tropical and subtropical species is probably less correlated to mature (spawning) biomass than has been observed for species from higher latitudes (Fitzhugh et al. Citation2011; Lowerre-Barbieri et al. Citation2011; Klibansky and Scharf Citation2013).
Red Snapper Lutjanus campechanus are indeterminate spawners that inhabit the tropical and subtropical waters of the southeastern United States and Mexico. They are long lived (>50 years: Wilson and Nieland Citation2001) and considered to have a periodic life history (Winemiller and Rose Citation1992), yet maturity can occur at age 2 (SEDAR Citation2013). Full reproductive potential has been estimated to occur by about 12–15 years of age (Goodyear Citation1995; Woods Citation2003; Kulaw Citation2012). The Red Snapper population in the northern Gulf of Mexico has been harvested commercially since the mid 19th century and currently supports one of the most important recreational fisheries in the world. Perhaps not surprisingly, the assessment and management of this species has been highly controversial (Shipp and Bortone Citation2009). Even the U.S. Congress has weighed in on the matter, holding congressional hearings and sponsoring several bills specifically addressing Red Snapper (e.g., Senate Bill 157 and Congressional Bill H.R. 3099).
One area of active debate concerns the level of abundance to which the Red Snapper stock must rebuild, which depends partly on the perceived relationship between egg production and age or size, and which is tied directly into fisheries management by law through the Magnuson–Stevens Fishery Conservation and Management Act (MSFCMA). Recent stock assessments (Porch Citation2004, Citation2007) modeled Red Snapper egg production in the Gulf of Mexico as the product of batch fecundity and maturity at age. Porch et al. Citation(2007) acknowledged the need to account for age or size dependence in the number of annual spawns. Until recently, however, the available data were insufficient to determine whether such a relationship existed, let alone to quantify it.
In 2011 a congressionally supported supplemental survey, in which Red Snapper was one of the target species, was conducted throughout the northern Gulf of Mexico (Campbell et al. Citation2012). Nearly 2,500 Red Snapper were sampled from multiple vessels operating throughout the Red Snapper spawning season (April through October) and across all known spawning areas, resulting in the most spatially and temporally extensive survey of Red Snapper spawning ever conducted. Nevertheless, the survey was not perfectly synoptic in that all areas and depths were not sampled on the same dates. Previous work has suggested that the spawning intensity of Red Snapper and other species varies considerably during the course of the season and in different locations (Woods Citation2003; Porch et al. Citation2007; Brown-Peterson et al. Citation2009; Lowerre-Barbieri et al. Citation2011). For this reason, the perceived relationship between spawning frequency and age or size is likely to vary depending on when and where reproductive samples are taken (Lowerre-Barbieri et al. Citation2011). For example, since older Red Snapper tend to be caught in deeper water, sampling deep and shallow waters at different times of the spawning season could alter the apparent relationship between spawning frequency and age.
The objective of this paper was to use the samples obtained from the supplemental survey to develop unbiased estimates of the relationship between spawning frequency and body length or age. The proposed modeling framework attempted to “standardize” these estimates by explicitly accounting for the effects of any factors that may have varied systematically during the sampling.
METHODS
Field survey and laboratory processing
Red Snapper were caught by hook-and-line gear (vertical line and longline) deployed from Dry Tortugas, Florida, through Brownsville, Texas, and from the inner shelf (9 m) to a depth of about 180 m according to a stratified random design as described in Campbell et al. Citation(2012). The time of day of all catches were recorded as the gear was retrieved. All of the Red Snapper caught were measured for TL and FL (mm), weighed (kg), and dissected to extract otoliths and gonads. Gonads were sexed and macroscopically staged while on the vessel, and ovaries were frozen for further processing in the laboratory.
Red Snapper were aged from sectioned otoliths as described in Allman and Fitzhugh Citation(2007) and Allman et al. Citation(2012). Frozen ovaries were weighed (nearest 0.1 g) and subsamples were taken from the right posterior lobe and placed into 10% neutral buffered formalin (NBF) for histological preparation (hematoxylin and eosin-y stain; Mass Histology Services, Worcester, Massachusetts). Histological examination determined the presence–absence of spawning markers in females (postovulatory follicles and/or hydrated oocytes: Hunter and Macewicz Citation1985; Murua et al. Citation2003). During selected cruises, a sample of 50 Red Snapper ovaries were weighed and tissue subsamples fixed in fresh formalin at sea prior to freezing the remainder of the ovaries, for the purpose of quality assessment. A comparison test of histology sections from the paired freshly preserved and frozen–preserved ovarian tissue subsamples was conducted to determine whether spawning markers could be readily identified after freezing.
Models of spawning fraction
We defined spawning fraction as the proportion of females bearing spawning markers. This definition is similar to usage elsewhere (Priede and Watson Citation1993; Murua et al. Citation2003; Lowerre-Barbieri et al. Citation2009; Kurita et al. Citation2010; Kurita Citation2012), but differs in that we refer to the proportion of all sampled females in the survey without further distinction of active or mature females. In later calculations (see section below), we address the conversion of spawning fraction to daily probabilities of spawning. This separation of steps recognizes that various factors may govern the duration of spawning markers, which subsequently can affect daily probabilities and the estimated total number of spawns (Priede and Watson Citation1993; Kurita Citation2012).
Tabulations of the 2011 survey results appeared to confirm previous findings that the proportion of females with spawning markers (spawning fraction) increases with age or size () and varies with the time of year (). However, the size and age of the Red Snapper in the sample also tended to vary with the time of year, and somewhat older fish on average were sampled near the peak of the spawning season. Accordingly, the apparent relationship between spawning fraction and age (or length) may be confounded to some unknown degree by the relationship between spawning fraction and time of year, and it is therefore necessary to model the effects of age and time of year simultaneously in order to disentangle them. The situation is further complicated by observations of variations in spawning fraction by depth, gear type (vertical line or longline), and region (east or west of the Mississippi River).
TABLE 1. Number of Red Snapper females and fraction bearing histological spawning markers by age-class from the 2011 congressional supplemental survey.
TABLE 2. Summary of Red Snapper females from the 2011 congressional supplemental survey. Within each approximate 2-week period, the initial date of sampling is listed. The numbers of sampling dates and females and fraction of females bearing histological spawning markers are indicated, along with the mean and range of age. The survey covered a total period of 198 d.
Models are often used to help distinguish the response of a measure to an explanatory variable of interest from its response to other variables of perhaps less interest. This is particularly true where the sampling is unbalanced in the sense that more measurements are made for some levels of the explanatory variables than for others. In the present case we are particularly interested in distinguishing real changes in spawning fraction with age or length from perceived changes that result by sampling different stages under different average conditions as illustrated in . If the model is approximately correct, then important biophysical relationships should be reflected by parameter estimates that are statistically different from the corresponding null model (where the explanatory variable is assumed to have no effect) and substantially increase the fraction of the variance explained by the model.
TABLE 3. Plausible biophysical and sampling-related explanations for perceived trends in spawning frequencies of Red Snapper by stage, depth, region, gear, and season. If the model is approximately correct, biophysical causes for changes in spawning frequency should manifest as parameter estimates that are statistically different from the null model.
The standard approach for modeling dependent variables that can have only two possible outcomes (e.g., success or failure to detect spawning markers) is a binomial regression. The dependent variable is treated as the outcome of a Bernoulli trial such that the total number of successes from a series of like trials is approximately binomially distributed. The maximum likelihood estimate for the probability of observing a success (p) is therefore obtained by minimizing the negative log-likelihood expression:(1) where i denotes one of N observations, o is an indicator that takes on a value of 1 for a “success” (spawning markers present) and a value of 0 for a “failure” (spawning markers absent), and p is the probability of observing a spawning marker.
One of the most common forms of binomial regression is the logistic regression, which assumes that the probability p of observing a success (presence of spawning markers) can be well approximated by a logistic function of a linear combination of explanatory variables x:(2)
Equation (2) can be linearized by the logit transformation, ln[p/(1 − p)] and falls into the class of generalized linear models handled by many standard statistical packages. Probit regression is similar except it assumes p(x) follows the cumulative standard normal distribution. The two approaches tend to give similar results, but logistic regressions may be somewhat more robust to outliers inasmuch as the logistic function has thicker tails than the probit.
The logistic model above implies that the probability of observing an event changes monotonically with the value of the explanatory variable x, increasing to a maximum of one if β is negative and decreasing to a minimum of zero otherwise. It seems reasonable to expect a monotonic relationship between the probability of spawning (spawning fraction) and length or age, but even the largest, oldest females may not be in spawning condition at all times. For this reason it is advantageous to incorporate a scale parameter that allows the maximum spawning fraction to be something less than one, in which case the appropriate model would take the form(3)
We allow for the possibility that the relationship between spawning fraction and age or length may vary across regions and gear types by allowing π, α, and β to vary categorically with each of the four combinations of gear and region (effectively gear–region–stage interaction terms). The relationship between spawning fraction and depth, if present, is assumed to be monotonic. This would be consistent with observations that there is an ontogenetic shift in the distribution of Red Snapper, and older, large fish tend to move to deeper water (Patterson 2007; Mitchell et al. 2014). If the cause of this shift is related to spawning behavior, then one might expect spawning frequency to increase with depth.
Field observations suggest that Red Snapper spawning is seasonal, as has also been demonstrated for Pouting Trisopterus luscus, and may be common among other species (Alonso-Fernández and Saborido-Rey Citation2011; Lowerre-Barbieri et al. Citation2011). A seasonal pattern would imply that the relationship between spawning fraction and time of year is dome-shaped rather than monotonic. A dome-shaped relationship could be mimicked by the use of categorical variables that represent discrete intervals of time, say 1 month, in which case our model would take the form:(4)
where s is a continuous variable representing the stage of life (here either age or length), d is a continuous variable representing depth, m is a categorical variable representing the month, g is a categorical variable representing gear type (longline or vertical line), r is a categorical variable representing region (east or west of the Mississippi River), πgrm is the scaling parameter, which may vary categorically by gear, region, and month (absent a gear or regional effect, πgrt = πt), αgrt is the linear intercept parameter, which may vary categorically by gear, region and month (absent a gear or regional effect, αgrt = αt), βgr is the slope parameter for age or length, which may vary categorically by gear and region (absent a gear and regional effect, βgr = β), and δ is the slope parameter for depth.
An important drawback of the model described by Equationequation (4)(4) is the large number of parameters associated with the time intervals (months), some of which will be poorly estimated if few samples are available for that month (as was the case for some months in our Red Snapper example). Alternatively, one can use a functional form to represent the dependence of spawning fraction on time of year. We chose to use the flexible gamma function:
(5) where t is the fraction of the year elapsed beginning on January 1, µ is the mode (time of peak spawning) and the expression κ + γs represents the dispersion coefficient (which is allowed to increase linearly with age or length to accommodate the possibility of age or size dependence in the duration of the spawning season). Note that this form of the gamma density has been divided by the value at the mode such that the maximum value is always 1.0.
The full model used in this paper is given by the product of Equationequation (4)(4) (without the categorical month effects) and Equationequation (5)
(5) ; i.e.,
(6)
This model allows the duration of the spawning season to vary with age or length, but assumes that it is the same across regions, gear types, and depth. For completeness, we note that all of the models posed in Equationequations (1)–(6) treat the explanatory variables s (length or age) and d (depth) as though they are measured without error. In the case of length and depth, the measurements were made by scientific observers and the error is negligible for all practical purposes. The age of the fish is subject to reader error, but Allman et al. Citation(2005) suggested that this error is relatively small and unbiased for Red Snapper.
Statistical estimation and comparisons of alternative models
Equation (6) does not fall into the class of general linear models owing to the incorporation of the scaling parameters π and the gamma function of t. Nevertheless, the parameters are easily estimated by numerical minimization of the likelihood expression given by Equationequation (1)(1) , where pi is understood to be the probability of observing a spawning marker predicted by Equationequation (6)
(6) given the values of the explanatory variables s, t, d, g, and r associated with sample i. In this study the numerical minimization was accomplished using Excel Solver and AD Model Builder.
Statistical comparisons among alternative models were made using Akaike's information criterion (AIC; Akaike Citation1973):where n is the total number of parameters estimated and L is the measure of goodness of fit (e.g., likelihood function) being maximized. The AIC attempts to identify the most parsimonious explanation of the data by balancing the relative improvement in model fit against the number of parameters required to achieve that fit. The “best” model is considered to be the one with the lowest AIC. A “rule of thumb” is that differences in AIC (ΔAIC) of less than 2 constitute weak evidence that one model is better than another, differences between 3 and 10 are regarded as moderate evidence, and differences greater than 10 are regarded as strong evidence (Burnham and Anderson Citation2002). A pseudo-R2 statistic was also computed as the fraction of the variance explained by the regression:
where
is the mean of the observations and
is the maximum likelihood estimate of the probability that a fish will have spawning markers.
Conversion to total number of spawns
The average spawning fraction during the course of a year for age or length s is obtained as(7)
In cases where p(t|s) is estimated to be essentially 0 at t = 1,(8) where the right hand side of Equationequation (8)
(8) is simply the inverse of the gamma density evaluated at the mode. In cases where spawning occurs throughout the year so that p(t|s) > 0 at t = 1, the integral in Equationequation (8)
(8) can be evaluated numerically.
The prevalence of hydrated oocytes and postovulatory follicles (histological spawning markers) can be detected over a period of time and may be influenced by temperature and the diel spawning pattern. Following Priede and Watson Citation(1993), the average spawning fraction can be converted to a daily probability of spawning P:(9) where TM is the duration in hours that spawning markers can be detected. The expected number of spawns during the course of a year is therefore obtained from multiplying Equationequation (9)
(9) by the number of days as follows:
(10)
Final oocyte maturation and hydration have been estimated to begin during morning hours (0830 hours) and remain evident throughout the day (to about 1800 hours, or 10 h duration: Jackson et al. Citation2006). Field observations and spontaneous spawning in tanks have indicated that ovulation occurs in mid to late afternoon, peaking at about 1600 hours with spawning commonly occurring before sunset around 2000 hours in summer months (Papanikos et al. Citation2003, Citation2008; Jackson et al. Citation2006). While postovulatory follicles have been noted to appear as early as 1100 hours, new postovulatory follicles are not thought to be common until after 1600 hours as ovulation peaks and postovulatory follicle duration is not thought to exceed about 24 h in the Gulf of Mexico during summer (Nieland et al. Citation2002; Jackson et al. Citation2006). Together, these findings indicate that TM is about 34 h.
RESULTS
Survey Summary
The 2011 congressional supplemental survey was conducted over 846 sea-days (April to October) and included 1,171 longline (four vessels) and 1,939 vertical-line (two vessels) stations (Campbell et al. Citation2012). Histological analyses were conducted on 1,002 females, of which 992 were aged. Based on comparison of the 50 paired quality control histology sections (freshly fixed versus initially frozen) it was visually apparent that freezing reduced the ability to resolve oocyte details such as germinal vesicles, follicular membranes, lipid vacuoles, and yolk globules. However, spawning markers (hydrated oocytes, postovulatory follicles) could be distinguished in the frozen samples. Comparison of the leading oocyte stage (primary growth, cortical alveolar, vitellogenesis, and oocyte maturation including hydration) yielded 90% agreement and presence–absence of postovulatory follicles yielded 82% agreement.
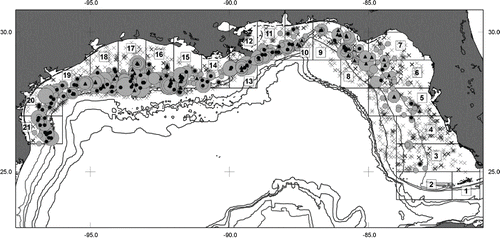
Females with spawning markers (n = 391) were distributed throughout the U.S. Gulf survey area, predominantly along the outer shelf at depths between 15 and 158 m (). The distribution of females with or without spawning markers overlapped spatially; females without markers were caught at an average depth of 60 m and females with markers were caught at an average depth of 62 m. These depths center on a discrete depth zone (50–100 m) that encompasses the high-relief paleoreefs found on the outer shelf of the southeastern United States (Koenig et al. Citation2000).
FIGURE 1. Distribution of sampling stations with zero (× symbol) and positive catches of Red Snapper during the 2011 supplemental survey (grey circle; progressively increasing size of circle indicates number landed per set: 1–4, 5–10, 10–13, 14–17, 18+). Solid dark symbols indicate capture locations of females with spawning markers (round symbols: bottom longline gear, triangles: bandit gear). The 50-, 100-, 200-, 1,000-, 1,300-, and 2,000-m isobaths are indicated. Statistical subareas are denoted from 1 to 21 within U.S. Gulf of Mexico waters. Numbers along horizontal and vertical borders of figure are longitude and latitude, respectively. Note that symbols are for graphical illustration and do not reflect scale of area fished per station.
Modeling the Spawning Fraction
Stepwise model building exercises were developed beginning with the null model (p = π), which assumed all fish have the same probability of being found in spawning condition. In the first step, the logistic age or length effects were added to the null model. The corresponding AIC values were decreased by more than 40 and about 6% of the residual variance was explained (). Accordingly, the statistical evidence for both the age and length effects was strong and these terms were retained in all subsequent models.
TABLE 4. Length-based and age-based models for Red Snapper spawning fractions developed during the stepwise model building procedure (based on the binomial regression of Equationequation 6(6) ). The shaded column highlights the final models. The use of t′ indicates the model allows the seasonal effect to vary with age or length. Subscripts refer to gear or region (subscripts in parentheses identify the gear–region combination when gear and region effects are estimated simultaneously); l = length, a = age, t = time elapsed, d = depth, g = gear type, r = region (see text for explanation), NA = not applicable.
In the second step, the spawning fraction was allowed to vary with time of year to accommodate the biological dynamics of the spawning season. Initially, the duration of the spawning season was assumed to be independent of age or length (i.e., γ = 0). In that case the AIC values were further reduced by over 200 and about 30% of the residual variance was explained. Accordingly, the statistical evidence for a seasonal effect on the spawning fraction was strong and these terms were retained in all subsequent models.
In the third step, the potential variation in the duration of the spawning season with age or length was investigated by estimating the parameter γ. The resulting AIC values suggested little evidence for a variation in season duration with age (ΔAIC < 3) and only moderate evidence for a variation in season duration with length (ΔAIC < 10). Moreover, the percentage of variation explained by the model was negligibly improved.
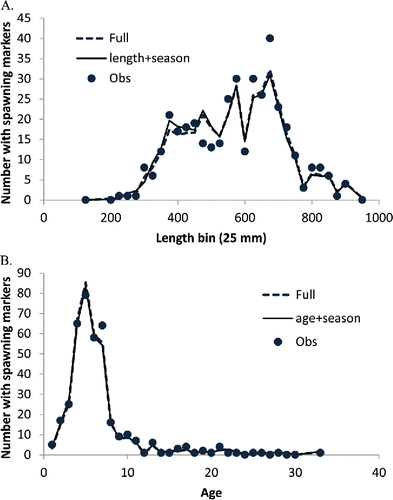
Finally, the effects of incorporating the covariates depth, gear, and region were examined by adding the corresponding parameters to the seasonal model derived from step 2 (one covariate at a time). The model fits suggested little evidence for gear effects (ΔAIC < 3), but strong evidence for either a regional or depth effect (ΔAIC > 10). None of these models, however, contributed to explaining a substantial fraction of the variance. Even the full model incorporating all parameters did not substantially improve the explanatory power of the regression (). For the length-based analysis, the r2 for the full model was 33% compared with 30% for the length + season model. For the age-based analysis, the r2 for the full model was 32% compared with 29% for the age + season model. Accordingly, the final model included only age or length and time of year.
FIGURE 2. Observed number of Red Snapper with spawning markers compared with the corresponding predictions of the final model (length + season, or age + season) and full model (all covariates included). Panels show the results for (A) length and (B) age.
The spawning season was estimated in the final model to occur primarily from early April through late October, with a peak in July (). The spawning fraction was estimated to increase rapidly with length or age (), starting at very low values for fish under 300 mm (age 1) and beginning to level off around 500 mm (age 6–7). There were no obvious trends in the residuals (difference between model fit and observed data) to suggest that the model was not fitting the oldest (or youngest) age-classes (). The asymptotic estimates of the variance of the parameters and associated correlation matrix (obtained from the Hessian via the application in AD Model Builder) are shown in .
FIGURE 3. Average observed spawning fraction of Red Snapper (primary axis) compared with model predictions of relative proportion of females with spawning markers (secondary axis) by time of year for age-based and length-based models.
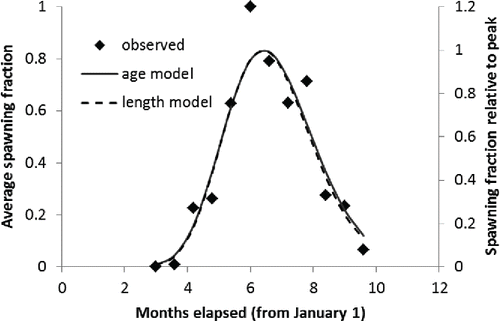
FIGURE 4. Predicted relationships between spawning fraction of Red Snapper (proportion of females with spawning markers) and (A) length (mm) or (B) age (years).
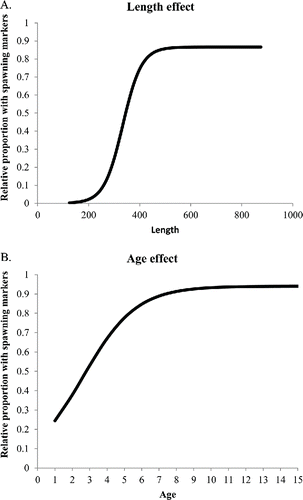
TABLE 5. Correlation matrix for final models to determine Red Snapper spawning fractions.
Conversion to Total Number of Spawns
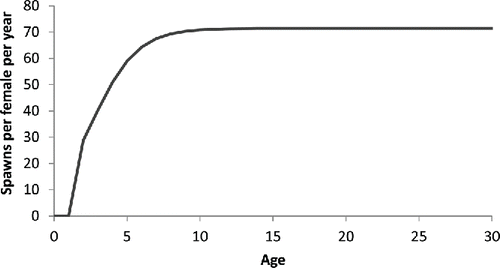
The expected number of spawns per year was computed according to Equationequation (10)(10) . The duration of the spawning season did not vary with age or size in the base models (the terms not being significantly different from zero); therefore, the integral of p(t|s) was a constant equal to 0.295 for all ages and equal to 0.288 for all lengths. The value of p(s) for the base model followed the logistic functions specified by the estimates for the parameters π, α, and β in . The value of TM, the time during which the prevalence of hydrated oocytes and postovulatory follicles (histological spawning markers) in Red Snapper can be detected, was set to 34 h (see discussion below). The resulting ogive indicates that 2-year-old Red Snapper spawn an average of 29 times per year, while the largest, oldest Red Snapper spawn an average of about 71 times per year ().
DISCUSSION
Our estimates of spawning fraction and related quantities such as spawning frequency are intended to reflect the population of sampled Red Snapper females regardless of whether they are active or not. Others have expressed these quantities in terms of active females only (Priede and Watson Citation1993; Stratoudakis et al. Citation2006; Kurita Citation2012). The two sets of metrics will lead to equivalent perceptions of the target population as long as the units are consistent. For example, the total number of spawning events in the population can be computed equivalently as either the product of spawning frequency per female and the number of females or the spawning frequency per active female and the number of active females. More importantly, neither set of metrics necessarily represented the average values observed in the population at large owing to the size selection of the gear and spatial variations in the availability of different size- and age-classes of the target species. It is possible in principle to adjust our estimates of spawning fraction to account for variations in selection and availability, but these are not as well understood for Gulf of Mexico Red Snapper as they are for a few other species (see Lowerre-Barbieri et al. Citation2009). Alternatively, population-level estimates of spawning frequency may be obtained by multiplying the estimated spawning frequency at size or age by independent estimates of the size or age structure of the female population (as might be obtained by a stock assessment). In this sense the procedure is similar to multiplying an age–length key by size composition data to determine the age structure of a population.
The estimates of spawning frequency (number of spawns per year) for Red Snapper from this study increase with age from 29 events per year for 2-year-old fish to about 71 per year for fish age 10 and older, corresponding to interspawning intervals ranging between 6.5 and 2.5 d. Not surprisingly, this range brackets previous estimates of interspawning intervals that were averaged across multiple age-classes (3.97–5.95 d: Collins et al. Citation1996; 3.4–4.2 d: Woods Citation2003; 4.3 d: Brown-Peterson et al. Citation2009) and is consistent with the frequency of spontaneous spawning observed under experimental aquaculture conditions (Papanikos et al. Citation2008). There was no evidence that spawning frequency decreased for the oldest fish sampled, but even our oldest fish (34 years) was considerably younger than the maximum age of Red Snapper (over 50 years). While it is possible that senescence may occur at some point, it is unlikely to be an important factor to consider in stock assessments as such old fish are very rare in the population.
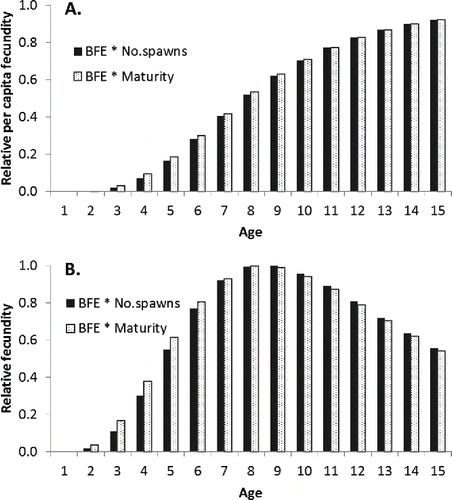
More importantly, the fact that younger mature Red Snapper do not spawn as often as older mature fish implies that they contribute less to the reproductive potential of the population than might be inferred from their maturity alone (; Porch Citation2004). For this reason, the per-capita fecundity of each age- or size-class of Red Snapper should be modeled as the product of spawns per female per year and batch fecundity rather than as the product of maturity and batch fecundity. The effect of replacing maturity with spawns per female on the perception of the per-capita fecundity of young fish is somewhat mitigated by the rather low batch fecundity of younger Red Snapper (). This is true even when one accounts for the fact that young Red Snapper tend to be much more abundant than larger, older fish. If, for example, the per-capita fecundity at age is multiplied by the equilibrium age structure associated with the maximum sustainable yield (SEDAR Citation2013), one finds that fish between 2 and 4 years old account for only 3% of the total egg production based on spawning frequency compared with 5% based on maturity ().
FIGURE 6. Comparison of maturity of Red Snapper and relative spawning frequency at age (years). Female maturity was based upon the presense of vitellogenic or maturing oocytes during the peak reproductive months of June, July, and August. The label on the vertical axis, relative fraction, refers to the value for maturity at age or spawning frequency at age divided by their respective maximum values (to put the two measures on the same scale).
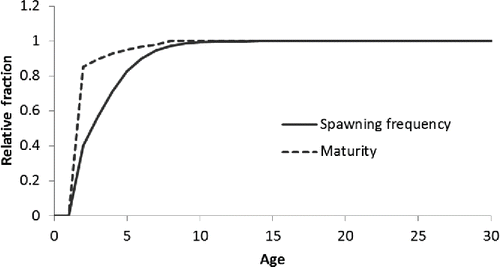
FIGURE 7. Trends in (A) relative per-capita fecundity at age (years) and (B) relative population fecundity at age (combined fecundity of all members in the age-class) in Red Snapper when the calculated number of spawns per female per year is used (solid bars) compared with the trends when spawning frequency is assumed to be proportional to maturity (open bars). The label “BFE * No. Spawns” refers to the calculation where age-specific estimates of batch fecundity are multiplied by age-specific estimates of the number of spawns per year. Similarly, the label “BFE * Maturity” refers to the calculation where age-specific estimates of batch fecundity are multiplied by age-specific estimates of maturity. The relative (population) fecundity is obtained by multiplying the per-capita fecundity at age by the relative abundance of each age-class at the equilibrium level associated with fishing at the maximum sustainable yield.
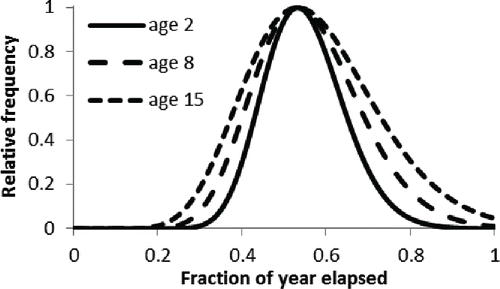
The seasonality of spawning was in general agreement with previous studies. Spawning begins in late April or early May, reaches a peak in midsummer, and declines by fall. It is possible that some spawning may occur outside the time period sampled during this study (April–October) when conditions are favorable; however, this has not been detected during several prior studies spanning decades (Collins et al. Citation1996; Woods Citation2003). The data also hint that the spawning season of 2- and 3-year-old Red Snapper is shorter than that of older Red Snapper; no 2- and 3-year-old fish were found in spawning condition before late May and very few after August. The season durations predicted by the models where the parameter γ was estimated are consistent with this observation (), but the statistical evidence was not strong and little additional variance was explained. It is possible, therefore, that this apparent trend is spurious. Nevertheless, the matter is of sufficient importance to merit further sampling. As Wright and Trippel Citation(2009) pointed out, an increase in the duration of the spawning season with age has implications beyond merely increasing the total number of annual spawning opportunities. By expanding the duration of the spawning season, older fish may increase their chance of spawning during favorable conditions, thereby reducing the variance and increasing the mean of progeny survival.
FIGURE 8. Estimated variation in the duration of the Red Snapper spawning season with age (i.e., ages 2, 8, and 15 years) corresponding to the final model, p(a,t′), in .
Spawning frequency was also estimated to increase with depth and to vary east and west of the Mississippi River. However, while the AIC indicated strong support for including either depth or region in the model, the support for including both simultaneously was weak. This implies that the two effects may be somehow correlated. The average depth sampled in the west (63 m) was slightly deeper than in the east (57 m), so it is possible that perceived regional differences may be partly attributable to differences in average depth. Nevertheless, it is important to point out that depth and region together contributed to explain less than 3% of the total variance and it remains possible that the estimated trends are spurious. This seems particularly likely in the case of the regional model where spawning frequency in the east was estimated to drop to essentially zero for fish below 400 mm largely as a consequence of having very few samples of fish below that size.
The existence of regional differences in the productivity of Red Snapper could have important implications for the management of Red Snapper. The highest spawning frequencies in our samples were exhibited by females caught in areas of the western Gulf associated with outer shelf banks and reef tracts (the western Louisiana shelf and central to the southern Texas shelf) which also happen to be the areas with the greatest concentration of Red Snapper eggs and larvae (Lyczkowski-Shultz and Hanisko Citation2007). The results of our study indicate that the regional effects were small, implying that the apparently higher spawning frequencies observed in these areas is mostly a reflection of the greater average size of fish and the time of year when the samples are taken, rather than any intrinsic differences in individual fish behavior. It is possible of course that spawning frequency does vary over different spatial and temporal scales owing to differences in ambient conditions. Nevertheless, it is clear that any comparisons must also account for possible differences in local population age structure and the time of year that the collections are made. A population that appears to enjoy a higher per-capita spawning frequency may simply have a higher proportion of older, larger fish. Similarly, the differences in the apparent spawning frequency of Red Snapper caught on longlines and vertical lines were attributable primarily to the differences in the size and age of fish caught by those gears.
The reproductive tissues used in this study were initially frozen, as opposed to the more common practice of preserving them first in 10% buffered formalin (but see Young et al. Citation2003). This occurred because the survey utilized commercial vessels working over extended days at sea with contracted samplers and, early in the study design, there were concerns about the routine use of formalin under such conditions. We tested the ability to discern spawning markers from frozen samples by comparing the results from blind reads of paired subsamples (one subsample initially frozen, one subsample initially fixed as fresh tissue) from 50 females. The results indicated 90% agreement for the leading oocyte stage and 82% agreement for presence–absence of postovulatory follicles, suggesting the ability to detect spawning markers may be slightly lower for frozen samples than for those fixed initially in formalin. Young et al. Citation(2003) encountered similar sampling circumstances, but reported no difference between samples that were frozen and those that were fixed in formalin in terms of the classification of oocyte stage, presence or absence of postovulatory follicles, or degree of atresia. In any case, we have no reason to expect that the degree of underestimation we observed would vary in time and space. Therefore, while it is possible that spawning frequency could be somewhat higher than estimated here, the statistical inferences and estimated relative trends in spawning frequency would not be affected.
Previous studies have demonstrated a clear relationship between the batch fecundity of Red Snapper and size or age (Collins et al. Citation1996; Woods Citation2003; Porch et al. Citation2007; Kulaw Citation2012). The present study has further demonstrated that spawning frequency also increases with size or age and is consistent with the idea that it is important to conserve old ages in the population (Hixon et al. Citation2014). What remains is to determine how egg quality may vary with age and other factors. For example, egg quality and fertilization success in Red Snapper are known to be related to adult nutrition (Papanikos et al. Citation2003). Moreover, the abundance of Red Snapper has been increasing rapidly and is expected to continue increasing over the next few years. This raises the possibility that surplus energy for growth and reproduction may be reduced (Goodwin et al. Citation2006; McBride et al. Citation2015). Thus, there is a need to continue monitoring Red Snapper reproductive ecology while remaining mindful of important temporal and spatial scales.
ACKNOWLEDGMENTS
Thanks are due to Melissa Cook, the sampling team at the National Marine Fisheries Service (NMFS) Pascagoula Laboratory, and the charter vessel crews. Beverly Barnett and Hanna Lang organized and proofed the data from the biological collections. Robert Allman and the NMFS Panama City aging team provided the age data. Nikolai Klibansky, Matthew Campbell, and two anonymous reviewers gave many helpful comments that improved the manuscript.
REFERENCES
- Akaike, M. 1973. Information theory and an extension of the maximum likelihood principle. Pages 267–281 in B. Petrov and F. Csaki, editors. Proceedings of the 2nd international symposium on information theory. Akademia Kiado, Budapest.
- Allman, R., B. Barnett, H. Trowbridge, L. Goetz, and N. Evou. 2012. Red Snapper (Lutjanus campechanus) otolith ageing summary for collection years 2009–2011. Southeast Data Assessment and Review, SEDAR31-DW05, North Charleston, South Carolina.
- Allman, R. J., and G. R. Fitzhugh. 2007. Temporal age progressions and relative year-class strength of Gulf of Mexico Red Snapper. Pages 311–328 in W. F. Patterson III, J. H. Cowan Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red Snapper ecology and fisheries in the U. S. Gulf of Mexico. American Fisheries Society, Symposium 60, Bethesda, Maryland.
- Allman, R. J., G. R. Fitzhugh, K. J. Starzinger, and R. A. Farsky. 2005. Precision of age estimation in Red Snapper (Lutjanus campechanus). Fisheries Research 73:123–133.
- Alonso-Fernández, A., and F. Saborido-Rey. 2011. Maternal influence on the variation of the reproductive cycle of Trisopterus luscus (Gadidae). Ciencias Marinas 37:619–632.
- Brown-Peterson, N., K. M. Burns, and R. M. Overstreet. 2009. Regional differences in Florida Red Snapper reproduction. Proceedings of the Gulf and Caribbean Fisheries Institute 61:149–155.
- Burnham, K. P., and D. R. Anderson 2002. Model selection and multimodal inference: a practical information-theoretic approach, 2nd edition. Springer-Verlag, New York.
- Campbell, M. D., A. Pollack, T. Henwood, J. Provaznik, and M. Cook. 2012. Summary report of the Red Snapper (Lutjanus campechanus) catch during the 2011 congressional supplemental sampling program. Southeast Data Assessment and Review, SEDAR31-DW17, North Charleston, South Carolina.
- Collins, L. A., A. G. Johnson, and C. P. Keim. 1996. Spawning and annual fecundity of the Red Snapper (Lutjanus campechanus) from the northeastern Gulf of Mexico. Pages 174–188 in F. Arreguin-Sanchez, J. L. Munro, M. C. Balgos, and D. Pauly, editors. Biology, fisheries and culture of tropical groupers and snappers. International Center of Living Aquatic Resources Management, Conference Proceedings 48, Manila.
- Cooper, W. T., L. R. Barbieri, M. D. Murphy, and S. K. Lowerre-Barbieri. 2013. Assessing reproductive potential in species with indeterminate fecundity: effects of age truncation and size-dependent reproductive timing. Fisheries Research 138:31–41.
- Fitzhugh, G. R., K. W. Shertzer, G. T. Kellison, and D. M. Wyanski. 2011. Review of size- and age-dependence in batch spawning: implications for stock assessment of fish species exhibiting indeterminate fecundity. U.S. National Marine Fisheries Service Fishery Bulletin 110:413–425.
- Goodwin, N. B., A. Grant, A. L. Perry, N. K. Dulvey, and J. D. Reynolds. 2006. Life history correlates of density dependent recruitment in marine fishes. Canadian Journal of Fisheries and Aquatic Sciences 63:494–509.
- Goodyear, C. P. 1995. Red Snapper in U.S. waters of the Gulf of Mexico. Southeast Fisheries Center, Contribution MIA-95/96-05, Miami.
- He, X., J. C. Field, S. G. Beyer, and S. M. Sogard. 2015. Effects of size-dependent relative fecundity specifications in fishery stock assessments. Fisheries Research 165:54–62.
- Hixon, M. A., D. W. Johnson, and S. M. Sogard. 2014. BOFFFFs: on the importance of conserving old-growth age structure in fishery populations. ICES Journal of Marine Science 71:2171–2185.
- Hunter, J. R., and B. J. Macewicz. 1985. Measurement of spawning frequency in multiple spawning fishes. NOAA Technical Report NMFS 36:79–94.
- Jackson, M. W., D. L. Nieland, and J. H. Cowan 2006. Diel spawning periodicity of Red Snapper Lutjanus campechanus in the northern Gulf of Mexico. Journal of Fish Biology 68:695–706.
- Klibansky, N., and F. S. Scharf. 2013. Size-dependent and temporal variability in batch number and fecundity of Red Porgy, a protogynous, indeterminate spawner, in the U.S. South Atlantic. Marine and Coastal Fisheries: Dynamics, Management and Ecosystem Science [online serial] 5:39–52.
- Koenig, C. C., F. C. Coleman, C. B. Grimes, G. R. Fitzhugh, K. M. Scanlon, C. T. Gledhill, and M. Grace. 2000. Protection of fish spawning habitat for the conservation of warm-temperate reef-fish fisheries of shelf-edge reefs of Florida. Bulletin of Marine Science 66:593–616.
- Kulaw, D. 2012. Habitat- and region-specific reproductive biology of female Red Snapper (Lutjanus campechanus) in the Gulf of Mexico. Master's thesis. Louisiana State University, Baton Rouge.
- Kurita, Y. 2012. Revised concepts for estimation of spawning fraction in multiple batch spawning fish considering temperature-dependent duration of spawning markers and spawning time frequency distribution. Fisheries Research 117–118:121–129.
- Kurita, Y., Y. Fujinami, and M. Amano. 2010. The effect of temperature on the duration of spawning markers−migratory-nucleus and hydrated oocytes and postovulatory follicles−in the multiple-batch spawner Japanese flounder (Paralichthys olivaceus). U.S. National Marine Fisheries Service Fishery Bulletin 109:79–89.
- LaPlante, L. H., and E. T. Schultz. 2007. Annual fecundity of tautog in Long Island Sound: size effects and long-term changes in a harvested population. Transactions of the American Fisheries Society 136:1520–1533.
- Lowerre-Barbieri, S. K., K. Ganias, F. Saborido-Rey, H. Murua, and J. R. Hunter. 2011. Reproductive timing in marine fishes: variability, temporal scales, and methods. Marine and Coastal Fisheries: Dynamics, Management and Ecosystem Science [online serial] 3:71–91.
- Lowerre-Barbieri, S. K., N. Henderson, J. Llopiz, S. Walters, J. Bickford, and R. Muller. 2009. Defining a spawning population (Spotted Seatrout, Cynoscion nebulosus) over temporal, spatial, and demographic scales. Marine Ecology Progress Series 394:231–245.
- Lyczkowski-Shultz, J., and D. S. Hanisko. 2007. A time series of observations on Red Snapper larvae from SEAMAP surveys, 1982–2003: seasonal occurrence, distribution, abundance and size. Pages 3–23 in W. F. Patterson III, J. H. Cowan Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red Snapper ecology and fisheries in the U. S. Gulf of Mexico. American Fisheries Society, Symposium 60, Bethesda, Maryland.
- McBride, R. S., S. Somarakis, G. R. Fitzhugh, A. Albert, N. A. Yaragina, M. J. Wuenschel, A. Alonso-Fernández, and G. Basilone. 2015. Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish and Fisheries 16:23–57.
- Mehault, S., R. Dominguez-Petit, S. Cervino, and F. Saborido-Rey. 2010. Variability in total egg production and implications for management of the southern stock of European Hake. Fisheries Research 104:111–122.
- Mitchell, W. A., G. T. Kellison, N. M. Bacheler, J. C. Potts, C. M. Schobernd, and L. F. Hale. 2014. Depth-related distribution of post juvenile Red Snapper in southeastern U.S. Atlantic Ocean waters: ontogenetic patterns and implications for management. Marine and Coastal Fisheries: Dynamics, Management and Ecosystem Science [online serial] 6:143–155.
- Murua, M., G. Kraus, F. Saborido-Rey, P. R. Witthames, A. Thorsen, and S. Junquera. 2003. Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. Journal of Northwest Atlantic Fisheries Science 33:33–34.
- Nieland, D. L., G. Thomas, and C. A. Wilson. 2002. Age, growth, and reproduction of Spotted Seatrout in Barataria Bay, Louisiana. Transactions of the American Fisheries Society 131:245–259.
- Papanikos, N., R. P. Phelps, D. A. Davis, A. Ferry, and D. Maus. 2008. Spontaneous spawning of captive Red Snapper, Lutjanus campechanus, and dietary lipid effect on reproductive performance. Journal of the World Aquaculture Society 39:324–338.
- Papanikos, N., R. P. Phelps, K. Williams, A. Ferry, and D. Maus. 2003. Egg and larval quality of natural and induced spawns of Red Snapper, Lutjanus campechanus. Fish Physiology and Biochemistry 28:487–488.
- Patterson, W. F. III. 2007. A review of movement in Gulf of Mexico Red Snapper: implications for population structure. Pages 245–261 in W. F. Patterson III, J. H. Cowan Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red Snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society, Symposium 60, Bethesda, Maryland.
- Porch, C. E. 2004. Batch-fecundity and maturity estimates for the 2004 assessment of Red Snapper in the Gulf of Mexico, revised. Southeast Data Assessment and Review, SEDAR7-AW-5, North Charleston, South Carolina.
- Porch, C. E. 2007. An assessment of the Red Snapper fishery in the U.S. Gulf of Mexico using a spatially-explicit age-structured model. Pages 355–384 in W. F. Patterson III, J. H. Cowan Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red Snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society, Symposium 60, Bethesda, Maryland.
- Porch, C. E., G. R. Fitzhugh, M. S. Duncan, L. A. Collins, and M. W. Jackson. 2007. Modeling the dependence of batch fecundity on size and age for use in stock assessments of Red Snapper in U.S. Gulf of Mexico waters. Pages 229–244 in W. F. Patterson III, J. H. Cowan Jr., G. R. Fitzhugh, and D. L. Nieland, editors. Red Snapper ecology and fisheries in the U.S. Gulf of Mexico. American Fisheries Society, Symposium 60, Bethesda, Maryland.
- Priede, I. G., and J. J. Watson. 1993. An evaluation of the daily egg production method for estimating biomass of Atlantic Mackerel (Scomber scombrus). Bulletin of Marine Science 53:891–911.
- SEDAR (Southeast Data Assessment and Review). 2013. Gulf of Mexico Red Snapper stock assessment report, SEDAR 31. SEDAR, North Charleston, South Carolina.
- Shipp, R. L., and S. A. Bortone. 2009. A perspective of the importance of artificial habitat on the management of Red Snapper in the Gulf of Mexico. Reviews in Fisheries Science 17:41–47.
- Stratoudakis, Y., M. Bernal, K. Ganias, and A. Uriarte. 2006. The daily egg production method: recent advances, current applications and future challenges. Fish and Fisheries 7:35–57.
- Wilson, C. A., and D. L. Nieland. 2001. Age and growth of Red Snapper, Lutjanus campechanus, from the northern Gulf of Mexico off Louisiana. U.S. National Marine Fisheries Service Fishery Bulletin 99:653–664.
- Winemiller, K. O., and K. A. Rose. 1992. Patterns of life-history diversification in North American fishes: implications for population regulation. Canadian Journal of Fisheries and Aquatic Sciences 49:2196–2218.
- Woods, M. K. 2003. Demographic differences in reproductive biology of female Red Snapper (Lutjanus campechanus) in the northern Gulf of Mexico. Master's thesis. University of South Alabama, Mobile.
- Wright, P. J., and E. A. Trippel. 2009. Fishery-induced demographic changes in the timing of spawning: consequences for reproductive success. Fish and Fisheries 10:283–284.
- Young, J., A. Drake, M. Brickhill, J. Farley, and T. Carter. 2003. Reproductive dynamics of Broadbill Swordfish, Xiphias gladius, in the domestic longline fishery off eastern Australia. Marine and Freshwater Research 54:315–332.