Abstract
Diet analysis is critical in understanding the flow of energy within marine food webs and is necessary for trophic ecosystem modeling and subsequent ecosystem-based management recommendations. This study represents the first comprehensive diet description for the Barndoor Skate Dipturus laevis, the largest rajid species found on the continental shelf in the northwestern Atlantic Ocean. Stomach contents were extracted from 273 individual skate caught as bycatch in the commercial scallop fishery on Georges Bank and a total of 31 prey species were identified. The Barndoor Skate feeds primarily upon sand shrimp Crangon septemspinosa, the rock crab Cancer irroratus, the Acadian hermit crab Pagurus acadianus, and teleost fish. Length-specific analysis revealed four significant feeding groups (ANOVA: P < 0.01). Skate < 35 cm TL were specialized feeders foraging solely on caridean shrimp, and as size increased (35–75 cm TL), they began to feed upon rock crab and then the Acadian hermit crab. At lengths ranging from 85 to 105 cm TL, no caridean shrimp were found in the skate's diet and the prevalence of crustaceans decreased. Large skate (>105 cm TL) began to prey heavily upon teleost fish, yet also continued to consume larger crustaceans. Significant sex-specific differences in food habits were also observed in the biggest skate (>105 cm TL): males fed primarily on teleost fish (∼80%); however, females maintained a diet of approximately equal amounts of fish and crustaceans. These sex-specific feeding patterns and differential food niche utilization may be mitigated by sexually dimorphic dentition.
Received November 18, 2014; accepted June 11, 2015
The Barndoor Skate Dipturus laevis is the largest member of the family Rajidae found on the continental shelf in the northwestern Atlantic Ocean, reaching a maximum length of 152 cm and a weight of 20 kg (Bigelow and Schroeder Citation1953). The species has been reported to range from Cape Hatteras, North Carolina, to the Grand Banks of Newfoundland, Gulf of St. Lawrence, and Nova Scotia (Leim and Scott Citation1966; McEachran and Musick Citation1975). It ranges from shallow coastal waters to depths greater than 450 m and tolerates water temperatures ranging from 1.2°C to 20°C (Bigelow and Schroeder Citation1953; McEachran and Musick Citation1975; Kulka et al. Citation2002). While the Barndoor Skate has no commercial value, it is often taken as a bycatch species on Georges Bank and overfishing has been reported to be threatening the survival of the species as a whole (Casey and Myers Citation1998). As an elasmobranch, the Barndoor Skate was believed to be particularly vulnerable to fishing pressure due to its large size and presumed late maturation. Over the last 10 years, however, a dramatic recovery in the population has been observed (Gedamke Citation2006). Although a reduction in fishing pressure is clearly a critical component in the recovery of the species, it may only be a single factor in a more complex picture. To accurately evaluate the population dynamics of the Barndoor Skate, more information about its life history and trophic interactions (i.e., predator–prey relationships) must be incorporated into future analyses.
Skate represent the most diverse group of chondrichthyan fishes with nearly 250 described species (Ebert and Compagno 2007), yet skate are poorly studied compared with other marine elasmobranchs (Orlov Citation1998). Skate are believed to play important roles in structuring demersal marine communities due to their wide distribution and high abundance (Ebert and Bizzarro Citation2007), yet studies pertaining to the diet composition and trophic roles of skate are few (Garrison and Link 2000; Bulman et al. 2001; Davenport and Bax 2002; Morato et al. Citation2003; Braccini and Perez Citation2005). The Barndoor Skate is no exception. In 2007, Ebert and Bizzarro Citation(2007) reported the standardized trophic level for 60 different species of skate, including the Barndoor Skate, and their trophic level calculations were based on stomach contents from three specimens. There is clearly a paucity of information pertaining to the food habits and trophic role of the Barndoor Skate.
Over the last 40 years, Georges Bank and the Gulf of Maine have undergone major changes (Fogarty and Murawski Citation1998; Collie and Delong 1999). Commercially important species such as Atlantic Cod Gadus morhua, Haddock Melanogrammus aeglefinus, and hake Urophycis spp. were replaced with elasmobranchs of lower market value including dogfishes and skate; meanwhile Atlantic Mackerel Scomber scombrus and Atlantic Herring Clupea harengus populations grew to historic levels (Collie and Delong 1999). While the decline in the groundfish community was largely attributed to overfishing, predation can be a large contributor to prerecruit mortality on Georges Bank (Sissenwine et al. 1984), further emphasizing the need to expand our knowledge of trophic interactions on Georges Bank.
The overall life history of the Barndoor Skate has only recently been investigated (Gedamke et al. Citation2005), yet very little is known about their food habits or their ecological role in the Gulf of Maine. Understanding the feeding habits of the Barndoor Skate can bring valuable insight into predator–prey relationships and can contribute to future studies of trophic interactions (Caddy and Sharp Citation1986), with the hope that future studies will not be weakened by limited data as in the Ebert and Bizzarro Citation(2007) study. The present study will provide vital information concerning the trophic role of the Barndoor Skate and will assist in the designation of essential fish habitat, which is defined legally as “those waters and substrate necessary to fish spawning, breeding, feeding, or growth to maturity (16 U.S.C. 1802[10]).” Our work addresses a research need identified in both the 2001 stock assessment report (NEFMC Citation2001) and the essential fish habitat source documents (NOAA Citation2003) to “investigate trophic interactions between skate species in the complex, and between skates and other groundfish.”
This study represents the first comprehensive analysis of the food habits of the Barndoor Skate in the Gulf of Maine. The goals of this study are threefold: (1) quantify the diet composition of the barndoor skate, (2) evaluate the possible ontogenetic shifts in prey items, and (3) explore the possibility that sexually dimorphic mature dentition influences prey selection.
METHODS
Field collections.
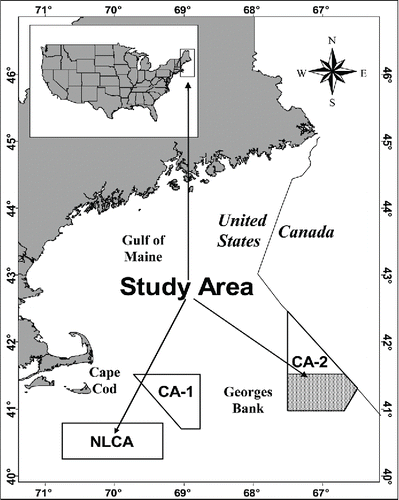
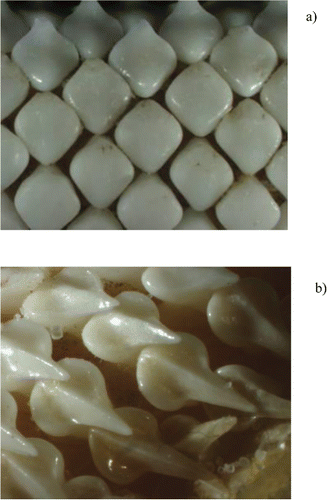
All specimens included in this study were collected onboard commercial scallop vessels on Georges Bank. A majority of the specimens were collected in the southern portion (south of 41°30′N) of closed area II while a limited number were collected from the Nantucket Lightship closed area (). Both of these areas were closed to the use of mobile fishing gear in December 1994 in an effort to rebuild groundfish stocks. In June 1999, access to the closed areas began on a limited basis as the development of a rotational management strategy was being explored in the commercial scallop fishery. Data were collected on eight trips between June and November in 1999, 2000, and 2003. Vessels fished with two 4.6-m New Bedford style dredges for sea scallops Placopecten magellanicus (Posgay Citation1957) constructed with a 25.4-cm twine top and either 8.9- or 10.2-cm ring bags. Gear was towed in 55–73 m of water at an average speed of 9.2 km/h. This gear captured a broad range of skate sizes ranging from 20 to 133.5 cm TL. Total length, sex, and maturity stage (mature versus immature) were recorded for all Barndoor Skate specimens upon capture. Sexual maturity was determined by the development of the testes or the presence of eggs in the ovaries along with other criteria (Gedamke et al. Citation2005). Furthermore, since tooth morphology in the males develops from molariform (i.e., plate-like) to cuspidate dentition (i.e., pointed teeth) at maturity, the presence or development of this secondary sexual character was also noted ().
Laboratory processing.
Entire skate stomachs were preserved in a solution of 10% phosphate-buffered formalin and then transferred to 70% ethanol prior to sorting. Stomachs were rinsed to remove all contents and then each prey item was identified to the lowest taxonomic level possible. To assist in the identification of teleost fishes, the museum collection at the Virginia Institute of Marine Science was used for comparative morphology and verification of initial identification. In addition, many of the invertebrates and fish species caught as bycatch were preserved to act as a reference library for the identification of stomach contents. The number of individual prey items was also recorded. In cases where prey were unrecognizable by gross morphology, remaining body parts (i.e., eyes, vertebrae, shell fragments) were used to make individual prey counts (Chipps and Garvey Citation2007). Following sorting, samples were weighed to obtain wet weights, placed in a drying oven for at least 24 h, and then reweighed to obtain dry weights. Plots and regressions of wet weights versus dry weights were evaluated to determine any differential patterns in the use of one metric versus the other.
Diet analysis.
Percent by weight (%W) was used to indicate which prey items were energetically important to the Barndoor Skate and was calculated as the weight of a given prey item divided by the weight of all prey items. Percent frequency of occurrence (%O) was used to indicate which prey items were routinely utilized by the Barndoor Skate and was calculated as the number of stomachs that contained a particular prey item divided by the total number of stomachs (Chipps and Garvey Citation2007; Graham et al. 2007).
A total of 31 prey items were recorded () and prey items were grouped into three logical ecological groupings: “crabs,” “shrimp,” and “fish.” These three prey groups accounted for 99.77% of the prey items by weight and were dominated by five key species: rock crab Cancer irrotatus, Acadian hermit crab Pagurus acadianus, sand shrimp Crangon septemspinosa, Ocean Pout Macrozoarces americanus, and Atlantic Herring (). Barndoor Skate were pooled into 10-cm length groups and a single-factor ANOVA was used to test the effect of skate TL on the mean proportion by weight for each of the three major prey categories. Prior to testing, proportional weight data were logit-transformed, as this transformation has been demonstrated to be more appropriate than the arcsine transformation for proportion data (Warton and Hui Citation2011). The logit transformation cannot transform proportions equal to zero or one, so the smallest nonzero value was added to the numerator and denominator of the logit function for zero values. Similarly, the smallest nonzero value was subtracted from the numerator and denominator of the logit function for values equal to one (Warton and Hui Citation2011). Levene's test for homogeneity of variance indicated equal variances when the smallest length classes (<35 cm TL) were removed, as these length groupings fed solely on shrimp (100% by weight); thus, there was no variance. After transformation, Shapiro–Wilk tests indicated nonnormality for some of the diet data; however, ANOVA is robust to the normality assumption and the validity of the analysis is only slightly affected by a nonnormal distribution (Zar Citation1999). Proportions were compared using post hoc Tukey's multiple comparison tests to determine at which lengths significant diet shifts occurred. An alpha value of 0.05 (α = 0.05) was used for all significance testing. Once significant length-groups were identified, single-factor ANOVA were used to compare the effect of sex on the proportion contribution by weight for each of the three major prey categories within each significant length grouping.
FIGURE 3. Percent by weight contribution of individual species of Barndoor Skate prey to our broader ecological groupings: (a) shrimp, (b) crabs, and (c) fish.
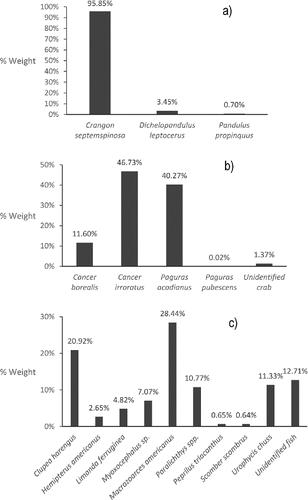
TABLE 1. Dietary composition of all Barndoor Skate sampled (n = 273) on Georges Bank, displayed as percent by number (%N), weight (%W), and frequency of occurrence (%O).
Compound indices such as the index of relative importance (IRI) incorporate multiple single indices and are believed to provide a more balanced understanding of the dietary importance of different prey types (Pinkas et al. Citation1971; Bigg and Perez Citation1985; Cortes Citation1997); however, they are not without controversy. Some authors claim that compound indices are redundant and add little to single indices (MacDonald and Green Citation1983), and others note that the arbitrary nature of IRI metrics complicates comparisons among species and different food types (Cortes Citation1997). More recently, IRI has been demonstrated to be intrinsically flawed as it combines mathematically dependent measures; this causes frequently occurring prey items to be overemphasized while rare prey items are underemphasized (Ortaz et al. Citation2006; Brown et al. Citation2012). Making matters worse, IRI is nonadditive across taxonomic levels; therefore, IRI values are arbitrarily controlled by the taxonomic resolution chosen by the researcher, which further complicates comparisons among studies (Brown et al. Citation2012). The intrinsic flaws associated with the IRI have caused researchers to develop a more appropriate compound index known as the prey-specific index of relative importance (PSIRI; Brown et al. Citation2012). This new index corrects the mathematical flaws associated with IRI and enables researchers to draw comparisons among studies regardless of the taxonomic level chosen by the researchers. The values for %PSIRI were calculated for all the three major prey categories and significant length-classes; %PSIRI is defined as
where %Oi is the frequency of occurrence for prey type i, %PNi is the prey-specific percent by number, or the percent by number of prey type i in all stomachs containing prey type i, and %PWi is the prey-specific percent by weight, or the percent by weight of prey type i in all stomachs containing prey type i.
RESULTS
Stomach samples were taken from a total of 273 Barndoor Skate, of which 267 (97.8%) contained prey items. A majority (n = 256) of the specimens were collected in the southern portion (south of 41°30′N) of closed area II, whereas a limited number (n = 17) were collected from the Nantucket Lightship closed area. Samples were taken from 137 females and 136 males ranging from 20 cm to 133.5 cm TL. Linear regressions of wet versus dry weights showed no pattern to the residuals, so only wet weights were used in subsequent analyses.
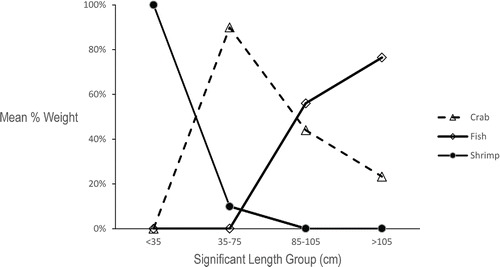
Ontogenetic diet shifts were immediately obvious upon plotting the 10-mm length-groups versus mean percent weight for the three major prey categories (), and statistical analyses indicated significant ontogenetic diet shifts for all three prey categories (ANOVA: P < 0.001). Tukey's multiple comparison tests allowed us to stratify skate lengths into four functional feeding groups based on significant dietary shifts in the mean proportion by weight of the major prey categories: <35 cm TL, 35–75 cm TL, 85–105 cm TL, and >105 cm TL. Until skate reached a size of approximately 35 cm, individual stomach samples contained only one species of caridean shrimp. Samples taken from Georges Bank closed area II contained only Crangon septemspinosa while samples from the Nantucket Lightship closed area contained only Dichelopandalus leptocerus. At lengths ≥ 35 cm TL, skate exhibited a significant shift from the sole utilization of small shrimp to a more diverse diet including rock crab and the Acadian hermit crab (). At lengths > 75 cm TL, skate no longer fed upon caridean shrimp and the prevalence of the rock crab in the diet began to decline in both males and females. At lengths > 105 cm TL, skate incorporated more fish into their diet, though crustaceans were still regularly consumed. A visual comparison of the diet composition for each 10-mm length-group () with the diet composition of the four significant length-groups () demonstrates that this simplification adequately captured the major dietary shifts.
FIGURE 4. Percent by weight contribution of the major prey categories when Barndoor Skate are pooled into 10-cm length-groups.
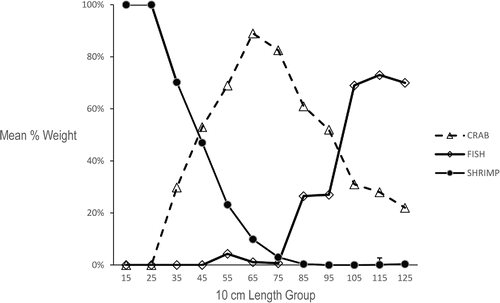
FIGURE 5. Mean percent weight of each major prey category for all statistically significant length groupings of Barndoor Skate.
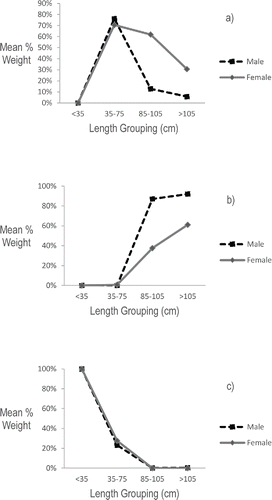
Sex-specific analyses revealed significant feeding patterns for the largest male and largest female skate (ANOVA: P < 0.01). Both sexes fed primarily on shrimp when small, and as they grew, they began to include other larger crustaceans in their diet (). At lengths greater than 75 cm TL, however, males began to prey more heavily on teleost fish than did females and this sexually dimorphic feeding pattern became significant for skate > 85 cm TL (ANOVA: P < 0.01).
FIGURE 6. Sex-specific feeding patterns of the major prey categories used by Barndoor Skate. Mean percent weight of (a) crabs, (b) fish, and (c) shrimp for the four significant length groupings (<35 cm TL, 35–75 cm TL, 85–105 cm TL, and >105 cm TL).
Prey-specific relative importance values were calculated for the three major prey categories when all stomach samples were pooled, and %PSIRI values were also calculated for the four significant length groupings (). When stomach samples were pooled, crabs represented the most important prey item for the Barndoor Skate (%PSIRI = 43.43%), followed by shrimp and fish (). The relative importance of prey items for each length-group largely reflected the results of the gravimetric length-specific analysis, with some exceptions. The same general pattern remained for the smaller skate: shrimp were the most important prey for small skate and crabs were the most important prey type for intermediate-sized skate; however, crabs remained the most important prey item for all but the largest skate (>105 cm TL), which differed from the percent by weight indices ().
FIGURE 7. Percent prey-specific index of relative importance (%PSIRI) for the major prey categories and the four significant length-groups of the Barndoor Skate.
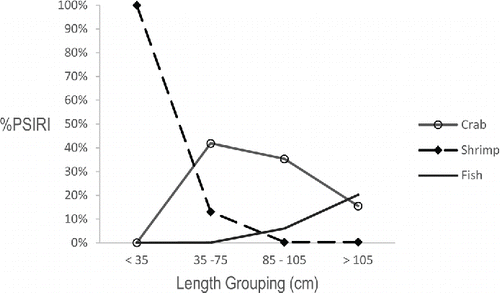
TABLE 2. Percent prey-specific index of relative importance (%PSIRI) for the major prey categories and the significant length-groups of the Barndoor Skate.
DISCUSSION
The diet of Barndoor Skate was dominated by a limited number of prey items with clear ontogenetic shifts in food habits. Smaller skate relied entirely on benthic invertebrates while larger skate began including more fish in their diet. Previous studies on skate have elucidated similar patterns, but the behavior does not appear to be consistent, even for species studied in similar geographic regions. In a study of six skate species off the South African coast, Small and Cowley Citation(1992) described three species as crustacean feeders (Raja miraletus, R. clavata, and Cruriraja parcomaculata), one as a specialist piscivore throughout its size range (R. alba) and two having ontogenetic changes in feeding habits. These two species, R. wallacei and R. pullopunctata, exhibited a pattern consistent with our results and fed primarily on crustaceans when they were small and then became mainly piscivorous when they became large. Ontogenetic changes in diet have also been described for a number of other batoid fishes (McEachran et al. Citation1976; Ajayi Citation1982; Platell et al. 1988; Orlov Citation1998) and have been attributed to morphological constraints (e.g., limited gape, tooth morphology) or better mobility, strength, and overall foraging ability of larger fish.
Our results for smaller skate would appear to support the hypothesis of morphological constraints as distinct shifts in diet were observed. The smallest individuals were specialized feeders foraging entirely on small carid shrimp, although other small prey such as crabs (Cancer spp.) would have been available. Individuals began to include other larger crustaceans in their diet once they exceeded 35 cm TL. A similar shift to include Acadian hermit crabs was observed at approximately 45 cm. Considering that all three of these prey are relatively slow moving, relatively common in our study area, and should not require great predatory swimming speeds to capture, there must be other factors limiting the Barndoor Skate from utilizing these food sources. Although the size of the mouth may play a role initially, many of the smaller Cancer spp. crabs and hermit crabs should be available as prey items. The simplest (but not the only) explanation may be found in the relationship between growth and the increased crushing power of the jaw required to crush thicker-shelled prey. However, very few shell fragments were observed in our samples indicating that the shells of Acadian hermit crabs were crushed prior to being ingested as prey.
The most interesting aspect of the ontogenetic changes in feeding habits we observed is the sex-specific habits of the largest skate. At lengths greater than 105 cm, male skate appeared to preferentially utilize teleost fish as prey (>80% by weight), while large females still preyed heavily upon larger crustaceans.
This sex-specific change in feeding habits became most pronounced at approximately the same size as when the skate reached maturity. Although there are a number of potential causes, the correlation between the size at maturity (males at 108 cm, females at 116 cm: Gedamke et al. Citation2005) and divergent sex-specific feeding habits is striking. One factor that is likely to play a role is sexual dimorphism in tooth structure. At maturity, female skate retain their molariform (i.e., plate like) teeth while males develop cuspidate dentition (i.e., pointed teeth). The dimorphism in tooth structure in the Barndoor Skate was apparent by even a cursory examination of sampled jaws. We noted that the development of cuspidate dentition in the males coincided with the development of other secondary sexual characteristics (i.e., allometric growth of claspers and development of alar thorns).
In the elasmobranchs, the role of sexual dimorphic dentition is generally attributed to the reproductive behavior of the group and the ability of males to grasp and hold females during mating. Males will bite prospective mates in courtship behavior and during mating to facilitate insertion of the clasper and to maintain intromission (Springer Citation1960; Tricas and LeFeuvre Citation1985; Carrier et al. Citation1994). This behavior has been documented in a number of the batoids including the Atlantic Stingray Dasyatis sabina (Kajiura et al. Citation2000), the Eagle Ray Aetobatis narinari (Tricas Citation1980), the Roughtail Stingray D. centroura (Reed and Gilmore Citation1981), and the Round Stingray Urolophus halleri (Nordell Citation1994). Evolutionarily, the development of sexually dimorphic tooth morphology was likely to have evolved from not only the selective pressures of maximizing reproductive success but also from the selective pressures on both sexes for feeding efficiency.
Feduccia and Slaughter Citation(1974) suggested that the strongly dimorphic tooth morphology in the rajids represents differential niche utilization between the sexes. This phenomenon has been demonstrated in bird, anole, and freshwater fish populations and results in reduced intraspecific competition for food, benefiting the population as a whole (Feduccia and Slaughter Citation1974). A number of authors studying the food habits of skate have suggested or shown that dentition plays a role in feeding habits (McEachran et al. Citation1976; Ebert et al. Citation1991; Smale and Cowley Citation1992), but as far as we are aware, this has not been confirmed for any elasmobranch.
If prey categories are of importance in determining trophic level, our study, along with that of Ebert and Bizzarro Citation(2007), suggests that the Barndoor Skate falls into the upper trophic-level predator category within their ecosystem and therefore may influence the relative abundance and diversity of co-occurring demersal species (Beddington Citation1984; Rogers et al. Citation1999). Due to the ontogenetic shifts observed in our study, we suggest that future trophic-level calculations incorporate different size-classes. This would be beneficial because it would take into account the shift in prey selection with increasing body size. Because prey selection appears to become sexually dichotomous once skate reach maturity, future trophic level calculations should also consider the differences in diet composition by sex. Accurate calculations and comparisons of diet composition for different size-classes, sexes, and life histories are important and would be beneficial in determining the ecological role of the Barndoor Skate within the community food web. This is especially important in the George's Bank region because there is evidence that trophic levels vary between, and within, different ecosystems (Morato et al. Citation2003). It is possible that the higher proportion of teleosts found in the diets of mature males could place them in a significantly higher trophic level (Holden and Tucker Citation1974; Quiniou and Andriamirado Citation1979; Ajayi Citation1982; Ellis et al. Citation1996). This is particularly important in the region of George's Bank where skate predation may negatively influence recruitment of commercially important groundfish species (Murawski Citation1991; Mayo et al. Citation1992; Fogarty and Murawski Citation1998). While there seems to be little evidence that Barndoor Skate are impacting important groundfish species, placement of the Barndoor Skate into an accurate trophic role would help determine their function in structuring the demersal marine communities in which they occur.
Although the correlation between divergent sex-specific feeding habits and sexually dimorphic dentition of Barndoor Skate at maturity was evident, we have not proven the case. Although sexually dimorphic tooth structure is the simplest and most likely explanation, this difference could have been due to other factors including depth segregation, nutritional needs, and/or size differences. For example, mature females may simply have different dietary needs than males, or the benthic feeding strategy of females may conserve energy that can be used for reproduction (Hanchet Citation1991; Stillwell and Kohler Citation1993; Simpfendorfer et al. Citation2001). Caution must be used before generalizing from our results because (1) all samples were collected from a small geographic area, (2) all samples were collected between June and November, and (3) samples were collected on commercial vessels actively fishing in the region. The small geographic area makes the interpretation of our results easier due to a limited number of prey species. Greater variability would have been observed if samples had been taken from a larger geographic area. For example, our samples from the Nantucket Light Ship closed area contained a different species of caridean shrimp than those from Georges Bank closed area II. Both of the prey species, Crangon septemspinosa and Dichelopandalus leptocerus, are morphologically similar and only reach maximum carapace lengths of 12 and 20 mm, respectively (Squires Citation1990). In each area, the smallest Barndoor Skate fed on only one species exhibiting clear specialization. If samples had been taken from a larger number of areas, a larger number of prey species would have been recorded for each size-class and interpreting the results may have been more difficult. As such, food habit studies should carefully consider the spatial aspects of sampling and resulting differences in prey availability.
Similarly, samples were only collected between June and November. Feeding patterns may be different at other times of the year. In fact, even our hypothesis pertaining to tooth morphology may have been more difficult to address if samples had been pooled over the entire year. In the Atlantic Stingray, Kajiura and Tricas Citation(1996) showed that the molariform morphology of the teeth in females is stable while male dentition shows a periodic shift from a female-like molariform to a recurved cuspidate form during the reproductive season.
Finally, while the opportunity to sample onboard commercial vessels allowed us to obtain a large number of samples, a significant amount of bycatch was also introduced into the environment. Although this may have facilitated the capture of teleost prey used by our sampled Barndoor Skate population, the differential utilization by males and females would have persisted. Males and females were captured simultaneously in very similar abundances, and prey availability would have been constant for both sexes.
The analytical methods applied in this study were carefully chosen not only to address the problems with pooling data over large spatial scales, but also to deal with the significant limitations of pooling data over a wide range of size-classes. No one method can provide an accurate picture of the feeding habits of a species (Hyslop Citation1980); thus our application of the common metrics (%N, %W, and %O) to the entire sample set allowed us to identify common prey items, but the details of size-specific prey selection were obscured. Only after a careful analysis of length-specific feeding patterns and reanalysis over distinct size-classes did the ontogenetic shifts in feeding habits become apparent. The combined use of a length-specific graphic analysis and pooled metrics not only allowed for the primary population-wide food sources to be identified but also extracted a compelling picture of the specificity of food preferences at the different life stages of the Barndoor Skate.
ACKNOWLEDGMENTS
We thank D. Rudders, J. D. Lange Jr., B. Carroll, and R. Harshbarger for their collection efforts at sea and the captains and crews of the FV Celtic, FV Alpha Omega, FV Barbara Ann, FV Heritage, FV Tradition, and the FV Mary Anne. We also thank the reviewers whose input greatly improved previous drafts of this manuscript. This research was made possible by funds generated from the 1% set-aside of the scallop total allowable catch (TAC) under Framework Adjustment 13 of the Sea Scallop Fishery Management Plan (FMP) and Framework Adjustment 34 to the Northeast Multispecies FMP.
REFERENCES
- Ajayi, T. O. 1982. Food and feeding habits of Raja species (Batoidei) in Carmarthen Bay, Bristol Channel. Journal of the Marine Biology Association of the UK 62:215–223.
- Beddington, J. R. 1984. The response of multispecies systems to perturbations. Pages 209–225 in R. M. May, editor. Exploitation of marine communities. Springer, Berlin.
- Bigelow, H. B., and W. C. Schroeder. 1953. Fishes of the western North Atlantic. Sawfishes, guitarfishes, skates and rays. Yale University, Sears Foundation for Marine Research Memoir I Part 2, New Haven, Connecticut.
- Bigg, M. A., and M. A. Perez. 1985. Modified volume: a frequency-volume method to assess marine mammal food habits. Marine Mammals and Fisheries 1985:277–283.
- Braccini, J. M., and J. E.Perez. 2005. Feeding habits of the sandskate Psammobatis extenta (Garman, 1913): sources of variation in dietary composition. Marine and Freshwater Research 56:395–403.
- Brown, S. C., J. J. Bizzarro, G. M. Cailliet, and D. A. Ebert. 2012. Breaking with tradition: redefining measures for diet description with a case study of the Aleutian Skate Bathyraja aleutica (Gilbert 1896). Environmental Biology of Fishes 95:3–20.
- Bulman, C., F. Althaus, X. He, N. J. Bax, and A. Williams. 2001. Diets and trophic guilds of demersal fishes of the southeastern Australian shelf. Marine and Freshwater Research 52:537–548.
- Caddy, J. F., and G. D. Sharp. 1986. An ecological framework for marine fishery investigations. FAO (Food and Agriculture Organization of the United Nations) Fisheries Technical Paper 283.
- Carrier, J. C, H. L. Pratt, and L. K. Martin. 1994. Group reproductive behaviors in free-living Nurse Sharks, Ginglymostoma cirratum. Copeia 1994:646–656.
- Casey, J. M., and R. A. Myers. 1998. Near extinction of a large, widely distributed fish. Science 281:690–692.
- Chipps, S. R., and J. E. Garvey, 2007. Quantitative assessment of food habits and feeding patterns. Pages 473–513 in C. Guy and M. Brown, editors. Analysis and interpretation of freshwater fisheries data. American Fisheries Society, Bethesda, Maryland.
- Collie, J. S., and A. K. DeLong. 1999. Multispecies interactions in the Georges Bank fish community. Pages 187–210 in B. Baxter, S. Keller, and C. Kaynor, editors. Ecosystem approaches for fisheries management. Alaska Sea Grant College Program, AK-SG-99-01, Anchorage.
- Cortes, E. 1997. A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Sciences 54:726–738.
- Davenport, S. R., and N. J. Bax. 2002. A trophic study of a marine ecosystem off southeastern Australia using stable isotopes of carbon and nitrogen. Canadian Journal of Fisheries and Aquatic Sciences 59:514–530.
- Ebert, D. A., and J. J. Bizzarro. 2007. Standardized diet compositions and trophic levels of skates (Chondrichthyes: Rajiformes: Rajoidei). Environmental Biology of Fishes 80:221–37.
- Ebert, D. A., and L. J. V. Compagno. 2007. Biodiversity and systematics of skates (Chondrichthyes: Rajiformes: Rajoidei). Environmental Biology of Fishes 80:111–124.
- Ebert, D. A., P. D. Cowley, and L. J. Compagno. 1991. A preliminary investigation of the feeding ecology of skates (Batoidea: Rajidae) off the west coast of South Africa. South African Journal of Marine Science 10:71–81.
- Ellis, J. R., M. G. Pawson, and S. E. Shackley. 1996. The comparative feeding ecology of six species of shark and four species of ray (Elasmobranchii) in the north-east Atlantic. Journal of the Marine Biology Association of the UK 76:89–106.
- Feduccia, A., and B. H. Slaughter. 1974. Sexual dimorphism in skates (Rajidae) and its possible role in differential niche utilization. Evolution 28:164–168.
- Fogarty, M. J., and S. A. Murawski. 1998. Large-scale disturbance and structure of marine systems: fishery impacts on Georges Bank. Ecological Applications 8:S6–S22.
- Garrison, L. P., and J. S. Link. 2000. Fishing effects on spatial distribution and trophic guild structure of the fish community in the Georges Bank region. ICES Journal of Marine Science 57:723–730.
- Gedamke, T. 2006. Development of a stock assessment for the Barndoor Skate (Dipturus laevis) on Georges Bank. Doctoral dissertation. College of William and Mary, Virginia Institute of Marine Science, Gloucester Point, Virginia.
- Gedamke, T., W. D. DuPaul, and J. A. Musick. 2005. Observations on the life history of the Barndoor Skate, Dipturus laevis, on Georges Bank (western North Atlantic). Journal of Northwest Atlantic Fishery Science 35:67–78.
- Graham, B. S., D. Grubbs, K. Holland, and B. N. Popp. 2007. A rapid ontogenetic shift in the diet of juvenile Yellowfin Tuna from Hawaii. Marine Biology 150:647–658.
- Hanchet, S. 1991. Diet of Spiny Dogfish, Squalas acanthias Linnaeus, on the east coast, South Island, New Zealand. Journal of Fish Biology 39:313–323.
- Holden, M. J., and R. N. Tucker. 1974. The food of Raja clavata Linnaeus 1758, Raja montagui Fowler 1910, Raja naevus Mueller and Henle 1841, and Raja brachyura LaFont 1873 in British waters. Journal du Conseil Permanent International pour l'Exploration de la Mer 35:189–193.
- Hyslop, E. J. 1980. Stomach content analysis—a review of methods and their application. Journal of Fish Biology 17:411–429.
- Kajiura, S. M., A. P. Sebastian, and T. C. Tricas. 2000. Dermal bite wounds as indicators of reproductive seasonality and behavior in the Atlantic Stingray, Dasyatis sabina. Environmental Biology of Fishes 58:23–31.
- Kajiura, S. M., and T. C. Tricas. 1996. Seasonal dynamics of dental sexual dimorphism in the Atlantic Stingray, Dasyatis sabina. Journal of Experimental Biology 199:2297–2306.
- Kulka, D. W., K. T. Frank, and J. E. Simon. 2002. Barndoor Skate in the Northwest Atlantic off Canada: distribution in relation to temperature and depth based on commercial fisheries data. Department of Fisheries and Oceans, Atlantic Fisheries Research Document 2002/073, Ottawa.
- Leim, A. H., and W. B. Scott. 1966. Fishes of the Atlantic coast of Canada. Fishery Research Board of Canada Bulletin 155.
- Macdonald, J. S., and R. H. Green. 1983. Redundancy of variables used to describe importance of prey species in fish diets. Canadian Journal of Fisheries and Aquatic Sciences 40:635–637.
- Mayo, R. K., M. J. Fogarty, and F. M. Serchuk. 1992. Aggregate fish biomass and yield on Georges Bank, 1960–87. Journal of Northwest Atlantic Fisheries Science 14:59–78.
- McEachran, J. D., D. F. Boesch, and J. A. Musick. 1976. Food division within two sympatric species - pairs of skates (Pisces: Rajidae). Marine Biology 35:301–317.
- McEachran, J. D., and J. A. Musick. 1975. Distribution and relative abundance of seven species of skates (Pisces: Rajidae) which occur between Nova Scotia and Cape Hatteras. U.S. National Marine Fisheries Service Fishery Bulletin 73:110–136.
- Morato, T., E. Sola, and M. P. Gros. 2003. Diets of Thornback Ray (Raja clavata) and Topeshark (Galeorhinus galeus) in the bottom longline fishery of the Azores, northeastern Atlantic. U.S. National Marine Fisheries Service Fishery Bulletin 101:590–602.
- Murawski, S. A. 1991. Can we manage our multispecies fisheries? Fisheries 16(5):5–13.
- NEFMC (New England Fisheries Management Council). 2001. Stock assessment and fishery evaluation (SAFE) report for the northeast skate complex. NEFMC, Newburyport, Massachusetts.
- NOAA (National Oceanic and Atmospheric Administration). 2003. Essential fish habitat source document: Barndoor Skate, Dipturus laevis, life history and habitat characteristics. NOAA Technical Memorandum NMFS-NE-173.
- Nordell, S. E. 1994. Observations of the mating behavior and dentition of the round stingray, Urolophus halleri. Environmental Biology of Fishes 39:219–229.
- Orlov, A. M. 1998. The diets and feeding habits of some deep-water benthic skates (Rajidae) in the Pacific waters off the northern Kuril Islands and southeastern Kamchatka. Alaska Fisheries Research Bulletin 5:1–17.
- Ortaz, M., P. B. Von Bach, and R. Candia. 2006. The diet of the neotropical insectivorous fish Creagrutus bolivari (Pisces: Characidae) according to the “graphic” and “relative importance” methods. Revista de Biologia Tropical 54:1227–1239.
- Pinkas, L., M. S. Oliphant, and L. K. Iverson. 1971. Food habits of Albacore, Bluefin Tuna, and Bonito in California waters. California Department of Fish and Game Fish Bulletin 152.
- Platell, M. E., I. C. Potter, and K. R. Clarke. 1988. Resource partitioning by four species of elasmobranchs (Batoidea: Urolophidae) in coastal waters of temperate Australia. Marine Biology 131:719–734.
- Posgay, J. A. 1957. Sea scallop boats and gear. U.S. Fish and Wildlife Service Fishery Leaflet 442.
- Quiniou, L., and G. R. Andriamirado. 1979. Variations of the diet of three species of rays from Douarnenez Bay (Raja montagui Fowler, 1910; Raja brachyura Lafont, 1873; Raja clavata L.,1758). Cybium 3:27–39.
- Reed, J. K., and R. G. Gilmore. 1981. Inshore occurrence and nuptial behavior of the Roughtail Stingray, Dasyatis centroura (Dasyatidae), on the continental shelf, east central Florida. Northeast Gulf Science 5:59–62.
- Rogers, S. I., K. R. Clarke, and J. D. Reynolds. 1999. The taxonomic distinctness of coastal bottom-dwelling fish communities of the Northeast Atlantic. Journal of Animal Ecology 68:769–782.
- Simpfendorfer, C. A., A. B. Goodreid, and R. B. McAuley. 2001. Size, sex and geographic variation in the diet of the Tiger Shark, Galeocerdo cuvier, from western Australian waters. Environmental Biology of Fishes 61:37–46.
- Sissenwine, M. P., E. B. Cohen, and M. D. Grosslein. 1984. Structure of the Georges Bank ecosystem. Rapports et Proces-Verbaux des Reunions du Conseil International pour l’Exploration de la Mer 183:243–254.
- Smale, M. J., and P. D. Cowley. 1992. The feeding ecology of skates (Batoidea: Rajidae) off the Cape South Coast, South Africa. South African Journal of Marine Science 12:823–834.
- Small, M. J., and P. D. Cowley. 1992. The feeding ecology of skates (Batoidea: Rajidae) off the cape south coast, South Africa. South African Journal of Marine Science 12:823–834.
- Springer, S. 1960. Natural history of the Sandbar Shark, Eulamia milberti. U.S. National Marine Fisheries Service Fishery Bulletin 61:1–38.
- Squires, H. J. 1990. Decapod crustacea of the Atlantic coast of Canada. Canadian Bulletin of Fisheries and Aquatic Sciences 221.
- Stillwell, C. E., and N. E. Kohler. 1993. Food habits of the Sandbar Shark Carcharhinus plumbeus off the U.S. northeast coast, with estimates of daily ration. U.S. National Marine Fisheries Service Fishery Bulletin 91:138–150.
- Tricas, T. C. 1980. Courtship and mating-related behaviors in myliobatid rays. Copeia 1980:553–556.
- Tricas, T. C., and E. M. LeFeuvre. 1985. Mating in the Reef White-tip Shark Triaenodon obesus. Marine Biology 84:233–237.
- Warton, D. I., and F. K. Hui. 2011. The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10.
- Zar, J. H. 1999. Biostatistical analysis, 4th edition. Pearson Education, Delhi, India.