Abstract
Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus were recently listed as threatened in the Gulf of Maine and endangered in the rest of their U.S. range. Continued research priorities include long-term population monitoring, identifying the species’ spawning and nursery grounds, and determining its use of estuaries and marine coastal waters. Although recent and ongoing research is filling in knowledge gaps, the Atlantic Sturgeon’s life history and its severely depleted populations make this a challenging species to fully characterize. Our goal was to compile data collected over 7 years from fish captured in the Penobscot River estuary, Maine, to inform management decision making. Atlantic Sturgeon were captured (n = 199), recaptured (n = 16), and passively telemetered (n = 32 that were analyzed here) from 2006 to 2013. Captured individuals were predominantly subadults, and data from telemetry indicated repeated use of a 5-km reach of the mesohaline portion of the estuary. Subadults predictably emigrated from the river each fall (mean date ± SD, August 31 ± 43.5 d) and immigrated back each spring to early summer (May 15 ± 27.8 d), with most individuals (>95% [31 of 32]) returning one or more years after tagging. Marine detections of these subadults were common (81.25% [26 of 32]) and spanned the geographic extent of both the threatened and endangered U.S. distinct population segments and into international waters, e.g., from the Hudson River, New York, to Minas Basin, Nova Scotia. However, they were more typically detected by receivers in the Gulf of Maine; 77% (20 of 26) were only detected in the Gulf of Maine when not in the river. These data indicate that, based on the temporal and spatial predictability of habitat use, the estuary of the Penobscot River is important for subadult Atlantic Sturgeon of the Gulf of Maine. The wider movement patterns emphasize the need for conservation and management across regions and international boundaries.
Received June 23, 2016; accepted January 4, 2017
The Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus is a long-lived anadromous species that historically ranged from Hamilton Inlet, Labrador, to the Saint Johns River, Florida (ASSRT Citation2007). Adults can grow to be over 4 m long and live to be 60 years old (Scott and Crossman Citation1973; LeBreton and Beamish Citation2004). Males typically mature around 12 years of age, at approximately 135 cm TL and 27 kg, and spawn every 1–5 years. Females typically mature around 15 years of age, at approximately 180 cm TL and 34 kg, and spawn once every 3–5 years (Van Eenennamm et al. Citation1996; Bain Citation1997; ASSRT Citation1998). Spawning usually occurs in major coastal rivers in early to mid-summer (Bain Citation1997; Kynard and Horgan Citation2002) upstream of the salt wedge in the freshwater portion of the river. Juvenile Atlantic Sturgeon remain within their natal river until they are between 2 and 6 years of age and are often found near the freshwater end of the brackish water transition zone (Døvel and Berggren Citation1983; Bain Citation1997; ASSRT Citation2007). Subadults (late-stage juveniles/immature individuals >100 cm; Bain Citation1997) tend to overwinter and make extensive movements in marine habitats and return to rivers, estuaries, and nearshore marine habitats in the warm summer months (Døvel and Berggren Citation1983; Bain Citation1997; Greene et al. Citation2009; Dunton et al. Citation2010).
Marine habitat use is not well described for most subadult and adult Atlantic Sturgeon, but patterns have been described (Laney et al. Citation2007; Nelson et al. Citation2013; Beardsall et al. Citation2016; Taylor et al. Citation2016). Between 1972 and 1996, Northeast Fisheries Science Center bottom trawl surveys captured 139 Atlantic Sturgeon from Canada to South Carolina (NEFSC, unpublished data; Savoy and Pacileo Citation2003). Commercial bycatch data suggest that Atlantic Sturgeon remain near the coastline, on the continental shelf, and in shallower habitats (Moser et al. Citation1998; Stein et al. 2004; Laney et al. Citation2007). While deeper, more offshore habitats have been shown to be important during winter months (Beardsall et al. Citation2016; Taylor et al. Citation2016), in spring and summer months subadult Atlantic Sturgeon in the mid-Atlantic and further south have been documented (using mark–recapture and telemetry data) making northward coastal movements and then southward movements in fall and winter (Døvel Citation1979; Smith Citation1985; Shirey Citation1995; Savoy and Pacileo Citation2003).
Atlantic Sturgeon were once very abundant throughout their range (ASMFC Citation1998; Armstrong and Hightower Citation2002) but are currently at low abundance. Their populations once supported a large fishery for caviar in the United States. The industry was established in 1870, peaked in 1890, and collapsed in 1901 (Van Eenennamm et al. Citation1996; Smith and Clugston Citation1997; Secor and Waldman Citation1999). Industrialization further contributed to Atlantic Sturgeon declines, particularly through river damming and water pollution (Metcalf and Eddy Citation1994; Van Eenennamm et al. Citation1996; ASMFC Citation1998). Bycatch in commercial fisheries, degraded water quality, and limited access to suitable spawning grounds and estuarine nursery habitat currently inhibit restoration efforts (Collins et al. Citation1996; ASMFC Citation1998; Secor and Gunderson Citation1998; Laney et al. Citation2007).
U.S. coastwide management of Atlantic Sturgeon began in 1988 when the National Marine Fisheries Service and U.S. Fish and Wildlife Service listed Atlantic Sturgeon as a species of concern. Two years later all states either closed the Atlantic Sturgeon fishery in their waters or instituted a size limit that achieved conservation equivalency (ASMFC Citation1990). In 1998, the ASMFC instituted a 40-year moratorium on the harvest of Atlantic Sturgeon in all U.S. waters (ASMFC Citation1998). Fourteen years later (2012), Atlantic Sturgeon were listed under the federal Endangered Species Act (ESA) in the United States. The Gulf of Maine (GOM) distinct population segment (DPS) was listed as threatened, and the New York Bight, Chesapeake Bay, Carolina, and South Atlantic DPSs were listed as endangered. Although there remains a commercial fishery for Atlantic Sturgeon in the St. Lawrence River, Canada (Apostle et al. Citation2013), it is tightly regulated, with individual annual quotas that limit overall fishing mortality to 60 tons per season (DFO Citation2013).
Listing of the GOM DPS as threatened means that it is at significant risk of becoming endangered in the foreseeable future (NOAA Citation2013). Before the listing, multiple studies were initiated in response to the Atlantic Sturgeon Status Review Team’s (ASSRT Citation2007) identification of research priorities, which included long-term population monitoring, estimating of spawning population abundance, characterizing population genetics, estimating bycatch and bycatch mortality, identifying spawning and nursery grounds, determining toxic contaminant impacts and thresholds, and determining fish passage. These studies resulted in the discovery of three spawning areas in the Kennebec River system, which includes the Kennebec, Androscoggin, and Sheepscot Rivers (Wippelhauser and Squiers Citation2015; Wippelhauser et al. Citation2017), and the identification of several potential foraging areas in the lower Kennebec River estuary (Wippelhauser and Squiers Citation2015), Saco River (Novak et al. Citation2017), and Penobscot River estuary (Dzaugis et al., University of Maine, unpublished data).
Current regulations protect Atlantic Sturgeon in the United States, with the understanding that they can mix with Atlantic Sturgeon from other designated DPSs and Canadian stocks in the marine environment. Approximately 10% of the Atlantic Sturgeon captured in the marine habitats of the Delaware–New York Bight region were identified (genetically) as being of GOM origin (NOAA Citation2013). This is consistent with Wirgin et al.’s (Citation2015b) observation that approximately 8% of the individuals in the mid-Atlantic region were of GOM origin. In addition, approximately 6% of the fish caught in the rivers (in waters with a salinity of <0.5‰) of this region were identified as likely being of GOM origin (NOAA Citation2013). Similarly, GOM Atlantic Sturgeon have been reported from Canada, with the summer assemblage sampled in Minas Basin or the Bay of Fundy having a 34–46% contribution from the GOM (Wirgin et al. Citation2012).
In the Penobscot River, Atlantic Sturgeon were historically documented as far upstream as the falls at Milford (rkm 62; Knight Citation1985; Fernandes et al. Citation2010). Although recent work confirms that Atlantic Sturgeon still inhabit the lower Penobscot River (Fernandes Citation2008; Fernandes et al. Citation2010), the construction of three dams starting in 1874 limited them to the lower 42 rkm until 1995, when the lowermost dam was breached. When research was initiated in the Penobscot estuary, it was unknown whether Atlantic Sturgeon actually inhabited the estuary and, if so, what areas of the estuary they used. Fernandes (Citation2008) documented the movements and demographic features of 35 Atlantic Sturgeon from Bangor (rkm 40) to the marine environment. Most (34) of these fish appeared to be subadults (71.2–131.7 cm FL and 2.5–18 kg), and one was a potential mature adult at 166.2 cm. In the first year of sampling, considerable effort (1,554 netting hours) was expended to capture relatively few (n = 7) Atlantic Sturgeon (Fernandes et al. Citation2010). Once it became clear that Atlantic Sturgeon preferred a narrow reach of the estuary, particularly during the summer months, sampling efforts became more efficient. Even those fish captured and tagged upstream of rkm 30 (n = 5) showed clear philopatry for the reach between rkm 20 and rkm 25 and returned to that reach shortly after tagging as well as the following year.
Consistent with the research priorities of the ESA and those identified by the Atlantic Sturgeon Status Review Team (ASSRT Citation2007), more data are needed concerning demography and fish presence in estuaries and marine coastal waters, particularly in the GOM and associated river systems. Our goal was to compile data collected over 7 years in the Penobscot River and the broader GOM to address the need for more data to inform decision making. More specifically, our objectives were to examine (1) the demographic characteristics of the Atlantic Sturgeon using the Penobscot River estuary and bay; (2) their movements within the Penobscot estuary and bay, highlighting spatial and temporal patterns to help identify critical habitat; and (3) their coastal movements outside the Penobscot estuary and bay to better characterize their marine habitat use.
Study Area
The Penobscot River and its tributaries constitute the largest watershed in Maine, draining an area of roughly 22,300 km2. The Penobscot River watershed has had a long history of industrial use, primarily by the lumber and pulp and paper industries. Mills and lumber transportation have negatively impacted water quality on the river. Following implementation of the Clean Water Act (Citation1972), conditions have greatly improved, although legacy impacts on water quality, substrate, and hydrography remain (Haefner Citation1967; Shorey Citation1973; Dionne Citation2010).
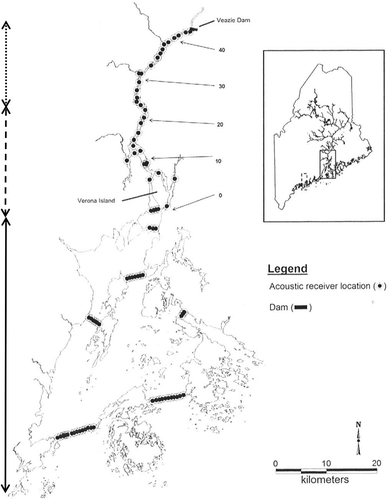
The area of the Penobscot River of interest for this study was the estuary and bay. We classified the upstream end of the estuary as the head of tide at rkm 46, as in Fernandes et al. (Citation2010), Dionne et al. (Citation2013), and Stich et al. (Citation2015). From 1833 until the summer of 2013, this was the upriver extent of Atlantic Sturgeon habitat due to the presence of the now-removed Veazie Dam. Also consistent with Fernandes et al. (Citation2010) and Dionne et al. (Citation2013), the southernmost point of Verona Island was considered the downstream end of the estuary (rkm 0). Penobscot Bay extends from this point south, roughly 90 km (). Although tidal, the Penobscot estuary is freshwater from the head of tide (at ~rkm 46) to between rkm 24 and 20, depending on tidal cycles and discharge. Salinity in this mesohaline (3.1–17‰) section of the estuary tends to vary vertically and horizontally based on discharge and tidal cycles. However, the lower limit of the mesohaline section of the estuary typically ranges from Bucksport (rkm 10) to somewhere in Penobscot Bay. Marine water (>30‰) can be present as far upstream as Bucksport at extreme low discharge and high tide but is more consistently present in Penobscot Bay south of Verona Island (Haefner Citation1967; Stich et al. Citation2015).
FIGURE 1. Map of the Penobscot River estuary with river kilometers delineated. Rkm 0 is at Verona Island and denotes the transition from Penobscot Bay to Penobscot estuary. The former Veazie Dam site (rkm 48) was the upper limit for Atlantic Sturgeon movement in the Penobscot River during the study period. The two southern gates and the southeastern gate were deployed from April to July 2005–2012. Map is from Fernandes et al. (Citation2010). The arrows to the left were added to indicate the river (dots), estuary (long dashes), and bay (solid) segments.
The study area extended outside of the Penobscot River because of opportunistic detections of acoustic tags in multiple collaborative networks. A collaborative agreement with the National Marine Fisheries Service (NMFS) ensured that we received detection information from the NOAA telemetry array deployed in Penobscot Bay and throughout the GOM (Goulette et al. Citation2014). Additionally, researchers that partnered with us through the Atlantic Cooperative Telemetry (ACT) Network (from Florida to Canada) and the Ocean Tracking Network (OTN; globally) shared detection information outside of the GOM as it was processed and became available.
Methods
Capture and tagging
The capture, handling, and tagging of Atlantic Sturgeon complied with NMFS protocols for sturgeons (Kahn and Mohead Citation2010) and researchers’ section 10 permit conditions (permits 1595 and 16526). Atlantic Sturgeon were captured in the Penobscot estuary during targeted sampling with gill nets that were either 15.24 or 30.48 m long and 2.4 m high, with 305-mm stretch mesh. They were also captured as bycatch during sampling for Shortnose Sturgeon Acipenser brevirostrum with 2.4-m-high, 152-mm-stretch-mesh gill nets. The 305-mm mesh nets were primarily used in the beginning of the study (until 2009) and in early summer to avoid capturing endangered Atlantic Salmon Salmo salar, which inhabit and spawn in the Penobscot River. All nets were set parallel to shore and placed in locations that avoided log booms, ledges, mudflats, and tidal creeks. The shorter nets were placed in locations where space was restricted. Sampling was conducted in the Penobscot estuary between rkm 7 and 46 for 0.2–23.8 h from May through November in 2006 and 2007 and between rkm 20 and 42 for 0.2–6.5 h from May through November in 2008 through 2013 (Fernandes et al. Citation2010; Dionne et al. Citation2013; Altenritter Citation2015). Catch per unit effort was calculated for each sampling year by dividing the total number of Atlantic Sturgeon caught by the total soak hours for all gillnetting for the season.
To examine the demographic features of this population, we recorded multiple measurements from each of the Atlantic Sturgeon captured from 2006 to 2013. The fish were removed from the gill net and placed in a floating net pen (2 m long, 1 m wide, and 1 m deep) attached to the side of the research vessel. An individual was removed from the net pen and immobilized in a canvas sling held in a sampling trough filled with water from the collection location. Each fish was then weighed in the sling (kg), and its total length (cm), fork length (cm), interorbital width (mm), and mouth width (mm) were measured. The fish was scanned for the presence of a passive integrated transponder (PIT) tag with an AVID Power Tracker VIII reader. If no tag was found, a 14-mm 134.2-kHz PIT tag was injected just below the base of the dorsal fin, above the lateral row of scutes. A small tissue sample was taken from the tip of the dorsal fin from each newly captured fish and preserved in ethanol for subsequent genetic analysis. An external tag with a unique identification number was attached to each fish just below and anterior to the base of the dorsal fin (anchor tags: dangler or dart). An endoscopic examination with a borescope was performed on each fish to identify females with visible eggs, following Kynard and Kieffer (Citation2002).
A subset of the Atlantic Sturgeon captured in the Penobscot estuary (n = 46) were implanted surgically with Vemco acoustic transmitters (). Surgery was performed only on fish that appeared to be in excellent health and when the water temperature was between 7ºC and 25ºC and dissolved oxygen was ≥5 mg/L. Each fish was anaesthetized with MS-222 (tricaine methanesulfonate) and immobilized in a canvas sling held in a water-filled sampling trough with aeration supply; ESA-approved surgery procedures were followed as described in Fernandes et al. (Citation2010). Each tagged fish was allowed to recover in the floating net pen for approximately 15 min and was released within 500 m of the site of capture after it showed clear signs of recovery.
TABLE 1. Summary information for coded acoustic tags used for Atlantic Sturgeon tagging in the Penobscot River and estuary, Maine, 2006–2011.
Acoustic receiver array
Annually, an array of between 80 and 120 VR2 and VR2W acoustic receivers (Vemco, Halifax, Nova Scotia) were deployed. The receivers were anchored to 45.4-kg mooring blocks and placed at designated locations less than 1 km from each other. The array consisted of approximately 40 sites in the Penobscot estuary (rkm 0–46), with the remaining receivers being deployed throughout Penobscot Bay (rkm 0 to –49) either singly or as “gates” (i.e., multiple regularly spaced receivers situated to detect low-powered V9 tags over the entire width of the river) spanning areas between islands where individuals could pass during emigration from the Penobscot River (Renkawitz et al. Citation2012). The southern three gates () were deployed from April until July annually prior to 2013. The entire receiver array was maintained as a collaborative effort between the University of Maine, the U.S. Geological Survey, and NMFS. In areas where the river width was greater than 500 m, two or more receivers were placed on each side of the channel to provide full monitoring coverage (receiver range typically varies from 500 to 800 m depending on tidal stage and other factors). Receivers typically were deployed in April and retrieved between November and the beginning of December, but not all receivers were successfully recovered in each year of the study.
Detections and analysis
To examine the movement patterns of the Atlantic Sturgeon, receiver locations in the Penobscot estuary were identified by river kilometer relative to the downstream end of the estuary at Verona Island. Data were downloaded from the receivers throughout the deployment period and a final time when the receivers were retrieved for the year. Detection data from 2007 through 2013 were analyzed. Data were sorted by transmitter number, date, and time. We then removed any single-code detections (defined as single readings for ≥24 h at the same receiver) from the data set to eliminate potential false detections. Fish positions (rkm) were plotted against date for each tagged fish to reveal movement patterns. Data from the year in which a fish was tagged were excluded to ensure a complete phenology of movement patterns based on a full “season.” Any tags that were not detected in the estuary after the year in which they were deployed (n = 5) or that remained stationary for more than 60 d (n = 5) were not analyzed due to the possibility that they represented nonfunctional or dropped tags. A full sampling year or more (up to 5 years) of detection information was analyzed for 32 Atlantic Sturgeon. A sampling year was defined as the span of time during which the acoustic receiver array was deployed in the estuary for a given calendar year (typically mid spring to late fall or early winter).
To quantify habitat use in the Penobscot River estuary, the length of the study area was binned into 5-rkm sections from rkm 0 to rkm 40. The number of detections of each fish was determined for each day. We then used the percent daily detections for all tagged Atlantic Sturgeon in the respective bins to determine whether the observed proportions were different from the null hypothesis that detections would be equivalent across all rkm bins (chi-square test).
We classified the movements of individual fish in and out of the Penobscot River estuary based on the categories used in Dionne et al. (Citation2013), including emigration and immigration. Consistent with Dionne et al. (Citation2013), emigration was defined as occurring when a tag placed in an Atlantic Sturgeon in the Penobscot estuary was either detected outside of the estuary or not detected again in the estuary for a minimum of 14 d. Immigration was defined as occurring when a tag placed in a fish determined to have emigrated was again detected in the Penobscot estuary and remained there for at least 14 d. The emigration date was the last date on which a tagged fish was detected upstream of rkm 0; the immigration date was the first date on which a tagged fish was detected upstream of rkm 0 after a previously documented emigration.
Marine (generally coastal) movements outside of the Penobscot estuary were examined using opportunistic detections of tags on receivers located beyond the Penobscot River and estuary. Several Atlantic Sturgeon tagged in the Penobscot estuary were detected by Vemco receivers on buoys maintained by the Northeastern Regional Association of Coastal and Ocean Observing Systems and other systems, both within and outside the GOM, via the Gulf of Maine Coastal Tracking Network. These detections were shared by the owners of the receivers through the ACT Network or OTN. When data were received from a collaborator through one of these networks, we first compared the data with our own detection data for consistency (i.e., to ensure that the fish were not detected in the Penobscot when they were detected elsewhere). We then removed any single-code detections (single reads over a period of 24 h or more on the same receiver) from the data set to eliminate potential false detections and plotted the detections over time and space.
Results
Demography
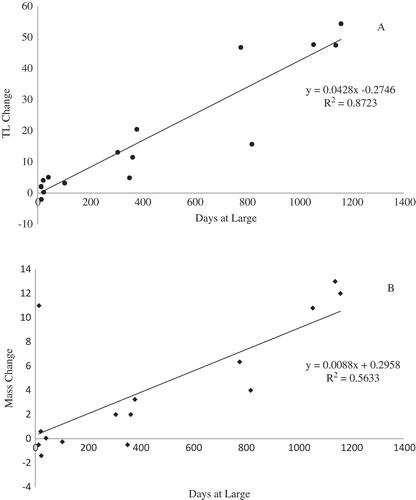
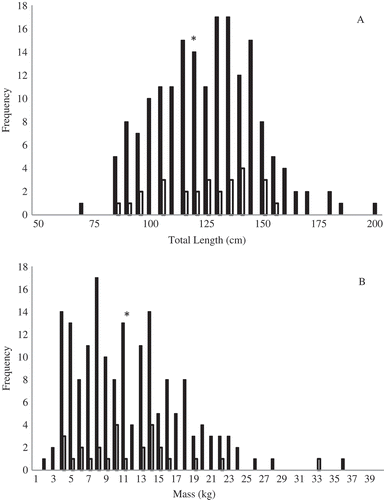
Between 2006 and 2013, we captured 199 Atlantic Sturgeon in the Penobscot River over a total of 2,523.62 netting hours (). Total catch per unit effort for all years was 0.08 fish per netting hour, with a yearly average of 0.24 (SD, 0.23) fish per netting hour. These sturgeon ranged in size from 65.6 to 196.4 cm TL (n = 163) and from 1.6 to 36.0 kg (n = 161) (). Of these fish, 26.3% (n = 43) were tagged with acoustic transmitters and 32 provided movement data that met the criteria noted above. Sixteen of these fish were recaptured and re-measured during the course of the study, of which 11 were at large for more than 30 d (509 ± 412.73) and which grew an average of 0.05 cm (SD, 0.08) and gained an average of 0.04 kg (SD, 0.01). The fish showed significant positive growth (linear regression, P < 0.001) in both length and weight for increased time at large between initial capture and recapture (). This includes individuals implanted with acoustic tags. One was recaptured after 3 months and had grown 3.2 cm, and two others were recaptured after approximately 1 year and had grown 4.8 and 8.5 cm, respectively.
TABLE 2. Catch per unit effort (CPUE; number captured per hour of soak time) of Atlantic Sturgeon captured in the Penobscot estuary by sampling year and summary statistics.
FIGURE 2. Frequency histograms for (A) total length and (B) mass of Atlantic Sturgeon captured in the Penobscot estuary between 2006 and 2013. The black bars indicate size at first capture and the white bars the lengths of recaptured individuals. Average length and mass are denoted on each histogram by an asterisk.
Movement Patterns within the Penobscot Estuary
On average, acoustically tagged Atlantic Sturgeon entered the Penobscot estuary (immigrated) on May 15 (SD, 27.8 d) and left the estuary (emigrated) on August 31 (SD, 43.5 d). Atlantic Sturgeon entered the river as early as the end of March and stayed as late as mid-October. Total time spent in the river was highly variable, with an average duration of 98 d (SD, 45.6). Of the 32 sturgeon that were tagged in the estuary and analyzed for movement patterns, most were detected in the estuary in one or more sampling years after the year in which they were tagged (this includes sturgeon tagged in the summer or fall of one calendar year and then detected the following spring). Thirty-one sturgeon were detected for 1 year after tagging, 22 for 2 years, 14 for 3 years, 6 for 4 years, and 1 for 5 years past its tagging year.
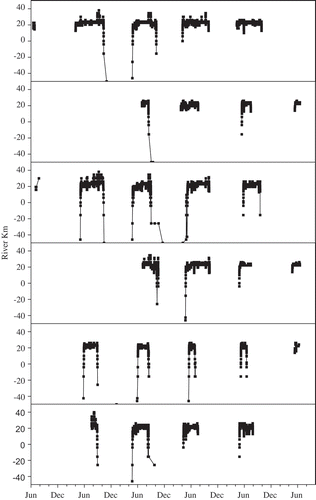
The acoustic telemetry array allowed for nearly continuous detection of acoustically tagged Atlantic Sturgeon within the study area of the estuary. Generally, sturgeon moved quickly (within the same 24-h period) through Penobscot Bay and the lower estuary when immigrating and emigrating (). Tagged sturgeon (n = 30) spent the majority of their time (70–84% annually; ) in the mesohaline reaches of the estuary, typically between rkm 20 and 30, occasionally moving into the upper reaches of the estuary (upstream of the salt wedge) or back into the lower estuary and bay, but often only for short periods. When plotted against river kilometer, these detections revealed distinct movement patterns. Although movement patterns within the Penobscot estuary differed among individual sturgeon, and even for the same individuals over multiple years, there was a predominant overall pattern of quick movements upriver to rkm 20–25 in the spring and quick movements back out in the late summer and fall (). Sturgeon tagged with multiyear tags allowed us to observe movement patterns throughout the estuary for up to 4 (n = 4) or 5 (n = 2) years. Six sturgeon reentered the bay two or more times during the same sampling year (as far upstream as rkm 42.5), followed by another movement farther up into the river (as far as rkm 40), but the amount of this “wandering” behavior varied among individuals and even for the same individuals from year to year. Although three Atlantic sturgeon made occasional quick trips into the freshwater section of the river and were detected as far upstream as rkm 40, they were still most commonly detected in the mesohaline reaches of the estuary, between rkm 15 and 30. A chi-square test revealed that the probability of detection in the 5-rkm sections was significantly different from the hypothesized equal detection probability in all sections (χ2 = 334.90; P < 0.001). The reach between rkm 20 and 25 had the most detections among all sampling years, with 67–84% of the detections in the river each year and 73% of the detections overall ().
TABLE 3. Proportions of acoustic detections of Atlantic Sturgeon (means ± SDs) by river kilometer (rkm 0 = mouth of river) in the Penobscot River estuary by year and over the entire study. The values for rkm 20 are in bold italics to denote the fact that a significantly higher proportion of individuals used this reach (χ2 = 334.90, P < 0.001).
FIGURE 4. Movement patterns of six acoustically tagged Atlantic Sturgeon in the Penobscot estuary and bay. The same basic pattern was characteristic of 21 of the 30 acoustically tagged sturgeon analyzed: fish moved quickly through the bay and into the estuary in one immigration movement in the spring, remained in the estuary (primarily between rkm 20 and 30) until fall, and then emigrated from the estuary and bay in one quick movement.
Movements outside the Penobscot Estuary
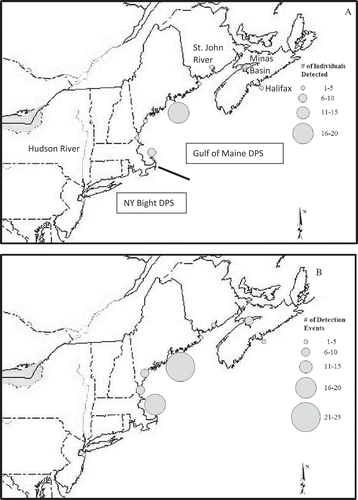
Twenty-six Atlantic Sturgeon acoustically tagged in the Penobscot estuary were detected by collaborators with acoustic receivers deployed in the GOM and other coastal systems in the Northwest Atlantic (). Most of these detections (18, or 60%) were of single individuals seen only once, in 1 year at one location. No individuals were detected in multiple locations outside of the river in the same year. One Atlantic Sturgeon tagged in the Penobscot estuary was detected in the St. John River in New Brunswick, four were detected in the Minas Passage in the Bay of Fundy, two were detected off the coast of Halifax, Nova Scotia, six were detected in the Saco River in southern Maine, seven were detected in the Merrimack River in New Hampshire, ten were detected in the coastal waters of Massachusetts near Cape Cod, and two were detected in the Hudson River and along Long Island, New York (). Additionally, 26 Atlantic Sturgeon tagged in the Penobscot estuary were detected by NMFS’s array of receivers deployed at coastal locations in the GOM (attached to either coastal research buoys or in lobster traps; Goulette et al. Citation2014). While Atlantic Sturgeon tagged in the Penobscot estuary were detected in regions outside the estuary throughout the year, they were most commonly detected in these regions during October (n = 17 [71%]) and November (n = 10 [38%]) (). Some of these individuals returned to these locations several times, often over the course of several years. For example, one sturgeon returned to the Minas Passage 3 years in a row, and another returned 2 years in a row (). Neither fish returned to the Penobscot River estuary between detections in the Minas Passage. Both were detected in the Minas Passage in the winter (December and January) and the summer (July). Four sturgeon repeatedly passed receivers on the coast of Massachusetts in two separate years, most commonly in November (n = 3). Seven sturgeon were each repeatedly detected by NMFS’s GOM receivers in two different years, most commonly in October (n = 5).
TABLE 4. Numbers of Atlantic Sturgeon acoustically tagged in the Penobscot River that were detected in other areas along the coast of the Northwest Atlantic Ocean. Also shown is the number of detection “events,” which are defined as the number of times that an Atlantic sturgeon was detected by receivers in a particular geographic area. For example, a fish could have been detected by several receivers over the course of several days or weeks or by one receiver on a single day. This information is meant to reflect sturgeons’ passing through an area on several different occasions, usually in different years. The data were obtained opportunistically from collaborators through telemetry data sharing networks such as the ACT Network and OTN.
TABLE 5. Months in which acoustically tagged Atlantic Sturgeon were detected by acoustic receivers in other areas of the Northwest Atlantic Ocean. The numbers in the table represent the number of sturgeon that were present at each location during those months. The table combines data collected over all study years; if an individual remained at a location for several months in a row, each month is included separately.
FIGURE 5. (A) Numbers of individual Atlantic Sturgeon acoustically detected in the Penobscot River estuary and (B) numbers of detection events by marine acoustic receivers. The solid line east of Cape Cod, Massachusetts, indicates a rough division between the Gulf of Maine and New York Bight distinct population segments.
Discussion
Data from the Atlantic Sturgeon captured, recaptured, and telemetered in the Penobscot River estuary can inform conservation efforts for this species, particularly with respect to identifying potential critical habitat for the GOM DPS in the United States. In-river captures were dominated by subadult Atlantic Sturgeon, and acoustic telemetry indicated repeated and predominant use of a specific 5-km reach in the mesohaline portion of the estuary for approximately 3 months (June–August) every year since 2007. These subadults departed the river each fall and spent their winters in marine habitats. During that time a small number of them were detected in locations as far away as Minas Basin and the Hudson River, but more often they were detected in the GOM from Massachusetts to eastern Maine. These extra-estuarine movements of subadults through the marine environment appear to be common and to include both the threatened (GOM DPS) and endangered (New York DPS) regions within the species’ range. Data collected over 7 years from the Penobscot River indicate the importance of this river and multiple marine regions to subadult Atlantic Sturgeon based on the temporal and spatial repeatability of their use of these habitats—consistent with the definition of critical habitat (Endangered Species Act of 1973)—and underscore the need for conservation across regions and international boundaries.
It is likely that the majority of the Atlantic Sturgeon captured in the Penobscot estuary were subadults since male Atlantic Sturgeon are reported to mature between 135 and 204 cm TL and 27 kg and females tend to mature between 180 and 254 cm TL and 34 kg (Van Eenennamm et al. Citation1996; Bain Citation1997). Although 29% of the Atlantic Sturgeon captured in the Penobscot estuary were over 133 cm, only two (0.01%) were over 180 cm and 27 kg and none were over 34 kg. While it is possible that we captured at least a few mature male Atlantic Sturgeon (based on TL), it is unlikely that a mature female was captured in the Penobscot estuary. Interestingly, the size of the Atlantic Sturgeon captured in the Penobscot estuary is very different from that reported for the neighboring Kennebec River (Wippelhauser and Squiers Citation2015). Individuals captured in the Penobscot were intermediate in size (120 cm) to the two predominant size-classes captured in the Kennebec (size peaks at 85 and 150 cm). Given that the captures in the Kennebec River were made with similar gear (6–8-in [152–203-mm] and 12-in [305-mm] gill nets) and over a similar period of time (2006–2011), the intermediate size structure that we found could indicate that while spawning and early growth occur in the Kennebec River, many subadults leave the Kennebec for alternative habitats, such as the Penobscot estuary, for further growth. This hypothesis is consistent with the movement patterns presented here and could be verified if the smaller size-classes of Atlantic Sturgeon captured in the Kennebec River were implanted with acoustic tags and monitored using the available GOM acoustic telemetry arrays (Goulette et al. Citation2014; Wippelhauser et al. Citation2015).
Atlantic Sturgeon were rarely seen in the freshwater sections of the Penobscot River during the expected spawning season of late May through mid-June (n = 3; Van Eenennamm et al. Citation1996). Also, spawning has not been documented in the Penobscot River in 7 years of egg and early-life-stage sampling efforts (Wegener Citation2012; Altenritter Citation2015), so it is unlikely that Atlantic Sturgeon are moving into the Penobscot River for spawning purposes. However, Atlantic Sturgeon spawning has been documented in the nearby Kennebec system (Wippelhauser et al. Citation2017), which might serve as primary spawning habitat in the GOM, much like it does for Shortnose Sturgeon (Wippelhauser et al. Citation2015).
In our study we document a repeated pattern of annual returns to the Penobscot estuary, suggesting some combination of consistent habitat preference or philopatry. Annual use of the mesohaline portion of the estuary is consistent with observations of subadults in other river–estuary systems (Døvel and Berggren Citation1983; Bain Citation1997; Greene et al. Citation2009; Dunton et al. Citation2010). For those individuals with long-lived tags (n = 32), over 95% returned to the Penobscot estuary one or more years after tagging and 43.75% returned three or more years (up to 5 years in a row for one individual), and we expect to see this pattern repeated in future years.
It is likely that Atlantic Sturgeon are using the Penobscot estuary for foraging based on their very specific summer use and some preliminary diet analyses. Acoustically tagged Atlantic Sturgeon spent on average 67–84% of their time each year in the narrow mesohaline section of the estuary between rkm 20 and rkm 25. Additionally, 90–98% of the detections each year occurred in this section or two immediately adjacent sections (rkm 15–19 and 20–24 or 20–24 and 25–29). In 2012, when over 75% of the detections in the Penobscot estuary were concentrated in rkm 25–30, eight individuals from this stretch of the river were gastrically lavaged and had, on average, more than 200 spionid polychaete worms Marenzelleria viridis in their stomachs. The diet of all eight was composed of 99–100% spionid polychaete worms (one individual had over 3,300 in its stomach; Dzaugis Citation2013). This stretch of the estuary has muddy substrate and a maximum density of spionid polychaete worms of 17,500 individuals/m2, which is two orders of magnitude greater than the densities in other parts of the river (Dzaugis Citation2013). This is similar to the forage base in the Minas Basin of the Bay of Fundy, where McLean et al. (Citation2013) found a high presence of polychaete worms (99.7%) in the stomachs of aggregating Atlantic Sturgeon. The presence density of this food source, along with the amount of time that individual sturgeon spend in this part of the estuary and the prevalence of polychaetes in their diets, suggest that Atlantic Sturgeon are attracted to this region for feeding. The limited geographic extent of this resource may imply significant value when designating critical habitat as defined in section 3 of the Endangered Species Act (Endangered Species Act Citation1973).
Information on Atlantic Sturgeon movements and their use of habitat in the marine environment is limited, and obtaining it has been identified as a research priority (Laney et al. Citation2007) from a species protection perspective (Apostle et al. Citation2013; NOAA Citation2013). Several other studies, including those using bycatch data from commercial fisheries and long-term fisheries independent data, indicate that subadult Atlantic Sturgeon inhabit shallow inshore areas, particularly during cold-weather months (Stein et al. Citation2004a, Citation2004b; Laney et al. Citation2007; Dunton et al. Citation2010; Oliver et al. Citation2015). Dunton et al. (Citation2010) found that subadult Atlantic Sturgeon aggregations tended to occur at the mouths of large bays (Chesapeake and Delaware) or estuaries (Hudson and Kennebec River) during the spring and fall months. Atlantic Sturgeon, particularly adults, have occasionally been captured in deeper offshore waters (Timoshkin Citation1968; Collins and Smith Citation1997; Stein et al. Citation2004a, Citation2004b; Beardsall et al. Citation2016; Taylor et al. Citation2016). Our data substantiate some of these observations, since detections occurred in regions with many of these characteristics (Minas Passage [25–115 m deep]: Stokesbury et al. Citation2016; Massachusetts Bay [~30–80 m]: Wippelhauser et al. Citation2017). Recent studies using satellite tags revealed winter use of marine habitats 5–21 km from shore in water >75 m deep and temperatures as cold as 4.9°C (Beardsall et al. Citation2016; Taylor et al. Citation2016), suggesting that telemetry receiver coverage should be expanded to deeper waters of the GOM.
Far-ranging movements by tagged subadult Atlantic Sturgeon have been documented in the past (Døvel and Berggren Citation1983; Dadswell Citation2006) and are being discovered more frequently (Taylor et al. Citation2016), particularly as researchers collaborate and share telemetry detection data. Gulf of Maine Atlantic Sturgeon tagged in the Merrimack, Saco, and Kennebec rivers have been detected in the coastal waters near Boston, Massachusetts, and in the Minas Passage in the Bay of Fundy (Wippelhauser et al. Citation2017). It appears that Atlantic Sturgeon tagged in the Penobscot estuary range as far north (Minas Passage) but might travel even farther south (e.g., the Hudson River) than documented for other GOM sturgeon (). It is also possible that Atlantic Sturgeon use certain marine corridors for long-range movements. Some of the Atlantic Sturgeon tagged in the Penobscot estuary were repeatedly detected by the same marine receivers around the same time several years in a row. These detections suggest that movements inside and outside of the GOM expose sturgeon to repeated localized risks, such as bycatch mortality associated with coastal fisheries activities (Collins et al. Citation1996; Stein et al. Citation2004b; Dunton et al. Citation2010; Beardsall et al. Citation2013).
Recent research on the genetic origins of Atlantic Sturgeon (particularly subadults) captured in various coastal marine waters indicates that there is extensive mixing of DPS sources in these habitats. For example, only 60–70% of the individuals captured in and near New York waters were from the New York Bight DPS (Dunton et al. Citation2012; Waldman et al. Citation2013), whereas 40% of those captured from the GOM to Cape Hatteras, North Carolina, were from the New York Bight DPS (Wirgin et al. Citation2015b). To the north, genetic mixing has also been reported in Minas Basin, where more than 40% of the individuals were genetically assigned to the GOM stock (Wirgin et al. Citation2012). While only two of the Atlantic Sturgeon acoustically tagged in the Penobscot River estuary were detected in the New York Bight region, those fish represented between 4.3% and 5.2% of the available tagged sturgeon for those years. While the telemetry and genetic data suggest mixing, it is important to keep in mind that relatively few fish from the GOM region are acoustically tagged (or sampled for genetic analysis) and that both sets of data are obtained opportunistically, requiring further validation.
Several areas for continued research have been identified in the United States (ASSRT Citation2007) and Canada, including long-term population monitoring, estimating spawning populations, determining population genetics, estimating bycatch and bycatch mortality, identifying spawning and nursery grounds, determining toxic contaminant impacts and thresholds, and determining fish passage. Recent and current research on Atlantic Sturgeon is filling in these gaps, and this will be vital for the species’ conservation. However, the lengthy, complicated life history of this species, its severely depleted population size, and the lack of transboundary cooperation in managing it (Apostle et al. Citation2013) make it a challenging species to fully characterize and manage. Ongoing research should help identify important habitats that can be improved and conserved, such as the habitat identified in the Penobscot estuary. Acoustic transmitters that are continuing to be deployed in Atlantic Sturgeon captured in the Penobscot estuary will allow for long-term population monitoring in the GOM. In addition, habitat improvements in the Penobscot River, such as the removal of dams through the Penobscot River Restoration Project (Opperman et al. Citation2011), will hopefully lead to the availability of more critical sturgeon habitat. However, these efforts in the U.S. portion of the GOM must still be considered in the broader context of the species’ full range to facilitate conservation of this species.
Acknowledgments
We thank Steve Fernandes, Phillip Dionne, Matthew Wegener, Kevin Lachapelle, Matthew Altenritter, and Catherine Johnston for capturing and tagging the Atlantic Sturgeon and maintaining the receiver array as part of their graduate work and Joseph Zydlewski for his contributions on graduate student committees and supply of field assistance and infrastructure.
We also thank the following Gulf of Maine Coastal Tracking Network, Atlantic Cooperative Telemetry Network, and Dalhousie University Ocean Tracking Network (OTN) researchers, staff, and partner organizations that maintained receivers outside the four study rivers and provided data: Jay Barthelotte, Duncan Bates, Rod Bradford, Robert Branton, Jeremy Broome, Nicholas Buchan, Michael Dadswell, Keith Dunton, Michael Frisk, Graham Goulette, James Hawkes, William Hoffman, Adrian Jordaan, Richard Karsten, Micah Kieffer, John Kocik, Matthew Litvak, Kim McKown, Paul Music, Anna Redden, Peter C. Smith, Michael Stokesbury, and James Sulikowski, as well as Acadia University, Dalhousie University, Department of Fisheries and Oceans Canada, Massachusetts Division of Marine Fisheries, Mount Allison University, National Marine Fisheries Service, New York Department of Environmental Conservation, OTN field and data teams, Stony Brook University, University of Massachusetts–Amherst, U.S. Geological Survey Conte Anadromous Fish Research Center, and University of New England.
This project was conducted under the University of Maine Institutional Animal Care and Use Committee protocols A2008-07-01, A2011-06-11, and A2014-05-06. The research was funded by NOAA-Fisheries award NA07NMF4720053 to the Maine Department of Marine Resources. This is Maine Agricultural and Forest Experiment Station publication 3526. All procedures were conducted under NOAA ESA Section 10 permits 1595 and 16526 for taking protected species for scientific purposes. The views expressed herein are those of the authors and do not necessarily reflect the views of the Penobscot River Restoration Trust, NOAA, USGS, or any of their members or subagencies.
References
- Altenritter, M. E. 2015. Shortnose Sturgeon (Acipenser brevirostrum) in the Gulf of Maine: local population dynamics and metapopulation implications. Doctoral dissertation. University of Maine, Orono.
- Apostle, R., M. J. Dadswell, C. Engler-Palma, M. K. Litvak, M. F. McLean, M. J. W. Stokesbury, A. D. Taylor, and D. L. VanderZwaag. 2013. Sustaining Atlantic Sturgeon: stitching a stronger scientific and governance net. Journal of International Wildlife Law and Policy 16:170–197.
- Armstrong, J. L., and J. E. Hightower. 2002. Potential for restoration of the Roanoke River population of Atlantic Sturgeon. Journal of Applied Ichthyology 18:475–480.
- ASMFC (Atlantic States Marine Fisheries Commission). 1990. Interstate fishery management plan for Atlantic Sturgeon. ASMFC, Fisheries Management Report 17, Washington, D.C.
- ASMFC (Atlantic States Marine Fisheries Commission). 1998. Atlantic Sturgeon stock assessment. ASMFC, Peer Review Report, Washington, D.C.
- ASSRT (Atlantic Sturgeon Status Review Team). 1998. Status review of Atlantic Sturgeon (Acipenser oxyrinchus oxyrinchus). Report to the National Marine Fisheries Service, Gloucester, Massachusetts.
- ASSRT (Atlantic Sturgeon Status Review Team). 2007. Status review of Atlantic Sturgeon (Acipenser oxyrinchus oxyrinchus). Report to the National Marine Fisheries Service, Gloucester, Massachusetts.
- Bain, M. B. 1997. Atlantic and Shortnose sturgeons of the Hudson River: common and divergent life history attributes. Environmental Biology of Fishes 48:347–358.
- Beardsall, J. W., M. F. McLean, S. J. Cooke, B. C. Wilson, M. J. Dadswell, A. M. Redden, and M. J. Stokesbury. 2013. Consequences of incidental otter trawl capture on survival and physiological condition of threatened Atlantic Sturgeon. Transactions of the American Fisheries Society 142:1202–1214.
- Beardsall, J. W., M. J. W. Stokesbury, L. M. Logan-Chesney, and M. J. Dadswell. 2016. Atlantic Sturgeon Acipenser oxyrinchus Mitchill, 1815 seasonal marine depth and temperature occupancy and movement in the Bay of Fundy. Journal of Applied Ichthyology 32:809–819.
- Clean Water Act. 1972. U.S. Code, volume 33, section 1251.
- Collins, M. R., S. G. Rogers, and T. I. J. Smith. 1996. Bycatch of sturgeons along the southern Atlantic coast of the USA. North American Journal of Fisheries Management 16:24–29.
- Collins, M. R., and T. J. Smith. 1997. Distributions of Shortnose and Atlantic sturgeons in South Carolina. North American Journal of Fisheries Management 17:995–1000.
- Dadswell, M. J. 2006. A review of the status of Atlantic Sturgeon in Canada, with comparisons to populations in the United States and Europe. Fisheries 31:218–229.
- DFO (Department of Fisheries and Oceans). 2013. Recovery potential assessment for the Atlantic Sturgeon, St. Lawrence population. Canadian Science Advisory Secretariat Science Advisory Report 2013/040.
- Dionne, P. E. 2010. Shortnose Sturgeon of the Gulf of Maine: the importance of coastal migrations and social networks. Master’s thesis. University of Maine, Orono.
- Dionne, P. E., G. B. Zydlewski, M. T. Kinnison, J. Zydlewski, and G. S. Wippelhauser. 2013. Reconsidering residency: characterization and conservation implications of complex migratory patterns of Shortnose Sturgeon (Acipenser brevirostrum). Canadian Journal of Fisheries and Aquatic Sciences 70:119–127.
- Døvel, W. J. 1979. The biology and management of Shortnose and Atlantic sturgeon of the Hudson River. New York State Department of Environmental Conservation, AFS-9-R, New York.
- Døvel, W. L., and T. J. Berggren. 1983. Atlantic Sturgeon of the Hudson estuary, New York. New York Fish and Game Journal 30:140–172.
- Dunton, K. J., D. Chapman, A. Jordaan, K. Feldheim, S. J. O’Leary, K. A. McKown, and M. G. Frisk. 2012. Genetic mixed-stock analysis of Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus in a heavily exploited marine habitat indicates the need for routine genetic monitoring. Journal of Fish Biology 80:207–217.
- Dunton, K. J., A. Jordaan, K. A. McKown, D. O. Conover, and M. G. Frisk. 2010. Abundance and distribution of Atlantic Sturgeon (Acipenser oxyrinchus) within the Northwest Atlantic Ocean, determined from five fishery-independent surveys. U.S. National Marine Fisheries Service Fishery Bulletin 108:450–465.
- Dzaugis, M. P. 2013. Diet and prey availability of sturgeons in the Penobscot River, Maine. Undergradute thesis. University of Maine, Orono.
- Endangered Species Act. 1973. U.S. Code, volume 16, sections 1531–1544.
- Fernandes, S. J. 2008. Population demography, distribution, and movement patterns of Atlantic and Shortnose sturgeons in the Penobscot River estuary, Maine. Master’s thesis. University of Maine, Orono.
- Fernandes, S. J., G. B. Zydlewski, J. D. Zydlewski, G. S. Wippelhauser, and M. T. Kinnison. 2010. Seasonal distribution and movements of Shortnose and Atlantic sturgeon in the Penobscot River estuary, Maine. Transactions of the American Fisheries Society 139:1436–1449.
- Goulette, G. S., J. P. Hawkes, J. F. Kocik, J. P. Manning, P. A. Music, J. P. Wallinga, and G. B. Zydlewski. 2014. Opportunistic acoustic telemetry platforms: benefits of collaboration in the Gulf of Maine. Fisheries 39: 441–450.
- Greene, K. E., J. L. Zimmerman, R. W. Laney, and J. C. Thomas-Blate. 2009. Atlantic coast diadromous fish habitat: a review of utilization, threats, recommendations for conservation, and research needs. Atlantic States Marine Fisheries Commission, Habitat Management Series 9, Washington, D.C.
- Haefner, P. A. 1967. Hydrography of the Penobscot River (Maine) estuary. Journal of the Fisheries Research Board of Canada 24:1553–1571.
- Kahn, J., and M. Mohead. 2010. A protocol for the use of Shortnose, Atlantic and Gulf, and Green sturgeons. NOAA Technical Memorandum NMFS-OPR-45.
- Knight, J. A. 1985. Differential preservation of calcified bone at the Hirundo site, Alton, Maine. Master’s thesis. University of Maine, Orono.
- Kynard, B., and M. Horgan. 2002. Ontogenetic behavior and migration of Atlantic Sturgeon, Acipenser oxyrinchus oxyrinchus, and Shortnose Sturgeon, A. brevirostrum, with notes on social behavior. Environmental Behavior of Fishes 63:137–150.
- Kynard, B., and M. Kieffer. 2002. Use of a borescope to determine the sex and egg maturity stage of sturgeons and the effect of borescope use on reproductive structures. Journal of Applied Ichthyology 18:505–508.
- Laney, R. W., J. E. Hightower, B. R. Versak, M. F. Mangold, W. W. Cole, and S. E. Winslow. 2007. Distribution, habitat use, and size of Atlantic Sturgeon captured during cooperative winter tagging cruises, 1988–2006. Pages 167–182 in J Munro, D. Hatin, J. E. Hightower, K. McKown, K. J. Sulak, A. W. Kahnle, and F. Caron, editors. Anadromous sturgeons: habitats, threats, and management. American Fisheries Society, Symposium 56, Bethesda, Maryland.
- LeBreton, G. T. O., and F. W. H. Beamish. 2004. Growth, bioenergetics, and age. Pages 195–215 in G. T. O. LeBreton, F. W. H. Beamish, and R. S. McKinley, editors. Sturgeons and paddlefish of North America. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- McLean, M. F., M. J. Dadswell, and M. J. Stokesbury. 2013. Feeding ecology of Atlantic Sturgeon, Acipenser oxyrinchus Mitchell, 1815 on the fauna of intertidal mudflats of Minas Basin, Bay of Fundy. Journal of Applied Ichthyology 29:503–509.
- Metcalf and Eddy, Inc. 1994. Biological assessment for the Shortnose Sturgeon (Acipenser brevirostrum) in the lower Penobscot River. U.S. Environmental Protection Agency, Boston.
- Moser, M. L., J. B. Bichy, and S. B. Roberts. 1998. Sturgeon distribution in North Carolina. U.S. Army Corps of Engineers, Wilmington, North Carolina.
- Nelson, T. C., P. Doukakis, S. T. Lindley, A. D. Schreier, J. E. Hightower, L. R. Hildebrand, R. E. Whitlock, and M. A. H. Webb. 2013. Research tools to investigate movements, migrations, and life history of sturgeons (Acipenseridae), with an emphasis on marine-oriented populations. PLOS (Public Library of Science) ONE [online serial] 8:e71552.
- NOAA (National Oceanic and Atmospheric Administration). 2013. Endangered and threatened species; protective regulations for the Gulf of Maine distinct population segment of Atlantic Sturgeon. Federal Register 50:223(19 November 2013):69310–69315.
- Novak, A. J., A. E. Carlson, C. R. Wheeler, G. S. Wippelhauser, and J. A. Sulikowski. 2017. Critical foraging habitat of the Atlantic Sturgeon based on feeding habits, prey distribution, and movement patterns in the Saco River estuary, Maine. Transactions of the American Fisheries Society 146:308–317.
- Oliver, M. J., M. W. Breece, D. A. Fox, D. E. Haulsee, J. T. Kohut, J. Manderson, and T. Savoy. 2015. Shrinking the haystack: using an AUV in an integrated ocean observatory to map Atlantic Sturgeon in the coastal ocean. Fisheries 38:210–216.
- Opperman, J. J., J. Royte, J. Banks, L. R. Day, and C. Apse. 2011. The Penobscot River, Maine, USA: a basin-scale approach to balancing power generation and ecosystem restoration. Ecology and Society [online serial] 16(3):7.
- Renkawitz, M. D., T. F. Sheehan, and G. S. Goulette. 2012. Swimming depth, behavior, and survival of Atlantic Salmon postsmolts in Penobscot Bay, Maine. Transactions of the American Fisheries Society 141:1219–1229.
- Savoy, T., and D. Pacileo. 2003. Movements and important habitats of subadult Atlantic Sturgeon in Connecticut waters. Transactions of the American Fisheries Society 132:1–8.
- Scott, W. B., and E. J. Crossman. 1973. Freshwater fishes of Canada. Fisheries Research Board of Canada Bulletin 184.
- Secor, D. H., and T. E. Gunderson. 1998. Effects of hypoxia and temperature on survival, growth, and respiration of juvenile Atlantic Sturgeon (Acipenser oxyrinchus). U.S. National Marine Fisheries Service Fishery Bulletin 96:603–613.
- Secor, D. H., and J. R. Waldman. 1999. Historical abundance of Delaware Bay Atlantic Sturgeon and potential rate of recovery. Pages 203–216 in J. A. Musick, editor. Life in the slow lane: ecology and conservation of long-lived marine animals. American Fisheries Society, Symposium 23, Bethesda, Maryland.
- Shirey, C. A. 1995. Delaware River Atlantic Sturgeon tagging program. Delaware Division of Fish and Wildlife, Dover.
- Shorey, W. K. 1973. Macrobenthic ecology of a sawdust-bearing substrate in the Penobscot River estuary (Maine). Journal of the Fisheries Research Board of Canada 30:493–497.
- Smith, T. I. J. 1985. The fishery, biology, and management of Atlantic Sturgeon, Acipenser oxyrinchus, in North America. Environmental Biology of Fishes 14:61–62.
- Smith, T. I. J., and J. P. Clugston. 1997. Status and management of Atlantic Sturgeon, Acipenser oxyrinchus, in North America. Environmental Biology of Fishes 48:335–346.
- Stein, A. B., K. D. Friedland, and M. Sutherland. 2004a. Atlantic Sturgeon marine distribution and habitat use along the northeastern coast of the United States. Transactions of the American Fisheries Society 133:527–537.
- Stein, A. B., K. D. Friedland, and M. Sutherland. 2004b. Atlantic Sturgeon marine bycatch and mortality on the continental shelf of the northeast United States. North American Journal of Fisheries Management 24:171–183.
- Stich, D. S., G. B. Zydlewski, J. F. Kocik, and J. D. Zydlewski. 2015. Linking behavior, physiology, and survival of Atlantic Salmon smolts during estuary migration. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science [online serial] 7:68–86.
- Stokesbury, M. J., L. M. Logan-Chesney, M. F. McLean, C. F. Buhariwalla, A. M. Redden, J. W. Beardsall, J. E. Broome, and M. J. Dadswell. 2016. Atlantic Sturgeon spatial and temporal distribution in Minas Passage, Nova Scotia, Canada, a region of future tidal energy extraction. PLOS (Public Library of Science) ONE [online serial] 11(7):e0158387.
- Taylor, A. D., K. Ohashi, J. Sheng, and M. K. Litvak. 2016. Oceanic distribution, behaviour, and a winter aggregation area of adult Atlantic Sturgeon, Acipenser oxyrinchus oxyrinchus, in the Bay of Fundy, Canada. PLOS (Public Library of Science) ONE [online serial] 11(4):e0152470.
- Timoshkin, V. P. 1968. Atlantic Sturgeon (Acipenser sturio L.) caught at sea. Journal of Ichthyology 8:598.
- Van Eenennamm, J. P., S. I. Doroshov, G. P. Moberg, J. G. Watson, D. S. Moore, and J. Linares. 1996. Reproductive conditions of the Atlantic Sturgeon (Acipenser oxyrinchus) in the Hudson River. Estuaries 19:769–777.
- Waldman, J. R., T. King, T. Savoy, L. Maceda, C. Grunwald, and I. Wirgin. 2013. Stock origins of subadult and adult Atlantic Sturgeon, Acipenser oxyrinchus, in a nonnatal estuary, Long Island Sound. Estuaries and Coasts 36:257–267.
- Wegener, M. T. 2012. Reproduction of Shortnose Sturgeon in the Gulf of Maine: a modeling and acoustic telemetry assessment. Master’s thesis. University of Maine, Orono.
- Wippelhauser, G. S., and T. S. Squiers. 2015. Shortnose Sturgeon and Atlantic Sturgeon in the Kennebec River system, Maine: a 1977–2001 retrospective of abundance and important habitat. Transactions of the American Fisheries Society 144:591–601.
- Wippelhauser, G. S., J. Sulikowski, G. B. Zydlewski, M. A. Altenritter, M. Kieffer, and M. T. Kinnison. 2017. Movements of Atlantic Sturgeon of the Gulf of Maine inside and outside the geographically defined distinct population segment. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science [online serial] 9:1–15.
- Wippelhauser, G. S., G. B. Zydlewski, M. Kieffer, J. Sulikowski, and M. T. Kinnison. 2015. Shortnose Sturgeon in the Gulf of Maine: use of spawning habitat in the Kennebec system and response to dam removal. Transactions of the American Fisheries Society 144:742–752.
- Wirgin, I., M. W. Breece, D. A. Fox, L. Maceda, K. W. Wark, and T. King. 2015a. Origin of Atlantic Sturgeon collected off the Delaware coast during spring months. North American Journal of Fisheries Management 35:20–30.
- Wirgin, I., L. Maceda, C. Grunwald, and T. L. King. 2015b. Population origin of Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus bycatch in U.S. Atlantic coast fisheries. Journal of Fish Biology 86:1251–1270.
- Wirgin, I., L. Maceda, J. R. Waldman, S. Wehrell, M. Dadswell, and T. King. 2012. Stock origin of migratory Atlantic Sturgeon in Minas Basin, Inner Bay of Fundy, Canada, determined by microsatellite and mitochondrial DNA analyses. Transactions of the American Fisheries Society 141:1389–1398.