Abstract
Anesthetizing fish facilitates handling procedures such as hormone injections to induce spawning, reduces risk of injuries, and may reduce stress response. We conducted two experiments with wild Hickory Shad Alosa mediocris caught by angling during spawning season. Induction and recovery times of fish anesthetized with tricaine methanesulfonate (MS-222 at 50–100 mg/L of water), 2-phenoxyethanol (PhE at 25–400 mg/L), and eugenol (Aqui-S, 25–100 mg/L) were compared in the first experiment, while physiological responses in fish following anesthesia were compared in the second experiment. Total time to induce and recover from anesthesia in fish was lowest with MS-222 at 100 mg/L and eugenol at 100 mg/L. External signs of stress (muscle spasms, coughing, decreased respiration) were evident in fish during anesthesia with highest concentration of PhE (400 mg/L), while physiological indicators of toxicity were evident in fish anesthetized with a lower concentration of PhE (100 mg/L) compared with controls (i.e., decreased total proteins and increased hemolysis, plasma lactate, and glucose). Reduced stress response (lower glucose and plasma lactate) was evident in fish treated with MS-222 (100 mg/L) at 1 h postinjection and with eugenol (100 mg/L) at 6 h. Both MS-222 and eugenol were effective in rapidly anesthetizing wild Hickory Shad with fewer signs of stress than PhE; however, more information is needed regarding effects of these anesthetics on spawning, gamete quality, and fertilization success in hickory shad and other species.
Received December 16, 2016; accepted April 14, 2017
Commercial landings of Hickory Shad Alosa mediocris, an anadromous species found along the Atlantic coast of North America, declined in Chesapeake Bay from 1959 to 1977, similar to other clupeid species (Klauda et al. Citation1991). In response to stock declines, the state of Maryland closed commercial and recreational landings of Hickory Shad in 1981 (Speir Citation1987). Declines have been attributed to overfishing, blockage of spawning rivers by dams, and degradation of habitat in spawning areas (Speir Citation1987; Klauda et al. Citation1991). Recently, there has been a resurgence in Hickory Shad spawning runs in some upper Chesapeake Bay tributaries, including the Susquehanna River (Maryland Department of Natural Resources, unpublished data). This increased abundance in spawning fish has allowed Maryland Department of Natural Resources (DNR) to implement a captive propagation program aimed at restocking fish in tributaries with poor spawning runs, such as the Choptank River.
Wild fish collected for propagation purposes are subject to a number of potential stressors stemming from capture, transport, and handling. Physical injuries from netting, angling or electrofishing (Chopin and Arimoto Citation1995; Siepker et al. Citation2007; Panek and Densmore Citation2013), crowding and deterioration in water quality in holding tanks, handling procedures, and air-exposure can stimulate or contribute to a series of nonspecific physiological changes in fish (Carmichael et al. Citation1984; Arends et al. Citation1999; Acerete et al. Citation2004). Stress response in fish involves an initial neuroendocrine-stimulated release of catecholamines and corticosteroids into circulation (Wendelaar Bonga Citation1997; Barton Citation2002) and can lead to changes in metabolism, hydro-mineral balance, cardiovascular, and respiratory and immune functions (Barton and Iwama Citation1991; Martínez-Porchas et al. Citation2009). The physiological changes induced in fish during a stress response are adaptive mechanisms that provide a burst of energy and enhance the ability of fish to maintain homeostasis (Mazeaud et al. Citation1977; Martínez-Porchas et al. Citation2009), but those responses may be harmful or lethal if the stressors are sufficiently severe or prolonged.
Anesthetic drugs are often used to calm or immobilize fish for data or tissue collection procedures and can reduce some indications of secondary stress response and help minimize injuries to the fish and biologist (Ross and Ross Citation2008). There are, however, few anesthetic choices available for fish research, and the effects of anesthesia in many fish species are unknown (Bowker et al. Citation2015). Tricaine methanesulfonate (MS-222) has been one of the most widely used anesthetics in fisheries research. Currently, MS-222 is the only approved anesthetic drug in the USA, but there are limitations for its use including a 21-d withdrawal period before fish can be released into the wild or used for human consumption. Additional anesthetics under review for approval by the U.S. Food and Drug Administration include isoeugenol, the active ingredient in Aqui-S (50% isoeugenol), eugenol, the active ingredient in Aqui-S 20E (10% eugenol), and benzocaine, the active ingredient in Benzoac (20% benzocaine). Other compounds have been used as anesthetics in fish research including 2-phenoxyethanol (PhE), quinaldine, metomidate, and carbon dioxide (Ross and Ross Citation2008). Chemical anesthetics can elicit a number of physiological side effects in fish, including hematologic changes, changes in plasma chemistry, hypoxia, hypercapnia, and hyperglycemia (Summerfelt and Smith Citation1990; Ross and Ross Citation2008; Gause et al. Citation2012; Trushenski et al. Citation2012b). Additionally, anesthesia may have detrimental effects on a variety of reproductive functions such as sperm motility, fertilization, and egg hatching success (Campbell et al. Citation1992; Schreck et al. Citation2001; Wagner et al. Citation2002; Gabriel et al. Citation2015).
The ability to immobilize Hickory Shad is an important component of current methods to handle shad and other species for captive propagation in Maryland and elsewhere. The calming effect of anesthetics facilitates data collection and injection of fish with gonadotropin-releasing hormone analog to stimulate spawning (Mylonas et al. Citation1995). Although previously used by Maryland DNR, PhE and other anesthetics commonly used (MS-222) or under current investigation for approval (eugenol) have not been evaluated for use in Hickory Shad. The goal of this study was to evaluate the efficacy of MS-222, PhE, and eugenol to achieve anesthesia and to evaluate physiological responses in fish to these anesthetics using select hematology (hematocrit [HCT] and hemoglobin [HGB]) and plasma chemistry (total protein [TP], glucose, and lactate) analytes. Anesthesia was defined here as stage III, plane 1 light anesthesia according to Zahl et al. (Citation2012). At this stage, fish exhibit loss of equilibrium, slow movement of pectoral and caudal fins, loss of ability to swim, steady ventilation, and lack of response to general handling.
METHODS
Field sampling.—Hickory shad were collected for this study over three consecutive days during spawning season (April 2014) via angling along the banks of the Susquehanna State Park (Susquehanna River), Maryland. Artificial lures known as “shad darts” were used to catch fish. All fish were hooked in the mouth parts and time to land fish was typically <20 s. Landed fish were transferred immediately to one of two holding tanks (2.6 m in diameter), which were in close to the angling area and contained river water that was aerated with oxygen and circulated in a closed loop with water pumps. Holding tanks were filled with water (4,000 L each) each morning prior to angling effort.
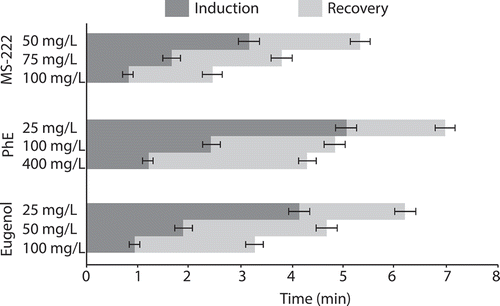
Experiment 1: anesthesia induction and recovery.—Angling occurred over a period of about 60 min on the morning of day 1 until 81 fish were stocked into holding tanks. Dose response trials were conducted using three different concentrations of each anesthetic drug: MS-222 at 50, 75, and 100 mg/L of water (Syndel USA, Ferndale, Washington); PhE at 25, 100, and 400 mg/L of water (Acros Organics, Waltham, Massachusetts); and Aqui-S 20 E at 25, 50, and 100 mg/L of water (Aqui-S, New Zealand, Lower Hutt, New Zealand). Solutions of each anesthetic were prepared from a single stock solution, and sodium bicarbonate (50–100 mg/L) was added to each MS-222 treatment tank as a pH buffer. Sodium bicarbonate was predissolved in river water to allow carbon dioxide to off-gas before inducing fish. Fish inductions were performed using one anesthetic drug at a time. The anesthetic concentrations were prepared in three fiberglass tank (1 m diameter), each containing 225 L of river water, and a fourth tank was used to recover fish from anesthesia. After treatment tanks were prepared with an anesthetic drug, the solutions were gently aerated with oxygen, and fish were netted from the holding tanks, three at a time, and placed, one fish each, in the three treatment tanks. Fish were observed and elapsed time until anesthetization was recorded. Once anesthetized, fish were weighed (g), total length measured (mm), and sex was noted by gamete expression from the vent. Fish were then transferred to a recovery tank, and time for fish to re-established equilibrium was recorded. Two additional batches of three fish were netted from the holding tanks and anesthetized, one fish at a time, in each of the three anesthetic treatment concentrations. Treatment and recovery tanks were drained, rinsed, and refilled with river water after every three rounds of fish inductions (three fish/treatment concentration). Anesthetic drugs were alternated during experiment 1 until a total of nine fish were induced in each of the three concentrations for each drug. Alternating with fresh anesthetic solutions served to reduce handling or treatment bias, minimize fouling of tanks, and thus, ensured anesthetic efficacy. Each fish was induced only once and portable water quality instruments (Hydrolab, OTT Hydromet, Loveland, Colorado) were used to record water quality information from the river during angling and in tanks (holding, treatment, and recovery) throughout experiment 1.
Experiment 2: effects of anesthesia on blood values.—On the morning of the second consecutive day of this study, two holding tanks were filled with river water and aerated with oxygen. Angling effort resumed for about 90 min until 64 male and 64 female hickory shad were collected and placed into holding tanks. Four identical treatment tanks (225 L each) were prepared with the following solutions in river water: tank 1 had no additive (control), tank 2 had MS-222 at 100 mg/L of water buffered with 100 mg/L sodium bicarbonate, tank 3 had PhE at 100 mg/L of water; and tank 4 had eugenol at 100 mg/L of water. Anesthetic concentrations were determined based on results of experiment 1. Four identical recovery tanks (1,100 L each) were filled with river water, and all tanks were gently aerated with oxygen. Groups of three fish of the same sex were netted from the holding tanks, and one group of three fish was placed in each of the treatment tanks. Fish were observed until anesthesia was induced in each fish (for the three anesthetic treatments); the control group was observed for 1 min (based on average time to anesthesia in experiment 1). Once anesthetized, one fish from each of the four treatment tanks was removed, 2 mL of blood was collected from the caudal vessels and transferred to a blood collection tube containing lithium heparin (Vacuette, Greiner Bio-One, Monroe, North Carolina), and the fish was euthanatized. The remaining two fish from each treatment group received a color-coded string tied around the caudal peduncle and were placed in one of the four recovery tanks associated with each treatment tank. The purpose of the colored string was to allow identification and timely blood specimen collection at select intervals without causing physical injury to the fish. One fish was netted from each of the recovery tanks 1 h following anesthesia and was bled and euthanatized; the second fish was removed 6 h following anesthesia and was bled and euthanatized. Any reaction by fish to needle insertion was recorded. Intervals of one and 6 h for specimen collection were based on preliminary work with Hickory Shad that indicated significant changes in some blood values (e.g., hematocrit and hemoglobin) by 1 h and delayed changes (e.g., glucose and lactate) by 6 h following capture and handling. Anesthesia treatments alternated between groups of male and female fish until four fish of each sex were bled at each of the select time intervals (0, 1 h, and 6 h postanesthesia). Water and anesthetic solutions were exchanged after every 12 fish, and water quality was monitored in each tank throughout experiment 2. During experiment 2, four male and four female fish were netted from the general population in the holding tanks at 2 h intervals (0–6 h), bled, and euthanatized to assess effects of stress-related changes that might occur in the holding tanks over time. On day 3, an additional 64 male and 64 female fish were captured to repeat the process of day 2 anesthesia treatments, blood specimen collection at select intervals (0, 1 h and 6 h following anesthesia), and sub-sampling fish for blood specimen collection from the holding tanks at 2-h intervals.
Blood specimens.—Each specimen was placed on wet ice and processed within 60 min following collection. We determined HCT by a manual reader following microcentrifugation of two tubes for 5 min at 12,162 × g. A spectrophotometer was used to measure HGB concentration (Hb 201+, HemoCue, Brea, California), lactate concentration (Lactate Plus, Nova Biomedical, Waltham, Massachusetts), and glucose concentration (Ultra 2 OneTouch, Lifescan, Wayne, Pennsylvania). A clinical refractometer (Westover Scientific, Bothell, Washington) was used to measure plasma TP. Duplicate blood smears were prepared with whole blood, air dried, and stained with PROTOCOL Hema-3 (Fisher Scientific, Waltham, Massachusetts) to assess morphology of blood cells following treatments.
Statistical analyses.—Data analyses were performed using SAS Enterprise Guide 4.1 (Davis Citation2007). Blood value data and fish weights and lengths were normally distributed (Kolmogorov–Smirnov cumulative distribution test) and homoscedastic (Bartlett’s test). Effects of sex on anesthesia induction and recovery times were tested with multiple t-tests. Analysis of variance (ANOVA) and general linear models were used to test effects of sampling day, sex, and time post anesthesia (0, 1 h and 6 h) on blood values in fish. Interaction terms for sex × anesthetic and time × anesthetic were included in models. Fish were anesthetized in groups of three in experiment 2, and therefore groups were considered the experimental unit for statistical tests, rather than individual fish. Water quality data (temperature, DO, and pH) for each type of tank (holding and treatment) were tested using ANOVA for effects of day and differences among tanks of the same type (e.g., holding and treatment tanks). Least squares linear regressions were used to test effects of time (0, 2, 4, and 6 h) on blood values of fish in holding tanks; significant change in blood values over time was indicated when slope m was significantly different from 0. Differences were considered significant at α = 0.05.
RESULTS
Water Quality
Water quality in the Susquehanna River was relatively consistent over the 3 d of this study, with a slight increase in mean temperature from 11.5°C (range, 11.1–12.8°C) on day 1 to 12.4°C (12.4–13.0°C) on day 3. Dissolved oxygen decreased slightly from day 1 (range, 10.4–10.7 mg/L) to day 3 (10.0–10.3 mg/L); pH also decreased from day 1 (range, 7.8–8.0) to day 3 (7.5–7.7). Water temperatures were slightly higher and DO was slightly lower in holding tanks than in treatment tanks because water was exchanged more frequently in treatment tanks throughout each day of the study. Difference in mean water temperatures, DO, and pH were not significant each day among the holding tanks (P > 0.3412) and treatment tanks (P > 0.1677). Air temperatures increased during working hours each day of this study (8–16°C on day 1, 10–18°C on day 2, and 10–16°C on day 3).
Anesthesia Induction and Recovery
Induction times decreased with increasing anesthetic concentrations for MS-222, PhE, and eugenol (), and there was no effect of sex on induction or recovery times (P > 0.1268). Induction times were significantly shorter for 100 mg/L MS-222 and 100 mg/L eugenol than all other anesthetic concentrations (P < 0.0141). Fish induced with 100 and 400 mg/L PhE exhibited coughing, mild twitching of the body or spasms in lateral musculature and cessation of ventilation in some fish; these responses were more pronounced in fish treated with 400 mg/L PhE. Times until fish regained equilibrium (recovery) were variable (). Recovery time in fish was significantly less when anesthetized with 100 mg/L MS-222 than fish induced with all other anesthetic concentrations (P = 0.0034), and total handling time (induction + recovery) was significantly less for 100 mg/L MS-222 and 100 mg/L eugenol than all other anesthetic concentrations (P < 0.0295). The pattern of recovery times in fish varied by anesthetic. In general, recovery times of fish anesthetized with MS-222 decreased with increasing anesthetic concentration, while recovery times of fish anesthetized with PhE increased with increasing anesthetic concentration ().
Physiological Changes following Anesthesia
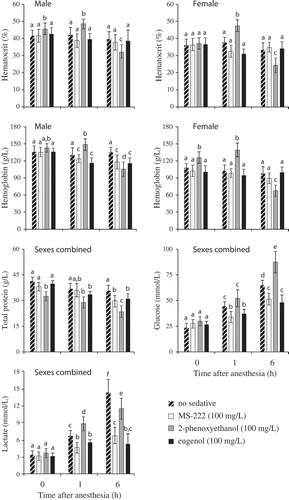
Fish anesthetized with MS-222 and eugenol did not react visibly to needle insertion for blood draws, while fish anesthetized with PhE occasionally exhibited a mild response (slight twitch or curling of tail). There was a significant effect of sex on HCT and HGB (); mean values of these analytes were greater in males than in females (). There was also a significant effect of both time postanesthesia and type of anesthetic on all blood analytes () indicating variable response among the anesthetic drugs. Mean values of HCT, HGB, and TP generally declined over 6 h postanesthesia for MS-222 and eugenol, while glucose and lactate generally increased in mean value over 6 h for all anesthetics (). In contrast, mean HCT and HGB in PhE-induced fish were significantly greater than other treatments at 1 h and significantly lower than other treatments at 6 h (). Concentrations of blood values in fish were not significantly different on day 3 than day 2 of experiment 2. There was a significant interaction of time × anesthetic for HCT, HGB, and glucose (). Examination of blood smears indicated that in general the amount of cell debris and ghost RBC, indicating hemolysis, was greatest in fish anesthetized with PhE and least in fish anesthetized with eugenol. Blood smears from PhE-anesthetized fish indicated moderate hemolysis at 1 h and extensive hemolysis at 6 h postanesthesia. Hemolysis in blood smears was moderate at 6 h from fish anesthetized with MS-222 and was mild at 6 h from eugenol anesthetized fish.
Table 1. Results (P-values) of mixed model ANOVA for blood values in Hickory Shad following sedation (MS-222, PhE, and eugenol). Significant effects (P < 0.05) are in bold italics.
Physiologic Changes in Untreated Fish in Holding Tanks
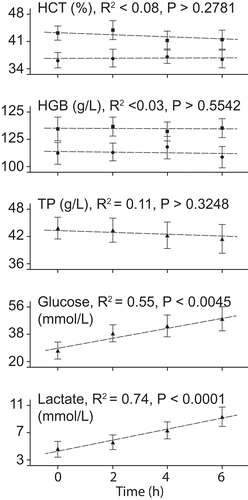
Slope of regression lines for glucose and lactate in fish held unanesthetized for 0–6 h in holding tanks were significantly > 0 (), indicating that mean concentrations of lactate and glucose increased significantly in fish over time.
FIGURE 3. Regression results for blood values of Hickory Shad in holding tanks prior to anesthesia treatments. Separate regressions for males (squares) and females (circles) were performed for hematocrit (HCT) and hemoglobin (HGB), but sexes were combined (triangles) for regressions of total protein (TP), glucose, and lactate. Significant changes in blood values over time indicated when P ≤ 0.05.
DISCUSSION
The results of this study indicate that 75–100 mg/L MS-222, 400 mg/L PhE, and 50–100 mg/L eugenol are effective in anesthetizing wild Hickory Shad in <2 min to a level of anesthesia sufficient for handling (stage III plane 1; Zahl et al. (Citation2012)). Hickory Shad appear to be more sensitive to MS-222 and PhE than another clupeid species. Alewives Alosa pseudoharengus, exhibited extended induction (10 min) and recovery times (14 min) to 100 mg/L MS-222 and required 550 mg/L PhE to achieve a comparable plane of anesthesia (Berlinsky et al. Citation2016). In contrast, adult and juvenile Alewives may be more sensitive to eugenol (35–40 mg/L clove oil) than adult Hickory Shad (Berlinsky et al. Citation2016). Dose response on induction times to stage III, plane 1 anesthesia in juvenile Alewives (decrease from 4.2 to 1.1 min) was similar to adult Hickory Shad (this study) for 75–100 mg/L MS-222; however the recovery times (time to regain equilibrium) from anesthesia in juvenile Alewives were approximately twice that of adult Hickory Shad (Berlinsky et al. Citation2016). Alewives were anesthetized in warmer water (17–18°C) and probably lower dissolved oxygen (Berlinsky et al. Citation2016) than Hickory Shad (this study), which can complicate comparisons.
Effective concentrations and sensitivity to anesthetic drugs can vary among fish species. Doses of 50–150 mg/L MS-222 were commonly used in a wide variety of species to achieve a comparable depth of anesthesia to that of fish in this study, which had induction times of typically <3 min (Ryan Citation1992; King et al. Citation2005; Palić et al. Citation2006; Weber et al. Citation2009; Maricchiolo and Genovese Citation2011). Concentrations >150 mL/L MS-222 have been used to achieve deeper anesthesia or to decrease induction times (Matsche Citation2011) in fish; however, toxicity often increases, and the safety margin decreases with increasing concentration (Topic Popovic et al. Citation2012). Eugenol is widely used to anesthetize fish, both in the form of clove oil and in the synthetic product Aqui-S 20E. Clove oil contains approximately 70–90% eugenol, while Aqui-S 20E contains 10% eugenol (Ross and Ross Citation2008). Concentrations of clove oil used to induce anesthesia in fish have generally varied between 20 and 100 mg/L, or approximately 14–90 mg/L eugenol (Keene et al. Citation1998; King et al. Citation2005; Mylonas et al. Citation2005; Roubach et al. Citation2005). Isoeugenol, formulated in Aqui-S, is often more efficacious than clove oil; eugenol at only 2–30 mg/L is commonly needed to induce anesthesia in fish (Small and Chatakondi Citation2005; Ross and Ross Citation2008; Woods et al. Citation2008). Concentrations of PhE required to induce anesthesia in <2 min (comparable times to this study) ranged from 300 mg/L in European Bass Morone labrax (Mylonas et al. Citation2005), 600 mg/L in juvenile Freshwater Angelfish Pterophyllum scalare (Mitjana et al. Citation2014) and Senegalese Sole Solea senagalensis (Weber et al. Citation2009), to 900 mg/L in Wels Silurus glanis (Velisek et al. Citation2007). There are a number of abiotic factors (e.g., water temperature, pH, salinity) and biotic factors (e.g., species, age, size, weight) that can influence anesthetic drug efficacy (Ross and Ross Citation2008; Javahery et al. Citation2012; Topic Popovic et al. Citation2012).
Choice of anesthetic drug and level of anesthesia induced in fish should be commensurate with procedures to be performed. The anesthetic drug and dose should induce the desired level of anesthesia rapidly and provide analgesic properties for invasive procedures but should not result in marked physiological indicators of stress (Zahl et al. Citation2012). Anesthesia in Hickory Shad to a stage III, plane 1 or light anesthesia (i.e., swimming ability, fight-or-flight response and reaction to stimuli from general handling is lost, ventilation is normal and fish exhibit fin and slight body movements) is sufficient to allow biologists to quickly and easily weigh, measure and inject fish prior to transport for spawning. Stage II (excitatory) and higher planes of anesthesia (Zahl et al. Citation2012) are not appropriate for Hickory Shad procedures because swimming activity and sensitivity to handling are increased and fish are more likely to suffer injuries during handling. Anesthesia (III, plane 2) may be appropriate for more invasive surgical procedures, such as coeliotomy, but typically results in shallow or no ventilation, decreased heart rate, increased recovery times, and decreased margin of safety in fish (Ross and Ross Citation2008). Reaction of fish to needle insertion under anesthesia in this study may indicate reduced efficacy of PhE compared with MS-222 and eugenol.
Transient changes in HCT and circulating glucose, lactate, and other indicator analytes are typically observed in fish in response to chemical anesthesia, either as a result of stimulation of a general stress response or toxic effects of the anesthetic drug (McKim et al. Citation1987; Ross and Ross Citation2008; Topic Popovic et al. Citation2012; Zahl et al. Citation2012). Types and magnitude of hematological changes vary by species, anesthetic drug, and other factors. Anesthesia in fish is often associated with an initial increase in HCT, either from swelling of RBC (Ryan Citation1992; Velisek et al. Citation2007; Gomulka et al. Citation2008; Matsche Citation2011), hemo-concentration from osmotic changes in gill tissues (Marino et al. Citation2001; Sladky et al. Citation2001), or influx of circulating RBC from the spleen to increase respiratory capacity (Wells and Weber Citation1990; Pearson and Stevens Citation1991; Ryan Citation1992). Declines in HCT are also observed in fish following anesthesia, typically 6–24 h postinduction, and may indicate hemolysis from the anesthetic drug (Gomulka et al. Citation2008; Kristan et al. Citation2012) or hemodilution from stresses associated with confinement or repeated sampling following anesthetic treatment (Iwama et al. Citation1989). In our study, increase in HCT and HGB with a decrease in TP at 1 h following PhE anesthesia was probably the result of RBC infusion from splenic reservoir, while subsequent decline in HCT and HGB at 6 h following PhE anesthesia was from extensive hemolysis and hemodilution. Confinement-related stresses over time in holding tanks in this study likely had little impact on HCT, HGB, and TP, but did elicit increases in circulating glucose and lactate. Rapid increase in glucose is induced by catecholamine or corticosteroids and is commonly observed following anesthesia and a wide variety of stressors, including net capture, air exposure, and confinement (Arends et al. Citation1999; Martínez-Porchas et al. Citation2009; Kristan et al. Citation2012; Trushenski et al. Citation2012a). Blood lactate accumulation is the result of anaerobic metabolism, and is often evident following anesthesia (Molinero and Gonzalez Citation1995; Trushenski et al. Citation2012b). Lactate increases in circulation may be associated with hypoxic effects of decreased respiration during anesthesia or hyperactivity immediately preceding anesthesia. Lactate accumulation often occurs 0.5–2 h following anesthesia (Trushenski et al. Citation2012b); some studies may fail to detect transient lactate increases because of inadequate blood sampling intervals (Kristan et al. Citation2012; Lepic et al. Citation2014). A decrease in circulating lactate has also been observed following anesthesia in some studies (Velisek et al. Citation2011).
Separating the physiological effects of anesthetic drugs from physical aspects of handling (e.g., capture methods and types of confinement) can be important to properly evaluate and develop fish handling practices for captive spawning or other purposes (Matsche Citation2013). Air exposure, confinement, and a variety of procedures such as phlebotomy can induce and contribute to stress response from anesthesia (Arends et al. Citation1999; Barton and Iwama Citation1991; Acerete et al. Citation2004). In our study, fish capture from angling occurred over a 90-min period preceding anesthetic treatments, which likely contributed to the overall stress response and introduced additional variability in the blood data. Comparison of blood values from anesthetized fish to controls indicated that handling and confinement resulted in mild or no hematologic changes, but a marked increase in glucose and lactate. Stresses from confinement in holding tanks probably contributed to the increases in glucose and lactate concentrations in fish at 6 h postinduction. The anesthetics MS-222 and eugenol significantly reduced some indicators of stress response (glucose and lactate), while PhE anesthesia contributed to a significantly greater stress response above that of handling and confinement. In some fish, PhE may elicit significant stress response, decrease respiratory capacity, have poor analgesic properties, irritate mucous membranes, adhere to gill tissue, and have a low safety margin (Velíšek and Svobodova Citation2004; Velisek et al. Citation2007; Mitjana et al. Citation2014). Adverse effects for other anesthetic drugs such as MS-222 have been reported, including hypoxia, hypercapnia, hyperglycemia, elevated cortisol, respiratory failure, and cardiovascular changes; the severity of response and safety margin can vary markedly by species, life stage, water quality, and other conditions (Ross and Ross Citation2008). Preliminary dose response tests are advised before anesthetic drugs are used for fisheries research.
The condition and sex-specific differences in hickory shad on spawning grounds in the Susquehanna River and elsewhere are the result of a culmination of stresses and physiological changes that occur in fish in preparation of spawning activity during a long-distance migration from the Atlantic Ocean. Upstream migration is energetically demanding and stressful, perhaps more so for female shad (Leonard and McCormick Citation1999a, Citation1999b). Capture and handling shad for captive propagation after upstream migration will compound physiological stress. The purpose of Hickory Shad sampling, however, is captive propagation, and therefore effective anesthetics, such as MS-222 and eugenol, need to be further evaluated for potential effects on gamete function and fertilization success.
ACKNOWLEDGMENTS
This project was made possible by the assistance of the staffs of Fish and Wildlife Health Program, Anadromous Fish Restoration Program, and the Joseph Manning State Fish Hatchery, Maryland DNR. Funding for this project was provided by Maryland DNR. The author declares no conflict of interest including financial or otherwise with the companies, products, or organizations mentioned in this study.
References
- Acerete, L., J. C. Balasch, E. Espinosa, A. Josa, and L. Tort. 2004. Physiological responses in Eurasian Perch (Perca fluviatilis, L.) subjected to stress by transport and handling. Aquaculture 237:167–178.
- Arends, R., J. Mancera, J. Munoz, S. W. Bonga, and G. Flik. 1999. The stress response of the Gilthead Sea Bream (Sparus aurata L.) to air exposure and confinement. Journal of Endocrinology 163:149–157.
- Barton, B. A. 2002. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integrative and Comparative Biology 42:517–525.
- Barton, B. A., and G. K. Iwama. 1991. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annual Review of Fish Diseases 1:3–26.
- Berlinsky, D. L., M. T. Watson, M. A. DiMaggio, and T. S. Breton. 2016. The use of tricaine methanesulfonate, clove oil, metomidate, and 2-phenoxyethanol for anesthesia induction in alewives. North American Journal of Aquaculture 78:84–91.
- Bowker, J. D., J. T. Trushenski, D. C. Glover, D. G. Carty, and N. Wandelear. 2015. Sedative options for fish research: a brief review with new data on sedation of warm-, cool-, and coldwater fishes and recommendations for the drug approval process. Reviews in Fish Biology and Fisheries 25:147–163.
- Campbell, P., T. Pottinger, and J. Sumpter. 1992. Stress reduces the quality of gametes produced by Rainbow Trout. Biology of Reproduction 47:1140–1150.
- Carmichael, G., J. Tomasso, B. Simco, and K. Davis. 1984. Confinement and water quality-induced stress in largemouth bass. Transactions of the American Fisheries Society 113:767–777.
- Chopin, F. S., and T. Arimoto. 1995. The condition of fish escaping from fishing gears—a review. Fisheries Research 21:315–327.
- Davis, J. 2007. Statistics using SAS enterprise guide. SAS Institute, Cary, North Carolina.
- Gabriel, U., S. Deekae, O. Akinrotimi, and O. Orokotan. 2015. Assessment of efficacy and egg hatchability in African catfish (Clarias gariepinus) exposed to anaesthetic metomidate. ARC Journal of Animal and Veterinary Sciences 1:24–31.
- Gause, B. R., J. T. Trushenski, J. C. Bowzer, and J. D. Bowker. 2012. Efficacy and physiological responses of Grass Carp to different sedation techniques: I. Effects of various chemicals on sedation and blood chemistry. North American Journal of Aquaculture 74:560–566.
- Gomulka, P., T. Wlasow, J. Velíšek, Z. Svobodová, and E. Chmielinska. 2008. Effects of eugenol and MS-222 anaesthesia on Siberian Sturgeon Acipenser baerii Brandt. Acta Veterinaria Brno 77:447–453.
- Iwama, G. K., J. C. McGeer, and M. P. Pawluk. 1989. The effects of five fish anaesthetics on acid-base balance, hematocrit, cortisol and adrenaline in Rainbow Trout. Canadian Journal of Zoology 67:2065–2073.
- Javahery, S., H. Nekoubin, and A. H. Moradlu. 2012. Effect of anaesthesia with clove oil in fish (review). Fish Physiology and Biochemistry 38:1545–1552.
- Keene, J., D. Noakes, R. Moccia, and C. Soto. 1998. The efficacy of clove oil as an anaesthetic for Rainbow Trout, Oncorhynchus mykiss (Walbaum). Aquaculture Research 29:89–101.
- King, W., B. Hooper, S. Hillsgrove, C. Benton, and D. L. Berlinsky. 2005. The use of clove oil, metomidate, tricaine methanesulphonate and 2-phenoxyethanol for inducing anaesthesia and their effect on the cortisol stress response in Black Sea Bass (Centropristis striata L.). Aquaculture Research 36:1442–1449.
- Klauda, R. J., S. A. Fischer, L. W. Hall Jr., and J. A. Sullivan. 1991. American Shad and Hickory Shad. Pages 9–1 to 9–27 in S. Funderburk, J. Mihursky, S. Jordan, and D. Riley, editors. Habitat requirements for Chesapeake Bay living resources, 2nd edition. U.S. Environmental Protection Agency, Chesapeake Bay Program, Annapolis, Maryland.
- Kristan, J., A. Stara, J. Turek, T. Policar, and J. Velisek. 2012. Comparison of the effects of four anaesthetics on haematological and blood biochemical profiles in pikeperch (Sander lucioperca L.). Neuroendocrinology Letter 33:66–71.
- Leonard, J., and S. McCormick. 1999a. The effect of migration distance and timing on metabolic enzyme activity in an anadromous clupeid, the American Shad (Alosa sapidissima). Fish Physiology and Biochemistry 20:163–179.
- Leonard, J. B., and S. D. McCormick. 1999b. Effects of migration distance on whole-body and tissue-specific energy use in American Shad (Alosa sapidissima). Canadian Journal of Fisheries and Aquatic Sciences 56:1159–1171.
- Lepic, P., A. Stara, J. Turek, P. Kozak, and J. Velisek. 2014. The effects of four anaesthetics on haematological and blood biochemical profiles in Vimba Bream, Vimba vimba. Veterinární Medicína 59:81–87.
- Maricchiolo, G., and L. Genovese. 2011. Some contributions to knowledge of stress response in innovative species with particular focus on the use of the anaesthetics. Open Marine Biology Journal 5:24–33.
- Marino, G., P. Di Marco, A. Mandich, M. Finoia, and S. Cataudella. 2001. Changes in serum cortisol, metabolites, osmotic pressure and electrolytes in response to different blood sampling procedures in cultured sea bass (Dicentrarchus labrax L.). Journal of Applied Ichthyology 17:115–120.
- Martínez-Porchas, M., L. R. Martínez-Córdova, and R. Ramos-Enriquez. 2009. Cortisol and glucose: reliable indicators of fish stress? Pan-American Journal of Aquatic Sciences 4:158–178.
- Matsche, M. A. 2011. Evaluation of tricaine methanesulfonate (MS-222) as a surgical anesthetic for Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus. Journal of Applied Ichthyology 27:600–610.
- Matsche, M. A. 2013. Relative physiological effects of laparoscopic surgery and anesthesia with tricaine methanesulfonate (MS-222) in Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus. Journal of Applied Ichthyology 29:510–519.
- Mazeaud, M., F. Mazeaud, and E. Donaldson. 1977. Primary and secondary effects of stress in fish: some new data with a general review. Transactions of the American Fisheries Society 106:201–212.
- McKim, J. M., P. K. Schmieder, R. W. Carlson, E. P. Hunt, and G. J. Niemi. 1987. Use of respiratory-cardiovascular responses of Rainbow Trout (Salmo gairdneri) in identifying acute toxicity syndromes in fish: part 1. Pentachlorophenol, 2, 4-dinitrophenol, tricaine methanesulfonate and 1-octanol. Environmental Toxicology and Chemistry 6:295–312.
- Mitjana, O., C. Bonastre, D. Insua, M. V. Falceto, J. Esteban, A. Josa, and E. Espinosa. 2014. The efficacy and effect of repeated exposure to 2-phenoxyethanol, clove oil and tricaine methanesulphonate as anesthetic agents on juvenile Angelfish (Pterophyllum scalare). Aquaculture 433:491–495.
- Molinero, A., and J. Gonzalez. 1995. Comparative effects of MS-222 and 2-phenoxyethanol on Gilthead Sea Bream (Sparus aurata L.) during confinement. Comparative Biochemistry and Physiology A 111:405–414.
- Mylonas, C. C., G. Cardinaletti, I. Sigelaki, and A. Polzonetti-Magni. 2005. Comparative efficacy of clove oil and 2-phenoxyethanol as anesthetics in the aquaculture of European Sea Bass (Dicentrarchus labrax) and Gilthead Sea Bream (Sparus aurata) at different temperatures. Aquaculture 246:467–481.
- Mylonas, C. C., Y. Zohar, B. M. Richardson, and S. P. Minkkinen. 1995. Induced spawning of wild American Shad Alosa sapidissima using sustained administration of gonadotropin-releasing hormone analog (GnRHa). Journal of the World Aquaculture Society 26:240–251.
- Palić, D., D. Herolt, C. Andreasen, B. Menzel, and J. Roth. 2006. Anesthetic efficacy of tricaine methanesulfonate, metomidate and eugenol: effects on plasma cortisol concentration and neutrophil function in Fathead Minnows (Pimephales promelas Rafinesque, 1820). Aquaculture 254:675–685.
- Panek, F. M., and C. L. Densmore. 2013. Frequency and severity of trauma in fishes subjected to multiple-pass depletion electrofishing. North American Journal of Fisheries Management 33:178–185.
- Pearson, M., and E. Stevens. 1991. Size and hematological impact of the splenic erythrocyte reservoir in Rainbow Trout, Oncorhynchus mykiss. Fish Physiology and Biochemistry 9:39–50.
- Ross, L., and B. Ross. 2008. Anaesthetic and sedative techniques for aquatic animals. Blackwell Publishing, Oxford, UK.
- Roubach, R., L. C. Gomes, F. A. Leão Fonseca, and A. L. Val. 2005. Eugenol as an efficacious anaesthetic for Tambaqui, Colossoma macropomum (Cuvier). Aquaculture Research 36:1056–1061.
- Ryan, S. 1992. The dynamics of MS-222 anaesthesia in a marine teleost (Pagrus auratus: sparidae). Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 101:593–600.
- Schreck, C. B., W. Contreras-Sanchez, and M. S. Fitzpatrick. 2001. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 197:3–24.
- Siepker, M., K. Ostrand, S. Cooke, D. Philipp, and D. Wahl. 2007. A review of the effects of catch-and-release angling on black bass, Micropterus spp.: implications for conservation and management of populations. Fisheries Management and Ecology 14:91–101.
- Sladky, K., C. Swanson, M. Stoskopf, M. Loomis, and G. Lewbart. 2001. Comparative efficacy of tricaine methanesulfonate and clove oil for use as anesthetics in Red Pacu (Piaractus brachypomus). American Journal of Veterinary Research 62:337–342.
- Small, B. C., and N. Chatakondi. 2005. Routine measures of stress are reduced in mature channel catfish during and after AQUI-S anesthesia and recovery. North American Journal of Aquaculture 67:72–78.
- Speir, H. J. 1987. Status of some finfish stocks in the Chesapeake Bay. Water, Air, and Soil Pollution 35:49–62.
- Summerfelt, R., and L. Smith. 1990. Anesthesia, surgery, and related techniques. Pages 213–272 in C. B. Schreck and P. Moyle, editors. Methods for fish biology. American Fisheries Society, Bethesda, Maryland.
- Topic Popovic, N., I. Strunjak-Perovic, R. Coz-Rakovac, J. Barisic, M. Jadan, A. Persin Berakovic, and R. Sauerborn Klobucar. 2012. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. Journal of Applied Ichthyology 28:553–564.
- Trushenski, J. T., J. D. Bowker, B. R. Gause, and B. L. Mulligan. 2012a. Chemical and electrical approaches to sedation of hybrid Striped Bass: induction, recovery, and physiological responses to sedation. Transactions of the American Fisheries Society 141:455–467.
- Trushenski, J. T., J. D. Bowker, B. L. Mulligan, and B. R. Gause. 2012b. Induction, recovery, and hematological responses of Largemouth Bass to chemo-and electrosedation. North American Journal of Aquaculture 74:214–223.
- Velisek, J., A. Stara., Z.-H. Li, S. Silovska, and J. Turek. 2011. Comparison of the effects of four anaesthetics on blood biochemical profiles and oxidative stress biomarkers in Rainbow Trout. Aquaculture 310:369–375.
- Velíšek, J., and Z. Svobodova. 2004. Anaesthesia of Common Carp (Cyprinus carpio L.) with 2-phenoxyethanol: acute toxicity and effects on biochemical blood profile. Acta Veterinaria Brno 73:247–252.
- Velisek, J., T. Wlasow, P. Gomulka, Z. Svobodova, and L. Novotny. 2007. Effects of 2-phenoxyethanol anaesthesia on Sheatfish (Silurus glanis L.). Veterinární Medicína 52:103–110.
- Wagner, E., R. Arndt, and B. Hilton. 2002. Physiological stress responses, egg survival and sperm motility for Rainbow Trout broodstock anesthetized with clove oil, tricaine methanesulfonate or carbon dioxide. Aquaculture 211:353–366.
- Weber, R., J. Peleteiro, L. Martín, and M. Aldegunde. 2009. The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese Sole (Solea senegalensis Kaup 1858). Aquaculture 288:147–150.
- Wells, R., and R. Weber. 1990. The spleen in hypoxic and exercised Rainbow Trout. Journal of Experimental Biology 150:461–466.
- Wendelaar Bonga, S. 1997. The stress response in fish. Physiological Reviews 77:591–625.
- Woods, L. C. III, D. D. Theisen, and S. He. 2008. Efficacy of Aqui-S as an anesthetic for market-sized Striped Bass. North American Journal of Aquaculture 70:219–222.
- Zahl, I. H., O. Samuelsen, and A. Kiessling. 2012. Anaesthesia of farmed fish: implications for welfare. Fish Physiology and Biochemistry 38:201–218.