Abstract
Chinook Salmon Oncorhynchus tshawytscha from western Alaska have experienced recent declines in abundance, size, and age at maturity. Declines have led to hardships for the region’s subsistence and commercial salmon harvesters, prompting calls to better understand factors affecting the life history of these populations. Western Alaskan Chinook Salmon are thought to spend their entire marine residency in the Bering Sea. The Bering Sea ecosystem demonstrates high interannual variability that is largely driven by the annual extent of sea ice. However, warming is expected to supersede interannual variability in the next several decades as a consequence of climate change. We investigated the influence of sea surface temperatures (SSTs) on the life history of western Alaskan Chinook Salmon by using information from two regional populations subject to long-term monitoring. We found strong correlations between early marine growth and SSTs. Warmer SSTs appeared to lead to a younger age at maturity, largely through the vector of augmented growth. However, we also present evidence that warmer SSTs may additionally decrease the average age of male recruits through reduced growth thresholds for early male maturation. Our results suggest that the anticipated warming of the Bering Sea will lead to higher early marine growth and a younger average age of maturation for western Alaskan Chinook Salmon.
Received March 30, 2017; accepted July 4, 2017
Populations of Chinook Salmon Oncorhynchus tshawytscha in western Alaska have declined in abundance since the late 1990s. At the same time, decreases in the size and age of fish have also been observed (Lewis et al. Citation2015), causing concern that diminishing individual reproductive success might be contributing to low returns. Low returns have led to restrictions on commercial and subsistence harvests as managers aim to meet escapement goals. The Chinook Salmon subsistence fisheries in the Yukon–Kuskokwim River delta region are the largest in Alaska (Fall Citation2016), and commercial fisheries provide a unique source of income in regional rural communities (Howe and Martin Citation2009). Thus, restrictions have led to substantial hardships for the region’s rural residents, culminating in recent disaster declarations (Alaska Department of Fish and Game Citation2013). There is accordingly considerable interest in better understanding the factors controlling the life history of western Alaskan Chinook Salmon.
A changing marine environment has been proposed as a possible source of population declines (Myers et al. Citation2010; Schindler et al. Citation2013). The physical environment (e.g., sea surface temperature [SST] and salinity) can directly influence rates of the physiological processes involved in salmon survival, growth, and maturation. Additionally, the marine environment can have a profound impact on the food web that supports salmon (Aydin and Mueter Citation2007; Eisner et al. Citation2014; Hertz et al. Citation2016). Western Alaskan Chinook Salmon are thought to spend their entire marine residency in the Bering Sea (Myers et al. Citation2010). Climate patterns in the Bering Sea over the last century have demonstrated interannual and decadal-scale variability characterized by warm and cold periods. These periods represent substantial shifts in air temperatures, SSTs, and sea ice extent (Stabeno et al. Citation2012). The Bering Sea climate has been shown to exert a profound impact on the survival and recruitment of zooplankton taxa (Eisner et al. Citation2014), with demonstrated cascading effects on higher taxa. Climate affects growth and condition of Chinook Salmon (Farley et al. Citation2009; Myers et al. Citation2010); their competitors, such as Walleye Pollock Gadus chalcogrammus (Hunt et al. Citation2011) and juvenile Pink Salmon O. gorbuscha and Chum Salmon O. keta (Wechter et al. Citation2016); and their prey, such as Capelin Mallotus villosus and Pacific Herring Clupea pallasii (Andrews et al. Citation2016). Accordingly, an investigation of how Chinook Salmon have responded to this climate variability in the past may help to illuminate the causes of recent population declines.
An understanding of how western Alaskan Chinook Salmon have responded to ecosystem variability in the past may also provide insights into how they will respond to future climate change. Climate model projections predict that major reductions in sea ice and increases in SST will occur in the Bering Sea during the 21st century (Wang et al. Citation2012). Although considerable natural climatic variability in the Bering Sea makes distinguishing the effects of climate change difficult on a short time scale, warming of the ocean surface is expected to supersede natural variability by mid-century (Wang et al. Citation2010). Consequently, Chinook Salmon in the Bering Sea will experience an altered ecosystem with unprecedented conditions in coming decades.
There is accumulating evidence that Chinook Salmon growth in the Bering Sea has been limited by temperature (Farley et al. Citation2009; Myers et al. Citation2010; McPhee et al. Citation2016). In ectotherms such as salmon, warmer temperatures increase both maintenance metabolic demands and growth potential. If prey are readily available to meet augmented energetic demands, then warmer temperatures below detrimental levels will lead to higher growth. Conversely, if prey are limited, energetic demand may outweigh energy intake, and growth will diminish at higher temperatures (e.g., Daly and Brodeur Citation2015). The Bering Sea represents the northern extent of the species’ range; thus, temperatures tend to be cooler than those in other important marine rearing areas, such as the Gulf of Alaska. Myers et al. (Citation2010) found that the first year of marine growth of Chinook Salmon in the Bering Sea was positively correlated with warm El Niño events and that second-year marine growth was positively correlated with the Pacific Decadal Oscillation and direct measures of SST in the eastern Bering Sea. These correlations suggest that Chinook Salmon in the Bering Sea have not been limited by the quantity and quality of available prey during warmer periods and thus have been able to capitalize on higher growth potentials due to warmer temperatures.
Growth-dependent survival during the first year at sea is thought to be a common driver of productivity in salmon populations (Beamish and Mahnken Citation2001). Larger fish are better able to avoid predators as a result of faster swimming speeds and outgrowing the gapes of predators. Previous work in the Yukon River detected size-selective mortality in the first summer of marine growth by comparing the distribution of marine-caught juveniles to back-calculated juvenile lengths from surviving adults in the Canadian-origin population (Murphy et al. Citation2013). Thus, warmer marine temperatures may lead to increased survival of western Alaskan Chinook Salmon as a consequence of a decrease in size-selective mortality with augmented growth (Farley et al. Citation2009).
The analysis of factors affecting the life history of western Alaskan Chinook Salmon is limited by the lack of accurate stock information. Long-running escapement monitoring weirs used for tributary populations provide an opportunity to analyze population dynamics at a finer resolution than is possible for the combined Yukon River and Kuskokwim River populations. Sampling at weirs is designed to produce unbiased estimates of the age and size distributions of the escapement (Williams and Shelden Citation2011; Mears Citation2013). Covariation of life history traits between monitored populations implies that they may be representative of the larger region. Therefore, the congruent analysis of two or more populations may be a useful tool with which to demonstrate regionwide trends and determinants of life history variability.
In this study, we explore the interplay between the marine environment (as described by SST), population productivity (recruits per spawner), and sex-specific measures of growth, average age at maturation, and maturation reaction norms (Siegel Citation2017) in two western Alaskan Chinook Salmon populations that have been subject to long-term monitoring: the East Fork Andreafsky River and the Kogrukluk River (tributaries of the Yukon and Kuskokwim rivers, respectively). Coastal populations of western Alaskan Chinook Salmon, including the study populations, are genetically distinct from mid-river and Canadian stocks in the Yukon River (Templin et al. Citation2011). Thus, the results of our investigation may best represent these coastal stocks of western Alaska. Siegel (Citation2017) previously completed run reconstructions estimating total returns in the study populations by combining escapement data from weirs with harvest data from the Alaska Department of Fish and Game. Siegel (Citation2017) found that thresholds for maturation had decreased over time in western Alaskan populations by using a new measure of probabilistic maturation reaction norms that accounts for growth history—the probability of maturation with average growth (PMAG). This result suggested that the documented declines in the age at maturation within the populations may represent an adaptive response. However, it was noted that environmental factors, including temperature, could affect the probability of maturation beyond the effects of growth and thus should be accounted for before strong inferences can be made regarding the cause of changing age at maturity.
Here, we use correlation matrices and extended Ricker stock–recruit analysis to inform a conceptual model describing the effects of ocean temperatures on the above-described life history characteristics of the East Fork Andreafsky and Kogrukluk River populations. Specifically, we addressed the following predictions: (1) warmer temperatures will correlate with higher growth in our study populations, (2) warmer temperatures during the early marine period will lead to higher productivity through the vector of increased growth decreasing size-selective mortality, and (3) warmer temperatures will lead to earlier maturation as a consequence of faster-growing individuals maturing earlier (McPhee et al. Citation2016; Siegel Citation2017). Additionally, we examined whether maturation thresholds (i.e., PMAGs) are directly affected by temperature in addition to temperature-mediated effects on growth (Tobin and Wright Citation2011).
METHODS
Study Populations
We analyzed populations from the East Fork Andreafsky River (hereafter, “Andreafsky River”), a tributary of the lower Yukon River basin; and the Kogrukluk River, a tributary of the middle Kuskokwim River basin (; see also McPhee et al. Citation2016). Both populations have been subject to long-term monitoring with escapement weirs, starting in 1994 for the Andreafsky River and 1981 for the Kogrukluk River. Weir operations are designed to produce escapement estimates and to monitor escapement characteristics, including age, sex, and length distributions. Sex is determined visually by weir crews on both rivers using secondary characteristics. Weir methods are described in more detail by Mears (Citation2013) for the Andreafsky River and by Williams and Shelden (Citation2011) for the Kogrukluk River.
FIGURE 1. Map showing the locations of the Chinook Salmon study populations (Andreafsky and Kogrukluk rivers) in Alaska and the Bering Sea area (54.3–60.0°N, 178.1°E–170.6°W) for which sea surface temperature data were extracted.
Providing the basis for our life history metric estimates, run reconstructions estimating total returns in the study populations were previously completed by combining escapement data from weirs with harvest data from the Alaska Department of Fish and Game (Siegel Citation2017; methods and results are included in Supplement A available separately online). Escapement estimates produced for the Andreafsky and Kogrukluk rivers by using a Bayesian approach to estimate missed migration were acquired from the Alaska Department of Fish and Game (Z. Liller, personal communication) and the U.S. Fish and Wildlife Service (J. Mears, personal communication), respectively. These estimates are considered the best available for both systems, although substantial uncertainty remains for years in which the weirs were largely nonoperational due to high water (Andreafsky River: brood year 2001; Kogrukluk River: brood years 1982, 1987, 1989, 2007, and 2012).
Both study populations are subject to harvest in terminal commercial, subsistence, and sport fisheries (terminal fisheries are those that catch mature fish returning to spawn within the river system). To estimate population-specific harvest, it was assumed that (1) the Andreafsky River population was harvested proportional to the lower Yukon River stock group in the fisheries below the confluence with the Yukon River and (2) the Kogrukluk River population was harvested proportional to the entire Kuskokwim River (Siegel Citation2017). Harvest was estimated to account for an average of 17% and 42% of the total returns in the Andreafsky and Kogrukluk rivers, respectively.
Sea Surface Temperature
We used the average April–December SST over the polygon spanning 60.0–54.3°N and 178.1°E–170.6°W to characterize annual Bering Sea SST (; Table S.B.1 available separately online in Supplement B). Monthly averaged SST data from the National Oceanic and Atmospheric Administration’s (NOAA) reanalysis data sets (Kalnay et al. Citation1996) were downloaded for the years 1975–2013 (NOAA, Earth System Research Laboratory, Physical Sciences Division; https://www.esrl.noaa.gov/psd/cgi-bin/data/timeseries/timeseries1.pl). The mean of monthly averaged temperatures from April through December was taken to create a single annual metric for SST. The SST during the first year of marine residency is notated as SST1, the SST during the second year of marine residency is notated as SST2, and so on.
FIGURE 2. (a) Monthly sea surface temperature (SST; °C) in the central Bering Sea (54.3–60.0°N, 178.1°E–170.6°W) averaged from 1975 to 2013, with 2-SD confidence intervals for monthly averages; and (b) average April–December SST from the Bering Sea polygon for the years 1975–2013. In panel a, the shaded area (January–March) represents the time period affected by sea ice (those data were excluded from calculation of the annual temperature metric). In panel b, the darker shaded area represents the period of escapement data (1994–2012) for the Andreafsky River Chinook Salmon returns; the lighter shaded area represents the period of escapement data (1981–2013) for the Kogrukluk River returns.
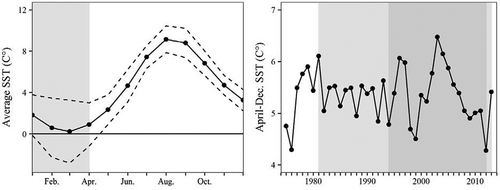
Although western Alaskan populations are thought to spend their first summer and fall at sea on the eastern Bering Sea shelf (Murphy et al. Citation2009), the polygon consisted of a largely ice-free area of the Bering Sea where older individuals are thought to reside (Myers et al. Citation2010). We found that the April–December SST in this area was strongly correlated (r > 0.50) with SSTs in areas further east and west, which these Chinook Salmon might also occupy. While sea ice is less influential in our chosen polygon than in other areas, it commonly encroaches into the northeastern region of the SST polygon during midwinter, creating negative temperature readings that are not representative of the temperatures that the fish would be experiencing. Accordingly, we removed January–March data from our annual metric, leaving us with the mean April–December SST (). The SSTs from adjacent months were well correlated across years, whereas temperatures in the spring (April and May) were largely independent from temperatures in the late summer and early fall (August–October; r < 0.27; Table S.B.2).
Life History Metrics
Growth
Retrospective scale analysis allows one to estimate age and growth for each year of a fish’s life. Annual growth increments of individual recruits were estimated by using retrospective analysis of scale samples collected at the Andreafsky River (return years 1994–2012) and Kogrukluk River (return years 1981–2013) weirs, following the methods detailed by McPhee et al. (Citation2016). We use the European notation (e.g., age 1.4) for age-classes (Koo Citation1962), with the first number representing the number of winters spent in freshwater and the second number (i.e., after the decimal) denoting the number of winters spent in salt water. The Andreafsky and Kogrukluk River Chinook Salmon populations are primarily composed of females maturing at ages 1.3, 1.4, and 1.5 and males maturing at ages 1.2, 1.3, and 1.4 (Siegel Citation2017). We only analyzed scales from these age × sex combinations, which represented over 97% of each population on average. Annual growth zone measurements were defined using the notation presented by Ruggerone et al. (Citation2007), where FW1 is first-year freshwater growth, SW1 is first-year marine growth, SW2 is second-year marine growth, and so on.
To produce a single estimate of mean cohort growth for each annual growth increment, the mean growth of each age × sex combination was weighted by its proportional representation in the returns (escapement plus harvest) previously estimated in run reconstruction (Siegel Citation2017). Estimates of growth for the total population and for males and females separately were analyzed by brood year (Andreafsky River: brood years 1990–2005; Kogrukluk River: brood years 1977–2006). To facilitate the interpretation of biological significance, we fitted a linear relationship between scale radius and mid-eye-to-fork length (mm) to translate scale growth into estimates of somatic growth (Figure S.B.1). The intercept of this relationship was fixed at an estimate of the size at first scale formation (40 mm; Rich Citation1920), leading to the following equation:
where l is the length of fish i; and R is the total scale radius, representing the sum of all annual growth increments. Individual growth increments were subsequently back-calculated using
where g is the annual growth increment at age a; and r is the scale growth increment width (with the exception of the freshwater growth increment, which was included the intercept term; Table S.B.1). Instead of using the generally preferred methods that account for individual variation in scale size (Francis Citation1990), we chose the linear method due to frequent reabsorption of accrued scale growth during the return migration, which created uncertainty around the original total scale length.
Average maturation age of recruits
The average age of recruits by brood year was previously calculated by summing the product of each age at maturity and its proportional representation in the return (Siegel Citation2017; Table S.B.1). Estimates of the number of recruits included the estimates of escapement (fish that survived to migrate past monitoring weirs) plus the estimates of fish harvested in the terminal fisheries of both populations.
Reaction norms for age at maturity
For a measure of maturation reaction norms, we used the PMAG, which was estimated for these populations by Siegel (Citation2017). The PMAG was proposed as an improvement over the midpoint of the probabilistic maturation reaction norm method because it accounts for growth history. To estimate PMAGs, Siegel (Citation2017) used logistic models that were informed by annual growth estimates from retrospective scale analysis. In the prediction of PMAGs, growth at each annual stage was held constant at the population-level mean growth value, and the probability of maturation for fish with this history of growth was estimated for each year by using the estimated annual fixed effect for each brood year. The PMAGs for two maturation decision points per sex were estimated for every cohort in both populations: the male age-1.2 and age-1.3 maturity decisions and the female age-1.3 and age-1.4 maturity decisions (Table S.B.1).
Statistical Analysis
Life history dynamics
We used Pearson’s product-moment correlation matrices to examine the relationship between SSTs and the above-described life history metrics. The relationships among life history metrics were also compared by using correlation matrices. Because we previously found that males and females differed in their relationship between growth and age at maturity (Siegel Citation2017), we investigated male- and female-specific life history metrics in addition to those of the total population. This analysis and all subsequent analyses were performed using the statistical program R version 3.1.2 (R Core Team Citation2014).
Productivity modeling
We used residuals of the Ricker stock–recruit model (Ricker Citation1954) to investigate the relationship between life history metrics and population productivity, defined as the number of returning adult recruits per spawner. We defined the number of spawners as the number of fish in the escapement as measured at the escapement weirs (Siegel Citation2017). Recruits mature and return across a range of return years at different ages. Our estimate of recruitment was the sum of estimated escapement and the estimated number of fish harvested in the terminal fisheries, indexed by brood year (Siegel Citation2017).
We used the linearized version of the extended Ricker stock–recruit model (Quinn and Deriso Citation1999) to model the relationship between recruits and spawners,
where Ry is the number of recruits from brood year y; Sy is the number of spawners that spawned in brood year y; E is an optional explanatory variable, such as growth or temperature; a is the productivity parameter; b is the inverse capacity parameter; c is the magnitude of the effect of the explanatory variable; and εy is the error term. The a and b parameters were estimated by fitting the basic Ricker model (i.e., excluding E) to the stock–recruit data from the run reconstructions. Once a and b had been estimated, the yield producing maximum returns (Smax = 1/b), the equilibrium yield (Seq = loge[a]/b), the maximum predicted recruits (Rmax = a/b e–1), and the maximum sustainable yield (Smsy = Seq × [0.5 – 0.07 loge{a}]) were calculated (Hilborn and Walters Citation1992).
After generating results for the simple linear Ricker model, we extended the model to include additional growth and SST variables. Growth and SST variables were analyzed separately since we expected them to be correlated. We focused our analysis on the first 2 years of marine residency due to the hypothesized effects of early marine growth on survival and the demonstrated importance of SW2 on the age at maturity of individual fish (Siegel Citation2017). Analyzed growth variables included SW1, SW2, and the sum of SW1 and SW2 as a single variable (SWsum). Analogous SST models including an effect of SST1, SST2, and the average of SST1 and SST2 as a single variable (SSTavg) were analyzed separately. To attain comparability of the coefficients, we standardized the explanatory variables to units of SDs from the mean. All analyzed models for each system were assessed in a model weighting table based on Akaike’s information criterion corrected for small sample sizes (AICc) calculated by using the AICcmodavg package (Mazerolle Citation2016).
RESULTS
Sea Surface Temperatures
The mean (±SD) April–December SST in the central Bering Sea during the study period 1975–2013 was 5.35 ± 0.50°C (). The maximum value during this period was 6.48°C in 2003, and the minimum was 4.28°C in 2012. Average SSTs were cool in the years 1975 and 1976 before oscillating around the mean value through the mid-1990s. Average SSTs were cool again in 1998 and 1999 before climbing to a peak in 2003, which was followed by a continual decline for six consecutive years. Relatively cool conditions persisted from 2007 to 2013.
Growth, Maturation, and Sea Surface Temperature
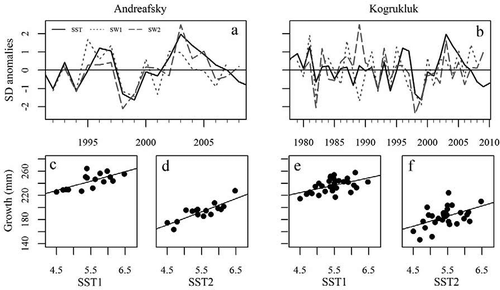
All measures of growth for each sex in both populations during the first 2 years of marine residency (SW1 and SW2) significantly increased with SST during the year in which growth occurred (). Relationships were stronger for the Andreafsky River population than for the Kogrukluk River population. The SST was not significantly correlated with any measure of later growth in either population. The positive relationship between SST and SW1 and SW2 growth appeared to be largely consistent across the time series in both populations, except during the late 1980s for the Kogrukluk River (). Additionally, growth estimates from the Andreafsky and Kogrukluk River populations were highly correlated, suggesting a shared environmental experience ().
TABLE 1. Pearson’s product-moment correlation coefficient matrix, showing the relationship between April–December average sea surface temperature (SST; SST1–SST4 = SST during the first year through fourth year of marine rearing) in the central Bering Sea and life history metrics (PMAG = probability of maturation with average growth [maturation decisions at ages 1.2 and 1.3 for males and at ages 1.3 and 1.4 for females]) of the Andreafsky River and Kogrukluk River Chinook Salmon populations (*P < 0.05, **P < 0.01, ***P < 0.005). The SSTs were correlated with growth increments accrued during the same year (SST1 with first-year marine growth [SW1]; SST2 with second-year marine growth [SW2]; etc.).
TABLE 2. Pearson’s product-moment correlation coefficient matrix, showing the relationship between life history metrics (FW1 = first-year freshwater growth; SW1–SW4 = first-year through fourth-year marine growth) of the Andreafsky River and Kogrukluk River Chinook Salmon populations (*P < 0.05, **P < 0.01, ***P < 0.005). Sex-specific recruit ages were correlated with sex-specific growth estimates. River comparison column shows the correlation of analogous growth increments between the two populations.
FIGURE 3. Line graphs demonstrating the variability of average April–December sea surface temperature (SST) in the central Bering Sea over time and first- and second-year marine growth (SW1 and SW2) occurring during corresponding years in Chinook Salmon populations from the (a) Andreafsky River and (b) Kogrukluk River. Scatterplots with linear fit lines between first- and second-year growth and corresponding SSTs for the (c), (d) Andreafsky River and (e), (f) Kogrukluk River populations are also presented.
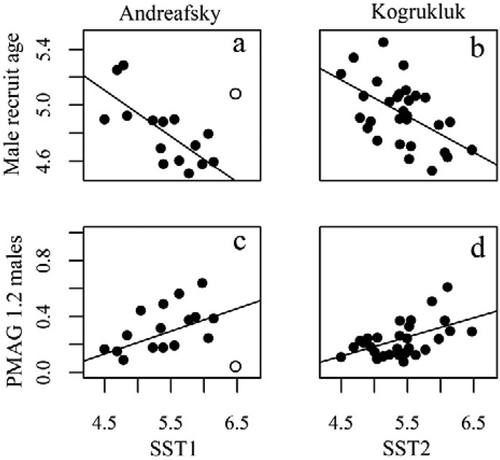
Warmer SST was generally associated with younger recruit age (). The ages of all recruits (r = −0.57, P = 0.021) and male recruits (r = −0.48, P = 0.059) in the Andreafsky River were negatively correlated with SST1 (). For the Andreafsky River, brood year 2001 was an anomalous year (studentized residual = 3.22; ), as these fish experienced the warmest SST1 during the time series (growth year 2003) but also expressed a relatively old average age of male maturation. When this outlier was excluded, the significance of the relationship between male recruit age and SST1 increased (r = −0.73, P = 0.002). Female recruit age in the Andreafsky River was negatively correlated with SST3 (r = −0.59, P = 0.015). For the Kogrukluk River population, the ages of all recruits and of male recruits () were negatively correlated with SST2 (all recruits: r = −0.45, P = 0.011; male recruits: r = −0.53, P = 0.002). Female recruit age in the Kogrukluk River was not significantly correlated with SST during any growth year, although correlation coefficients with SST1 to SST3 were negative.
FIGURE 4. Scatterplots with linear fit lines showing the relationship between sea surface temperature (SST) and male recruit age as well as the probability of maturation with average growth (PMAG) for the age-1.2 male maturity decision in the (a), (c) Andreafsky River and (b), (d) Kogrukluk River Chinook Salmon populations. The outlier in the Andreafsky River graphs (brood year 2001; open circle) was excluded from the presented linear fits.
The relationships between marine growth (SW1, SW2, etc.) and male recruit age were similar to those between SSTs and male recruit age, suggesting that much of the effect of SST on recruit age was through increased growth (). As with SST, the total age of all recruits and of male recruits declined with increasing SW1 for the Andreafsky River (all recruits: r = −0.53, P = 0.033; male recruits: r = −0.62, P = 0.001) and with increasing SW2 for the Kogrukluk River (all recruits: r = −0.50, P = 0.005; male recruits: r = −0.52, P = 0.004). Second-year marine growth was negatively correlated with earlier maturity of females in both the Andreafsky River (r = −0.55, P = 0.029) and the Kogrukluk River (r = −0.41, P = 0.025). In contrast, average SW4 for the Kogrukluk River population, which is only accrued by age-1.4 and age-1.5 fish, was found to be positively correlated with recruit age (r = 0.42, P = 0.020).
Warmer temperatures were associated with lower growth thresholds for early male maturity in both populations (; ). In the Andreafsky River population, brood year 2001 also appeared as an outlier in the relationship between the PMAG for the male age-1.2 maturity decision and SST1 (studentized residual = −2.86; ), with few males returning at age 1.2 in 2005 despite experiencing the warmest SST1 during the time series (i.e., in 2003; Siegel Citation2017). When this outlier was excluded, a significant positive correlation between the PMAG for the male age-1.2 maturity decision and SST1 was detected (r = 0.56, P = 0.027). For the Kogrukluk River population, the PMAG for the male age-1.2 maturity decision was positively correlated with SST2 (r = 0.51, P = 0.003), and the PMAG for the female age-1.3 maturity decision was positively correlated with SST1 (r = 0.39, P = 0.027).
Productivity Modeling
Productivities of the two populations were significantly correlated during the overlapping years of analysis (brood years 1994–2005; r = 0.86, P < 0.001). Both populations experienced three continuous brood years below replacement level from 1994 to 1996 and exhibited peaks in productivity during 2000: 4.4 and 10.6 recruits per spawner in the Andreafsky and Kogrukluk rivers, respectively (, ). During 1983, the Kogrukluk River population experienced another spike in productivity of 14.8 recruits per spawner. The Kogrukluk River was found to be about three times as productive as the Andreafsky River according to stock–recruit metrics estimated from the basic Ricker model ().
TABLE 3. Ricker stock–recruit parameters (a = productivity parameter; b = inverse capacity parameter; Smax = number of spawners producing maximum returns; Rmax = maximum number of predicted recruits; Seq = equilibrium yield; Smsy = maximum sustainable yield) for Chinook Salmon populations from the Andreafsky River (brood years 1990–2005) and Kogrukluk River (brood years 1977–2006).
FIGURE 5. (a), (b) Bar plots of productivity (recruits per spawner) by brood year and (c), (d) scatterplots of spawners and recruits with basic Ricker stock–recruit relationships (solid lines) for Chinook Salmon populations in the Andreafsky and Kogrukluk rivers. In bar plots, even years are plotted in white, and odd years are plotted in black. Replacement level is shown by the dashed line in all panels.
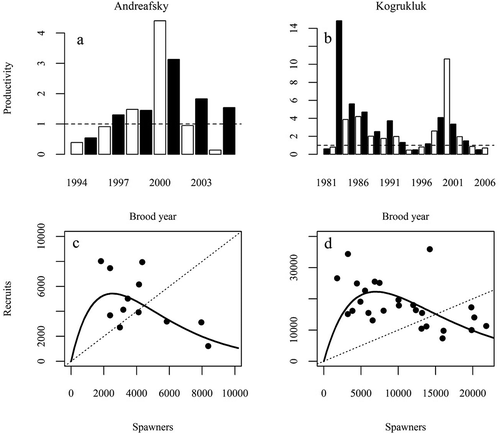
Productivity of the Andreafsky River population increased in the brood years experiencing warmer SSTs during early marine residency (). Ricker residuals for the Andreafsky River population were positively correlated with SST1 (r = 0.58, P = 0.047) and SST2 (r = 0.67, P = 0.017). In contrast, Ricker residuals for the Kogrukluk River were not significantly related to SST during any year of marine growth (). We detected no significant correlations between Ricker residuals and any measure of growth (from FW1 to SW4) in either river, although the correlation coefficients were all positive for the Kogrukluk River (the sign was variable for the Andreafsky River; ). Ricker residuals tended to be greater for brood years with a younger recruit age in both populations; however, this relationship was only significant for female recruit age in the Kogrukluk River population ().
For the Andreafsky River, extended Ricker model analysis supported the hypothesis that warmer SSTs and greater growth during the first 2 years of marine residency led to increased survival. A model including an effect of SSTavg was the best model for the Andreafsky River (). Additionally, separate models including effects of SST1, SST2, and SWsum had more support than the basic Ricker model. Support for an effect of SSTs on productivity in the Kogrukluk River was minimal. For the Kogrukluk River, no extended models surpassed the basic Ricker model according to AICc values (). However, separate models including an effect of SW1 and an effect of SWsum had nearly as much support as the basic Ricker model (AICc difference [ΔAICc] = 0.23 and 0.61, respectively).
TABLE 4. Model weighting tables based on Akaike’s information criterion corrected for small sample sizes (AICc), used to evaluate the Ricker and extended Ricker models that included sea surface temperature (SST) and growth variables for the Andreafsky River and Kogrukluk River Chinook Salmon populations (logL = log likelihood; ΔAICc = AIC difference; weight = Akaike weight; S = number of spawners; SW1, SW2 = first- and second-year marine growth; SWsum = sum of SW1 and SW2; SST1, SST2 = SST during the first and second years of marine rearing; SSTavg = average of SST1 and SST2; a colon represents an interaction between terms). To achieve comparability of the coefficients, all explanatory variables were analyzed as SD anomalies.
DISCUSSION
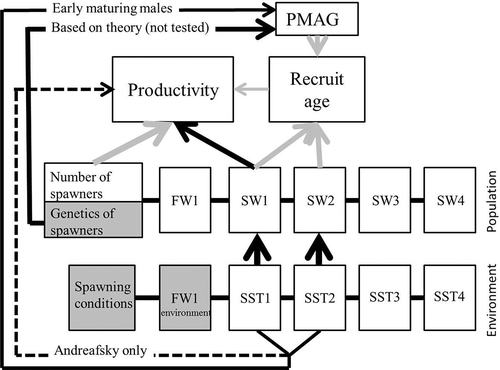
Our results suggest that ocean temperatures influence the life history of western Alaskan Chinook Salmon. We found strong correlations between warmer ocean temperatures in the central Bering Sea and (1) higher growth during the first 2 years of marine residency; (2) higher productivity in the Andreafsky River population (although no relationship was found for the Kogrukluk River population); and (3) earlier maturation. Results suggest that fish mature earlier at warm temperatures not only due to the effect of temperature on growth but also as a consequence of a decrease in early male maturation thresholds (i.e., PMAGs). We summarize our results in a conceptual model of the effect of SST on the life history and productivity of western Alaskan Chinook Salmon (). Although there is some uncertainty in the data used for our run reconstructions (mainly in the estimates of harvest and visual sex identification and for years with missed weir sampling due to high water), the strong correlations we found are unlikely to be due to inaccuracies in the data. Growth and productivity were highly correlated between the two study populations, suggesting that our results may be representative of Chinook Salmon from the coastal western Alaska region. Although Bering Sea SSTs were strongly correlated with age at maturation of western Alaskan Chinook Salmon, a lack of a temporal trend in SST over the period of analysis (1977–2013), combined with the temperature independence of most measures of PMAG, suggests that annual temperature alone cannot explain the documented age and size declines.
FIGURE 6. Conceptual model of the relationship between sea surface temperatures (SSTs) in the central Bering Sea and the growth and life history metrics of western Alaskan Chinook Salmon (PMAG = probability of maturation with average growth; FW1 = first-year freshwater growth; SW1–SW4 = first-year through fourth-year marine growth; SST1–SST4 = SST during the first year through fourth year of marine rearing). Black arrows demonstrate positive relationships, and gray lines represent negative relationships. Gray boxes represent recognized variables that were not directly tested in these analyses. The thickness of each arrow demonstrates the strength of the relationship. Dashed arrow connecting early marine SSTs to productivity represents a relationship observed only for the Andreafsky River population.
Our finding of greater growth when waters are warmer during early marine residency suggests that Chinook Salmon have not been limited by the availability of prey in the Bering Sea during their first 2 years of marine rearing and thus have been able to capitalize on higher growth potentials in warmer years. Chinook Salmon prey consumption in the Bering Sea is variable and has been linked to climate conditions. In an analysis of juveniles on the eastern Bering Sea shelf, Farley et al. (Citation2009) found that prey composition during the first marine year was dominated by fish and squid in the relatively warm years of 2002–2005, while euphausiids were dominant in the relatively cold year of 2006. The year 2006 was associated with distribution changes and generally poorer condition of juvenile Chinook Salmon. Larger squid and fish prey in the Bering Sea are more calorically dense than alternative prey items (Davis et al. Citation1998), potentially stimulating greater growth of Chinook Salmon beyond the direct effects of warmer temperatures.
The relationship between SST and growth was found to break down after the second year in the marine environment. This result may be due to shifts in the drivers of marine growth as fish age. In each of our study populations, growth of fish from consecutive brood years rearing at the same time in the marine environment was significantly correlated, whereas growth of fish separated by two or more years of age was not (). This may be a consequence of fish consuming different prey and/or occupying different habitats as they increase in size and age. For example, younger Chinook Salmon on the Bering Sea shelf have been shown to eat more fish in comparison to higher proportions of squid consumed by generally older fish over the shelf break and in the Bering Sea basin (Davis et al. Citation2003).
However, it also must be noted that our estimates of SW3 and SW4 are not completely independent measures because variable portions of the population mature before the third year of marine residency. Although growth rate is largely determined by the environment, there is likely a genetic effect driven by differences in behavior and the allocation of energy (e.g., Berejikian et al. Citation2011). Thus, if a larger number of faster-growing fish matures earlier, this could have a negative effect on our estimates of later growth because the faster-growing fish are removed from the population. This may explain the positive correlation between SW4 and recruit age observed in the Kogrukluk River (). Consequently, we cannot draw strong conclusions about the relationship between marine temperatures and SW3 and SW4 from our analyses alone.
TABLE 5. Pearson’s product-moment correlation coefficient values calculated between average annual growth estimates (FW1 = first-year freshwater growth; SW1–SW4 = first-year through fourth-year marine growth) from the same growth year (i.e., different-aged cohorts growing at the same time) for the Chinook Salmon populations of the Andreafsky River (brood years 1990–2005) and the Kogrukluk River (brood years 1977–2006; *P < 0.05, **P < 0.01, ***P < 0.005).
We also found evidence that higher SSTs during the first 2 years of marine residency may lead to lower growth thresholds for early male maturity (PMAG for age 1.2) in both populations, whereas no such consistent relationship was detected for the other maturity decisions. Because the PMAG already accounts for growth, this suggests that any additional effects of temperature on maturation (beyond the effect of temperature on growth) might be limited largely to the male early maturity decision. The reason for this is unclear, but it could be related to the fitness consequences of body size for salmon. Large size tends to confer fitness advantages to females and for males adopting the dominant mating tactic; therefore, maturing at a smaller size could result in diminished reproductive success for those individuals. The smallest males (which tend to be the youngest) are likely to adopt a peripheral, sneaking (“jack”) mating tactic for which larger size at age may not confer a great advantage. A plastic response to temperature that leads to maturation at a smaller size within the youngest age-class might have limited fitness costs and thus may be less canalized compared to other maturity decisions. However, evaluation of this hypothesis was beyond the scope of our study.
Changes in Chinook Salmon age composition caused by warmer ocean temperatures are likely to have ramifications for the population and evolutionary ecology of these stocks. Younger-maturing recruits face less exposure to mortality as a consequence of shorter marine residency before maturation. Reduced marine mortality would result in a higher number of recruits per spawner, although this effect could be counteracted by the reduced fecundity of younger, smaller females. Fewer older individuals would shorten the generation time of these populations, resulting in a lower genetically effective population size for a given number of annual spawners (Waples Citation1990) and potentially leading to a reduction in genetic diversity if enhanced marine survival of younger-maturing fish (i.e., more spawners per brood year) does not outweigh the effects of shortened generation time. However, a larger number of males returning at the youngest age should reduce the average reproductive success of the frequency-dependent jack tactic (Berejikian et al. Citation2010), so a directional trend toward reduced age at maturity might ultimately be counteracted by natural selection.
Extended Ricker model stock–recruit analysis revealed evidence to support our hypothesis that warmer early marine SSTs lead to higher productivity in the Andreafsky River (). For the Andreafsky River population, the average combined temperature during the first 2 years of marine residency (SSTavg) explained 53% of the variation in productivity that was unexplained by spawner density in the basic Ricker model (R2 increased from 0.81 to 0.91). The positive relationship between productivity and SST may be a consequence of higher growth during warmer years leading to reduced size-selective mortality. Size-selective mortality was found to occur in the Canadian stock group, which likely occupies the same rearing areas on the shelf as Andreafsky River fish during the first year of marine rearing (Murphy et al. Citation2013). Earlier maturation due to higher growth at warmer temperatures—and thus less exposure to potential marine mortality—is likely a contributing factor.
However, despite similar relationships between growth and SST for both populations, we were unable to find strong evidence for a relationship between SSTs and Kogrukluk River productivity. The inability to relate marine conditions to productivity in the Kogrukluk River may be attributable to inadequate resolution of our data. With 82% of the variability explained by the basic Ricker model, our ability to describe the remaining unexplained variance may have been limited by the precision of our productivity estimates from run reconstructions. Although we have high confidence in the weir escapement estimates used for most years (a few years had lower confidence due to missed sampling from high water), there may be more inaccuracy in our harvest estimates due to the assumptions we were forced to make in run reconstructions. This is likely a larger source of error for the Kogrukluk River due to a higher exploitation rate (estimated average exploitation ± SD was 17 ± 9% for the Andreafsky River and 42 ± 11% for the Kogrukluk River).
Conversely, the increased importance of SST for survival in the Andreafsky River population relative to the Kogrukluk River population could also be a consequence of the Yukon River’s more northerly location. It has been speculated that fish from the Yukon River could become entrained in the ice buildup, reducing survival during colder years (Murphy et al. Citation2013); this provides a possible source of additional mortality during colder years beyond reduced growth. Ice begins to build up on the northern shelf in rearing areas during November (Murphy et al. Citation2013), while the southeastern Bering Sea generally remains ice free for a longer period. Thus, fish in the Kuskokwim River may be less likely to experience ice-related mortality.
Additionally, the effect of higher marine temperatures on productivity in the Kogrukluk River could also be obscured by processes during freshwater rearing that drive variation in productivity. Numerous freshwater processes can affect egg-to-smolt mortality, which is generally substantial and can be highly variable. There is limited published information on the freshwater ecology of western Alaskan Chinook Salmon; however, freshwater processes have been shown to influence productivity in other Yukon River tributary populations (Neuswanger et al. Citation2015). The good fit of the basic Ricker models (Andreafsky River: R2 = 0.81; Kogrukluk River: R2 = 0.82) suggests that density-dependent processes in freshwater may be dominant drivers of productivity in both populations. We did not analyze the effect of the freshwater environment in our investigation because we were unable to find consistent and reliable environmental data (e.g., water temperature and discharge) for these populations. Quality environmental time series of data describing the freshwater environment in western Alaska would be quite valuable for investigating this phenomenon.
Conclusions
Our results have substantial implications for the future of Chinook Salmon in western Alaska as a consequence of climate change. Major reductions in sea ice and increases in SST of around 3°C compared to 1980–1999 averages are predicted for the Bering Sea in the 21st century (Wang et al. Citation2012). Consequently, Chinook Salmon in the Bering Sea will experience unprecedentedly warm conditions during the coming decades. Changes may already be occurring; in the available record (1948 to present), SSTs in the central Bering Sea were highest during the years just after our period of analysis (average April–December SSTs in the central Bering Sea during 2014 and 2016 were 6.75°C and 6.81°C, respectively). Our results suggest that western Alaskan Chinook Salmon will respond with higher growth and a younger average age at maturity, particularly in males. These populations have historically represented some of the oldest-maturing populations of Chinook Salmon; however, they may become younger, with age structures similar to those of more southerly populations.
Caution must be used when applying retrospective correlations to predict future responses in complicated ecosystems. As the Bering Sea enters an unprecedented physical state, the food web that supports Chinook Salmon is likely to change significantly as well. Therefore, past environmental relationships determining the expression of life history traits may break down, and new ones may form as the species adapts. For example, the one outlier observed in our relationship between SST and male maturation in the Andreafsky River (brood year 2001) occurred during the warmest temperatures in the time series. Because there was only one such outlier, we can draw no strong conclusions on whether it represents data inaccuracy, a stochastic event, or a change in ecosystem dynamics at anomalously high temperatures. However, this result demonstrates the need to monitor the relationships described here to determine whether the drivers of growth, survival, and maturation change as these high temperatures become more common. Because of the uncertainty inherent in forecasting biological responses to unprecedented conditions, management should be responsive and adaptable to change (see Schindler et al. Citation2008).
Siegel_et_al_Supplement_A.pdf
Download PDF (534 KB)ACKNOWLEDGMENTS
Funding for this work was provided by the Alaska Sustainable Salmon Fund (Project Number 44903), the Pollock Cooperative Conservation and Research Center, and the University of Alaska Fairbanks’ Global Change Student Research Grant Competition. The views expressed in this paper do not necessarily reflect those of the funding entities. We thank J. Leon, who measured the female scale samples and performed analyses that laid the foundation for this work; Z. Liller, L. Dubois, T. Hamazaki, and J. Mears, who assisted with the acquisition of unpublished data; and L. Wilson and B. Agler, who provided scale reading training and oversight. R. Brown provided insight and helpful comments on earlier versions of the manuscript.
References
- Alaska Department of Fish and Game. 2013. Low runs of Chinook Salmon in Alaska: hot topics and issues. Alaska Department of Fish and Game. Available: http://www.adfg.alaska.gov/index.cfm?adfg=hottopics.lowchinookruns_info (September 2016).
- Andrews, A. G., W. W. Strasburger, E. V. Farley, J. M. Murphy, and K. O. Coyle. 2016. Effects of warm and cold climate conditions on Capelin (Mallotus villosus) and Pacific Herring (Clupea pallasii) in the eastern Bering Sea. Deep Sea Research Part II: Topical Studies in Oceanography 134:235–246.
- Aydin, K., and F. Mueter. 2007. The Bering Sea—a dynamic food web perspective. Deep Sea Research Part II: Topical Studies in Oceanography 54:2501–2525.
- Beamish, R. J., and C. Mahnken. 2001. A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Progress in Oceanography 49:423–437.
- Berejikian, B. A., M. Bradford, D. M. Van Doornik, R. C. Endicott, T. L. Hoffnagle, E. P. Tezak, M. E. Moore, and J. Atkins. 2010. Mating success of alternative male phenotypes and evidence for frequency-dependent selection in Chinook Salmon, Oncorhynchus tshawytscha. Canadian Journal of Fisheries and Aquatic Sciences 67:1933–1941.
- Berejikian, B. A., D. M. Van Doornik, and J. J. Atkins. 2011. Alternative male reproductive phenotypes affect offspring growth rates in Chinook Salmon. Transactions of the American Fisheries Society 140:1206–1212.
- Daly, E. A., and R. D. Brodeur. 2015. Warming ocean conditions relate to increased trophic requirements of threatened and endangered salmon. PLOS (Public Library of Science) ONE [online serial] 10(12):e0144066.
- Davis, N. D., J. L. Armstrong, and K. W. Myers. 2003. Bering Sea salmon food habits: diet overlap in fall and potential for competition. Yukon River Drainage Fisheries Association, National Oceanic and Atmospheric Administration Award Number NA06FM0316, Anchorage, Alaska.
- Davis, N. D., K. W. Myers, and Y. Ishida. 1998. Caloric value of high-seas salmon prey organisms and simulated salmon ocean growth and prey consumption. North Pacific Anadromous Fish Commission Bulletin 1:146–162.
- Eisner, L. B., J. M. Napp, K. L. Mier, A. I. Pinchuk, and A. G. Andrews. 2014. Climate-mediated changes in zooplankton community structure for the eastern Bering Sea. Deep Sea Research Part II: Topical Studies in Oceanography 109:157–171.
- Fall, J. A. 2016. Regional patterns of fish and wildlife harvests in contemporary Alaska. Arctic 69:47–64.
- Farley, E. V., J. Murphy, J. Moss, A. Feldman, and L. B. Eisner. 2009. Marine ecology of western Alaska juvenile salmon. Pages 1–23 in C. C. Krueger and C. E. Zimmerman, editors. Pacific salmon ecology and management of western Alaska’s populations. American Fisheries Society, Symposium 70, Bethesda, Maryland.
- Francis, R. I. C. C. 1990. Back calculation of fish length: a critical review. Journal of Fish Biology 36:883–902.
- Hertz, E., M. Trudel, S. Tucker, T. D. Beacham, C. Parken, D. Mackas, and A. Mazumder. 2016. Influences of ocean conditions and feeding ecology on the survival of juvenile Chinook Salmon (Oncorhynchus tshawytscha). Fisheries Oceanography 25:407–419.
- Hilborn, R., and C. G. Walters. 1992. Quantitative fisheries stock assessment: choice, dynamics and uncertainty. Chapman and Hall, New York.
- Howe, L., and S. Martin. 2009. Demographic change, economic conditions, and subsistence salmon harvests in Alaska’s Arctic–Yukon–Kuskokwim region. University of Alaska, Munich Personal RePEc (Research Papers in Economics) Archive, Paper 33575, Anchorage.
- Hunt, G. L., K. O. Coyle, L. Eisner, E. V. Farley, R. Heintz, F. Mueter, J. M. Napp, J. E. Overland, P. H. Ressler, S. Sale, and P. J. Stabeno. 2011. Climate impacts on eastern Bering Sea food webs: a synthesis of new data and an assessment of the oscillating control hypothesis. ICES Journal of Marine Science 68:1230–1243.
- Kalnay, E., M. Kanamitsy, R. Kistler, W. Collins, D. Deaven, L. Gandin, M. Iredell, S. Saha, G. White, J. Woollen, Y. Zhu, M. Chelliah, W. Ebisuzaki, W. Higgins, J. Janpwiak, K. C. Mo, C. Ropelewski, J. Wang, A. Leemaa, R. Reynolds, R. Jenne, and D. Joseph. 1996. The NCEP/NCAR 40-year reanalysis project. Bulletin of the American Meteorological Society 77:437–471.
- Koo, T. S. Y. 1962. Age designation in salmon. Pages 41–48 in T. S. Y. Koo, editor. Studies of Alaska Red Salmon. University of Washington, Publications in Fisheries, New Series 1, Seattle.
- Lewis, B., W. S. Grant, R. E. Brenner, and T. Hamazaki. 2015. Changes in size and age of Chinook Salmon Oncorhynchus tshawytscha returning to Alaska. PLOS (Public Library of Science) ONE [online serial] 10(6):e0130184.
- Mazerolle, M. J. 2016. AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 2.1-0. Available: https://cran.r-project.org/package=AICcmodavg. (September 2017).
- McPhee, M. V., J. M. Leon, L. I. Wilson, J. E. Siegel, and B. A. Agler. 2016. Changing growth and maturity in western Alaskan Chinook Salmon Oncorhynchus tshawytscha, brood years 1975–2005. North Pacific Anadromous Fish Commission Bulletin 6:307–327.
- Mears, J. D. 2013. Abundance and run timing of adult Pacific salmon in the East Fork Andreafsky River, Yukon Delta National Wildlife Refuge, Alaska, 2012. U.S. Fish and Wildlife Service, Alaska Fisheries Data Series 2013-9, Fairbanks, Alaska.
- Murphy, J., K. Howard, L. Eisner, A. Andrews, W. Templin, C. Guthrie, K. Cox, and E. Farley. 2013. Linking abundance, distribution, and size of juvenile Yukon River Chinook Salmon to survival in the northern Bering Sea. North Pacific Anadromous Fish Commission Technical Report 9:25–30.
- Murphy, J. M., W. D. Templin, E. V. Farley Jr., and J. E. Seeb. 2009. Stock-structured distribution of western Alaska and Yukon juvenile Chinook Salmon (Oncorhynchus tshawytscha) from United States BASIS surveys, 2002–2007. North Pacific Anadromous Fisheries Commission Bulletin 5:51–59.
- Myers, K. W., R. V Walker, N. D. Davis, J. L. Armstrong, and J. Wyatt. 2010. Climate-ocean effects on Arctic–Yukon–Kuskokwim Chinook Salmon. 2010 Arctic–Yukon–Kuskokwim Sustainable Salmon Initiative Project, Final Product SAFS-UW-10, Seattle.
- Neuswanger, J. R., M. S. Wipfli, M. J. Evenson, N. F. Hughes, and A. E. Rosenberger. 2015. Low productivity of Chinook Salmon strongly correlates with high summer stream discharge in two Alaskan rivers in the Yukon drainage. Canadian Journal of Fisheries and Aquatic Sciences 72:1125–1137.
- Quinn, T. J., and R. B. Deriso. 1999. Quantitative fish dynamics. Oxford University Press, New York.
- R Core Team. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
- Rich, W. H. 1920. Early history and seaward migration of Chinook Salmon in the Columbia and Sacramento rivers. U.S. Bureau of Fisheries Bulletin 37.
- Ricker, W. E. 1954. Stock and recruitment. Journal of the Fisheries Research Board of Canada 11:559–623.
- Ruggerone, G. T., J. L. Nielsen, and J. Bumgarner. 2007. Linkages between Alaskan Sockeye Salmon abundance, growth at sea, and climate, 1955–2002. Deep-Sea Research Part II: Topical Studies in Oceanography 54:2776–2793.
- Schindler, D. E., X. Augerot, E. Fleishman, N. J. Mantua, B. Riddell, M. Ruckelshaus, J. Seeb, and M. Webster. 2008. Climate change, ecosystem impacts, and management for Pacific salmon. Fisheries 33:502–506.
- Schindler, D. E., C. Krueger, P. Bisson, M. Bradford, B. Clark, J. Conitz, K. Howard, M. Jones, J. Murphy, M. K. M. Scheuerell, E. Volk, and J. Winton. 2013. Arctic–Yukon–Kuskokwim Chinook Salmon research action plan: evidence of decline of Chinook Salmon populations and recommendations for future research. Arctic–Yukon–Kuskokwim Sustainable Salmon Initiative, Anchorage, Alaska.
- Siegel, J. E. 2017. Determinants of life history variability in the Chinook Salmon (Oncorhynchus tshawytscha) of western Alaska. University of Alaska, Fairbanks.
- Stabeno, P. J., N. B. Kachel, S. E. Moore, J. M. Napp, M. Sigler, A. Yamaguchi, and A. N. Zerbini. 2012. Comparison of warm and cold years on the southeastern Bering Sea shelf and some implications for the ecosystem. Deep-Sea Research Part II: Topical Studies in Oceanography 65–70:31–45.
- Templin, W. D., J. E. Seeb, J. R. Jasper, A. W. Barclay, and L. W. Seeb. 2011. Genetic differentiation of Alaska Chinook Salmon: the missing link for migratory studies. Molecular Ecology Resources 11:226–246.
- Tobin, D., and P. J. Wright. 2011. Temperature effects on female maturation in a temperate marine fish. Journal of Experimental Marine Biology and Ecology 403:9–13.
- Wang, M., J. E. Overland, and N. A. Bond. 2010. Climate projections for selected large marine ecosystems. Journal of Marine Systems 79:258–266.
- Wang, M., J. E. Overland, and P. Stabeno. 2012. Future climate of the Bering and Chukchi seas projected by global climate models. Deep-Sea Research Part II: Topical Studies in Oceanography 65–70:46–57.
- Waples, R. S. 1990. Conservation genetics of Pacific salmon III: estimating effective population size. Heredity 81:277–289.
- Wechter, M. E., B. R. Beckman, A. G. Andrews, A. H. Beaudreau, and M. V. McPhee. 2016. Growth and condition of juvenile Chum and Pink Salmon in the northeastern Bering Sea. Deep-Sea Research Part II: Topical Studies in Oceanography 135:145–155.
- Williams, D. L., and C. A. Shelden. 2011. Kogrukluk River salmon studies, 2010. Alaska Department of Fish and Game, Fishery Data Series 10-49, Anchorage.
