Abstract
In a 2013 pilot study, acoustic tags were inserted into two species of river herring, Alewife Alosa pseudoharengus and Blueback Herring Alosa aestivalis. The primary objectives were to identify handling and tagging effects and assess in-river residence time. The secondary objective was to identify postspawn coastal migration patterns. Fish were collected on spawning grounds in the upper portion of the Hudson River, New York. Vemco V7 acoustic transmitters were gastrically inserted into 25 river herring (13 Alewives and 12 Blueback Herring) that were in pre, active, or post spawning conditions. In-river acoustic data were collected from 23 of the 25 river herring. The majority of tagged fish exhibited some level of fallback (downstream migration) after the tagging event, all Blueback Herring and all male Alewives returning to spawning areas. The majority of female Alewives did not return to the spawning area after tagging, and this may be a result of when and where tagging events occurred. Both species of river herring exhibited similar in-river residence times of approximately 2–3 weeks and exited the system 3–6 d after spawning. Information on coastal movements of four Blueback Herring (two females and two males) was also obtained, spanning the south shore of Long Island, New York, to the mouth of Penobscot Bay, Maine. Coastal and in-river tag detections were reported by members of the Atlantic Cooperative Telemetry Network. In conclusion, this experiment can now be repeated with confidence on a larger scale with multiyear tags in order to identify unknown in-river spawning areas, provide information regarding spawning site fidelity, and bolster current knowledge of coastal migration patterns for both species.
Received October 6, 2016; accepted August 2, 2017
Anadromous river herring are composed of two closely related and jointly managed species, Alewife Alosa pseudoharengus and Blueback Herring Alosa aestivalis. Alewives range from Newfoundland to North Carolina, and Blueback Herring range from Cape Breton, Nova Scotia, to the St. John’s River in Florida (Collette and Klein-MacPhee Citation2002; NOAA Citation2007). Both species are highly migratory, euryhaline, schooling, coastal pelagic fish that spend most of their adult lives at sea returning to freshwater only to spawn in the spring. The onset of spawning runs for both species is related to water temperature; thus, it varies with latitude and may vary annually by 3–4 weeks in a given locality (Loesch Citation1987). Alewives generally initiate spawning migrations when water temperatures reach 5–10°C (Loesch Citation1987) and spawn in lentic environments over a wide range of substrates such as gravel, sand, detritus, and submerged vegetation (NOAA Citation2007). Blueback Herring generally initiate spawning migrations when water temperatures reach 10–15°C (Loesch Citation1987) and spawn in lotic environments over hard substrates actively avoiding lentic sites (Collette and Klein-MacPhee Citation2002). Adults of both species return to spawn for the first time between ages 3 and 5 and are iteroparous spawners throughout the majority of their respective ranges (Collette and Klein-MacPhee Citation2002).
The Hudson River below the Federal Dam at Troy, New York, has approximately 68 primary tributaries, most of which provide some spawning habitat for river herring (Schmidt and Lake Citation2000). River herring spawn in the entire freshwater portion of the Hudson River and its tributaries, up to the first impassible barrier, as well as in shallow waters of the main-stem river. However, Schmidt and Lake (Citation2000) concluded that virtually all of the reproducing river herring in Hudson River tributaries below the Federal Dam are, in general, Alewives. Although Alewife and Blueback Herring spawning grounds overlap, Blueback Herring generally spawn more northerly in the river compared with Alewives and are also known to spawn in the Mohawk River (Greeley Citation1935; Owens et al. Citation1998), the largest tributary to the Hudson River. Blueback Herring use navigation locks for passage (Owens et al. Citation1998).
In New York, river herring are an economically and culturally important species. Spawning populations are commercially and recreationally exploited for human consumption (Hattala et al. Citation2012) and as bait for recreational fishing.
Coastwide declines in both species of river herring resulted in the National Oceanic and Atmospheric Administration (NOAA) designating them as a species of concern in 2007, followed by a petition to list the species as endangered in 2011. In 2013, after a status review, NOAA determined that the listing was not warranted. Concurrently, the Atlantic State Marine Fisheries Commission produced a coastwide stock assessment in 2012, which was followed by the development and adoption of Amendment 2 to the Interstate Fisheries Management Plan for Shad and River Herring (ASMFC Citation2012). Prior to the assessment, several Atlantic states (Massachusetts, Rhode Island, Connecticut, and North Carolina) implemented a moratorium on the harvest or possession of river herring. After implementation of Amendment 2, New York, along with four other states (Maine, New Hampshire, North Carolina and South Carolina) allowed limited harvest of river herring under their respective Sustainable Fishery Management Plans. An underlying need identified in the New York River Herring Sustainable Fishery Management Plan (Hattala et al. Citation2011) was to quantify in-river habitat use and to better understand coastal movements, especially in relation to the vulnerability of river herring to ocean fisheries.
Management of river herring populations depends in part on identifying spatial distribution during spawning, length of spawning residence time, number of spawning events within native river systems, and identifying temporal and spatial congregation areas in coastal marine habitats where they have the potential to be harvested as bycatch. Tracking within-river Alosa movements of spawning adults is of special interest to many researchers and management agencies (Frank et al. Citation2009). Previous tagging studies of river herring (e.g., Chappelear and Cooke Citation1994; Smith et al. Citation2009) primarily focused on tagging methodologies and upstream passage efficiency at fishways. In order to gain a better understanding of river herring behavior during and after spawning events in the Hudson River and coastal environments, a pilot study using acoustic tags was initiated. The primary objectives were to identify handling and tagging effects and quantify in-river residence time. A secondary objective was to identify postspawn coastal migration patterns.
STUDY SITE
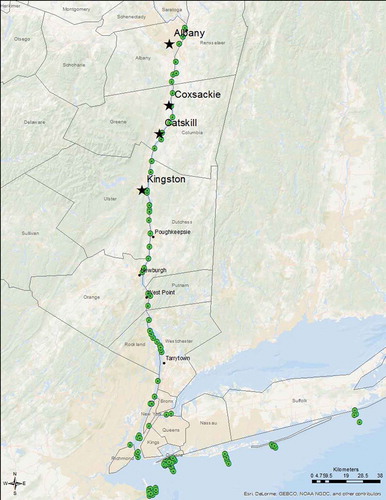
The study area for the handling and tagging and in-river residence time portion of the study was the tidal portion of the Hudson River from its confluence with the Atlantic Ocean at river kilometer (rkm) 0 in New York harbor to the Federal Dam in Troy (rkm 244; ). For the coastal migration portion, the Atlantic coast ().
METHODS
River herring were collected on the spawning grounds in the upper portion of the Hudson River estuary between rkm 146 and rkm 223 during the months of April and May 2013 in conjunction with the New York State Department of Environmental Conservation’s (NYSDEC) annual fisheries independent spawning stock survey. A 91-m haul seine with 5.08-cm stretch mesh was used to capture the fish. Alewives and Blueback Herring were distinguished via morphological characteristics described by Collette and Klein-MacPhee (Citation2002).
Once captured, the Alewives and Blueback Herring were immediately placed into an in-river holding pen and evaluated for condition. Fish determined to be in favorable condition were selected for tagging. Favorable condition was defined as normal upright swimming behavior with strong opercular movement, absence of bleeding from the gills or body, absence of any gross morphological abnormalities, such as body wounds, fin rot, fungus, blindness, or skeletal deformities and minimal scale loss (Normandeau Associates Citation2008). Fish determined to be in poor or stressed condition were excluded from the study.
Size selection was based on criteria outlined by Winter (Citation1996), who recommends that transmitter weight in air is not to exceed 2% of fish body weight in air. Weight in air of a Vemco V7-4L is 1.8 g; therefore, candidate fish selected had a minimum body mass of 90 g. For both species, mean weight-at-length tables were calculated using data collected from prior fisheries independent sampling surveys conducted in the Hudson River. Only Alewives >215 mm total length and Blueback Herring >225 mm total length were selected for tagging.
Tagging protocol.—A gastric tagging method was selected for this study based on results of Smith et al. (Citation2009) and Castro-Santos and Vono (Citation2013). Castro-Santos and Vono (Citation2013) reported 98% tag retention when Alewives were gastrically implanted with passive integrated transponder PIT tags. Smith et al. (Citation2009), who gastrically tagged river herring in controlled hatchery trials, had 100% tag retention and no mortality 14 d post tagging. Smith et al. (Citation2009) also reported that both tagged and untagged herring held in field cages experienced similar mortality, plasma cortisol, glucose, and chloride measured at 24 h, Lastly, Smith et al. (Citation2009) found no evidence that their tagging protocol adversely affected river herring compared with untagged fish that were subjected only to handling and holding.
River herring selected for tagging were removed from the in-river holding pen and placed on a wet measuring board where data on total length, sex, spawning condition, and scale samples were collected. Sex was determined by gently applying pressure to the abdomen until either milt or eggs exited the vent. A transmitter was gastrically inserted using methods as described in Liedtke and Rub (Citation2012) and Smith et al. (Citation2009). Gastric implantation of transmitters can be performed quickly, with limited or no use of anesthetics (McCleave et al. Citation1978; Pearcy Citation1992; Rivinoja et al. Citation2006; Smith et al. Citation2009; Dunning and Ross Citation2010). A small amount of mineral oil was placed on one end of the transmitter to ease insertion. Transmitters were gently but firmly inserted down the pharynx, past the cardiac sphincter and into the stomach using a small diameter tube until resistance was felt at the pyloric sphincter, similar to methods described in Smith et al. (Citation2009). Handling times was generally <5 min and and tagging time <30 s. Tagged fish were immediately released back into the river without posttagging observation to minimize additional stress (Castros-Santos and Haro Citation2011). A single experienced individual conducted the transmitter implantations to reduce tagger effect. All fish released were observed to be in favorable condition prior to release.
Acoustic data collection.—Transmitters (Vemco V7-4 L) were programmed to transmit on low power (136 dB) and emitted a unique, encoded signal every 30–90 s for the first 60 d (coinciding with the spawning season), and every 60–180 s for the subsequent 135 d (coinciding with the coastal migration period). These settings conserved battery power and allowed for an estimated tag life of 195 d, which was enough time to allow for detection during the coastal migration period. The estimated range for detection of a tag in seawater is between 122 to 292 m depending on environmental conditions (http://vemco.com/range-calculator/). When seawater and freshwater experience similar environmental conditions (e.g., wind velocity, water temperature, rainfall, and turbidity). Tag detection ranges in freshwater are presumed to be considerably greater (Dale Webber, Vemco, personal communication), as observed in other acoustic tagging surveys in the Hudson River (Higgs et al., Cornell University and New York State Department of Environmental Conservation, unpublished data).
Acoustic transmissions were collected by an extensive array of over 40 acoustic receivers spread throughout the Hudson River and New York Harbor (). The NYSDEC, State University of New York–Stony Brook and Delaware State University owned and maintained the Vemco VR2W receivers in this array. Receivers were primarily deployed in 8-km intervals on U.S. Coast Guard maintained navigational buoys and some bridges in the northern part of the Hudson River. Receivers were suspended approximately 5 m below the surface.
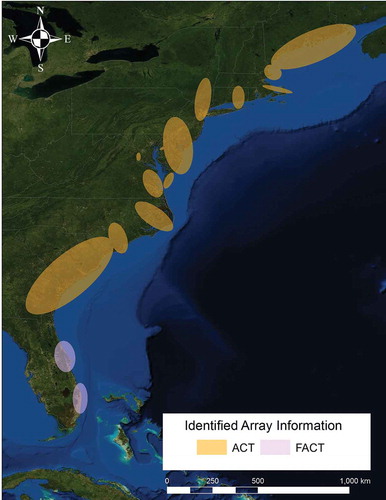
In-river receivers were deployed in early April prior to tagging events and removed in early November. Data were downloaded at various times from July through November. As a member of the Atlantic Cooperative Telemetry Network (http://www.theactnetwork.com/home; ), NYSDEC shares all tag codes with other coastal researchers who maintain coastal receiver arrays. Transmitter detections that occurred from coastal New York to the Gulf of Maine were reported by ACT network members from State University of New York–Stony Brook, Massachusetts Division of Marine Fisheries, and Maine Department of Marine Resources.
FIGURE 2. Map of Atlantic coastal receiver arrays. Map provided by the Atlantic Cooperative Telemetry Network (ACT).
All data transmissions were analyzed and detections were determined to be valid if there were at least three detections within a 24-h period. The wide extent of the in-river array () allowed behavior to be classified as described below.
Posttagging behavior.—Posttagging behaviors may have been affected at varying levels by the individual stresses associated with tagging events, which include capture, handling, and tagging. Fallback is defined as the downstream movement of an upstream migrating anadromous fish following tagging (Frank et al. Citation2009) and is used as a behavioral bioassay of adverse tag effects on alosines (Beasley and Hightower Citation2000; Hightower and Sparks Citation2003; Bailey et al. Citation2004; Olney et al. Citation2006). Once identified, fallback duration was calculated for both Alewives and Blueback Herring as the number of days until continuous up-river movement was observed. Fallback duration was only calculated for individuals that returned to or attempted to return to the spawning grounds and excluded individuals that lost complete spawning and (or) migratory motivation following tagging (). Complete loss of continued spawning or migratory motivation was defined as the lack of further upstream migration and (or) emigration without any attempt to return to spawning areas following tagging events. Henceforth, this behavior is termed terminal fallback. See for hypothetical examples of posttagging behavior designations. Cornell university and New York state department of Environmental conservation.
TABLE 1. Tagging data for individual Alewives and Blueback Herring acoustically tagged in the Hudson River, New York. Posttagging behavior (PTB) was classified as hesitant (H), motivated (M), spawning (S), or unidentified (U). Fallback was classified as having occurred (Y) or not (N), or terminal (T). The initial detection location is relative to the tagging location. A blank cell indicates a measure that is not applicable to that individual.
TABLE 2. Duration of posttagging behaviors (d; means ± SDs) of acoustically tagged Alewives and Blueback Herring in the Hudson River. Sample sizes are given in parentheses; NA = not applicable.
FIGURE 3. Examples of hypothetical posttagging behaviors representing spawning, motivated, or hesitant designations, and an example of a terminal fallback behavior (upper panels). Example of hypothetical posttagging behavior illustrating fallback, fallback duration, posttagging residence time, and postspawn emigration (bottom panel).
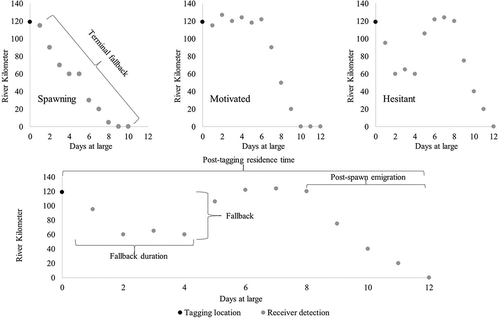
In addition to fallback and terminal fallback as metrics to describe posttagging behavior, tagged fish were segregated into three groups: spawning, motivated, or hesitant. Because fish were tagged on the spawning grounds, failure for fish to migrate upstream may be a result of spawning behavior instead of handling or tagging stresses (Bernard et al. Citation1999) and, thus, were grouped as spawning (; ). Fish that were first detected above the tagging location or exhibited some level of fallback but remained within the spawning area were classified as motivated. Fish first detected downstream of the tagging location that eventually returned to or continued migrating upstream of the tagging location were classified as hesitant (Bernard et al. Citation1999; ; ).
Posttagging residence time for each tagged river herring was calculated as the duration, in days, from its initial tagging event until its final in-river tag detection. Comparisons of posttagging residence time were made between species and sexes.
Postspawn emigration duration was calculated from the initial day an individual was detected moving downriver and exhibited continuous downriver movement until exiting the river at rkm 0. Six individuals disappeared during the study and were not included in either posttagging residence time or postspawn emigration analyses; however, they were included in fallback analysis as long as fallback was identified prior to disappearance. Any significant difference in posttagging behaviors was determined using Welch’s two sample t-test (Welch Citation1938).
RESULTS
In all, 25 river herring were tagged (13 Alewives and 12 Blueback Herring). Tags were evenly distributed between sexes and across a variety of length-classes (). Alewives were tagged and released on April 19, 22, and 23 between rkm 146 and 189; mean lengths were 273 mm for males and 283 mm for females. Blueback Herring were tagged and released on May 7, 8, and 9 between rkm 185 and 223; mean lengths were 242 mm for males and 259 mm for females.
After release, all 13 Alewives and 10 of 12 tagged Blueback Herring were subsequently detected. Six of the remaining 23 tagged fish disappeared during the study, three Alewives (one female, two males) and three Blueback Herring (one male, two females).
Posttagging Behavior
Two of the seven female Alewives (28.6%) exhibited fallback; one of these individuals (tag 8076) returned to the spawning grounds, and the other (tag 8079) disappeared (). The duration of fallback was 10 d and 11 d, respectively (). Four female Alewives (57.1%) exhibited terminal fallback after tagging. (). These fish moved several kilometers downriver following the initial tagging event, where they remained for a few days, then exited the river. The majority (71.4 %) of female Alewives were classified as spawning, 14.3% as hesitant, and 14.3% as motivated.
FIGURE 4. Detections at river kilometers (rkm) plotted over time for Alewives per tag identification number (ID) and sex. Posttagging behavior metrics for each individual fish are H = Hesitant, M = Motivated, or S = Spawning. Detections below rkm 0 are from receivers deployed in New York Harbor and along the south shore of Long Island.
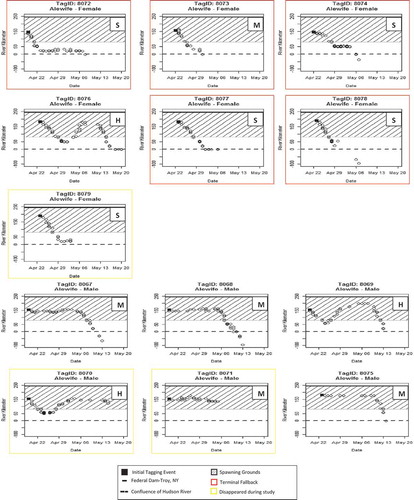
Male Alewives exhibited 100% fallback, 100% returning to the spawning grounds (). The mean duration of fallback was 6.0 d (SD = 2.4, N = 5;). Male Alewives were classified as 66.7% motivated, 33.3% hesitant, and 0% spawning.
Five Blueback males and six females exhibited similar posttagging behaviors with 80% fallback after the tag event (; ). While all males that fell back returned to the spawning grounds, only two females exhibiting fallback behavior returned to the spawning grounds. The remaining two females (tags 8081 and 8087) disappeared from the study before their postspawn behavior could be assessed. Two female Blueback Herring (tags 8082 and 8084) had a fallback durations of 10 and 7 d, respectively (). Male Blueback Herring had a mean fallback duration of 6.2 d (SD = 2.9, N = 5; ). Neither sex of Blueback Herring exhibited terminal fallback following tagging events. Male Blueback Herring were classified as 60.0% hesitant and 40.0% motivated, while female Blueback Herring were classified as 66.7% hesitant and 33.3% motivated. There were no significant differences in mean duration of fallback between species (t = 0.28, df = 11.9, P = 0.786) or between sexes of Blueback Herring (t = 1.15, df = 2.7, P = 0.34); however, there was a significant difference in mean fallback duration between male and female Alewives (t = 3.74, df = 4.9, P < 0.014), female Alewives averaging an additional 4.5 d during fallback events. Alewives and Blueback Herring classified as motivated had a mean maximum fallback distance of 8.3 and 14.5 km, respectively, while those classified as hesitant had a mean maximum fallback distance of 64.5 and 99.2 kilometers, respectively. One Alewife classified as spawning had a maximum fallback distance of 83 km.
FIGURE 5. Detections at river kilometers (rkm) plotted over time for Blueback Herring per tag identification number (ID) and sex. Posttagging behavior metrics for each individual are described as either H = Hesitant, M = Motivated, or S = Spawning. Detections below rkm 0 are from receivers deployed in New York Harbor and along the south shore of Long Island. Posttagging behaviors for tags 8081 and 8087 were unable to be classified due to disappearance during the study. No Blueback Herring exhibited terminal fallback.
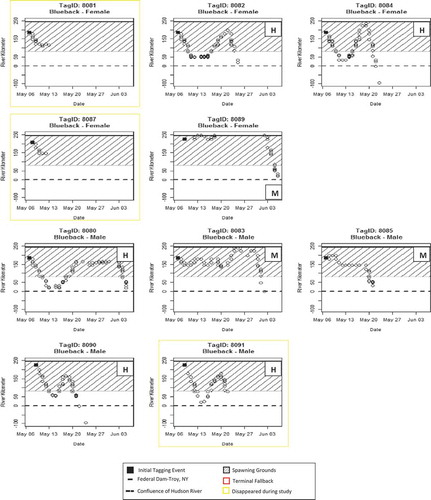
One female Blueback (tag 8089) appears to have moved above the Federal dam at Troy via the navigation lock, spawned, possibly in the Mohawk River, reappeared below the dam then emigrated to the ocean.
Posttagging Residence Time
Female Alewives had a mean posttagging residence time of 14.0 d (SD = 6.2, n = 6) and male Alewives 22.5 d (SD = 1.7, n = 4) (,). Female Blueback Herring had a mean posttagging residence time of 21.3 d (SD = 6.8, n = 3) and males 21.0 d (SD = 8.2, n = 4 (,). The difference in posttagging residence time between male and female Alewives was significant (t = –3.18, df = 6.1, P < 0.019).
There was no significant difference (t = –1.11, df = 12.3, P = 0.286) in posttagging residence time between Alewife and Blueback Herring. Alewives had a mean of 17.4 d (SD = 6.4, n = 10) and Blueback Herring a mean of 21.1 d (SD = 7.1, n = 7) ().
Both river herring species exhibited rapid postspawn emigration behavior (). Female Alewives had a mean postspawn emigration of 6.2 d (SD = 1.3, n = 5) and males 5.3 d (SD = 1.5, n = 4). Female Blueback Herring had a mean postspawn emigration of 3.3 d (SD = 0.6, n = 3) and males 3.3 d (SD = 0.5, n = 4). There were no significant differences in postspawn emigration between male and female Alewives (t = 1.00, df = 6.1, P = 0.356) or male and female Blueback Herring (t = 0.20, df = 4.0, P = 0.851), however there was a significant difference between Alewife and Blueback Herring (t = 4.98, df = 10.4, P < 0.0004) with Alewife averaging an additional 2.4 d to exit the river.
Coastal Movements
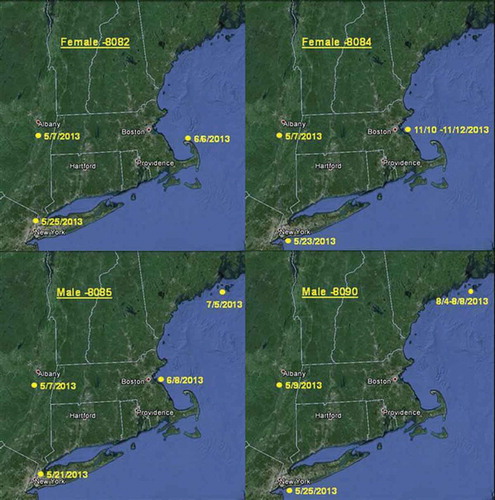
Data were collected on coastal movements of four Blueback Herring (two females and two males, see ). The four Blueback Herring were detected on receivers spanning the south shore of Long Island, New York to the mouth of Penobscot Bay, Maine. Coastal tag detections ranged from June 6th through November 12th. No data were received on coastal movements of Alewives.
DISCUSSION
Posttagging Behavior
Instances of zero posttagging detections are probably due to mortality; however, albeit unlikely, there is also the possibility of tag expulsion due to regurgitation or egestion. Other alosine tagging studies (Chappelear and Cooke Citation1994; Acolas et al. Citation2004; Bailey et al. Citation2004; Castro-Santos and Vono Citation2013) report high tag retention rates (>95%) when using gastric implantation methods. Five of the six tagged fish that disappeared during the study occurred in areas of heavy harvest from both commercial and recreational fishers. Another possible cause of disappearance is predation from piscivorous fishes (e.g., Striped Bass Morone saxatilis, black bass Micropterus spp.) or birds of prey (e.g., bald eagles Haliaeetus leucocephalus, ospreys Pandion haliaetus).
Terminal fallback exhibited by female Alewives may be an artifact of the timing and location of the tagging event. Female Alewives were tagged on the spawning grounds and were either in spawning condition (ripe and flowing) or very close to spawning condition. Thus, the resulting emigration could have been a result of typical spawning behavior. Alternatively, it is possible that stresses resulting from tagging altered normal spawning behavior, causing accelerated spawning (releasing of eggs) or spawning cessation followed by rapid emigration. Olney et al. (Citation2006) reported that American Shad Alosa sapidissima exhibited similar altered migratory behaviors following tagging events causing some individuals to cease or delay spawning runs. The only female Alewife that returned to the spawning grounds was classified as spent (finished spawning) prior to tag insertion. This is counterintuitive to the reported spawning behavior of female Alewives, which suggests that spent fish move rapidly downstream and pass later migrants on their way upstream to spawning grounds (Cooper Citation1961; Kissil Citation1974; Collette and Klein-MacPhee Citation2002). It is possible that this individual was not completely spent, and this behavior may be further evidence of batch spawning, as documented in Alewives by Ganais et al. (Citation2015) and similarly in American Shad (Olney et al. Citation2001; Maltais et al. Citation2010).
Male Alewives and both sexes of Blueback Herring behavior appeared less influenced by the tagging event. Although 100% of male Alewives exhibited fallback, the majority (66.7%) were classified as motivated and never left the spawning grounds during their respective fallback events. Both sexes of Blueback Herring were tagged earlier in the spawning run, probably in prespawning condition, and in locations where continued upstream migration was likely to occur.
Posttagging Residence Time
Results of this study suggest male Alewives and both sexes of Blueback Herring are less affected by timing and location of tagging than female Alewives (, ). Loesch (Citation1969) notes that Blueback Herring males tend to remain longer than females during spawning events and, after exiting spawning areas, some males may actually return with the succeeding wave of upstream migrants. This behavior may apply to male Alewives as well, given the similar migratory behaviors observed between male Alewives and Blueback Herring.
Coastal Movements
Blueback Herring tagged in the Hudson River migrated as far north as the Gulf of Maine following spawning similar to findings by Normandeau Associates (Citation2008). For many years, incidental bycatch in marine fisheries was a known but unquantified mortality source for river herring and shad. Bycatch estimation was identified as a high priority in the most recent American Shad stock assessment review (ASMFC Citation2007). A significant source of river herring incidental ocean bycatch in New England and the mid-Atlantic are the high-volume midwater trawl and small-mesh bottom trawl fisheries targeting Atlantic Herring Clupea harengus, Atlantic Mackerel Scomber scombrus, squid, and Butterfish Peprilus triacanthus. Between 1989 and 2010, observed annual Alewife landings ranged from 2.7 to 484 metric tons with an annual average of 119 metric tons, while landings for Blueback Herring were 19.6 metric tons to 1,803 metric tons with an annual average of 290 metric tons (ASMFC Citation2012). Detections of Blueback Herring along the northeast Atlantic coast during this study provides the confidence that the implementation of a large-scale tagging study investigating coastal migration patterns is feasible. With a better understanding of the spatiotemporal distribution of river herring during coastal migrations, incidental bycatch can be reduced.
RECOMMENDATIONS FOR FUTURE TAGGING STUDIES
This study illustrates the importance of selecting the appropriate location for and timing of tagging events on migratory anadromous species. Stresses induced by tagging can result in terminal fallback and may alter normal spawning behavior if tagging occurs late in the spawning run and on the spawning grounds. In order to obtain data most representative of normal spawning behaviors, we recommend tagging upstream-migrating anadromous fishes early in their migration and before they reach their spawning grounds. Additionally, when possible we recommend the use of multiyear transmitters. With the exception of the initial tagging year, the use of multiyear transmitters will eliminate stress induced behavior resulting from tagging, and it should be more representative of natural upstream migration and spawning behaviors. However, multiyear transmitters may require surgical implantation in lieu of gastric insertion to avoid the potential long-term negative impacts to feeding, stomach rupture, and transmitter loss (Liedtke and Rub Citation2012).
Based on the behavior of the female Blueback (tag 8089) that appeared to ascend the lock at the Federal Dam at Troy, any future studies in the Hudson River watershed should include additional receivers above the Federal Dam, as well as throughout the Mohawk River.
The study indicated high variability in fallback behavior (see ). While some individuals moved several kilometers downriver, others only moved minimal distances remaining within the spawning area. Following the above recommendation that fish be tagged early in their migration and before they reach their spawning grounds should reduce the variability observed in fallback behavior.
Researchers should take advantage of any opportunity that arises to examine fish after internal transmitter placement. The use of a secondary mark (i.e., external streamer tag) to identify internally tagged individuals can assist in assessing transmitter loss, transmitter location relative to placement, as well as the ability to document outcomes of transmitter placement (Liedtke and Rub Citation2012) in the event tagged individuals are recaptured. Individuals that disappeared in areas of heavy harvest from both commercial and recreational fishers could have provided additional information as described above during possible recapture events had they been fitted with a secondary mark. Secondary external tags were not used in this study because the extra handling time required to attach tags was thought to increase stress and, thus, would be detrimental to tagged individuals. However, results of this study indicate that mortality due to gastric tagging is minimal and, thus, suggest that river herring may be able to handle the added stress of an additional external tag. Additionally, Normandeau Associates (Citation2008) reported a tag retention rate of 100% for 90 Alewives and 94 Blueback Herring tagged with a T-bar style external streamer tag. To identify potential transmitter effects, as well as provide opportunity to assess an individual fish’s overall posttagging condition during recapture events, we recommend the use of a secondary T-bar style external streamer tag.
This study demonstrates the importance of the ACT network and its ability to connect researchers to data that would otherwise be unavailable. The ACT network is a grassroots effort to facilitate data sharing between researchers utilizing acoustic telemetry to gain a greater understanding of a wide variety of aquatic species. The ACT network began with 15 researchers in 2006 and now has over 120 members from Florida to Maine. It is strongly recommended that all researchers conducting acoustic tagging studies along the Atlantic coast become members of the ACT network. Existing and future ACT network members are strongly encouraged to actively seek out the owners of “unknown” transmitter codes that appear in their respective arrays. This experiment can now be repeated with confidence on a larger scale with multiyear tags in order to identify unknown in-river spawning areas, provide information regarding spawning site fidelity, and bolster current knowledge of coastal migration patterns for both species.
Acknowledgments
I thank all the employees of the Hudson River Fisheries Unit who helped collect and tag the fish for this study, as well as Pat Sullivan, K. Hattala, C. Standley, R. Adams, three anonymous reviewers, and my brilliant, beautiful wife, Jessica Best, for their helpful comments and edits to earlier versions of this manuscript. I also thank Dewayne Fox, Keith Dunton, the Massachusetts Division of Marine Fisheries and the Maine Department of Marine Resources for sharing transmitter detections from their acoustic receiver arrays along the Atlantic coast. I thank the Cornell University, the ACT network, New England Interstate Water Pollution Control Commission, New York State Department of Environmental Conservation and the Hudson River Estuary Program for their support during this project.
References
- Acolas, M. L., M. L. Bégout Anras, V. Vernon, H. Jourdan, M. R. Sabatie, and J. L. Bagliniere. 2004. An assessment of the upstream migration and reproductive behavior of Allis Shad (Alosa alosa L.) using acoustic tracking. ICES Journal of Marine Science 61:1291–1304.
- ASMFC (Atlantic States Marine Fisheries Commission). 2007. American Shad stock assessment report for peer review, volume 1. ASMFC, Stock Assessment Report 07-01, Arlington, Virginia.
- ASMFC (Atlantic States Marine Fisheries Commission). 2012. River herring benchmark stock assessment, volume 1. ASMFC, Stock Assessment Report 12-02, Arlington, Virginia.
- Bailey, M. M., J. J. Isely, and W. C. Bridges. 2004. Movement and population size of American Shad near a low-head lock and dam. Transactions of the American Fisheries Society 133:300–308.
- Beasley, C. A., and J. E. Hightower. 2000. Effects of a low-head dam on the distribution and characteristics of spawning habitat used by Striped Bass and American Shad. Transactions of the American Fisheries Society 129:1319–1330.
- Bernard, D. R., J. J. Hasbrouck, and S. J. Fleischman. 1999. Handling-induced delay and downstream movement of adult Chinook Salmon in rivers. Fisheries Research 44:37–46.
- Castros-Santos, T., and A. Haro. 2011. Passage of anadromous shad and river herring at barriers. U.S. Geological Survey, Study Plan 09069, Reston, Virginia.
- Castro-Santos, T., and V. Vono. 2013. Posthandling survival and PIT tag retention by Alewives—a comparison of gastric and surgical implants. North American Journal of Fisheries Management 33(4):790–794.
- Chappelear, S. J., and D. W. Cooke. 1994. Blueback Herring behavior in the tailrace of the St. Stephan Dam and fish lock. Pages 108–112 in J. E. Cooper, R. T. Eades, R. J. Klauda, J. G. Loesch, editors. Anadromous Alosa symposium. American Fisheries Society, Tidewater Chapter, Bethesda, Maryland.
- Collette, B. B., and G. Klein-MacPhee. 2002. Bigelow and Schroeder’s fishes of the Gulf of Maine, 3rd edition. Smithsonian Institution Press, Washington, D.C.
- Cooper, R. A. 1961. Early life history and spawning migration of the Alewife Alosa psuedoharengus. Master’s thesis. University of Rhode Island, Kingston.
- Dunning, D. J., and Q. E. Ross. 2010. Effect of radio-tagging on escape reactions of adult Blueback Herring to ultrasound. North American Journal of Fisheries Management 30:26–32.
- Frank, H. J., M. E. Mather, J. M. Smith, J. T. Finn, and S. D. McCormick. 2009. What is “fallback”?: metrics needed to assess telemetry tag effects on anadromous fish behavior. Hydrobiologia 653:237–249.
- Ganais, K., J. N. Divino, K. E. Gherard, J. P. Davis, F. Mouchlianitis, and E. T. Schultz. 2015. A reappraisal of reproduction in anadromous Alewives: determinate versus indeterminate fecundity, batch size, and batch number. Transactions of the American Fisheries Society 144:1143–1158.
- Greeley, J. R. 1935. Fishes of the watershed with annotated list. Pages 63–101 in E. Moore, editor. A biological survey of the Mohawk–Hudson watershed. New York Conservation Department, Supplement to the 24th Annual Report, Albany.
- Hattala, K., A. W. Kahnle, and R. D. Adams. 2011. Sustainable fishing plan for New York river herring stocks. Available: http://www.dec.ny.gov/docs/fish_marine_pdf/nyherrsfmp.pdf. (October 2017).
- Hattala, K., A. W. Kahnle, and R. D. Adams. 2012. Status of New York river herring stocks. Pages 291–366 in Stock Assessment Report 12-02 of the Atlantic States Marine Fisheries Commission: river herring benchmark stock assessment, volume II. Atlantic States Marine Fisheries commission, Arlington Virginia.
- Hightower, J. E., and K. L. Sparks. 2003. Migration and spawning habitat of American Shad in the Roanoke River, North Carolina. Pages 193–199 in K. E. Limburg and J. R. Waldman, editors. Biodiversity, status, and conservation of the world’s shads. American Fisheries Society, Symposium 35, Bethesda, Maryland.
- Kissil, G. W. 1974. Spawning of the anadromous Alewife, Alosa psuedoharengus, in Bride Lake, Connecticut. Transactions of the American Fisheries Society 103:312–317.
- Liedtke, T. L., and A. M. W. Rub. 2012. Techniques for telemetry transmitter attachment and evaluation of transmitter effects on fish performance. Pages 45–87 in N. S. Adams, J. W. Beeman, J. H. Eiler, editors. Telemetry techniques: a user guide for fisheries research. American Fisheries Society, Bethesda, Maryland.
- Loesch, J. G. 1969. A study of Blueback Herring, Alosa aestivalis (Mitchell). Master’s thesis. University of Connecticut, Storrs.
- Loesch, J. G. 1987. Overview of life history aspects of anadromous Alewife and Blueback Herring in freshwater habitats. Pages 97–103 in M. J. Dadswell, R. J. Klauda, C. M. Moffitt, R. L. Saunders, R. A. Rulifson, and J. E. Cooper, editors. Common strategies of anadromous and catadromous fishes. American Fisheries Society, Symposium 1, Bethesda, Maryland.
- Maltais, E., G. Daigle, G. Colbeck, and J. J. Dodson. 2010. Spawning dynamics of American Shad (Alosa sapidissima) in the St. Lawrence River, Canada–USA. Ecology of Freshwater Fish 19:586–594.
- McCleave, J. D., J. H. Power, and S. A. Rommel Jr. 1978. Use of radio telemetry for studying upriver migration of adult Atlantic Salmon (Salmo salar). Journal of the Fisheries Research Board of Canada 32:559–563.
- NOAA (National Oceanic and Atmospheric Administration). 2007. River herring species of concern. Available: www.nmfs.noaa.gov. (September 2017).
- Normandeau Associates. 2008. Spawning stock characteristics of Alewife (Alosa pseudoharengus) and Blueback Herring (Alosa aestivalis) in the Hudson River estuary and tributaries, including the Mohawk River. Final Report prepared for the New York State Department of Environmental Conservation, Albany.
- Olney, J. E., S. C. Denny, and J. M. Hoenig. 2001. Criteria for determining maturity stage in female American shad, Alosa sapidissima, and a proposed reproductive cycle. Bulletin Franc¸ais de la Peˆche et de la Pisciculture 362⁄363:881–901.
- Olney, J. E., R. J. Latour, B. E. Watkins, and D. G. Clarke. 2006. Migratory behavior of American Shad in the York River, Virginia, with implications for estimating in-river exploitation from tag recovery data. Transactions of the American Fisheries Society 135:889–896.
- Owens, R. W., R. O’Gorman, E. L. Mills, L. G. Rudstam, J. J. Hasse, B. H. Kulik, and D. B. MacNeil. 1998. Blueback Herring (Alosa aestivalis) in Lake Ontario: first record, entry route, and colonization potential. Journal of Great Lakes Research 24:723–730.
- Pearcy, W. G. 1992. Movements of acoustically-tagged Yellowtail Rockfish Sebastes flavidus on Heceta Bank, Oregon. U.S. National Marine Fisheries Service Fishery Bulletin 90:726–735.
- Rivinoja, P., K. Leorardsson, and H. Lundqvist. 2006. Migration success and migration time of gastrically radio-tagged v. PIT-tagged adult Atlantic Salmon. Journal of Fish Biology 69:304–311.
- Schmidt, R., and T. Lake. 2000 Alewives in hudson river tributaries, two years of sampling. Final report to the Hudson River Foundation from Hudsonia, Annandale, New York.
- Smith, J. M., M. E. Mather, H. J. Frank, R. M. Muth, J. T. Finn, and S. D. McCormick. 2009. Evaluation of a gastric radio tag insertion technique for anadromous river herring. North American Journal of Fisheries Management 29:367–377.
- Welch, B. L. 1938. The significance of the difference between two means when the population variances are unequal. Biometrika 29:350–362.
- Winter, J. 1996. Advances in underwater biotelemetry. Pages 555–590 in B. R. Murphy and D. W. Willis, editors. Fisheries techniques, 2nd edition. American Fisheries Society, Bethesda, Maryland.