ABSTRACT
Gasoline and heating oil travel through pipelines and are also distributed by truck to above-ground and underground storage tanks. Through its extensive use as a fuel oxygenate, methyl tert-butyl ether (MTBE) is found nearly ubiquitously throughout the environment. MTBE, a widely used gasoline additive, is recently being scrutinized for potential environmental damage in groundwater and in the atmosphere. We study the structural, total energy, thermodynamic, and conductive properties of absorption of MTBE on carbon nanotube (CNT), which converts to CO and H2O. There are four situations for MTBE to be near by single-walled nanotube (SWNT) that we have investigated its adsorption on CNT and convertion to other products. The thermodynamic properties are calculated for them. The calculations have quantum mechanical detail and are based on a semiempirical (MNDO method), which is applied to the evaluation of both the electronic structure and of the conductance.
INTRODUCTION
MTBE (methyl tert-butyl ether) is a chemical added to gasoline to increase octane. Its use began in the 1970s to replace lead in gasoline. After 1995, many metropolitan areas of the country with smog problems also added MTBE to gasoline because it helps to reduce harmful emissions from automobile exhaust. Adding MTBE to gas has been one way to meet the Environmental Protection Agency's (EPA's) oxygenate mandate.
The major concern about MTBE (and other oxygenate compounds) is focused on their occurrence in groundwater and drinking water supplies. In MTBE, oxygenates are used as octane enhancers, principally in replacement of alkyl-lead compounds.
Many processes will govern the exchange of MTBE between surface waters and the atmosphere. As a major process in removing MTBE from aquatic systems, atmospheric behavior and fates of the compound are clearly important.[ Citation 1 ]
Air-water exchange appears to be the major process in the removal of MTBE from the estuarine and coastal waters. Carbon nantube (CNT), however, is likely to contribute to the removal (albeit to a lesser extent).[ Citation 2 ]
Single-walled carbon nanotubes (SWNTs) have attracted great interest due to their unique electronic properties and nanometer size. Because of these unique properties, they are great potential candidates in many important applications, such as nanoscale electronic devices, chemical sensors, and field emitters. The effect of gas adsorption on the electrical resistance of a CNT has received great attraction because of fast response, good sensitivity of chemical environment gases, and low operating temperature.[ Citation 3 ] Theoretical studies have confirmed the remarkable change in electronic properties of CNT due to the detection and removed of gas molecules.[ Citation 4 , Citation 5 ] Most molecules are known to be an electron acceptor such as O2 or an electron donor such as MTBE and H2O, displaying relatively small charge transfer between molecules adsorbed weakly on the CNT wall. SWNTs are used for investigation, detection, and removal MTBE in air-water.
Interaction between MTBE molecules and SWNT is investigated using MNDO method by semiempirical methods. We study the structural, total energy, thermodynamic properties of passing MTBE and SWNT in room temperature. All the geometry optimization structures were carried out using Gaussian program package. Density functional theory (DFT) optimized intermediates and transient states of them. The results show a sensitivity enhancement in resistance and capacitance when MTBE is passing across SWNT.
THE COMPUTATIONAL METHODS
The geometry optimizations were performed using an all-electron linear combination of atomic orbital density functional theory (DFT) calculations using the Gaussian program package.
Another advantage is that for specific and well-parameterized molecular systems, these methods can calculate values that are closer to experiment than lower level ab initio techniques. The accuracy of a molecular mechanics or semiempirical quantum mechanics method depends on the database used to parameterize the method. Configuration Interaction (or electron correlation) improves energy calculations using CNDO, INDO, MINDO/3, MNDO, AM1, PM3, ZINDO/1, and ZINDO/S for these electron configurations.
The mechanism of conversion of MTBE to other products on SWNT are investigated with MNDO methods, because MNDO calculated heats of formation, molecular geometries, dipole moments, ionization energies, electron affinities, and other properties for them.[ Citation 6 , Citation 7 ]
The adsorption, electric, binding nuclear energy, root mean square (RMS) gradient, heat of formation, and Gibbs free energy are calculated by MNDO methods in semiempirical quantum in DFT. The electric resistance for them is following as:
RESULTS
MTBE moves quickly through soil, dissolves easily in water, and takes longer to break down than some other chemicals. Drinking water with MTBE levels of 20 to 40 “parts per billion” (acceptable taste and odor) would probably not pose health risks. MTBE at 20 ppb in water is about the same as one drop in 500 gallons of water. Therefore, farm and residential releases, car accidents, spills, boats, and storm water run off also release gasoline into the environment. MTBE moves quickly through soil, dissolves easily in water, and takes longer to break down than some other chemicals. We have used single-walled carbon nanotube (SWNT) for removed MTBE in environment.
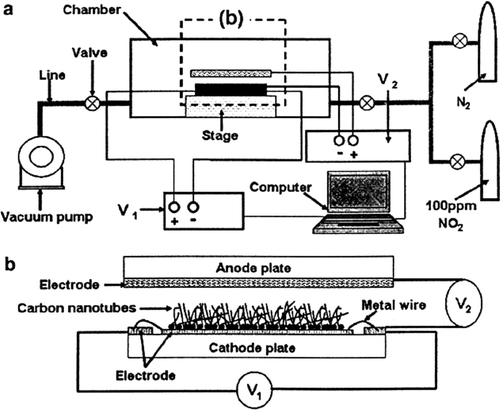
SWNTs have been used as the sensing material in pressure,[ Citation 8 , Citation 9 ], field emission effect,[ Citation 10 ] thermal,[ Citation 11 ] gas,[ Citation 12 ] optical,[ Citation 13 ] mass,[ Citation 4 ] position,[ Citation 14 ] stress,[ Citation 15 ], strain,[ Citation 16 ] chemical,[ Citation 17 ] and biological sensors,[ Citation 5 , Citation 18 ] as shown in . Upon exposure to gaseous molecules such as MTBE, the electrical resistance of a semiconducting SWNT is found to dramatically increase or decrease.
The main reaction products for MTBE-CNT in this interaction are methanol and t-butyl alcohol, with secondary product CO and H2O in air-water
To understand the effects of MTBE in SWNT, the thermodynamic properties were calculated by MNDO methods in semiempirical quantum with the Gaussian program package.
This type of interaction is more known as an acid-base interaction, where the H atom of the acid (in this case MTBE) interacts with carbons in end SWNT (the base). Simultaneously, the carbon site (acting as Lewis acid) interacts with the O (2p) orbital of the oxygen of the adsorbed MTBE molecule.
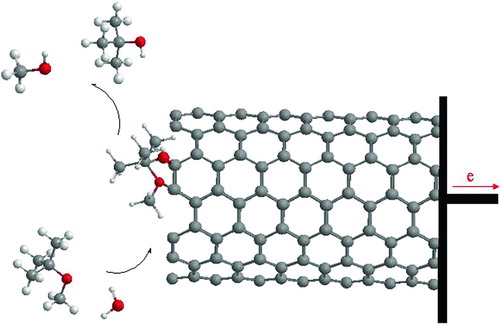
Formation of methanol and t-butyl alcohol by the oxidative dehydrogenation of MTBE and H2O depends critically upon the reaction step that requires the oxide surface to acquire a negative charge. It is well accepted that upon adsorption, the C–O–C bond of the ether dissociates heterolytically to yield a methoxid and a proton. Ball-and-stick models of the SWNT and MTBE are showed in .
In this work, the first situation is investigated for MTBE. The effects of MTBE on SWNT-based gas sensor are shown in .
TABLE 1 The thermodynamic properties of the interaction of MTBE–SWNT and conversion to alcohols in air-water at 298K
In this mechanism, the transition of electron is between MTBE and SWNT-H2O, those steps that are transient state converted MTBE to alcohols. Adsorption of MTBE and H2O-reduced on nanosurface has also been studied in air-water.
The total energy (12356.18 MJ/mol) and binding energy (14583.33 MJ/mol) for this adsorption has maximum distance of 2.76 Å to SWNT, which the heat of formation energy of them is the highest in this distance (14720.42 MJ/mol).
The enthalpy difference (Δ H) and Gibbs free difference (Δ G) energy for this reaction are −353.35 and −582 MJ/mol, respectively, which indicates that the interaction is exothermic and spontaneous and MTBE is separated from air-water. The dipole moment is 1.7 × 104 D when MTBE is converted to alcohols at 6.54 Å. RMS gradient (Kcal/mol· Å) is different for conversion of MTBE on SWNT in 298K.
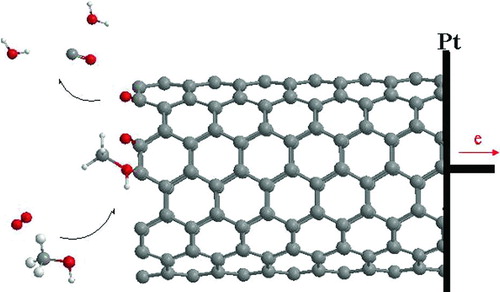
CO and H2O represent the second most important reaction products in our calculations. This sort of product formation from oxidation has been reported on the surfaces. shows ball-and-stick models of conversion of methanol to CO and H2O in air. Alcohol oxidation resulted in the peeling of the outer graphite layers from the nanotubes.
show thermodynamic properties at 298 K obtained this way for the pure oxides.
TABLE 2 The thermodynamic properties of interaction between methanol and t-butyl alcohol–SWNT and conversion to CO and H2O
In , the total energy different for this conversioni is negative (−527.70 MJ/mol), indicating that this interaction is exothermic. shows that their binding energy (E bin) and heat of formation energy increased until 2.49 Å, thus it decreased for this conversion on surface because electrons are translated between surface and alcohols molecule in transient state. shows that the Gibbs free energy is enhanced for intermediates and transient state and then it is reduced for the product. That nuclear energy is too. The dipole moment is 1.23 × 104 D when alcohols converted to CO and H2O in 5.54 Å.
The goal of this work is to improve the detection of surface species of SWNTs gas sensors under their working conditions using computer calculation, and to correlate the sensor signals with the relative changes of the electric resistance (Ω).
When uniform alcohol pressure was applied on the membranes, a change in electric resistance of the SWNTs was observed (). In the interaction, to correlate the sensor signals with the relative changes of the electric resistance (Ω), we have to convert calculation data (E ele(V)) to the electric resistance (Ω) (Equation Equation2), which is shown in .
FIGURE 4 The electric resistance: (a) MTBE converted to methanol and t-butyl alcohol; (b) methanol and t-butyl alcohol converted to CO and H2O.
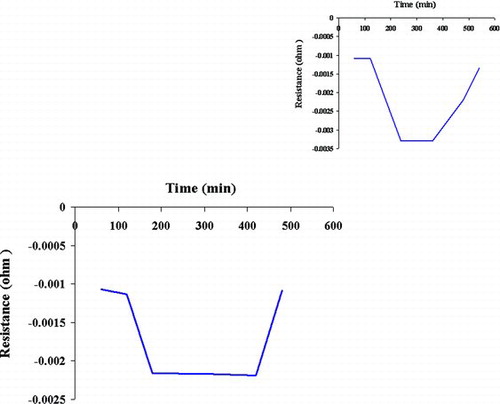
A current versus voltage curve recorded with a SWNT sample after time exposure to MTBE showed down-fold conductance depletion. Exposure to MTBE molecules increased the conductance of the SWNT sample.
The SWNT is a down hole-doped semiconductor, as can be gleaned from the current versus gate voltage curve shown in (middle curve), where the resistance of the SWNT is observed to decrease. The conductance of the SWNT with alcohols to CO and H2O () is more than MTBE to alcohols ().
The MTBE-air is injected into the nano-reactor containing either pure SWNT compound with a large fraction of MTBE that was found to be transformed to CH3OH and (CH3)3COH. It then can be adsorbed in the form of CO and H2O, producing an adsorbed oxygen atom. This transformed to formaldehyde (CH3O) forming an adsorbed hydrogen atom for methanol.
There are same results are found for t-butyl alcohol, which can use this method for removing or reducing MTBE in the environment.
CONCLUSION
We have used SWNT for decreasing MTBE in air-water. Because the SWNT is snared for MTBE and adsorption on wall is impossible, it is converted to CO and H2O. The results in confirms this idea.
The prominent peak corresponds to increasing distances (Å) of adsorption of MTBE on SWNT at 298 K, which is affected to electric properties SWNT.
The sensitivity could be measured when the detecting gas was mixed homogeneously with the air. The sensitivity depends on MTBE concentration at each work temperature. The sensitivity increases with increasing the MTBE gas concentration. The response and sensitivity of the MTBE-treated sensor was found to be very high when compared to the air-treated sensor.
REFERENCES
- Guitart , C. , Bayona , J. M. and Readman , J. W. 2004 . Sources, distribution and behaviour of methyl tert-butyl ether (MTBE) in the Tamar Estuary, UK . Chemosphere , 57 : 429 – 437 .
- Star , A. , Bradley , K. , Gabriel , J. Ch. P. and Grüner , G. 2004 . Carbon nanotube nanoelectronic devices for chemical detection in liquid hydrocarbons . Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. , 49 : 888 – 889 .
- Doo Lee , Y. , Cho , W. S. , Moon , S. I. , Lee , Y. H. , Kim , J. K. , Nahm , S. and Ju , B K . 2006 . Gas sensing properties of printed multiwalled carbon nanotubes using the field emission effect . Chem. Phys. Lett. , 433 : 105 – 109 .
- Jiang , L. , Colmenares , L. , Jusys , Z. , Sun , G. Q. and Behm , R. J. 2007 . Ethanol electrooxidation on novel carbon supported Pt/SnOx/C catalysts with varied Pt:Sn ratio . Electrochim. Acta , 53 : 377 – 389 .
- Sinha , N. , Ma , J. and Yeow , J. T. W. 2006 . Carbon nanotube-based sensors . J. Nanosci. Nanotechnol. , 6 : 573 – 590 .
- Dewar , M. J. S. and McKee , M. L. 1977 . Ground states of molecules. 41. MNDO results for molecules containing boron . J. Am. Chem. Soc. , 99 : 5231 – 5241 .
- Dewar , M. J. S. and Thiel , W. 1977 . Ground states of molecules. 38. The MNDO method. Approximations and parameters . J. Am. Chem. Soc. , 99 : 4899 – 4907 .
- Dharap , P. , Li , P. , Nagarajaiah , S. and Barrera , E. V. 2004 . Nanotube film based on nanotubes for strain sensing . Nanotechnology , 15 : 379 – 382 .
- Dag , S. , Ozturk , Y. , Ciraci , S. and Yildirim , T. 2005 . Adsorption and dissociation of hydrogen molecules on bare and functionalized carbon nanotubes . Phys. Rev. B , 72 : 1 – 8 . (155404)
- Lee , Y. D. , Cho , W. S. , Moon , S. I. , Lee , Y. H. , Kim , J. K. , Nahm , S. and Ju , B. K. 2006 . Gas sensing properties of printed multi walled carbon nanotubes using the field emission effect . Chem. Phys. Lett. , 433 : 105 – 109 .
- Smart , S. K. , Cassady , A. I. , Lu , G. Q. and Martin , D. J. 2006 . The biocompatibility of carbon nanotubes . Carbon , 44 : 1034 – 1047 .
- Kong , J. , Franklin , N. R. , Zhou , C. , Chapline , M. G. , Peng , S. , Cho , K. and Dai , H. 2000 . Nanotube molecular wires as chemical sensors . Science , 287 : 622 – 625 .
- Modi , A. , Koratkar , N. , Lass , E. , Wei , B. and Ajayan , P. M. 2003 . Miniaturized gas ionization sensors using carbon nanotubes . Nature , 424 : 171 – 174 .
- Silva , L. B. , Fagan , S. B. and Mota , R. 2004 . Ab initio study of deformed carbon nanotube sensors for carbon monoxide molecules . Nano Lett. , 4 : 65 – 67 .
- Fu , Q. and Liu , J. 2005 . Integrated single-walled carbon nanotube/microfluidic devices for the study of the sensing mechanism of nanotube sensors . J. Phys. Chem. B , 109 : 13406 – 13408 .
- Wang , J. , Musameh , M. and Lin , Y. 2003 . Solubilization of carbon nanotubes by nafion toward the preparation of amperometric biosensors . J. Am. Chem. Soc. , 125 : 2408 – 2409 .
- Li , J. , Lu , Y. , Ye , Q. , Cinke , M. , Han , J. and Meyappan , M. 2003 . Carbon nanotube sensors for gas and organic vapor detection . Nano Lett. , 3 : 929 – 933 .
- Qi , P. , Vermesh , O. , Grecu , M. , Javey , A. , Wang , Q. , Dai , H. , Peng , S. and Cho , K. J. 2003 . Toward large arrays of multiplex functionalized carbon nanotube sensors for highly sensitive and selective molecular detection . Nano Lett. , 3 : 347 – 351 .