ABSTRACT
The grafting reaction was carried out at 165°C with dibutyl peroxide (DTBP) as initiator (PEW:MA:DTBP = 100:10:2). The grafted product was analyzed by Fourier transform infrared (FTIR) spectroscopy. Hydrophobic CaCO3 particles were prepared in the process of modification by polyethylene wax grafting maleic anhydride (MA-g-PEW) coupling agent. The weight ratio of MA-g-PEW to CaCO3 changed from 1 to 2.5 wt%. By changing the weight ratio of MA-g-PEW to CaCO3, the surface property of CaCO3 particles was evidenced by active ratio, transmission electron microscope (TEM), scanning electron microscope (SEM), FTIR. The active ratio of CaCO3 reached 100%, it showed that the surface of CaCO3 was changed from hydrophilic to hydrophobic after modification. FTIR spectra of CaCO3 showed that the authors have succeeded in modification through chemical bond.
INTRODUCTION
The free-radical-initiated functionalization of polyolefins has received considerable attention in recent years and has become a method largely used from an industrial point of view.[ Citation 2 ] It is an efficient modifier of CaCO3 also. CaCO3 is one of the important fillers used in the industries of plastic, rubber, paint, and so on.[ Citation 3 ] They only lower the cost but also improve the tensile strength and stiffness of the base resin, leading to composites exhibiting a satisfactory performance improvement. Obviously, the filler must be well dispersed in the matrix to avoid zones of weaker cohesion where flaws and other defects will be initiated upon stressing.[ Citation 4 ] CaCO3, however, has some disadvantages, such as a poor acid resistance and a weaker surface strength[ Citation 5 ] that make it very difficult to be used widely. Surface modification of CaCO3 with hydrophobic species would lead to these shortcomings to be improved, because this treatment can endow CaCO3 particles with new properties. So there is widespread interest in the modification of calcium carbonate. In the literature, a number of different coupling agents and modifiers are reported for this system, such as various silanes and titanates as well as stearic acid.[ Citation 6 ] This modification leads to facilitation of dispersion in the polymer nonpolar matrix as a consequence of reduction of the calcium carbonate surface energy and its polarity.
We have succeed in preparing the grafted product of polyethylene wax grafting maleic anhydride (MA-g-PEW) in the presence of a free radical initiator at high temperature,[ Citation 7 ] which is adopted as the modifier of calcium carbonate. The effectiveness of different weight ratio of MA-g-PEW to calcium carbonate is discussed.
EXPERIMENTAL
Materials
Calcium carbonate (1.6 μm) was obtained from Puyuan Chemical, Hubei, China. Toluene (analytical reagent grade) and liquid olefin were purchased from Tianjin Chemical, Tianjin, China. Maleic anhydride (CR) was obtained from Beijing Chaoyang Chemical, Beijing, China. Di-tert-butyl peroxide (DTBP; chemical formula: CH3C(CH3)2OO(CH3)2CCH3), polyethylene, and polyethylene wax were purchased from Yanshan Oetrifaction, Hubei, China. Stearic acid was bought from Bodi Chemical, Tianjin, China. All of the chemical reagent used in this experiment were of analytical grade.[ Citation 1 ]
Apparatus
Apparatus include Bruker IFS 66 v/s infrared spectrometer for Fourier transform infrared (FTIR) spectroscopic analysis; NDJ-1 circumrotating viscidity meter; HAAKE Rheocord; and JEM-1200EX/S transmission electron microscope (TEM).
Preparing of MA-g-PEW
The grafted polyethylene wax grafting maleic anhydride (MA-g-PEW) was synthesized in a solution state. In the typical solution-grafting process, 60 g polyethylene wax was melted in a 250-mL round-bottom flask at 110°C under a nitrogen atmosphere for 40 min. After complete meltion of the PEW, maleic anhydride (6 g) dissolved in 5 mL acetone and the free radical initiator DTBP were added. The reaction was allowed to proceed at certain conditions and temperature. Moreover, there was continuous stirring to complete the reaction.[ Citation 8 ]
Purification of MA-g-PEW
The separation of the graft copolymers and other components in the raw grafted samples was performed as follows: several grams of raw MA-g-PEW samples were firstly packed into filter paper, and then boiled with refluxing xylene for 0.5 h in a round flask. The solution was precipitated in acetone in hot, then filtered by Buchnr funnel and repeatedly washed with fresh acetone, and then dried in a vacuum at 60°C to a constant weight. Thus, the sample without gel was obtained.[ Citation 9 ]
Acidity of MA-g-PEW
The quantity of MA grafted onto PEW molecules was determined by titration of acid groups derived from the anhydride functions. After dissolution of 0.3 g of MA-g-PEW in 20 mL of xylene at boiling temperature, .0.2 mL of distilled water was added to hydrolyze anhydride functions into carboxylic acid functions. The carboxylic acid concentration was determined by titration with 0.025 N potassium hydroxide in ethanol. The indicator used was phenolphthalein. The carboxylic acid concentration was converted to the acidity of MA-g-PEW as follows[ Citation 10 ]:
Modification of Calcium Carbonate
The procedure employed for the modification of CaCO3 was as follows. First 1.0 g MA-g-PEW and 100 mL toluene were transferred into a 250-mL three-necked flask.[ Citation 11 ] After the digestion, 100 g calcium carbonate was added to the above solution. The mixture was stirred vigorously for 1 h at 100°C using a mechanical stirrer in order to ensure that CaCO3 and MA-g-PEW could react completely. The final product was dried for 2 h in an oven at 100°C, resulting in a 1% modified calcium carbonate. In the same method, the ratio of MA-g-PEW to calcium carbonate was controlled at 1.5%, 2.0%, and 2.5%.
The Active Ratio of the Modified Calcium Carbonate
where G is the preset weight of modified sample, and W is the weight of the floated part after the preset modified sample has been dispersed in distilled water.[ Citation 12 ] The methods was as follows. Five grams of the final sample was added to 100 mL of distilled water. We measured the ratio of floated product to overall weight of sample after they were mixed in distilled water and stirred vigorously.[ Citation 3 ]
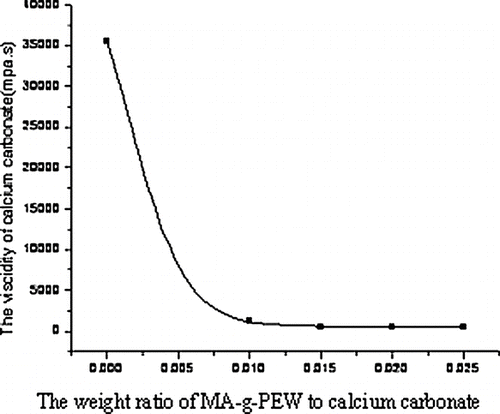
Viscidity of the Modified Calcium Carbonate
The viscidity of the modified calcium carbonate was recorded on the NDJ-1 circumrotating viscidity meter at room temperature using the solution of 120 g liquid olefin and 100 g modified calcium carbonate.
Rheology of Modified Calcium Carbonate
The results were obtained at 270°C for 15 min by the HAAKE Rheometer using the mixture of 0.5 g stearic, 2.5 g polyethylene wax, 12.5 g polyethylene, and 70 g modified calcium carbonate.
RESULTS AND DISCUSSION
Evidence of the Formation of MA-g-PEW
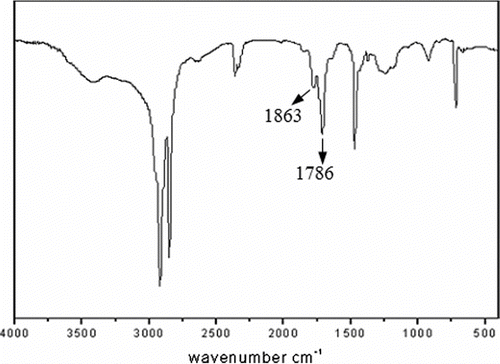
FTIR spectra of blank and MA-g-PEW samples were illustrated in . Compared with the spectra of blank sample, two new peaks at 1786 and 1863 cm−1, corresponding to the symmetric and asymmetric C=O stretching modes coming from MA, appeared. The presence of characteristic absorption bands of MA is taken as evidence of the grafting reaction. From 2.5% test, we obtained the acidity of MA-g-PEW to be 56.
IR Spectra of Modified Calcium Carbonate Surfaces
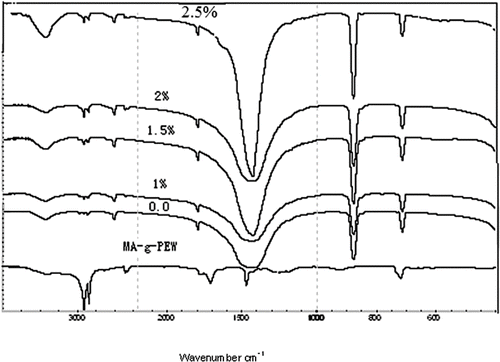
shows the IR bands of MA-g-PEW and the calcium carbonate modified with the weight ratio of PEW-g-MA to calcium carbonate ranging from 0% to 2.5%, respectively. From the figure we can see that a series of characteristic bands of calcium carbonate were found at 1420, 870, 711 cm−1, attributed to Ca-O stretching and bending vibrations,[ Citation 5 ] respectively. After modification, besides the characteristic bands of calcium carbonate, the absorption occurred around 2415 cm−1, which is consistent with the appearance of C=C groups from the MA-g-PEW.[ Citation 2 ] It also shows that with MA-g-PEW concentration increased, the intensities of IR bands at 2800 and 2900 cm−1, which are attributable to the CH2,CH3 stretching vibration, increased, whereas the IR band at 1420 cm−1, attributable to the Ca-O stretching vibration, decreased slowly due to the surface of calcium carbonate being coated by MA-g-PEW.
Morphology of the Modified Calcium Carbonate
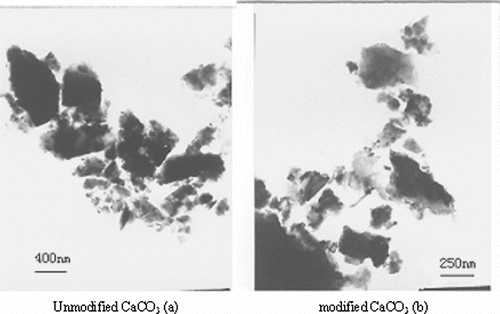
shows the transmission electron microscopy (TEM) morphology of calcium carbonate particles before and after modification. It can be seen that modified calcium carbonate becomes less agglomerate compared to unmodified calcium carbonate, because the surface of calcium carbonate is changed from hydrophilic to hydrophobic in the process of modification. At the same, there are some differences in the thickness of calcium carbonate before and after modification. It is clear that MA-g-PEW has grafted the surface of calcium carbonate.[ Citation 5 ]
The Effectiveness of Different Modifying Reagent Dosage
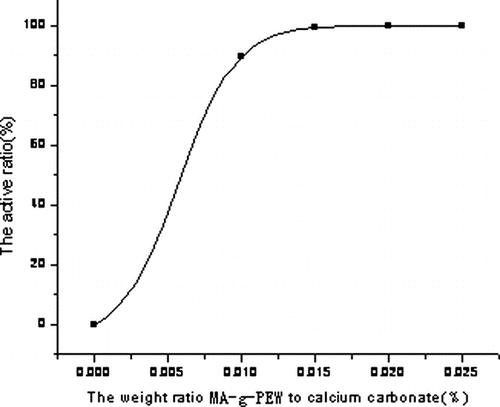
shows the variation of active ratio with different modifying reagent dosages. It can be seen that with the increase in MA-g-PEW dosage, active ratios reach their maximum value at PEW-g-MA dosage of 2.5%. The reason for a poorer effect at lower dosage is that if there is little MA-g-PEW in the mixture, the surface of calcium carbonate cannot be modified completely, the calcium carbonate is hydrophilic to some extent, seriously influencing the active ratio. Thus, the best MA-g-PEW dosage is 2.5% or a little more.
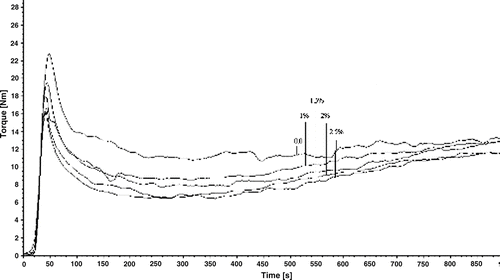
Evaluation of the Modified Calcium Carbonate by Rheology
shows that the shear force decreased with the amount of modifying agent; the shear force of unmodified calcium carbonate is very high, whereas that of modified calcium carbonate (2.5%) decreases a little compared to modified calcium carbonate (2%). The trend matches that of the viscidity of calcium carbonate (). Because with decreasing the viscidity, the resistance force necessarily declines. These can also be explained by the fact that the surface of unmodified calcium carbonate has huge specific energy and strong conglomeration ability attributed to its hydrophilic property. After modification, the surface energy of calcium carbonate drops a lot. It is hydrophobic to some extent. As the weight ratio of MA-g-PEW to calcium carbonate reaches 2.5%, the surface of calcium carbonate is changed from hydrophilic to hydrophobic completely.
CONCLUSIONS
We have succeeded in surface modification of calcium carbonate by MA-g-PEW. After modification, calcium carbonate particles changed from hydrophilic to hydrophobic completely. From the above results, we can see that high weight ratio of MA-g-PEW to calcium carbonate promotes the active ratio of calcium carbonate particles and an efficient weight ratio for the modification of calcium carbonate is about 2.5%.
REFERENCES
- Liu , P. , Liu , Y. S. and Su , Z. X. 2006 . Modification of poly(hydroethylacrylate)-grafted cross-linked poly(vinyl chloride) particles via surface-initiated atom-transfer radical polymerization (SI-ATRP). Competitive adsorption of some heavy metal ions on modified polymers . Ind. Eng. Chem. Res. , 45 : 2255 – 2260 .
- Knaus , S. , Spoljaric-Lukacic , L. , Liska , R. and Saf , R. 2005 . Peroxide-initiated grafting of maleimides onto hydrocarbon substrates . Eur. Polym. J , 41 : 2240 – 2254 .
- Price , G. J. and Ansari , D. M. 2004 . Surface modification of calcium carbonates studied by inverse gas chromatography and the effect on mechanical properties of filled polypropylene . Polym. Int. , 53 : 430 – 438 .
- Shui , M. 2003 . Polymer surface modification and characterization of particulate calcium carbonate fillers . Appl. Surf. Sci. , 220 : 359 – 366 .
- Kim , D. S. and Lee , C. K. 2002 . Surface modification of precipitated calcium carbonate using aqueous fluosilicic acid . Appl. Surf. Sci. , 202 : 15 – 23 .
- Akovali , G. and Akmant , M. A. 1997 . Mechanical properties of plasma surface-modified calcium carbonate-polypropylene composites . Polym. Int. , 42 : 195 – 202 .
- Qiu , W. , Endo , T. and Hirotsu , T. 2005 . A novel technique for preparing of maleic anhydride grafted polyolefins . Eur. Polym. J. , 41 : 1979 – 1984 .
- Nakason , C. , Kasaman , A. and Supasanthitikul , P. 2004 . The grafting of maleic anhydride onto natural rubber . Polym Test , 23 : 35 – 41 .
- Zhu , L. , Tang , G. , Shi , Q. , Cai , C. and Yin , J. 2006 . Neodymium oxide co-catalyzed melt free radical grafting of maleic anhydride onto co-polypropylene by reactive extrusion . React. Funct. Polym. , 66 : 984 – 992 .
- Li , H.-M. , Chen , H.-B. , Shen , Z.-G. and Lin , S. 2002 . Preparation and characterization of maleic anhydride-functionalized syndiotactic polystyrene . Polymer , 43 : 5455 – 5461 .
- Ai , R. K. , Hild , S. , Ziegler , A. and Marti , O. 2004 . Water-soluble terpolymer-mediated calcium carbonate crystal modification . Langmuir , 20 : 3123 – 3128 .
- Wu , W. and Lu , S.-C. 2003 . Mechano-chemical surface modification of calcium carbonate particles by polymer grafting . Powder Technol. , 137 : 41 – 48 .