Abstract
Genomics and ecology is becoming a popular combination. New variants of “omics” seem to be invented on a daily basis; older variants of omics are reused for different fields and described differently. Altogether it becomes increasingly difficult for people from other research disciplines and – especially – from outside the scientific community to make sense of genomics research, or to identify the findings relevant for their own profession, for instance in environmental management or decision making. In this article, we provide a framework for positioning the different omics studies related to ecology – which we will label “ecogenomics”. Furthermore, we give an overview of important articles that have shaped ecogenomics research and provide our readers with examples of ecogenomics promises for society.
1. Introduction
Where ecology and molecular biology meet, new fields of research with the suffix “omics” are invented. Because of this, many different types of omics related to (microbial) ecology flood the literature on ecology. However, what exactly counts as what kind of omics is often ill defined and consensus among authors is lacking. As a result it is difficult for people who are less familiar with molecular work to deal with the various omics articles, especially since the combination of ecology and molecular biology is relatively new (Thomas and Klaper Citation2004; Van Straalen and Roelofs 2006). Therefore, structure is needed.
This article aims to offer such a structure. We use the concept “ecogenomics” (sometimes referred to as ecological genomics) as a starting point and explain its relation to traditional ecology (section 2). Then we relate several omics studies in the literature that deal with both ecology and molecular approaches to ecogenomics (section 3), to demonstrate that ecogenomics can function as an umbrella descriptor and a starting point for understanding ecology-related molecular studies. Based on this structure we will give an overview of several influential ecogenomics research projects (section 4) and various promises of ecogenomics research for society (section 5), and we will end with a reflection (section 6).
2 Ecogenomics: where the largest and the smallest level of studying life meet
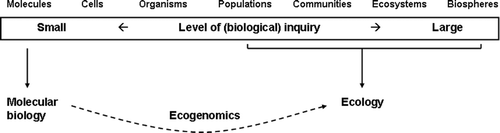
Life can be studied at many different levels, from proteins and nucleic acids (as in biochemistry and molecular biology) to cells (cell biology), to organisms (zoology, plant biology, etc.) and finally at the level of populations and communities up to ecosystems and biospheres. These latter levels – from populations to biospheres – are generally seen as the main topics of ecological inquiry.
Several authors agree that where ecology and molecular biology meet, one can speak of ecogenomics (e.g. Handelsman et al. Citation1998; Van Straalen and Roelofs 2006; Chariton et al. Citation2010; Maphosa et al. Citation2010). However, there is no consensus among them on exactly how these two are supposed to meet. Influential researchers state that ecogenomics uses a genomic explanation – the molecular level – to describe the relation and interaction between environment and phenotype – the macro-level of studying organisms (Feder and Mitchell-Olds Citation2003; Ouborg and Vriezen Citation2007). This implies that one can understand functional species-environment relationships when one studies molecular dynamics (Chapman Citation2001). For example: organisms have “stress genes”, genes that respond to fluctuations in the organism's direct environment. Some of these genes switch on or off in reaction to specific stressors (e.g. a pollutant), others respond to general stresses (e.g. heath, drought). Because of this, the on/off switching of certain genes – gene expression – potentially provides insight in the environment the organism encountered and can be indicative for an environmental condition, for instance the bioavailability of a pollutant. In this line of thought Van Straalen and Roelofs in 2006 published the first book fully dedicated to ecogenomics. They describe ecogenomics as “a scientific discipline that studies the structure and functioning of a genome with the aim of understanding the relationship between the organism and its biotic and abiotic environments” (Van Straalen and Roelofs, 2006 – emphasis added). One well-understood organism is the starting point from which a complex and unknown environment is explored.
However, this approach is not shared by all researchers who claim to perform ecogenomics research or work in ecogenomics programmes. Other researchers describe ecogenomics as the superimposed genetic material of all microorganisms present in a specific environment; they tend to define ecogenomics as if it is a synonym for metagenomics. An example of this is that one recovers all the genetic material (DNA or RNA) that is present in the sample of a certain location (e.g. a soil), and subsequently uses the collected material to identify the microbial inhabitants of that specific environment. Metagenomics can thus help to obtain a more holistic image of the microbial biodiversity at a certain site (e.g. DeLong Citation2004; Ward Citation2006; Torsten et al. Citation2007; Kakirde et al. Citation2010).
All ecogenomics researchers seem to share the perception that ecogenomics goes beyond making claims on gene functions in cells or individual organisms (which is the mission of more traditional molecular approaches such as genetics and genomics of a single organism or species). Central in ecogenomics research is the aim to generate insight in the relationship between organisms (including microbes) and their environment, using various molecular techniques ( ). Though molecular techniques evolve rapidly, the ecogenomics aim – to contribute to a more detailed picture of a specific environment – roughly remains the same.
3 Ecology, ecogenomics and the other omics
Ecology can be classified in several ways. Depending on the classification strategy, different ecological subdisciplines can be defined. For instance, when one categorises ecology on overall complexity, one can distinguish behavioural ecology, population ecology and community ecology. But it is also possible to categorise on organisms that are under study (e.g. animal ecology, plant ecology), or on the systems that are investigated (e.g. forest ecology, soil ecology). Furthermore, several specialist interdisciplinary branches of ecology exist, i.e. ecophysiology, ecotoxicology and evolutionary ecology.
When these different ecological subdisciplines – independent of the classification strategy – adopt molecular techniques, researchers tend to add the omics label. As a result, many different omics studies can be found in the literature and probably many more will follow. Examples include marine ecogenomics (Béjà Citation2004), ecotoxicogenomics (Snape et al. Citation2004) and evolutionary and ecological functional genomics (Feder and Mitchell-Olds Citation2003). A complicating factor is that the same subdiscipline – or subdisciplines that are highly related – is sometimes labelled differently by different authors. This is the case for metagenomics, for which microbial ecogenomics and environmental genomics are used as synonyms (Handelsman Citation2004; Xu Citation2006).
The understanding of ecogenomics furthermore is hindered by the tendency of academic researchers to label various molecular approaches with omics as well. A conceptual use of omics that we have not yet described in detail is related to the molecular biological analyses within (eco)genomics studies, i.e. genomics, transcriptomics, proteomics and metabolomics. Genomics in this sense relates to studying genes or DNA, transcriptomics refers to the transcripts of these genes once they are active (or “expressed”) like messenger RNA (mRNA), proteomics studies the products that are produced as a result of this gene expression like proteins, and metabolomics subsequently is used to identify the final products of proteomics after several metabolic processes like protein folding. Ecogenomics encompasses research from all these different types of analyses. Depending on the type of research question, a different analysis can be chosen. For example if one wants to detect species present in an environment, one uses genome regions that act as DNA barcodes unique for specific species (genomics). However, if one is interested in the specific stress caused by an environmental pollutant, one looks at the expression of “stress genes” (transcriptomics). In general, the analytical complexity increases from genomic to metabolomic analysis and advanced bioinformatics tools are necessary to deal with the vast amounts of data that are produced.
4 Ecogenomics: elaboration on two different approaches
In the second chapter already some insight was provided in two different approaches that currently dominate in the genomic activities on organism–environment interactions. For one of these the label “metagenomics” was proposed (by for instance Handelsman et al. Citation1998), and we propose to frame the other approach as “model organism ecogenomics (MOE)”. The main difference between these two ecogenomics approaches lies in the amount of attention that is paid to the organismic level of research. When the approach is not made explicit, it may cause confusion between researchers from different disciplines (like on the difference between metagenomics and ecogenomics in general) or between researchers and other professionals (e.g. from industry or government). Especially for non-academic professionals, background knowledge on the approach is important since rather different outcomes can be expected from the two approaches. This insight can for instance help research decision makers to manage expectations on potential societal output of ecogenomics research. But before we address the societal promises of ecogenomics (Section 5), in the following chapters we will first provide more insight in both approaches and briefly discuss influential papers that contributed to their development.
4.1 Key ecogenomics research with a metagenomics approach
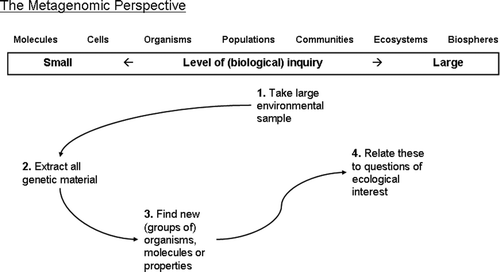
Metagenomics came into being when genomics tools revolutionised the domain of microbial ecology. It is the culture independent Footnote1 genomic analysis of a local patch of microorganisms (often focusing on Operational Taxonomic Units (OTUs) containing rRNA genes (16S, 18S) as unique genetic markers). Metagenomics enables the identification of new organisms, the construction of phylogenetic trees (to comprehend the evolutionary relatedness and classification of species) and the identification of new bioindicators. Metagenomics researchers take a meta-approach to research: they pay little attention to individual organisms or species and focus on the collective genomes in an environmental spot. As visualised in , there is only a modest role for the level of the individual organism as a third step in the process, when new (groups of) organisms are revealed in the project.
Microbial communities are found in various environments and – unsurprisingly – metagenomics can therefore be used to study a wide variety of environments. Until now most studies have focused on aquatic and soil habitats, although other environments – including larger organisms' digestive systems – today also are being explored.
4.1.1 Aquatic- or marine metagenomics
In less than a decade metagenomics has fundamentally changed our view of life in the ocean (Doney et al. Citation2004) and in other aquatic domains (Hofmann et al. Citation2005), and it continues to do so (Fuhrman Citation2009; Fonseca et al. Citation2010). Many researchers and environmental decision makers today agree that knowledge on microbial abundance, diversity and dynamics is required to develop a functional view of the ocean. For instance, knowledge about microbial interactions with marine animals and their impact on ecosystem functioning and animal populations is crucial if you wish to design accurate ecosystem-based conservation models (Azam and Worden Citation2004). Venter et al. performed one of the most extensive metagenomics studies in the aquatic domain when they researched microorganisms living in the Sargasso Sea near Bermuda. They identified 148 new bacterial phylotypes and 1.2 million previously unknown genes, and proved that a metagenomics approach was a suitable method for (among others) studying biodiversity (Venter et al. Citation2004). The study raised many questions relevant for ecology, such as “What ecological and evolutionary processes maintain such high microbial diversity in oceans?”, “How many new functional components are there?” and “Have we been missing major players?” (Falkowski and de Vargas 2004). Another metagenomics study indeed demonstrated that major players and processes had been missed before. Béjà et al. radically enhanced insights in marine ecosystems when they identified a new and unsuspected light-driven energy source called “rhodopsin” in an uncultured organism. They proved this photopigment to be broadly distributed among different taxa in oceanic surface waters (Béjà et al. Citation2000). Today, these studies are frequently used to illustrate and debate the value of metagenomics.
4.1.2 Soil metagenomics
How complex marine microbial diversity might seem, the soil's microbial diversity is even more complicated (Young and Crawford Citation2004). A single gram of soil contains up to 10 billion microorganisms that possibly represent many thousands distinct genomic species (Torsvik and Ovreas Citation2002). However, no more than 0.1–1% of the soil population can be cultured using standard cultivation techniques (Daniel Citation2004). Therefore, very little is known about the soil ecosystem and communities living in its micropores and the soil as living environment still largely is a black box. But today there is metagenomics, which is culture-independent. Metagenomics comes with possibilities to discover the hidden majority of microorganisms living in the soil, as well as their roles and functions in the ecosystem; it provides original, alternative ways to access the soil “metagenome” (the collective genome of the soil).
The idea of cloning the soil metagenome to identify new microbes, functions and products from previously uncultured soil microorganisms was coined by Handelsman et al. (Citation1998). Shortly thereafter, Rondon demonstrated how to realise this in practice: they constructed metagenomic libraries of DNA isolated directly from soil (using BAC vectors) (Rondon et al. Citation2000). Since then several scientists have researched the soil's microbial diversity and its capabilities (Tringe et al. Citation2005; Leveau Citation2007; Kakirde et al. Citation2010). In the Netherlands, between 2004 and 2010 a large, public-private research collaboration – the Ecogenomics Consortium – focused (among others) on identification of the soil's hidden microbial diversity (e.g. Kowalchuk et al. Citation2007).
One study needs to be mentioned that focused on an environment other than aquatic or soil. The study of Tyson et al. in an acid mine environment greatly impacted the development of metagenomics and the study of natural microbial communities in general. Tyson et al. used metagenomics to identify community structure and metabolism of microbial genomes from a low-complexity acid mine drainage biofilm (Tyson et al. Citation2004). They were among the first to culture-independently recover (two near-complete and three partial) microbial genomes from an original environmental sample.
4.2 Key ecogenomics research with a model organism approach
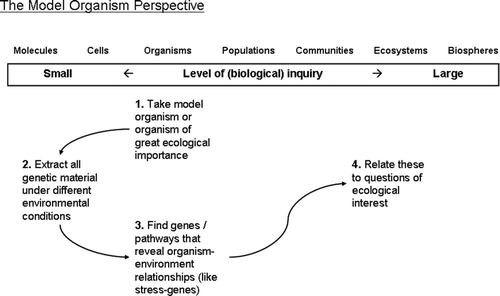
The other ecogenomics approach discussed here is MOE. Model organism ecogenomicists envision a much larger role in their research for the level of the individual organism than metagenomics researchers. MOE makes use of the principle that organisms, when exposed to various environments or variable environmental conditions, react at the molecular level through changes in gene expression. In traditional genetics and genomics studies, this principle is used to learn more about an organism. By selectively varying conditions in a well-known and controlled environment, genetics and genomics approaches enable researchers to study complex molecular systems of organisms. MOE approaches it the other way around: MOE often starts from a well-known (model) organism for which gene expression patterns are known. Model organism ecogenomicists subsequently aim to find out characteristics and systems in the (unknown, uncontrolled) environment to which they exposed their model organism. They thus use genes as minuscule bio-indicators for environmental characteristics and changes. MOE can provide insight in abiotic parts of the environment, but also on interactions between species (e.g. symbioses).
In MOE, an organism of ecological interest is used as the central, often starting point, of research. Detailed information on the genome of this specific organism needs to be present or collected at the start, Footnote2 since the approach depends on changes in the organism's gene expression under various environmental conditions. Schematically MOE researchers roughly have the following approach ( ).
A difficulty for MOE research is that most of today's popular model organisms originally were selected for motives other than their ‘ecological importance’ or their ‘relevance for environmental decision making’. Frequently, they were chosen as research models because they (molecularly) were not too complex, and/or because they could be kept and studied in a laboratory setting relatively easily. Because of this, and because of long periods of laboratorial inbreeding, the value of many common traditional model organisms is limited for ecology and ecosystem research (Feder and Mitchell-Olds Citation2003). Even today, relatively little molecular information is available for the organisms that are ecologically the most relevant ones. A first step in MOE, after selection of a relevant organism (that will serve as a new model), therefore often is to acquire detailed molecular insight in this organism. This requires substantial investments, both financially and in time.
Fortunately, at this moment the issue is to a certain extent resolved. Sequencing methods become cheaper and faster, which enables ecologists to obtain whole-genome sequences of species that are of greater ecological interest more easily. Also, new strategies are identified to overcome the limited availability of information and tools on ecologically relevant organisms (e.g. Travers et al. Citation2007). For example, in the last few years functional genomic tools became available to study stress caused by abiotic factors at the molecular level in ecologically relevant organisms (Bijlsma and Loeschcke Citation2005; Roelofs et al. Citation2008). Furthermore, today novel ideas are formulated to translate genomic findings between different species, and to look at similarities (for instance between stress responses in springtails and humans). Some hope that in the future a “universal stress transcriptome” will be identified that enables the translation of findings across different species (Van Straalen, personal communication). Presently, new genomic information of ecologically relevant organisms rapidly becomes available (e.g. Folsomia candida, Nota et al. Citation2008) and organisms of greater ecological importance like Daphnia spp. are being sequenced (Poynton et al. Citation2007; Shaw et al. Citation2007).
Like metagenomics, MOE can be used to study a wide variety of environments, depending on the organism selected as model. For aquatic environments, examples of new ecologically relevant model organisms include the zebrafish Danio rerio and the micro-crustacean Daphnia pulex. They are helpful in the assessment of environmental toxicology: ecotoxicogenomics (Shaw et al. Citation2007; Van Boxtel et al. Citation2008). For the terrestrial environment, molecular characteristics of Folsomia candida were revealed (Timmermans et al. Citation2007). This information is interesting for evolutionary biology (Timmermans et al. Citation2008) and for ecotoxicology in soils (Nota et al. Citation2008). Furthermore, Travers et al. used the grass species Andropogon gerardii directly in an ecological field study to observe the effects of variation in rainfall on gene expression (Travers et al. Citation2007).
However, more traditional model organisms have not fully lost their attractiveness in ecogenomics (Shimizu and Purugganan Citation2005; Landry et al. Citation2006; Li et al. Citation2006; Pieterse and Dicke Citation2007). The signal-transduction pathways in these organisms are relatively well known. Therefore, interactions between these organisms (or mutants) and the environment at the molecular level can reveal interesting new insights. Examples from the literature include insights in environmentally induced changes in gene expression (gene expression plasticity) in Caenorhabditis elegans (Li et al. Citation2006) and new perspectives on the insect-plant and microbe-plant communities via molecular approaches to Arabidopsis spp. (Dicke et al. Citation2004; Pieterse and Dicke Citation2007; Snoeren et al. Citation2007; Van Loon Citation2007).
5 Applied ecogenomics: promises for society
As demonstrated in the previous chapters, molecular approaches clearly are of added value for research on microbial communities and for ecology. Both metagenomics and MOE provide insight in important ecological issues related to for instance biodiversity, survival strategies and symbioses. Ecogenomics generates loads of input for new research. However, besides demonstrating the scientific value of ecogenomics research, most ecogenomics papers also hint at societal benefits. In this chapter, we discuss several popular “promises of ecogenomics for society”.
5.1 Detection of and dealing with beneficial and pathogenic microbes
Metagenomics can be a valuable strategy to rapidly, selectively and accurately identify pathogens (Roossinck et al. Citation2010). Microarrays – small glass slides that contain thousands specific pieces of DNA or RNA – for instance are very suitable for the simultaneous identification of many known pathogens (viruses, bacteria, fungi) in a single sample. Developments are ongoing and today even more advanced “next-generation sequencing (NGS)” technologies are being developed that can speed-up detection (Woon Roh et al. Citation2010). Microarray and NGS tools can be helpful when one wants to assess for instance aquaculture health (Wilson et al. Citation2005) or drinking water safety (e.g. to detect Legionella spp.). Furthermore, metagenomics can help to measure the presence and activity of pathogenic and beneficial microorganisms in rhizospheres of agricultural fields (e.g. disease suppressive soils) (Raaijmakers et al. Citation2009).
The risk of pathogenic infection may reduce the yield in aquaculture and other intensive farming and agricultural developments. Infection prevention is therefore of great economic importance. For this, beside metagenomic detection, increased insight in defence mechanisms of the cultured species (as a host) and pathogens is necessary. Here MOE, preferably using the farming species or closely related species as model, can provide this insight and help to control diseases (Wilson et al. Citation2005).
5.2 Nature conservation and bioindicator species
Ecogenomics has potential for nature conservation and for the assessment of ecosystem health status and -stability. With metagenomics, specific changes in the local biodiversity can be noticed in response to changes in environmental conditions (e.g. Chariton et al. Citation2010; Medinger et al. Citation2010). Furthermore, bioindicator species that are indicative of specific beneficial or problematic environmental conditions can be identified and monitored “on site” (Ficetola et al. Citation2008). As such, metagenomics can be used to find and monitor optimal conditions for intensive farming agriculture, and for nature conservation. Furthermore, MOE enables us to look at the expression differences of genes of a single organism (e.g. C. elegans) under various environmental conditions. Genes that respond to environmental stresses (such as fluctuations in temperature and oxygen availability or environmental pollutions) can act as early indicators for monitoring environmental quality and the biological availability of stressors. These can then be used as warning systems for changes in the environmental health (Ouborg et al. Citation2010).
MOE and metagenomics were relatively recently successfully combined in an effort to make nematodes more effective soil-health indicators. Morphologically, different nematode species strongly resemble to each other: it requires a trained eye, a microscope and patience to distinguish between them. Ecogenomics can simplify the procedure substantially. The nematodes' DNA can serve as a “barcode” that differentiates between different types of nematodes. With routine biomolecular techniques, one can analyse very large samples to obtain a metagenomics picture of all the nematodes, their abundance and the on-site distribution of different nematode species. This way differences in exact nematode community composition can be observed and related to soil conditions (Holterman et al. Citation2008; Porazinska et al. Citation2009). But DNA-barcoding is also used in other settings, for instance to differentiate between tropical trees. Barcoding here can be an important tool to establish and maintain tropical diversity (Dexter et al. Citation2010).
To determine how natural and human-caused changes in the environment affect ecosystems and specific species, it is important to understand how spatial and temporal distributions of organisms are controlled and affected. This especially holds for species that are of commercial importance, for those that hold vital roles in the ecosystem, and for endangered species and populations (Thakur et al. Citation2008). Ecogenomics techniques might help to realise more sustainable, more causal driven nature conservation strategies, for they provide insight in complex modes of (inter)actions and on species status and significance.
5.3 Pollution and bioremediation
A third domain of ecogenomics application is in the field of environmental pollution and bioremediation. Monitoring microbial community dynamics across different environmental settings can lead to the discovery of patterns associated with biodegradation. This can then help in the identification of species with bioremediation capacity and with the development of new bioremediation tools (Head et al. Citation2006; Maphosa et al. Citation2010). Also, by comparing metagenomes of relatively undisturbed sites (both terrestrial and aquatic) with their polluted counterparts, the types of contaminations and situations that cause real ecosystem disturbances can be identified. It might be well conceivable that metagenomics reveals relative ecosystem balance at many sites that are now labelled “polluted” because of suspected ecosystem health problems (and the reverse also holds true).
5.4 Ecosystem health
As the previous chapter points out, metagenomics might redefine ideas of “pollution” and related risks for ecosystems. This raises questions like: What is exactly “ecosystem health”? When is an ecosystem healthy and when is it not? And how can decision makers respond to such insights; is the system locked-in in a traditional physiological-chemical perception of pollution? We observed that metagenomics is already able to provide meaningful input to answer the first two questions. If pitched right at the research level and adopted by environmental decision makers, it can be of great value to answer the third as well.
5.5 Nature mining – tapping into the tremendous natural resource
The literature (and project proposals) on metagenomics often hint that metagenomes in soil and water can be “a tremendous untapped resource” of antibiotics, anticancer drugs, health-improving compounds and biologically active secondary metabolites, e.g. (Pettit Citation2004; Riesenfeld et al. Citation2004; Langer et al. Citation2006; Schmeisser et al. Citation2007; Demain and Adrio 2008; Oh et al. Citation2009; Barke et al. Citation2010; Kakirde et al. Citation2010). Sixty per cent of the drugs that became commercially available over the past 20 years originate from existing natural compounds, and not directly from the chemical industry (Lefevre et al. Citation2008). Microbes are therefore considered to be very promising sources of new chemical structures. “Nature mining” or “bioprospecting” (DeLong Citation2002) is the term used for tapping into this resource, and metagenomics is considered to be a potentially helpful field of research for handling complex problems around human health, like multidrug resistance and cancer.
Metagenomics findings can be relevant outside the medical world as well, for instance as strategy to identify new bioactive enzymes that are currently hidden in soil- and water metagenomes. Examples are biopolymers, ingredients for detergents and dairy and bakery products, bioplastics and biofuels (Lorenz and Eck Citation2005). Furthermore, metagenomics can contribute to the realisation of a “bio-based economy”. Metagenomics provides new options to study, on-site (e.g. in dedicated plants) monitor and enhance bio-redox conversions by populations of microorganisms and can help to improve the recycling of resources. An example is the efficient conversion of cellulose biomass; a key step in the production of second generation biofuels that do not compete with food production. More efficient bioconversion is needed, and metagenomics can help to identify new cellulose processing enzymes or organisms that are able to do this (Brune Citation2007; Warnecke et al. Citation2007; Ouborg and Kammenga Citation2008; Suen et al. Citation2010).
6 Conclusion: ecogenomics – directions, limitations and considerations
Leveau (Citation2007) identified several major (overlapping) directions for metagenomics, namely:
| 1. | to provide ecologists with basic clues on “Who is out there?” | ||||
| 2. | the follow-up question “Who is doing what out there?” | ||||
| 3. | to strive “for the discovery and mining of interesting new natural compounds” | ||||
| 4. | to obtain “a more holistic picture of the biotic environment that is assessed for instance by the mass sequencing of environmental samples”. | ||||
By our definition, ecogenomics can have three additional research directions that focus on the questions:
| 1. | “Who is interacting with what?” | ||||
| 2. | “Who is interacting with whom?” and | ||||
| 3. | “How does interaction specifically take place?” | ||||
Most ecogenomics research projects are organised around one or multiple of these questions. Exactly what questions are tackled and the order in which they can be addressed is dependent on the selected ecogenomics approach.
In metagenomics we noticed that especially the first two research directions are popular ( ). Impressive amounts of data are collected by research groups interested in knowing “who is out there?” (which, when single marker genes are used, can be referred to as “metagenetics” – Fonseca et al. Citation2010) and “who is doing what where?”. Despite the fact that the third research direction – relevant for nature mining – can be the main focus of metagenomics projects in each phase of a project (even already from the start), until today this direction is often left untouched or only present in the discussion section of the respective articles.
Figure 4. The upper part of this picture (a) illustrates the order in which research questions often occur when the metagenomics approach is selected. The lower part of this picture (b) illustrates various societal promises, and corresponds to questions in the upper half that first need to be answered. As depicted here, metagenomics allows a focus on nature mining in all phases of the research.
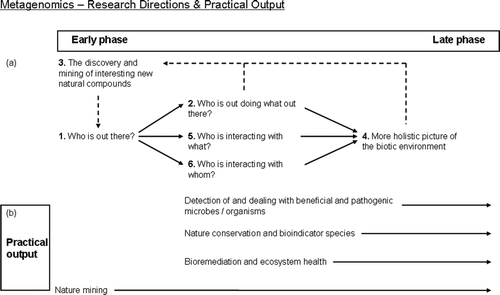
As demonstrated in , to realise metagenomics' application potential with respect to detection of and dealing with beneficial and pathogenic (micro)organisms, nature conservation and bioindicator species, and bioremediation and ecosystem health, researchers need to move beyond answering the question “Who is out there?”. A pitfall of metagenomics projects in relation to their societal value is that researchers might continue to discover new organisms and genes (Galperin and Koonin Citation2010) but shy away from a leap to a next phase that concerns the ecosystem function of these organisms and their interacting activities in relation to (societal pressing) ecological issues. This can result in a failure to realise the abovementioned application areas. Furthermore, the holistic picture then is not fully developed.
MOE often tends to focus on research direction 2, 5, 6 and/or 7 ( ). For various reasons – including lack of time, insufficient financial support and “more research needed” – until today most of these studies have not made it to direction 3 and 4.
Figure 5. As shown in Figure 4, the upper part (a) in this figure illustrates the order in which research questions often occur when MOE research is performed. The lower part of this picture (b) illustrates various societal promises, and corresponds to questions in the upper half that first need to be answered. As depicted here, nature conservation can be the focus directly at the start of these projects (but this depends heavily on the model organism selected). Nature mining is possible, but other questions need to be answered first.
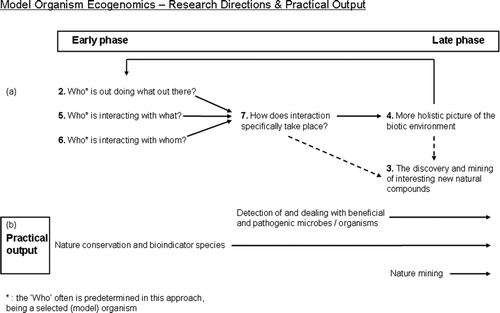
We believe that MOE can enhance nature conservation strategies. MOE can identify relevant bioindicator species and genes, and monitor early effects of ecological disasters (e.g. the Mississippi oil spill) and of nature management efforts (e.g. the deliberate “flooding” of certain regions in the Netherlands to create new habitats). A pitfall in this respect can be that a group's favourite model organism is selected at the start without critically taking into account its value for environmental assessment or its capacity to link up with current regulations. The primary aim might then no longer be to understand an ecological issue, but to learn more about the organism (traditional genomics research). Like metagenomics, MOE has a risk that researchers tend to remain focused on studying the organism and interaction itself without making the leap to zoom in on the environmental or societal relevance. In a field as new as ecogenomics, so much is still to be discovered that it can be tempting not to go beyond the exploratory phases.
In conclusion, we believe that ecogenomics is very capable of speeding up scientific discoveries and holds considerable promises for society. For example: nature mining (direction 3) can be interesting for industry, and the holistic ecosystem picture (direction 4) can be highly useful for environmental policy makers. However, we also demonstrated that “ecogenomics” an sich provides relatively little information on the precise aim and approach, on expected outcomes, on the main interdisciplinary opportunities and on (potential) societal relevance. This can lead to various challenges, for example at the level of ecogenomics communication and collaboration. Furthermore, we showed that there still is so much to be discovered via ecogenomics, that research directions that are of great societal relevance might not always easily be pursued. A challenge for ecogenomics can therefore be that the step from promise to (a concrete) product or service is big. Schmeisser et al. (Citation2007) pointed to this issue when they stressed that the detection of novel genes and enzymes is a lesser problem than the actual implementation in production processes. Fortunately, parallel to the rapid developments in ecogenomics research, also knowledge is becoming available on how the “societal promise” of emerging science and technology like ecogenomics can (further) be shaped and realised (Roelofsen et al. Citation2008; Kloet et al. Citation2010). A fulfilment of various ecogenomics promises might not be far away; there is substantial potential for both excellence and relevance.
Notes
1. Culture independent means that the approach does not require that organisms are grown under standardised laboratory conditions. For most microorganisms, especially inhabitants of soils, cultivation in laboratories is problematic. Metagenomics provides access to these “unculturable” microbes.
2. Sometimes organisms of which the genomes are not fully sequenced are selected, for instance because of their ecological relevance. In that case researchers often look at Expressed Sequence Tags (ESTs) that are less informative but quicker and cheaper to obtain than whole-genome sequences.
References
- Azam , F and Worden , A Z . 2004 . Oceanography: microbes, molecules, and marine ecosystems . Science , 303 : 1622 – 1624 .
- Barke , J , Seipke , R F , Grüschow , S , Heavens , D , Drou , N , Bibb , M J , Goss , R JM , Yu , D W and Hutchings , M I . 2010 . A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant. Acromyrmex octospinosus . BMC Biol , 8 : 109
- Béjà , O . 2004 . To BAC or not to BAC: marine ecogenomics . Curr Opin Biotechnol , 15 : 187 – 190 .
- Béjà , O , Aravind , L , Koonin , E V , Suzuki , M T , Hadd , A , Nguyen , L P , Jovanovich , S B , Gates , C M , Feldman , R A Spudich , J L . 2000 . Bacterial rhodopsin: evidence for a new type of phototrophy in the sea . Science , 289 : 1902 – 1906 .
- Bijlsma , R and Loeschcke , V . 2005 . Environmental stress, adaptation and evolution: an overview . J Evol Biol , 18 : 744 – 749 .
- Brune , A . 2007 . Woodworker's digest . Nature , 450 : 487 – 488 .
- Chapman , R W . 2001 . EcoGenomics – a consilience for comparative immunology? . Dev Comp Immunol , 25 : 549 – 551 .
- Chariton , A A , Court , L N , Hartley , D M , Colloff , M J and Hardy , C M . 2010 . Ecological assessment of estuarine sediments by pyrosequencing eukaryotic ribosomal DNA . Front Ecol Environ , 8 ( 5 ) : 233 – 238 .
- Daniel , R . 2004 . The soil metagenome – a rich resource for the discovery of novel natural products . Curr Opin Biotechnol , 15 : 199 – 204 .
- DeLong , E F . 2002 . Microbial population genomics and ecology . Curr Opin Microbiol , 5 : 520 – 524 .
- DeLong , E F . 2004 . Microbial population genomics and ecology: the road ahead . Environ Microbiol , 6 : 875 – 878 .
- Demain , A. Adrio J . 2008 . Contributions of microorganisms to industrial biotechnology . Mol Biotechnol , 38 : 41 – 55 .
- Dexter , K G , Pennington , T D and Cunningham , C W . 2010 . Using DNA to assess errors in tropical tree identifications . How often are ecologists wrong and when does it matter? Ecol Monogr , 80 ( 2 ) : 267 – 286 .
- Dicke , M , Van Loon , JJA and De Jong , PW . 2004 . Ecogenomics benefits community ecology . Science , 305 : 618 – 619 .
- Doney , S C , Abbott , M R , Cullen , J J , Karl , D M and Rothstein , L . 2004 . From genes to ecosystems: the ocean's new frontier . Front Ecol Environ , 2 : 457 – 466 .
- Falkowski , P G and de , Vargas C . 2004 . Shotgun sequencing in the sea: a blast from the past? . Science , 304 : 58 – 60 .
- Feder , M E and Mitchell-Olds , T . 2003 . Evolutionary and ecological functional genomics . Nat Rev Genet , 4 : 649 – 655 .
- Ficetola , G F , Miaud , C , Pompanon , F and Taberlet , P . 2008 . Species detection using environmental DNA from water samples . Biol Lett , 4 : 423 – 425 .
- Fonseca , V G , Carvalho , G R , Sung , W , Johnson , H F , Power , D M , Neill , S P , Packer , M , Blaxter , M L , Lambshead , J D Thomas , W K . 2010 . Second-generation environmental sequencing unmasks marine metazoan biodiversity . Nat Comm , 1 : 1 – 8 .
- Fuhrman , J A . 2009 . Microbial community structure and its functional implications . Nature , 459 : 193 – 198 .
- Galperin , M Y and Koonin , E V . 2010 . From complete genomics sequence to ‘complete’ understanding? . Trends Biotechnol , 28 : 398 – 406 .
- Handelsman , J . 2004 . Metagenomics: application of genomics to uncultured microorganisms . Microbiol Mol Biol Rev , 68 ( 4 ) : 669 – 685 .
- Handelsman , J , Rondon , M R , Brady , S F , Clardy , J and Goodman , R M . 1998 . Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products . Chem Biol , 5 : R245 – R249 .
- Head , I M , Jones , D M and Röling , W FM . 2006 . Marine microorganisms make a meal of oil . Nat Rev Microbiol , 4 : 173 – 182 .
- Hofmann , GE , Burnaford , J L and Fielman , K T . 2005 . Genomics-fueled approaches to current challenges in marine ecology . Trends Ecol Evol , 20 : 305 – 311 .
- Holterman , M , Rybarczyk , K , Van , den Elsen S , Van , Megen H , Mooyman , P , Santiago , R P , Bongers , T OM , Bakker , J and Helder , J . 2008 . A ribosomal DNA-based framework for the detection and quantification of stress-sensitive nematode families in terrestrial habitats . Mol Ecol Resour , 8 : 23 – 34 .
- Kakirde , K S , Parsley , L C and Liles , M R . 2010 . Size does matter: application-driven approaches for soil metagenomics . Soil Biol Biochem , 42 : 1911 – 1923 .
- Kloet , R R , De , Cock Buning T and Bunders , J FG . 2010 . Applying the multi-level perspective to understand research program dynamics . Submitted to Sci Pub Pol ,
- Kowalchuk , G A , Speksnijder , A GCL , Zhang , K , Goodman , R M and Van Veen , JA. 2007 . Finding the needles in the metagenome haystack . Microb Ecol , 53 : 475 – 485 .
- Landry , C R , Townsend , J P , Hartl , D L and Cavalieri , D . 2006 . Ecological and evolutionary genomics of Saccharomyces cerevisiae . Mol Ecol , 15 : 575 – 591 .
- Langer , M , Gabor , E M , Liebeton , K , Meurer , G , Niehaus , F , Schulze , R , Eck , J and Lorenz , P . 2006 . Metagenomics: an inexhaustible access to nature's diversity . Biotechnol J , 1 : 815 – 821 .
- Lefevre , F , Robe , P , Jarrin , C , Ginolhac , A , Zago , C , Auriol , D , Vogel , T M , Simonet , P and Nalin , R . 2008 . Drugs from hidden bugs: their discovery via untapped resources . Res Microbiol , 159 : 153 – 161 .
- Leveau , J . 2007 . The magic and menace of metagenomics: prospects for the study of plant growth-promoting rhizobacteria . Eur J Plant Pathol , 119 : 279 – 300 .
- Li , Y , Ãlvarez , O A , Gutteling , E W , Tijsterman , M , Fu , J , Riksen , J AG , Hazendonk , E , Prins , P , Plasterk , R HA Jansen , R C . 2006 . Mapping determinants of gene expression plasticity by genetical genomics in C. elegans . PLoS Genet , 2 : e222
- Lorenz , P and Eck , J . 2005 . Metagenomics and industrial applications . Nat Rev Microbiol , 3 : 510 – 516 .
- Maphosa , F , de , Vos WM and Smidt , H . 2010 . Exploiting the ecogenomics toolbox for environmental diagnostics of organohalide-respiring bacteria . Trends Biotechnol , 28 : 308 – 316 .
- Medinger , R , Nolte , V , Pandey , R V , Jost , S , Ottenwälder , B , Schlötterer , C and Boenigk , J . 2010 . Diversity in a hidden world: potential and limitation of next-generation sequencing for surveys of molecular diversity of eukaryotic microorganisms . Mol Ecol , 19 : 32 – 40 .
- Nota , B , Timmermans , M J , Franken , O , Montagne-Wajer , K , Mariën , J , De Boer , ME , De Boer , TE , Ylstra , B , Van Straalen , NM and Roelofs , D . 2008 . Gene expression analysis of collembola in cadmium containing soil . Environ Sci Technol , 42 ( 21 ) : 8152 – 8157 .
- Oh , D C , Poulsen , M , Currie , C R and Clardy , J . 2009 . Dentigerumycin: a bacterial mediator of an ant fungus symbiosis . Nat Chem Biol , 5 ( 6 ) : 391 – 393 .
- Ouborg , N J , Angeloni , F and Vergeer , P . 2010 . An essay on the necessity and feasibility of conservation genomics . Conserv Genet , 11 : 643 – 653 .
- Ouborg , N J and Kammenga , J E . Ecogenomics in the Netherlands: exploration of opportunities and necessities. Vision document of the Netherlands Ecogenomics Research Organisation (NERO) . Paper presented at: First National Ecogenomics Day . February 29 2008 , Ede, The Netherlands. Paper and more information available via the corresponding author
- Ouborg , N J and Vriezen , W H . 2007 . An ecologist's guide to ecogenomicsSoil DNA libraries for anticancer drug discovery . J. Ecol , 95 : 8 – 16 .
- Pettit , R K . 2004 . Soil DNA libraries for anticancer drug discovery . Cancer Chemother Pharmacol , 54 : 1 – 6 .
- Pieterse , C MJ and Dicke , M . 2007 . Plant interactions with microbes and insects: from molecular mechanisms to ecology . Trends Plant Sci , 12 : 564 – 569 .
- Porazinska , D L , Giblin-Davis , R M , Faller , L , Farmerie , W , Kanzaki , N , Morris , K , Powers , T O , Tucker , A E , Sung , W and Thomas , W K . 2009 . Evaluating high-throughput sequencing as a method for metagenomic analysis of nematode diversity . Mol Ecol Res , 9 : 1439 – 1450 .
- Poynton , H C , Varshavsky , J R , Chang , B , Cavigiolio , G , Chan , S , Holman , P S , Loguinov , A V , Bauer , D J , Komachi , K Theil , E C . 2007 . Daphnia magna ecotoxicogenomics provides mechanistic insights into metal toxicity . Environ Sci Technol , 41 : 1044 – 1050 .
- Raaijmakers , J M , Paulitz , T C , Steinberg , C , Alabouvette , C and Moënne-Loccoz , Y . 2009 . The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms . Plant Soil , 321 : 341 – 361 .
- Riesenfeld , C S , Goodman , R M and Handelsman , J . 2004 . Uncultured soil bacteria are a reservoir of new antibiotic resistance genes . Environ Microbiol , 6 : 981 – 989 .
- Roelofs , D , Aarts , M GM , Schat , H and Van Straalen , NM . 2008 . Functional ecological genomics to demonstrate general and specific responses to abiotic stress . Funct Ecol , 22 : 8 – 18 .
- Roelofsen , A , Broerse , J EW , De Cock , Buning T and Bunders , J FG . 2008 . Exploring the future of ecological genomics: integrating CTA with vision assessment . Technol Forecast Soc Change , 75 : 334 – 355 .
- Rondon , M R , August , P R , Bettermann , A D , Brady , S F , Grossman , T H , Liles , M R , Loiacono , K A , Lynch , B A , MacNeil , I A Minor , C . 2000 . Cloning the soil metagenome: a strategy for accessing the genetic and functional diversity of uncultured microorganisms . Appl Environ Microbiol , 66 : 2541 – 2547 .
- Roossinck , M J , Saha , P , Wiley , G B , Quan , J , White , J D , Lai , H , Chavarria , F , Shen , G and Roe , B A . 2010 . Ecogenomics: using massively parallel pyrosequencing to understand virus ecology . Mol Ecol , 19 : 81 – 88 .
- Schmeisser , C , Steele , H and Streit , W . 2007 . Metagenomics, biotechnology with non-culturable microbes . Appl Microbiol Biotechnol , 75 : 955 – 962 .
- Shaw , J , Colbourne , J , Davey , J , Glaholt , S , Hampton , T , Chen , C , Folt , C and Hamilton , J . 2007 . Gene response profiles for Daphnia pulex exposed to the environmental stressor cadmium reveals novel crustacean metallothioneins . BMC Genomics , 8 : 477
- Shimizu , K K and Purugganan , M D . 2005 . Evolutionary and ecological genomics of arabidopsis . Plant Physiol , 138 : 578 – 584 .
- Snape , J R , Maund , S J , Pickford , D B and Hutchinson , T H . 2004 . Ecotoxicogenomics: the challenge of integrating genomics into aquatic and terrestrial ecotoxicology . Aquat Toxicol , 67 : 143 – 154 .
- Snoeren , T AL , De Jong , PW and Dicke , M . 2007 . Ecogenomic approach to the role of herbivore-induced plant volatiles in community ecology – essay review . J Ecol , 95 : 17 – 26 .
- Suen , G , Scott , J J , Aylward , F O , Adams , S M , Tringe , S G , Pinto-Tomas , A A , Foster , C E , Pauly , M , Weimer , P J Barry , K W . 2010 . An insect-herbivore microbiome with high plant biomass-degrading capacity . PLoS Genet , 6 ( 9 ) : e1001129
- Thakur , N L , Jain , R , Natalio , F , Hamer , B , Thakur , A N and Müller , W EG . 2008 . Marine molecular biology: an emerging field of biological sciences . Biotechnol Adv , 26 : 233 – 245 .
- Thomas , M A and Klaper , R . 2004 . Genomics for the ecological toolbox . Trends Ecol. Evol , 19 : 439 – 445 .
- Timmermans , M , Roelofs , D , Marien , J and Van Straalen , NM. 2008 . Revealing pancrustacean relationships: Phylogenetic analysis of ribosomal protein genes places Collembola (springtails) in a monophyletic Hexapoda and reinforces the discrepancy between mitochondrial and nuclear DNA markers . BMC Evol Biol , 8 : 83
- Timmermans , M J , De Boer , ME , Nota , B , De Boer , TE , Marien , J , Klein-Lankhorst , R M , Van Straalen , NM and Roelofs , D . 2007 . Collembase: a repository for springtail genomics and soil quality assessment . BMC Genomics , 8 : 341
- Torsten , T , Suhelen , E , Dominic , B , Charmaine , N , Lily , T and Ricardo , C . 2007 . Integration of genomics and proteomics in marine microbial ecology . Mar Ecol Prog Ser , 332 : 291 – 299 .
- Torsvik , V and Ovreas , L . 2002 . Microbial diversity and function in soil: from genes to ecosystems . Curr Opin Microbiol , 5 : 240 – 245 .
- Travers , S E , Smith , M D , Bai , J , Hulbert , S H , Leach , J E , Schnable , P S , Knapp , A K , Milliken , G A , Fay , P A Saleh , A . 2007 . Ecological genomics: making the leap from model systems in the lab to native populations in the field . Front Ecol Environ , 5 : 19 – 24 .
- Tringe , S G , Von Mering , C , Kobayashi , A , Salamov , A A , Chen , K , Chang , H W , Podar , M , Short , J M , Mathur , E J Detter , J C . 2005 . Comparative metagenomics of microbial communities . Science , 308 : 554 – 557 .
- Tyson , G W , Chapman , J , Hugenholtz , P , Allen , E E , Ram , R J , Richardson , P M , Solovyev , V V , Rubin , E M , Rokhsar , D S and Banfield , J F . 2004 . Community structure and metabolism through reconstruction of microbial genomes from the environment . Nature , 428 : 37 – 43 .
- Van Boxtel , AL , Kamstra , J H , Cenijn , P H , Pieterse , B , Wagner , M J , Antink , M , Krab , K , Van der Burg , B , Marsh , G Brouwer , A . 2008 . Microarray analysis reveals a mechanism of phenolic polybrominated diphenylether toxicity in zebrafish . Environ Sci Technol , 42 : 1773 – 1779 .
- Van Loon , L. 2007 . Plant responses to plant growth-promoting rhizobacteria . Eur J Plant Pathol , 119 : 243 – 254 .
- Van Straalen , NM and Roelofs , D . 2006 . An introduction to ecological genomics , 1st ed. , Oxford : Oxford University Press .
- Venter , J C , Remington , K , Heidelberg , J F , Halpern , A L , Rusch , D , Eisen , J A , Wu , D Y , Paulsen , I , Nelson , K E Nelson , W . 2004 . Environmental genome shotgun sequencing of the Sargasso Sea . Science , 304 : 66 – 74 .
- Ward , N . 2006 . New directions and interactions in metagenomics research . FEMS Microbiol Ecol , 55 : 331 – 338 .
- Warnecke , F , Luginbuhl , P , Ivanova , N , Ghassemian , M , Richardson , T H , Stege , J T , Cayouette , M , McHardy , A C , Djordjevic , G Aboushadi , N . 2007 . Metagenomics and functional analysis of hindgut microbiota of a wood-feeding higher termite . Nature , 450 : 560 – 565 .
- Wilson , K , Thorndyke , M , Nilsen , F , Rogers , A and Martinez , P . 2005 . Marine systems: moving into the genomics era . Mar Ecol , 26 : 3 – 16 .
- Woon Roh , S , Abell , G CJ , Kim , K H , Nam , Y D and Bae , J W . 2010 . Comparing microarrays and next-generation sequencing technologies for microbial ecology research . Trends Biotechnol , 28 : 291 – 299 .
- Xu , J . 2006 . Microbial ecology in the age of genomics and metagenomics: concepts, tools, and recent advances . Mol Ecol , 15 : 1713 – 1731 .
- Young , I M and Crawford , J W . 2004 . Interactions and self-organization in the soil-microbe complex . Science , 304 : 1634 – 1637 .