Abstract
The alcohol distilleries are growing extensively worldwide due to widespread industrial applications of alcohol such as in chemicals, pharmaceuticals, cosmetics, beverages, food and perfumery industry, etc. The industrial production of ethanol by fermentation results in the discharge of large quantities of high-strength liquid wastes. Distillery wastewater is one of the most polluted waste products to dispose because of the low pH, high temperature, dark brown colour, high ash content and high percentage of dissolved organic and inorganic matter with high biochemical oxygen demand (BOD) and chemical oxygen demand (COD) values. Its characteristics are depending on the feed stock and various aspects of ethanol production process. Spent wash polluted the water bodies into ways; first, the highly coloured nature which can block out sunlight, thus reducing oxygenation of the water by photosynthesis and hence becomes detrimental to aquatic life. Second, it has a high pollution load which would result in eutrophication of contaminated water sources. Distillery wastewater, without any treatment can result in depletion of dissolved oxygen in the receiving water streams and poses serious threat to the aquatic flora and fauna. This review presents an account of the problems associated with distillery wastewater and a detailed study of existing biological treatment approaches. The role of various microorganisms and their enzymes in the wastewater treatment has been discussed to develop a better understanding of the phenomenon.
1. Introduction
Distilleries are one of the most polluting industries as 88% of its raw materials are converted into waste and discharged into the water bodies, causing water pollution. In the distillery, for every litre of alcohol produced, about 15 l of spent wash is released (Ravikumar et al. 2007). Alcohol serves as a basic chemical for a large number of chemical industries and therefore, demand for alcohol will see a great increase in future and distilleries are fulfilling this demand. The first distillery in India was set up at Kanpur (then Cawnpore) in 1805 for manufacturing rum for the army. At present, there are about 315 distilleries with a total capacity of 3250 million litres of alcohol per annum with 40.4 billion litres of effluent, annually (Mohana et al. Citation2009). Most of the distilleries co-exist with sugar mills and utilize the molasses from cane sugar manufacturing as the starting material for alcohol production.
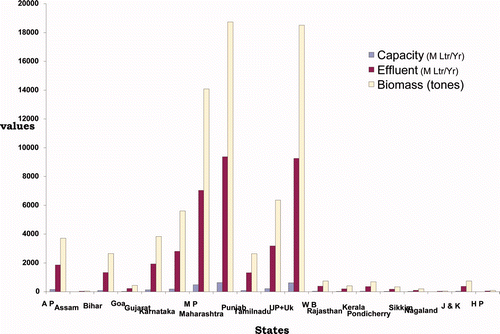
A maximum number of distilleries is present in Maharashtra (67) with an annual capacity of about 625 million litres of alcohol per year and an effluent generation of about 9367 million liter per year, approximately. Annual capacity and effluent generation of distillery industry in various States of India is shown in (Chauhan and Dikshit Citation2007).
2. Manufacturing process and wastewater generation
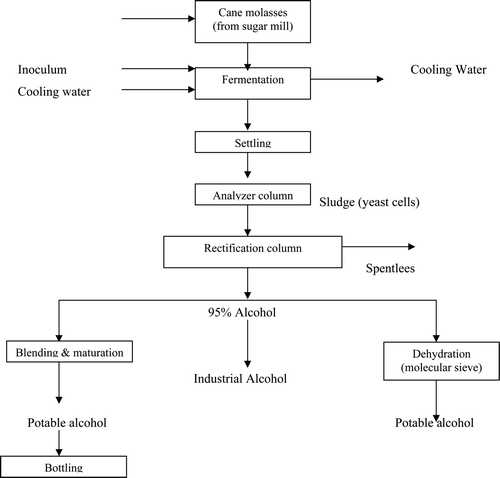
Alcohol (preferably ethanol) is mainly produced from cellulosic materials. Raw materials, mainly used in distilleries are sugarcane molasses, grains, grapes, sugarcane juice, and barley malt. Alcohol production in distilleries consists of four main steps viz. feed preparation, fermentation, distillation, and packaging. shows the various steps of manufacturing process in distillery industries. Diluted sugarcane molasses is inoculated with yeast and fermented in either batch or continuous mode to yield a broth containing 6–8% ethanol. In the continuous process, the cellulosic materials first delignified and hemi-cellulose and cellulose are subsequently acid hydrolyzed into simple sugars. These sugars are fermented in the presence of yeast to yield ethanol and carbon dioxide. The alcohol vapor is removed from the fermentation solution under reduced pressure and subsequently distilled. Carbon dioxide gas may be sparged throughout the fermenting solution in order to aid in the removal of the alcohol from the fermenting solution (Saha et al. Citation2005; Tewari et al. Citation2007; Satyawali and Balkrishnan 2008). The gaseous carbon dioxide is captured and utilized for the manufacture of additional quantities of ethanol or other basic chemicals.
3. Characteristics of wastewater generated during manufacturing process
Alcohol distilleries are highly water intensive units generating large volumes of high strength wastewater which pose a serious environmental concern. The quantum and characteristics of wastewater generated at various stages in the manufacturing process is provided in and their characteristics are shown in .
Table 1. Wastewater generation in various operations.
Table 2. Typical characteristics of different wastewater streams.
The distilleries have been generating huge quantities of high toxic effluents. The production and the characteristics of the spent wash are highly variable and dependent on the raw material used and various aspects of the ethanol production process (Wedzicha and Kaputo Citation1992; Pant and Adholeya Citation2007a, Citation2007b). The characteristics of different types of distillery wastewater are given in .
Table 3. Chemical characteristics of distillery wastewaters (Melamane et al. 2007).
The spent wash generated from distilleries has high chemical oxygen demand (COD) (80,000–100,000 mg/L) and biochemical oxygen demand (BOD) (40,000–50,000 mg/L), high temperature, is dark brown in colour having low pH (<4.0–4.5) (Central Pollution Control Board 1994). COD and BOD values of this spent wash are due to the presence of a number of organic compounds, such as polysaccharides, reduced sugars, lignin, proteins, melanoidin, waxes, etc. The amount of inorganic substances such as nitrogen, potassium, phosphates, calcium, and sulfate is also very high () (Melamane et al., Citation2007). Spent wash contains about 2% melanoidin which has an empirical formula of C17–18H26–27O10N and molecular weight between 5000 and 40,000 Da (Martin et al. 2002; Manisankar et al. Citation2004). These compounds have antioxidant properties, which render them toxic to many microorganisms such as those typically present in wastewater treatment processes (Kumar et al. Citation1997).
According to EPA Guidelines for Wineries and Distilleries (2004), some of the potential effects of the various constituents of liquid and solid waste by-products from the wine making process on the environment are summarized in .
Table 4. Potential environmental impacts of winery and distillery wastes.
Many technologies have been tried for the treatment of spent wash, however, none of these methods have been found effective and economically viable to achieve the standard norms () set by the Central Pollution Control Board, Government of India.
Table 5. Effluent standards for distilleries, maltries, and breweries.
4. Treatment options of distillery spent wash
Current treatment options used to treat distillery spent wash includes physical, chemical, physicochemical and biological methods before its disposal. The selection of treatment methods depends on various factors viz. treatment efficiency, treatment cost, local geography, climate, landuse, regulatory constraints, and public acceptance of the treatment.
4.1. Physical treatment
Physical treatment methods are used at the initial stage of effluent treatment. Various physical treatment methods currently being used in distillery wastewater treatment are screening, flow equalization mixing, flotation, and sedimentation (Thakur Citation2006). Adsorption is also a one of the most widely used physical method. Adsorption on activated carbon is widely employed for removal of colour and specific organic pollutants. Physical treatment is used to decrease suspended/settable solids from wastewater which may be removed inexpensively via sedimentation by using the force of gravity to separate suspended material, oil, and grease from the wastewater (Jayanti and Narayanan Citation2004). Hamoda et al. (Citation2004) reported removal of total suspended solids (TSS) by passing wastewater through a fine granular media such as sand, the particles are captured in the fine pores and sorbed on the surface of the granular media. Nataraj et al. (Citation2006) also reported that membrane based nano-filtration and reverse osmosis processes can be used to reduce the total dissolved solids (TDS), COD, and potassium (K+) content of distillery wastewater by 99.80, 99.90, and 99.99%, respectively.
4.2. Chemical treatment
During chemical wastewater treatment, compound like chlorine (Cl2), oxygen (O2), ozone (O3), and permanganate (MnO4) are added to the wastewater to oxidize the wastewater components into carbon dioxide (CO2), water, inorganic matter, and other harmless products (Benitez et al. Citation2003). Coagulation and flocculation processes are used for rapid and economical removal of suspended, inert, or undesirable colloidal materials in industrial wastewater (Tatsi et al. Citation2003). Mohana et al. (Citation2009) have reported that melanoidins can be decolourized by various physiochemical methods. Majority of these methods remove colour by either concentrating the colour into sludge or by partial or complete breakdown of the colour molecules. Adsorption is a physical process so discuss it in physical process paragraph. Adsorption on activated carbon is widely employed for removal of colour and specific organic pollutants.
Pikaev et al. (Citation2001) reported that treatment of distillery wastewater using iron sulfate (Fe2 (SO4)3) as a coagulant results, 40% removal of wastewater pollutants. Beltrain de Heredia et al. (Citation2005) also achieved 55% reduction in COD by using integrated Fenton-coagulant/flocculation process in distillery wastewater treatment. In another report, addition of ferrous sulfate, aluminum chloride, calcium oxide, ferric chloride along with coagulants reduced the colour to 95%, 74.4%, 80.2%, and 83%, respectively, while COD was reduced as 78%, 61.3%, 39.8%, and 55%, respectively (Chaudhari et al. Citation2007).
4.3. Biotechnological/biological processes
All biological treatment processes depends upon the natural growth and selection of microorganisms in a suspended culture or fixed film (Droste Citation1997; Lettinga et al. Citation1997), during treatment process, these microorganisms utilize pollutants for growth and convert the organic substrate in the wastewater into simpler substances such as CO2 and water, in the presence (aerobic) or absence (anaerobic) of O2 (Henry and Heinke Citation1996). Distillery wastewater needs to undergo intensive treatment in order to meet stipulated guidelines. Physical/chemical methods are invariably costly and are less employed in industries. While in recent years, biological wastewater treatment systems have attracted the attention of workers throughout the world and have helped in developing efficient and low-cost waste treatment systems (Naik et al. Citation2008). Biotechnology treatment encompasses a range of scientific and engineering techniques for applying biological systems to the manufacture or transformation of valuable materials or the elimination of problematic, often poisonous, liquid, solid, or gaseous wastes. There are a number of waste treatment systems which are based on aerobic and/or anaerobic microbial action to the wastewater (Willmott et al. Citation1998).
This approach can remove most of the biologically removable organics, CODs, and colour. Identification and optimization of biotechnological treatment methods is a necessity of the present time (Thakur Citation2006). There are several reports citing the potential of biological treatment for distilleries wastewater also. Both aerobic and anaerobic systems are commonly used to treat distillery spent wash. In view of potential applications, this section is discussed in details as biological treatment.
Various biotechnical/biological processes are being practiced successfully to remove the high pollution load from the distillery wastewater.
4.3.1. Anaerobic treatment
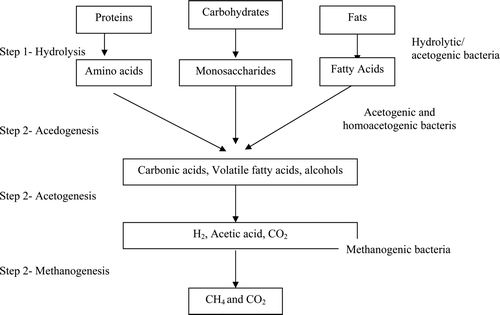
Anaerobic digestion is a natural process in which various microbial species work together, in the absence of O2, to transform organic wastes through a verity of intermediates into biogas (Mata-Alvarez Citation2003). During anaerobic digestion, biomass and biogas are produced, while pathogenic microorganisms and offensive organic matter are reduced. Therefore, anaerobic digestion can be used both as a depollution tool and to produce energy (Moletta Citation2005). Anaerobic digestion of high strength wastewater usually occurs in successive steps and is accomplished by four trophic groups of bacteria (Ranade et al. Citation1999). These bacteria groups function in a synergistic relationship and form a food chain, in which the final products are CH4 and CO2 (). There are a number of microorganisms that are involved in the process of anaerobic digestion including acetic acid-forming bacteria (acetogens) and methane-forming archaea (methanogens). There are four key biological and chemical stages of anaerobic digestion: hydrolysis, acidogenesis, acetogenesis, and methanogenesis.
Figure 3. Schematic diagram of anaerobic digestion indicating the process steps and the four bacteria groups involved in the process (Garcia-Heras 2003).
Various anaerobic treatment devices have been used, viz. anaerobic lagoons, anaerobic digester, anaerobic contact reactor, anaerobic filter, up flow UASB, anaerobic fluidized, and expended bed reactor.
Anaerobic digestion is the most suitable option for the treatment of high strength organic effluent. Anaerobic treatment is an accepted practice and various high rate anaerobic reactor designs have been tried at pilot and full-scale operation (Lata et al. Citation2002). The major advantages of anaerobic treatment using up flow UASB is that besides treatment of the wastewater, methane gas is also generated in this process which can be used as a fuel specially in boilers, thus anaerobic treatment processes not only helps in wastewater treatment but also lowers fuel consumption cost. Some of the anaerobic systems being applied for treatment of distillery wastewater are summarized in .
Table 6. Anaerobic methods employed for distillery effluent treatment.
The performance and treatment efficiency of anaerobic process can be influenced both by inoculum source and feed pre-treatment. Anaerobic lagoons are the simplest option for the anaerobic treatment of distillery spent wash. Rao (Citation1972) reported that employing two anaerobic lagoons in series resulted in final BOD levels up to 600 mg/l. Rao (Citation1972) carried out the pioneering research work in the field of distillery waste management by studying the application of anaerobic lagoon treatment in two pilot-scale lagoons in series, with overall BOD removal ranging from 82 to 92%. Treatment of winery wastewater was investigated using an anaerobic sequencing batch reactor (ASBR). The reactor was operated at an organic loading rate (OLR) of 8.6 kg COD m−3 d−1 with soluble COD removal efficiency greater than 98%, hydraulic retention time (HRT) of 2.2 days (Ruiz et al. Citation2002).
Garcia-Calderon et al. (Citation1998) reported the application of the down-flow fluidization technology for the anaerobic digestion of red wine distillery wastewater. The system achieved 85% total organic carbon (TOC) removal, at an organic loading rate of 4.5 kg TOC m3 day−1. Up flow UASB reactor is the most popular high rate digester that has been utilized for anaerobic treatment of various types of industrial wastewaters (Akunna and Clark Citation2000). Treatment by a UASB reactor resulted in 75% COD removal in sugarcane molasses spent wash (MSW). Wolmarans and De Villiers (Citation2002) have also reported COD removal efficiency of greater than 90% over three seasons in a UASB plant treating distillery wastewater. In another anaerobic treatment method, fluidized bed reactors contain an appropriate media such as sand, gravel, or plastics for bacterial attachment and growth. A two stage process with an anaerobic filter followed by a UASB reactor was investigated by Blonskaja et al. (Citation2003). The acidogenic and methanogenic phases were clearly separated ensuring better conditions for the methanogens. COD reduction was 54% and 93% in the first and second stage, respectively. The highest BOD removal is possible in open lagoon, whereas highest biomethane produced is in up flow UASB bioreactor. Compared to aerobic system, it has slow growth rate, mainly associated with methanogenic bacteria. Therefore, it requires a long retention time, and also only a small portion of the degradable organic waste is being synthesized to new cells (Pant and Adholeya, Citation2007).
4.3.2. Aerobic treatment
Aerobic processes are biological treatment processes that occur in the presence of oxygen. The aerobic environment in the reactor is achieved by the use of diffused or mechanical aeration, which maintain the mixed liquor in a completely mixed regime. Aerobic digestion is an alternative method of treating the organic sludge's produced from various treatment operations. In the conventional aerobic digestion (Coverti et al. Citation1993), the wastewater is aerated for an extended period of time in an open, unheated tank using conventional air diffusers, or surface aeration equipment (Hsu and Shich Citation1993). According to Metcalf and Eddy Inc. (1995), there are two variations in the aerobic digestion process, namely, conventional and pure oxygen. Aerobic treatment systems are used mainly to remove the BOD of these wastes. Partial reduction of BOD and COD is achieved in many distilleries using biological treatment (Jawed and Tare Citation1999; Laubscher et al. Citation2001; Wolmarans and De Villiers Citation2002; Coetzee et al. Citation2004).
Jackson et al. (Citation2007) used a bioreactor system to treat distillery wastewater with a two-week HRT. COD of the distillery wastewater was decreased from 2255 mg/l to a final value of < 150 mg/l. Some of the aerobic systems being applied for treatment of distillery wastewater are summarized in .
Table 7. Aerobic methods employed for distillery effluent treatment.
The aerobic treatment of industrial wastewaters usually depends on the oxidative activities of microorganisms. Although, a large number of microorganisms, such as bacteria, cynobacteria, yeast, fungi, etc. have been used for treatment of spent wash. Filamentous fungi can be of important sources of phenolic-degrading organisms, as they frequently grow on wood, utilizing lignin as a carbon source (Benitez et al. Citation1999; Coulibaly et al. Citation2003; Mendonca et al. Citation2004).
4.3.2.1. Fungal treatment
Fungi have shown potential for the treatment of various specific pollutants and mixed wastewaters, including dark-coloured, phenolic wastewaters such as molasses (Jimenez et al. Citation2003) and olive mill waste (Perez et al. Citation1998; Aggelis et al. Citation2003; Fenice et al. Citation2003; Mendonca et al. Citation2004; Ruiz et al. Citation2006), which means that fungal treatment of these wastewaters could be used as a pre-treatment step for anaerobic digestion. According to Coulibaly et al. (Citation2003), fungi can be used to treat wastewaters in a liquid environment, where bioreactors with wastewater can be exposed to the specific live fungus that is capable of degrading a single pollutant or preferably a mixture of pollutants. Another promising approach would be to use enzymes derived from fungi to treat the wastewater (Coulibaly et al. Citation2003). Some of the fungi applied for treatment of distillery wastewater are summarized below in .
Table 8. Fungi employed for the decolourization and COD removal of distillery effluent.
Four white-rot fungal cultures were examined for their ability to decolourize and bioremediate anaerobically digested molasses spent wash (DMSW) generated by biomethanation plants. Two cultures Coriolus versicolourand Phanerochaete chrysospotium showed an ability to decolourize and reduce the COD of diluted DMSW (125% v/v). Maximum decolourization (71.5 and 53.5%) and COD reduction (90.0 and 73.0%) were achieved in 6.25% (v/v) DMSW medium by C. versicolourand P. chrysospotium, respectively (Kumar et al. Citation1998). Phanerochaete chrysosporium and Trametes versicolour are the most widely studied among these. Phanerochaete chrysosporium JAG 40 resulted in 80% decolourization of diluted synthetic melanoidin (absorbance unit of 3.5 at 475 nm), as well as with 6.25% anaerobically digested spent wash (Kumar et al. Citation1998; Dahiya et al. Citation2001). Flavodon flavus (Klotzsch) Ryvarden, a basidiomycete (NIOCC strain 312) isolated from decomposing leaves of a sea grass, decolourized pigments in MSW by 80% after 8 days of incubation, when used at concentrations of 10% and 50% (Raghukumar and Rivonkar Citation2001). Aspergillus fumigatus has been found to be effective for decolourization of anaerobically treated distillery wastewater (Mohammad et al. Citation2006). Fungal consortium was employed in fluidized film aerobic system (FFAS). The analyzed effluent at the end of FFAS treatment showed a reduction of 70% in BOD and 63% in COD without causing any colour change (Ravikumar et al. Citation2007).
The fungi Geotrichum candidumroman Coriolus versicolourroman Phanerochaete chrysosporium, and Mycelia sterilia were screened for their ability to decolourize spent wash and to reduce the COD level. A 10 day pretreatment with Geotrichum candidum at 30°C resulted in reducing the COD by 53.17% and total phenols by 47.82%, enabling other bioremediating organisms to grow. Coriolus versicolour immobilized in a packed-bed reactor reduced the COD of spent wash by a further 50.3%, giving an overall reduction in COD of 77% to 15,780 mg/l (Fitzgibbon et al. Citation2007).
4.3.2.2. Bacterial treatment
Bacterial cultures are capable of bioremediation of distillery spent wash. Some of the bacteria applied for treatment of distillery wastewater are summarized below in . Pioneering work on spent wash decolourizarion by bacteria was done by Kumar et al. (Citation1997). They observed that two aerobic bacterial isolates LA-1 and D-2 brought about maximum decolourization (36.5% and 32.5%) and COD reduction (41% and 39%) under optimized conditions in eight days. The most prominent bacterial species isolated from the reactor liquid belonged to Pseudomonas, while Bacillus was isolated mostly from colonized carriers. Pseudomonas fluorescens, decolourized melanoidin wastewater (MWW) up to 76% under non-sterile conditions and up to 90% in sterile samples (Dahiya et al. Citation2001). Acetogenic bacteria are capable of oxidative decomposition of melanoidins. Cibis et al. (Citation2002) achieved biodegradation of potato slops (distillation residue) by a mixed population of bacteria under thermophilic conditions up to 600C. A COD removal of 77% was achieved under non-optimal conditions.
Table 9. Bacteria employed for the decolourization biodegradation of distillery effluent.
4.3.2.3. Algal treatment
The prokaryotic, oxygen-evolving photoautotrophs (cyanobacteria) have been reported to be useful for treatment of solid wastes and wastewaters containing phenol (Shashirekha et al. Citation1997). Since they are able to metabolize and use these compounds as nitrogen, phosphorus, carbon, and sulfur sources, respectively. Kalavathi et al. (Citation2001) reported degradation and metabolization of the melanoidin in distillery effluent by the marine cyanobacterium Oscillatoria boryana BDU 92181.They, the decolourization of pure melanoidin pigment (0.1% W/V) by about 75% and crude pigment in the distillery effluent (5% V/V) by about 60% in 30 days reported. It was observed that the strain Oscillatoria resulted in almost complete colour removal (96%), whereas Lyngbya and Synechocystis were less effective resulting in 81 and 26% colour reduction, respectively (Patel et al. Citation2001). The treatment of anaerobically treated 10% distillery effluent using the microalga Chlorella vulgaris followed by Lemna minuscula resulted in 52% reduction in colour (Valderrama et al. Citation2002).
4.3.2.4. Phytoremediation
Phytoremediation of effluents is a low cost technique is used to remediate sites, contaminated with heavy metals and toxic organic compounds. Phytoremediation takes advantage of plants, nutrients utilization processes transpire water through leaves, and act as transformation system to metabolize organic compounds such as oil and pesticides. They may also absorb and bioaccumulate toxic trace elements, such as the heavy metals like lead, cadmium, and selenium (Witten et al. Citation1997). Phytoremediation depends on the availability of plant species – ideally those native to the region of interest – able to tolerate and accumulate high concentrations of heavy metals (Baker and Whiting Citation2002). Some plants can accumulate remarkable levels of heavy metals: 100–1000-fold the levels normally found in most species. This striking phenomenon, known as metal hyperaccumulation (i.e. the ability to accumulate at least 0.1% of the leaf dry weight in a heavy metal), is only exhibited by <0.2% of angiosperms (Baker and Whiting Citation2002), making the selection of native species for phytoremediation efforts a difficult task. Many hyperaccumulating species, characterized by their tolerance to toxic levels of metals such as As, Co, Cu, Zn, Mn, Pb, Se, Ni, and Cd, are often endemic to metal-rich substrates and are rare in their distribution (Baker et al. Citation2000). There are about 420 species belonging to about 45 plant families recorded as hyperaccumulators of heavy metals (Cobbett Citation2003).
Aquatic plants have excellent capacity to reduce the level of toxic metals, BOD, and total solids from the wastewaters (Kumar and Chandra Citation2004). Aquatic weeds are known to accumulate metals and other pollutants from the contaminated water. Weeds like Hydrillaroman Agrostisroman Eichhorniaroman Pistia, etc. are known to remove considerable amounts of metals from the matter in which they are growing (Satyakala and Jamil, Citation1997). The water hyacinth (E. crassipes) has been found to be most effective an economical in removing many chemical pollutants, nutrients and heavy metals, from industrial wastewater (Rai et al. Citation1995).
Bioaccumulations of the four heavy metals Cr, Cu, Pb, and Zn in Lemna gibba (duckweed) as an environmental indicator of contaminated industrial wastewater were detected. Plant pigment contents (chlorophyll and carotenoids) were estimated. During the study, heavy metals were ranked according to the preference for bioaccumulation by L. gibba, Zn came in the first place followed by Cr, Pb, and Cu with bioaccumulation factors 13.9, 6.3, 5.5, and 2.5, respectively (Hegazy et al. Citation2009). The correlation between heavy metal contents and pigment contents in L. gibba fronds revealed negative relationships with chlorophyll contents and positive relationships with carotenoid content for all study heavy metals. The decrease in total chlorophyll content was mainly associated with the increase in contents of Zn, Cu, and Pb reaching correlation coefficients of −0.99, −0.97, and −0.92, respectively. Chlorophyll a degradation was mostly associated with the increase in Zn contents, while chlorophyll b degradation was influenced by the increase in contents of Cr, Cu, Zn, and Pb. The degreened stage was excluded from the correlation between heavy metal and carotenoid contents; otherwise, weak correlations were obtained. Apart from Zn and Pb, Cu and Cr contents, where significantly correlated with the increase in carotenoid content in L. gibba fronds attaining correlation coefficients of 0.99 and 0.98, respectively (Hegazy et al. Citation2009).
Many species of plants have been successful in absorbing contaminants such as lead, cadmium, chromium, arsenic, and various radionuclides from soils. One of phytoremediation categories, phytoextraction, can be used to remove heavy metals from soil using its ability to uptake metals which are essential for plant growth (Fe, Mn, Zn, Cu, Mg, Mo, and Ni). Some metals with unknown biological function (Cd, Cr, Pb, Co, Ag, Se, Hg) can also be accumulated (Cho-Ruk et al. Citation2006).
According to Sinha et al. (Citation2004), the plants act both as “accumulators” and “excluders”, accumulators survive despite concentrating contaminants in their aerial tissues. They biodegrade or biotransform the contaminants into inert forms in their tissues. The excluders restrict contaminant uptake into their biomass.
4.3.2.5. Enzymatic treatment of wastewaters
The most recent research has been focused on the development of enzyme processes for the treatment of wastewater (Karam and Nicell Citation1999). A large number of enzymes from a variety of different plants and microorganisms have been reported to play an important role in an array of waste treatment applications (Naik et al. Citation2008). Enzymes can act on specific recalcitrant pollutants by precipitation or transformation to other products. They can also change the characteristics of a given waste to render it more amenable to treatment or aid in converting waste material to value-added products (Karam and Nicell Citation1999). Various oxidative enzymes such as Peroxidases and/or phenoloxidases are mainly involved in biotransformation or bioremediation of recalcitrant compounds (Durán and Esposito, 2000).
Although the enzymatic system related with decolourization of melanoidins is yet to be completely understood, it seems greatly connected with fungal ligninolytic mechanisms. The white-rot fungi is reported to have a complex enzymatic system which is extracellular and non-specific, and under nutrient-limiting conditions is capable of degrading lignolytic compounds, melanoidins, and polyaromatic compounds that cannot be degraded by other microorganisms (Benito et al. Citation1997). Miyata et al. (Citation1998) used C. hirsutus pellets to decolourize a melanoidin-containing medium. It was elucidated that extracellular H2O2 and two extracellular peroxidases, a manganese-independent peroxidase (MIP) and manganese peroxidase (MnP) were involved in decolourization activity.
Fungi especially the white-rot fungi produce enzymes laccase, Mn peroxidase, and lignin peroxidase (LiP), which are involved in degradation of lignin in their natural lignocellulosic substrates. Lignin peroxidase is able to oxidize various aromatic compounds, while Manganese peroxidase oxidizes almost exclusively Mn(II) to Mn(III), which then degrades phenolic compounds. Laccases are copper-containing oxidases. They reduce molecular oxygen to water and oxidize phenolic compounds (Mester and Tien Citation2000). D'souza et al. (Citation2006), cultured a marine fungal isolate, NIOCC # 2a from decaying mangrove wood and reported 100% decolourization of 10% spent wash by a fungal isolate whose laccase production increased several folds in the presence of phenolic and non-phenolic inducers.
Enzymatic decolourization of molasses medium has also been tried using P. chrysosporium (Thakkar et al. Citation2006). Under stationary cultivation conditions, none of the strains could decolourize molasses nor produce enzymes lignin peroxidase, manganese peroxidase, and laccase. A combined treatment technique consisting of enzymatic hydrolysis, followed by aerobic biological oxidation was investigated for the treatment of alcohol distillery spent wash. Sangave and Pandit (Citation2006a) used cellulase enzyme for the pretreatment step with an intention of transforming the complex and large pollutant molecules into smaller molecules. Sangave and Pandit (Citation2006b) used irradiation and ultrasound combined with the use of an enzyme as pretreatment technique for treatment of distillery wastewater. The combination of the ultrasound and enzyme yielded the best COD removal efficiencies as compared to the processes when they were used as stand-alone treatment techniques.
Pant and Adholeya (Citation2007) isolated two fungal strains producing ligninolytic enzymes and having the potential to decolourize distillery effluent from the soil of a distillery effluent contaminated site. These two isolates along with one isolate of Pleurotus florida EM 1303 were assessed for their ligninolytic enzyme activity in culture filtrate as well as after solid state fermentation on two substrates wheat straw and corncob powder. Both P. pinophilum TERI DB1 and A. gaisen TERI DB6 were found to produce laccase, manganese-dependent peroxidases (MnP) and lignin peroxidases (LiP). The immobilized fungal biomass was then used for decolourization of the post biomethanated distillery wastewater and observed the reduction in colour up to the magnitude of 86, 50, and 47% with P. florida, P. pinophilum, and A. gaisen respectively. Some of the enzymes produced from the different from fungi are summarized in .
Table 10. Enzymes produced by fungi employed for the decolourization of distillery wastewater.
Bodalo et al. (Citation2006) has compared the two enzymes, horseradish peroxidase (HRP) and soybean peroxidase (SBP), the two most widely used commercial peroxidases for the removal of phenol from wastewater. Both enzymes achieved maximal removal efficiency in a neutral pH medium although they were active in a pH range of between 6.0 and 8.0.
5. Conclusions
Various treatment technologies such as physico-chemical treatment, composting, and biological treatment have been investigated by the researchers. The physical and chemical treatment methods remove organic pollutants at low level; they are highly selective to the range of pollutants removed (colour, turbidity, TSS or foul odors and COD). Nevertheless, the disadvantages associated with these methods are excess use of chemicals, sludge generation with subsequent disposal problems, high operational costs, and sensitivity to variable water input.
The conventional biological treatment methods (anaerobic digestion, anaerobic lagoons, activated sludge process, etc.) are not effective for complete colour and other pollutants removal from this stream. Various researchers have demonstrated that anaerobic processes enabling recovery of biogas appear to be the most promising technology for the treatment of spent wash. The technologies currently used by distilleries for treatment of wastewater are biomethanation followed by two-stage biological treatment and disposal in water courses or for utilization on land for irrigation, composting with or without biomethanation. These technologies treat the wastewater up to a certain level. However, there are limitations posed by these technologies for full compliance of prescribed standards. These limitations are either due to high cost of treatment or inherent inability of technology to remove certain pollutants like TDS and color, to safe and acceptable limits for disposal into surface water or on land. The use of an individual process alone may not treat the wastewater completely. An anaerobic treatment followed by aerobic biological oxidation is a common technique used for decolourizing wastewater. But these processes are not efficient enough to treat these large volumes of coloured wastewaters. There is a need of an ideal, cost effective, and commercial treatment scheme which should comprise of biomethanation as the primary step followed by physicochemical treatment and concluding with aerobic treatment.
The emerging treatment methods like enzymatic treatment have technological advantages and yet are in its infancy, requiring economical considerations in order to apply it on the plant scale. There appears to be a great potential for enzymes in a large number of waste treatment areas. The feasibility of application of the process to full-scale would need further research in this continuous culture set-up, in order to minimize the added nutrients and extend the biomass activity for a longer period.
Acknowledgments
The authors gratefully acknowledge the University School of Environment Management, Guru Gobind Singh Indraprastha University, Kashmiri Gate, Delhi 110406, India, for providing the research grants to support this work.
References
- Acharya , B K , Mohana , S , Madamwar and D . 2008 . Anaerobic treatment of distillery spent wash: a study on upflow anaerobic fixed film bioreactor . Bioresource Technol , 99 : 4621 – 4626 .
- Aggelis , G , Iconomou , D , Christou , M , Bokas , D , Kotzaillias , S , Christou , G , Tsagou , V and Papanikolaou , S . 2003 . Phenolic removal in a model olive mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process . Water Res , 37 : 3897 – 3904 .
- Akunna , J C and Clark , M . 2000 . Performance of a granular-bed anaerobic baffled reactor (GRABBR) treating whisky distillery wastewater . Bioresource Technol , 74 ( 3 ) : 257 – 261 .
- Angayarkanni , J , Palaniswamy , M and Swaminathan , K . 2003 . Biotreatment of distillery effluent using Aspergillus niveus . Bull Environ Contam Toxicol , 70 : 268 – 277 .
- Baker , AJM , McGrath , SP , Reeves , RD and Smith , JAC . 2000 . “ Metal hyperaccumlator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal polluted soils ” . In Phytoremediation of contaminated soil and water , Edited by: Terry , N and Bañuelos , G . 85 – 108 . Boca Raton , FL : Lewis .
- Baker , A JM and Whiting , S M . 2002 . In Search for the Holy Grail – another step in understanding metal hyperaccumulation? . New Phytol , 155 : 1 – 7 .
- Beltrain de Heredia , J , Domingues , J R and Partido , E . 2005 . Physico-chemical treatment for depuration of wine distillery wastewater (vinasses) . Water Sci Technol , 51 ( 1 ) : 159 – 166 .
- Benitez , F J , Beltran-Heredia , J , Real , F J and Gonzalez , T . 1999 . Aerobic and anaerobic purification of wine distillery wastewater in batch reactors . Chem Eng Technol , 22 : 165 – 172 .
- Benitez , F J , Leal , A I , Acero , J L , Garcia , J and Sanchez , M . 2003 . Kinetics of the ozonation and aerobic biodegradation of winw vinasses in discontinuous and continuous process . J Hazard Mater , 101 ( 2 ) : 203 – 218 .
- Benito , G G , Miranda , M P and Santos , D R . 1997 . Decolorization of wastewater from an alcoholic fermentation process with Trametes versicolour . Bioresource Technol , 61 : 33 – 37 .
- Biesterfeld , S , Farmer , G , Figueroa , L , Parker , D and Rissell , P . 2003 . Quantification of a denitrfication potential in carbonaceous trickling filters . Water Res , 37 ( 16 ) : 4011 – 4017 .
- Blonskaja , V , Menert , A and Vilu , R . 2003 . Use of two-stage anaerobic treatment for distillery waste . Adv Environ Res , 7 : 671 – 678 .
- Bodalo , A , Gomez , J L , Gomez , E , Bastida , J and Maximo , M F . 2006 . Comparison of commercial peroxidases for removing phenol from water solutions . Chemosphere , 63 ( 4 ) : 626 – 632 .
- Bories , A , Raynal , J and Bazile , F . 1988 . Anaerobic digestion of high strength distillery wastewater (cane molasses stillage) in a fixed-film reactor . Biol Waste , 23 ( 4 ) : 251 – 267 .
- Bories , A , Sire , Y and Colin , T . 2005 . Odorous compounds treatment of winery and distillery effluents during natural evaporation in ponds . Water Sci Technol , 51 ( 1 ) : 129 – 136 .
- Central Pollution Control Board . 2003 . Chapter on corporate responsibility for environmental protection, distillery Available from: http://www.cpcb.nic.in/Charter/charter5.htm
- Chaturvedi , S , Chandra , R and Rai , V . 2006 . Isolation and characterization of Phragmites australis (L.) rhizosphere bacteria from contaminated site for bioremediation of colored distillery effluent . Ecol Eng , 27 : 202 – 207 .
- Chaudhari , P K , Mishra , I M and Chandb , S . 2007 . Decolorization and removal of chemical oxygen demand (COD) with energy recovery: treatment of biodigester effluent of a molasses-based alcohol distillery using inorganic coagulants . Colloid Surface A , 296 : 238 – 247 .
- Chauhan , M S and Dikshit , A K . Decolorization of distillery wastewater in India: current status and future trends . Proceedings of the 10th International Conference on Environmental Science and Technology; . September 5–7 , Kos Island , Greece. pp. A220 – A227 .
- Cho-Ruk , K , Kurukote , J , Supprung , P and Vetayasuporn , S . 2006 . Perennial plants in the phytoremediation of lead-contaminated soils . Biotechnology , 5 ( 1 ) : 1 – 4 .
- Cibis , E , Kent , C A , Krzywonos , M , Garncarek , Z , Garncarek , B and Miskiewicz , T . 2002 . Biodegradation of potato slops from a rural distillery by thermophilic aerobic bacteria . Bioresource Technol , 85 : 57 – 61 .
- Cobbett , C . 2003 . Heavy metals and plants – model systems and hyperaccumulators . New Phytol , 159 : 289 – 293 .
- Coetzee , G , Malandra , L , Wolfaardt , G M and Viljoen-Bloom , M . 2004 . Dynamics of microbial biofilm in a rotating biological contactor for the treatment of winery effluent . Water SA , 30 ( 3 ) : 407 – 412 .
- Collins , G , Foy , C , McHugh , S , Maho , T and O'Flaherty , V . 2005 . Anaerobic biological treatment of phenolic wastewater at 15–18°C . Water Res , 39 : 1614 – 1620 .
- Contreras , E , Bertola , B and Zaritzky , N . 2001 . The application of different techniques to determine activated sludge kinetics parameters in food industry wastewater . Water SA , 27 ( 2 ) : 169 – 176 .
- Coulibaly , L , Gourene , G and Agathos , N S . 2003 . Utilization of fungi for biotreatment of raw wastewaters . Afr J Biotechnol , 2 : 620 – 630 .
- Coverti , A , Borghi , D M and Ferraido , G . 1993 . Influence of organic loading rate on the anaerobic treatment of high strength semi synthetic waste water in a biological fluidized bed . Chem Eng J , 52 : 21 – 28 .
- Dahiya , J , Singh , D and Nigam , P . 2001 . Decolorization of synthetic and spent wash melanoidins using the white-rot fungus Phanerochaete chrysosporium JAG-40 . Bioresource Technol , 78 ( 1 ) : 95 – 98 .
- Droste , R L . 1997 . Theory and practice of water and wastewater treatment , 547 – 549 . New York : John Wiley . 622–629, 650–652
- D'Souza , D T , Tiwari , R , Sah , A K and Raghukumar , C . 2006 . Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes . Enzyme Microb Technol , 38 : 504 – 511 .
- Durán , N and Esposito , E . 2000 . Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: a review . Appl Catal B: Eviron , 28 ( 2 ) : 83 – 99 .
- EPA Guidelines for Wineries and Distilleries . 2004 . South Australian Wine Industry Association Environment Committee. ISBN 1-876562-66-8
- Fahy , V , FitzGibbon , F J , McMullan , G , Singh , D and Marchant , R . 1997 . Decolorization of molsasses spent wash by Phanerochaete chrysosporium . Biotechnol Lett , 16 : 97 – 99 .
- Fenice , M , Sermani , G G , Federici , F and D'Annibale , A . 2003 . Submerged and solid-state production of laccase and Mn-peroxidase by Panus tigrinus on olive mill wastewater-based media . J Biotechnol , 100 : 77 – 85 .
- Fitzgibbon , F J , Nigam , P , Singh , D and Marchant , R . 2007 . Biological treatment of distillery waste for pollution-remediation . J Basic Microb , 35 ( 5 ) : 293 – 301 .
- Foresti , E . 2001 . Perspectives on anaerobic treatment in developing countries . Water Sci Technol , 44 ( 8 ) : 201 – 208 .
- Fujita , M , Era , A , Ike , M , Soda , S , Miyata , N and Hirao , T . 2000 . Decolorization of heat treatment liquor of waste sludge by a bioreactor using polyurethane foam immobilized white rot fungus equipped with an ultra membrane filtration unit . J Biosci Bioeng , 90 : 387 – 394 .
- Garcia-Calderon , D , Buffiere , P , Moletta , R and Elmaleh , S . 1998 . Anaerobic digestion of wine distillery wastewater in down-flow fluidized bed . Water Res , 32 ( 12 ) : 3593 – 3600 .
- Garcia-Heras , J L . 2003 . “ Reactor sizing, process kinetics, and modeling of anaerobic digestion of complex wastes ” . In Biomethanization of the organic fraction of municipal solid wastes , Edited by: Mata-Alvarez , J . 31 – 43 . London : IWA Publishing .
- Genovesi , A , Harmand , J and Steyer , J P . 2000 . Integrated fault detection and isolation: application to a winery's wastewater treatment plant . Appl Intell , 13 : 59 – 76 .
- Ghosh , M , Verma , S C , Mengoni , A and Tripathi , A K . 2004 . Enrichment and identification of bacteria capable of reducing chemical oxygen demand of anaerobically treated molasses spent wash . J Appl Microbiol , 96 : 1278 – 1286 .
- Goodwin , J AS , Finlayson , J M and Low , E W . 2001 . A further study of the anaerobic biotreatment of malt whisky distillery pot ale using an UASB system . Bioresource Technol , 78 : 155 – 160 .
- Guimaraes , C , Porto , P , Oliveira , R and Mota , M . 2005 . Continuous docolorization of a sugar refinery wastewater in a modified rotating biological contactor with Phanerochaete chrysosporium immobilized on polyurethane foam discs . Process Biochem , 40 : 535 – 540 .
- Hammer , M J and Hammer , M J . 1996 . Water and wastewater technology , 364 – 378 . New Jersey : Prentice-Hall . 382–384
- Hamoda , M F , Al-Ghusain , I and Al-JAsen , D M . 2004 . Application of granular media filtration in wastewater reclamation and reuse . J Environ Sci Health , 39 ( 2 ) : 385 – 395 .
- Harada , H , Uemura , S , Chen , A C and Jayadevan , J . 1996 . Anaerobic treatment of a recalcitrant distillery wastewater by a thermophilic UASB reactor . Bioresource Technol , 55 : 215 – 221 .
- Hegazy , A K , Kabiela , H F and Fawzy , M . 2009 . Duckweed as heavy metal accumulator and pollution indicator in industrial wastewater ponds . Desalination Water Treat , 12 : 400 – 406 .
- Henry , J B and Heinke , G W . 1996 . Environmental science and engineering , 2nd ed. , 440 – 441 . New Jersey : Prentice-Hall . 445, 452, 456–457
- Hsu , Y and Shich , W K . 1993 . Start up of anaerobic fluidized neutral with acetic acid as the substrate . Biotechnol Bioeng , 41 : 347 – 353 .
- Jackson , V A , Paulse , A N , Bester , A A , Neethling , J H , Du , Plessis KR and Khan , W . 2007 . The application of bioremediation: reduction of metal concentrations in river water and COD in distillery effluent . Water Sci Technol , 55 ( 3 ) : 183 – 186 .
- Jain , N , Minocha , A K and Verma , C L . 2002 . Degradation of predigested distillery effluent by isolated bacterial strains . Indian J Exp Bot , 40 : 101 – 105 .
- Jawed , M and Tare , V . 1999 . Microbial composition assessment of anaerobic biomass through methanogenic activity tests . Water SA , 25 ( 3 ) : 345 – 350 .
- Jayanti , S and Narayanan , S . 2004 . Computational study of particle-eddy interaction in sedimentation tank . J Environ Eng , 130 ( 1 ) : 37 – 49 .
- Jimenez , A M , Rafael , B , Martin and A . 2003 . Aerobic-anaerobic biodegradation of beet molasses alcoholic fermentation wastewater . Process Biochem , 38 : 1275 – 1284 .
- Kahraman , S and Yesilada , O . 2003 . Decolorization and bioremediation of molasses wastewater by white rot fungi in a semi solid state condition . Folia Microbiol , 48 : 525 – 528 .
- Kalavathi , D F , Uma , L and Subramanian , G . 2001 . Degradation and metabolization of the pigment-melanoidin in distillery effluent by the marine cyanobacterium Oscillatoria boryana BDU 92181 . Enzyme Microb Technol , 29 : 246 – 251 .
- Karam , J and Nicell , J A . 1999 . Potential applications of enzymes in waste treatment . J Chem Technol Biotechnol , 69 ( 2 ) : 141 – 153 .
- Kaushik , G and Thakur , I S . 2009 . Isolation and characterization of distillery spent wash colour reducing bacteria and process optimization by Taguchi approach . Int Biodeter Biodegr , 163 ( 1 ) : 12 – 25 .
- Keyser , M , Witthuhn , R C , Ronquest , L C and Britz , T J . 2003 . Treatment of winery effluent with upflow anaerobic sludge blanket (UASB) – granular sludges enriched with Enterobacter sakazakii . Biotechnol Lett , 25 ( 22 ) : 1893 – 1898 .
- Kivaisi , A K . 2001 . The potential of constructed wetlands for wastewater treatment and reuse in developing countries: a review . Ecol Eng , 16 ( 4 ) : 545 – 560 .
- Kumar , P and Chandra , R . 2004 . Detoxification of distillery effluent through Bacillus thuringiensis (MTCC 4714) enhanced phytoremediation potential of Spirodela polyrrhiza (L.) Schliden . Bull Environ Contam Toxicol , 73 : 903 – 910 .
- Kumar , V , Wati , L , FitzGibbon , F , Nigan , P , Banat , I M , Singh , D and Marchant , R . 1997 . Bioremediation and decolorization of anaerobically digested distillery spent wash . Biotechnol Lett , 19 : 311 – 313 .
- Kumar , V , Wati , L , Nigam , P , Banat , I M , Yadav , B S , Singh , D and Marchant , R . 1998 . Decolorization and biodegradation of anaerobically digested sugarcane molasses spent wash effluent from biomethanation plants by white-rot fungi . Process Biochem , 33 ( 1 ) : 83 – 88 .
- Lata , K , Kansal , A , Balakrishnan , M , Rajeswari , K V and Kishore , V VN . 2002 . Assessment of biomethanation potential of selected industrial organic effluents . Resour Conserv Recy , 35 : 147 – 161 .
- Laubscher , A CJ , Wentzel , M C , Le Roux , JMW and Ekama , G A . 2001 . Treatment of grain distillation wastewater in an upflow anaerobic sludge bed (UASB) system . Water SA , 27 ( 4 ) : 433 – 444 .
- Lettinga , G , Field , J , van Lier , J , Zeeman , G and Hulshoff , Pol LW . 1997 . Advanced anaerobic wastewater treatment in the near future . Water Sci Technol , 35 ( 1 ) : 5 – 12 .
- Manisankar , P , Rani , C and Viswanathan , S . 2004 . Effect of halides in the electrochemical treatment of distillery effluent . Chemosphere , 57 ( 8 ) : 961 – 966 .
- Martin , M A , Raposo , F , Borja , R and Martin , A . 2002 . Kinetic study of the anaerobic digestion of vinasse pretreated with ozone, ozone plus ultraviolet, and ozone plus ultraviolet light in the presence of titanium dioxide . Process Biochem , 37 : 699 – 706 .
- Mata-Alvarez , J . 2003 . “ Fundamentals of the anaerobic digestion process ” . In Biomethanization of the organic fraction on Municipal solid wastes , 1 – 20 . London: IWA : Publishing .
- Melamane , X L , Strong , P J and Burgess , J E . 2007 . Treatment of wine distillery wastewater: a review with emphasis on anaerobic membrane reactors . S Afr J Enol Vitic , 28 ( 1 ) : 25 – 36 .
- Mendonca , E , Martins , A and Anselmo , A M . 2004 . Biodegradation of natural phenolic compounds as single and mixed substrates by Fusarium flocciferum . Electron J Biotechnol , 7 ( 1 ) : 30 – 36 .
- Mester , T and Tien , M . 2000 . Oxidation mechanism of ligninolytic enzymes involved in the degradation of environmental pollutants . Int Biodeter Biodegr , 46 : 51 – 59 .
- Metcalf and Eddy , Inc . 1995 . Wastewater engineering treatment, disposal, and use , 3rd ed. , New York : McGraw-Hill .
- Miranda , P M , Benito , G G , Cristobal , N S and Nieto , C H . 1996 . Color elimination from molasses wastewater by Aspergillus niger . Bioresource Technol , 57 : 229 – 235 .
- Miyata , N , Iwahori , K and Fujita , M . 1998 . Manganese-independent and -dependent decolorization of melanoidin by extracellular hydrogen peroxide and peroxidases from Coriolus hirsutus pellets. J Ferment Bioeng , 85 : 550 – 553 .
- Miyata , N , Mori , T , Iwahori , K and Fujita , M . 2000 . Microbial decolorization of melanoidin containing wastewaters: combined use of activated sludge and the fungus Coriolus hirsutus . J Biosci Bioeng , 89 : 145 – 150 .
- Mohammad , P , Azarmidokht , H , Fatollah , M and Mahboubeh , B . 2006 . Application of response surface methodology for optimization of important parameters in decolorizing treated distillery wastewater using Aspergillus fumigatus UB2.60 . Int Biodeter Biodegr , 57 : 195 – 199 .
- Mohana , S , Bhavik , K A and Madamwar , D . 2009 . Distillery spent wash: treatment technologies and potential applications . J Hazard Mater , 163 ( 1 ) : 12 – 25 .
- Moletta , R . 2005 . Winery and distillery wastewater treatment by anaerobic digestion . Water Sci Technol , 51 ( 1 ) : 137 – 144 .
- Naik , N M , Jagadeesh , K S and Alagawadi , A R . 2008 . Microbial decolorization of spent wash: a review . Indian J Microbiol , 48 : 41 – 48 .
- Nataraj , S K , Hosamani , K M and Aminabhavi , T M . 2006 . Distillery wastewater treatment by the membrane-based nano-filtration and reverse osmosis processes . Water Res , 40 ( 12 ) : 2349 – 2356 .
- Nazaroff , W W and Alvarez-Cohan , L . 2001 . Environmental engineering science , 306 – 364 . New York : John Wiley .
- Ohmomo , S , Kaneko , Y , Sirianuntapiboon , S , Somchal , P , Atthasampunna , P and Nakamura , I . 1987 . Decolorization of molasses wastewater by a thermophilic strain, Aspergillus fumigatus G-2-6 . Agric Biol Chem , 51 : 3339 – 3346 .
- Pant , D and Adholeya , A . 2007a . Biological approaches for treatment of distillery wastewater: a review . Bioresource Technol , 98 : 2321 – 2334 .
- Pant , D and Adholeya , A . 2007b . Enhanced production of ligninolytic enzymes and decolorization of molassed distillery wastewater by fungi under solid state fermentation . Biodegradation , 18 : 647 – 659 .
- Patel , A , Pawar , R , Mishra , S and Tewari , A . 2001 . Exploitation of marine cyanobacteria for removal of colour from distillery effluent . Indian J Environ Prot , 21 ( 12 ) : 1118 – 1121 .
- Perez , M , Romero , L I and Sales , D . 1998 . Comparative performance of high rate anaerobic thermophilic technologies treating industrial wastewater . Water Res , 32 : 559 – 564 .
- Pikaev , A K , Ponimarev , A V , Bludenko , A V , Minin , V N and Elizarvar , L M . 2001 . Combined electron beam and coagulation purification of molasses distillery slopes . Radiat Phys Chem , 61 ( 1 ) : 81 – 87 .
- Raghukumar , C and Rivonkar , G . 2001 . Decolorization of molasses spent wash by the white rot fungi Flavadon flavus isolated from marine habitat . Appl Microbiol Biotechnol , 55 : 510 – 514 .
- Raghukumar , C , Mohandass , C , Kamat , S and Shailaja , M S . 2004 . Simultaneous detoxification and decolorization of molasses spent wash by the immobilized white rot fungus Flavodon flavus isolated from a marine habitat . Enzyme Microb Technol , 35 : 197 – 202 .
- Rai , U N , Sinha , R D , Tripathi , P and Chandra , P . 1995 . Wastewater treatability potential of some aquatic macrophytes; removal of heavy metals . Ecol Eng , 5 : 5 – 12 .
- Ramana , S , Biswas , A K and Yadava , R BR . 2002a . Relative efficiency of different distillery effluents on growth nitrogen fixation and yield of groundnut . Bioresource Technol , 81 ( 2 ) : 117 – 121 .
- Ramana , S , Biswas , A K and Yadava , R BR . 2002b . Effect of distillery effluent on some physiological aspects in maize . Bioresource Technol , 84 ( 3 ) : 295 – 297 .
- Ranade , D R , Dighe , A S , Bhirangi , S S , Panhalkar , V S and Yeole , T Y . 1999 . Evaluation of the use of sodium molybdate to inhibit sulphate reduction during anaerobic digestion of distillery waste . Bioresource Technol , 63 ( 3 ) : 287 – 291 .
- Rao , S . 1972 . A low cost waste treatment method for disposal of distillery waste (spent wash) . Water Res , 6 : 1275 – 1282 .
- Ravikumar , R , Saravanan , R , Vasanthi , N S , Swetha , J , Akshaya , N , Rajthilak , M and Kannan , K P . 2007 . Biodegradation and decolorization of biomethanated distillery spent wash . Indian J Sci Technol , 1 ( 2 ) : 1 – 6 .
- Ruiz , C , Torrijos , M , Sousbie , P , Lebrato , Martinez J , Moletta , R and Delgenes , J P . 2002 . Treatment of winery wastewater by an anaerobic sequencing batch reactor . Water Sci Technol , 45 : 219 – 224 .
- Ruiz , G , Jeison , D and Chamy , R . 2006 . Development of denitrifying and methanogenic activities in USB reactors for the treatment of wastewater: effect of COD/N ratio . Process Biochem , 41 : 1338 – 1342 .
- Saha , N K , Balakrishnan , M and Batra , V S . 2005 . Improving industrial water use: case study for an Indian distillery . Resour Conserv Recy , 43 : 163 – 174 .
- Sangave , P C and Pandit , A B . 2006a . Enhancement in biodegradability of distillery wastewater using enzymatic pretreatment . J Environ Manage , 78 : 77 – 85 .
- Sangave , P C and Pandit , A B . 2006b . Ultrasound and enzyme assisted biodegradation of distillery wastewater . J Environ Manage , 80 : 36 – 46 .
- Satyakala , G and Jamil , K . 1997 . Studies on the effect of heavy metal pollution of Pistia statioles (water lettuce) . Indian J Envirom Hlth , 39 ( 1 ) : 1 – 7 .
- Satyawali , Y and Balakrishanan , M . 2007 . Wastewater treatment in molasses-based alcohol distilleries for COD and colour removal: a review . J Environ Manage , 86 : 481 – 497 .
- Schubert , W. and Gunthert , F.W . 2001 . Particle size distribution in effluent filters and in humus tanks . Water Res , 35 ( 16 ) : 3993 – 3997 .
- Shashirekha , S , Uma , L and Subramanian , G . 1997 . Phenol degradation by the marine cyanobacterium Phormidium valderianum BDU 30501 . J Ind Microbiol Biotechnol , 19 : 130 – 133 .
- Shayegan , J , Pazouki , M and Afshari , A . 2004 . Continuous decolorization of anaerobically digested distillery wastewater . Process Biochem , 40 : 1323 – 1329 .
- Sinha , R K , Herat , S and Tandon , P K . 2004 . “ 14 phytoremediation: role of plants in contaminated site management ” . In In: Book of environmental bioremediation technologies , 315 – 330 . Berlin , , Germany : Springer .
- Sirianuntapiboon , S , Sihanonth , P , Somchai , P , Atthasampunna , P and Hayashida , S . 1995 . An adsorption mechanism for melanoidin decolorization by Rhizoctonia sp . Biosci Biotechnol Biochem , 59 : 1185 – 1189 .
- Tatsi , A A , Zouboulis , A L , Matis , K A and Samaras , P . 2003 . Coagulation, flocculation pretreatment of sanitary landfill leachates . Chemosphere , 53 ( 7 ) : 737 – 744 .
- Tewari , P K , Batra , V S and Balakrishnan , M . 2007 . Water management initiatives in sugarcane molasses based distilleries in India . Resour Conserv Recycl , 52 : 351 – 367 .
- Thakkar , A P , Dhamankar , V S and Kapadnis , B P . 2006 . Biocatalytic decolorisation of molasses by Phanerochaete chrysosporium . Bioresource Technol , 97 : 1377 – 1381 .
- Thakur , I S . 2006 . Industrial biotechnology: problems and remedies , 44 – 69 . New Delhi : I. K. International Pvt. Ltd .
- Vahabzadeh , F , Mehranian , M and Saatari , A R . 2004 . ‘Color removal ability of Phanerochaete chrysosporium in relation to lignin peroxidase and manganese peroxidase produced in molasses wastewater . World J Microbiol Biotechnol , 20 : 859 – 864 .
- Valderrama , L T , Del Campo , CM , Rodriguez , C M , de-Bashan , L E and Bashan , Y . 2002 . Treatment of recalcitrant wastewater from ethanol and citric acid production using the microalga Chlorella vulgaris and the macrophyte Lemna minuscula . Water Res , 36 ( 17 ) : 4185 – 4192 .
- Vlissidis , A and Zouboulis , AI . 1993 . Thermophilic anaerobic digestion of alcohol distillery wastewaters . Bioresource Technol , 43 ( 2 ) : 131 – 140 .
- Watanabe , Y , Sugi , R , Tanaka , Y and Hayashida , S . 1982 . Enzymatic decolorization of melanoidin by Coriolus sp. No. 20 . Agric Biol Chem , 46 : 1623 – 1630 .
- Wedzicha , B L and Kaputo , M T . 1992 . Melanoidins from glucose and glycine: composition, characteristics and reactivity towards sulphite ion . Food Chem , 43 ( 5 ) : 359 – 367 .
- Willmott , N , Guthric , J and Nelson , G . 1998 . The biotechnology approach to colour removal from textile effluent . J Soc Dyers Colour , 114 : 38 – 41 .
- Witten , P C , Terence , H , Copper , E and Gorham , M T . 1997 . Environmental encyclopedia , 2nd ed. , Mumbai , , India : Jaico Publishing House .
- Wolmarans , B and De Villiers , G . 2002 . Start up of a UASB effluent treatment plant on distillery wastewater . Water SA , 28 ( 1 ) : 63 – 68 .