Abstract
Rice cultivar is the most influential factor affecting methane emissions from double rice fields. A two-year field experiment was conducted at Huizhou, Guangdong province, South China, to identify from among nine cultivars those cultivars with high-yield potential and lower yield-scaled methane emissions (YSMEs). Methane emissions were measured using the static chamber – gas chromatograph method. Results indicate that the cultivars Qihuazhan (QH), Yexianzhan 8 (YX8) and Yue’erzhan (YE) provide higher rice grain yield (8.69%) with lower YSME (30.27%) compared to the other six cultivars (Yexianzhan 6, Yuejingsimiao, Hefengzhan, Huangsizhan, Huangruanzhan and Huangxiuzhan) (p < 0.05). In particular, QH has the highest yield potential (6777 kg ha−1) and lowest methane emission intensity (0.36 kg kg−1 yield) capacity. Methane emissions from the double rice field was found to be significantly (p < 0.05) and positively correlated with tiller number, culm biomass and soil organic matter, dissolved soil organic carbon and total carbon content, but negatively correlated (p < 0.05) with rice harvest index (HI), and root and panicle biomass, suggesting that organic source strength provides the substrate of methane production while the oxidation potential in the rhizosphere and the methane transport capacity of rice roots and culm dominate the emissions of methane from soil to the atmosphere. Multivariate decision regression tree (DRT) analysis showed a significant class difference between QH, YX8 and YE with the other six cultivars. These three cultivars are suitable for promotion of low carbon agriculture in South China. DRT analysis also successfully illustrated a potential way to identify rice varieties for low YSME by decisive parameters of tiller number (<15), HI (>0.43) and nitrogen assimilation of leaves (<40). These findings suggest that optimization of rice cultivars may represent an effective way to address both food demand and climate change concerns by improving rice yields while simultaneously minimizing the impact of climate change per unit yield.
1. Introduction
Food security and global warming are both high profile global concerns. Researchers and governments have made great efforts to mitigate global climatic change and its impact on food production worldwide. Methane (CH4) is an important greenhouse gas (GHG) with a global warming potential (GWP) per molecule 28 times greater than that of carbon dioxide (CO2) over a 100-year time horizon (IPCC Citation2013). Recent assessment of methane emissions from global rice (Oryza sativa L.) production ranges between 493 and 723 Mt CO2-e yr−1, which accounts for 11% of global methane emissions in 2010 (IPCC Citation2014). Methane emissions from rice fields may increase by up to 58% in the future as a result of warmer temperatures and higher atmospheric CO2 concentration. As a major agricultural producer, rice cultivation is especially important for China, feeding more than 60% of Chinese people as staple food while also contributing 30% of China’s agricultural GHG emissions (The People’s Republic of China, Citation2012). Methane dominates GHG emissions from China’s paddy rice fields, which contribute 11% of the global methane budget (Qin Citation2011). In Asia as a whole, where >90% of global rice is planted and produced (Maclean et al. Citation2002), increasing total rice grain yield while reducing methane emission is a significant challenge (Ma et al. Citation2010). The Chinese government has initiated a national low carbon economy programme. Practical policies and strategies should be made to reduce the global warming impact of Chinese agriculture, as part of efforts to develop a low carbon economy. Better understanding of mechanisms of methane dynamics in paddy soils is needed to improve methane emission predictions and to develop more effective mitigation and management strategies (Bodegom et al. Citation2001).
Methane is a gas emitted from irrigated rice fields affected by the processes of production, oxidation and transportation (Mer & Roger Citation2001; Wang & Li Citation2002). The emission of methane to the atmosphere consists of three pathways: molecular diffusion, ebullition and plant-mediated transport (Wassmann et al. Citation1996; Khosa et al. Citation2010). Among the climatic, environmental and field management factors influencing methane emissions from rice paddy, rice cultivar is one of the most influential factors (Mosier et al. Citation1990; Chen et al. Citation1997; Fu et al. Citation2009; Baruah et al. Citation2010; Su et al. Citation2015). Of the total methane emitted from a rice field during the growing season, 60–90% is transported through the rice plants rather than through molecular diffusion across water-air interfaces or through the release of gas bubbles (Holzapfel-Pschorn et al. 1986; Schütz et al. Citation1989; Wassmann & Aulakh Citation2000). Rice plant have three key functions regulating the methane budget (Setyanto et al. Citation2004; Zheng et al. Citation2013): (1) as a source of methanogenic substrate through root exudates and/or dead root cells (Wang et al. Citation1999; Kerdchoechuen Citation2005); (2) as a channel for methane through well-developed intercellular air spaces (aerenchyma) in leaf blades, leaf sheaths, culms and roots of rice plants, which provide an effective channel for gas exchange (methane transport capacity (MTC)) between the atmosphere and the anaerobic soil (Raskin & Kende Citation1985; Butterbach-Bahl et al. Citation1997; Shao & Li Citation1997; Aulakh et al. Citation2000; Fu et al. Citation2007); and (3) as an active methane oxidizing site in the rice rhizosphere by supporting oxygen counter transport through the aerenchyma system (Win et al. Citation2012; Gutierrez et al. Citation2014).
Research regarding the effect of rice cultivars on methane emissions has produced remarkably different findings (Neue et al. Citation1994; Wang et al. Citation1997; Wang et al. Citation1999). Previous studies suggested that the effect of rice cultivars on methane emissions is mostly related to rice growth performance, i.e. number of plant tillers, plant above and belowground biomass (Mariko et al. Citation1991; Wang et al. Citation1997; Xu et al. Citation1999; Aulakh et al. Citation2000). Although many studies found that there was a significant positive relationship between rice biomass and methane fluxes (Singh et al. Citation1997; Khosa et al. Citation2010), comparison of rice cultivars have produced different results (Yu et al. Citation2013; Qin et al. Citation2014), highlighted the role of nitrogen fertilizer use efficiency by rice plants (Adviento-Borbe et al. Citation2013; Pittelkow et al. Citation2013) and the effect of rice cultivar on root methanotrophic community composition (Lüke et al. Citation2011), suggesting that high-yield rice cultivars tend to possess a low MTC. This points to a potential strategy of breeding and screening rice cultivars to promote environmentally friendly rice production.
One way to integrate climate change concerns with food production objectives is to use a ‘yield-scaled’ or ‘greenhouse gas intensity’ approach when evaluating GHG emissions from agriculture (Mosier et al. Citation2006; Van Groenigen et al. Citation2010). In contrast to assessing GHG emissions on an area-scaled basis, yield-scaled emissions are expressed on an agronomic productivity basis, i.e. as an agricultural efficiency indicator where cumulative CO2 equivalent emissions are divided by crop yield (Herzog et al. Citation2006). Thus, the yield-scaled approach may provide a tool to address dual goals of increasing rice production and environmental sustainability (Pittelkow et al. Citation2014). Most previous studies have relied on pot or lab incubation (Ma et al. Citation2010; Gutierrez et al. Citation2014), and little long-term synthetic research on the yield-scaled GWP of rice production has been conducted (Zheng et al. Citation2013). This has impeded the demonstration of the low carbon technologies by optimizing rice varieties. Therefore, a two-year study was conducted with the objective of (1) identifying suitable rice cultivars with low yield-scaled methane emission (YSME) potential among nine cultivars commonly cultivated in the major rice production region of South China; and (2) excavating the decisive variables for low YSME from rice plant growth parameters and soil characteristics by conducting multivariate decision regression tree (DRT) analysis.
2. Material and methods
2.1. Site description and experimental design
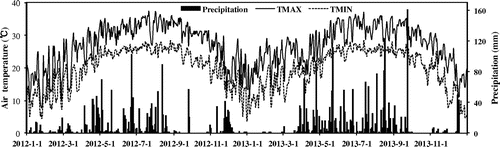
A two-year field experiment was conducted from 2012 to 2013 at the regional test site for rice cultivars in South China, which located in the Huizhou Institute of Agricultural Sciences, Guangdong province, China (114.34°E, 23.06°N). This is in the typical region for double rice production in South China. It has a temperate climate, with average precipitation of 1800 mm, mostly occurring between April and September. Mean annual temperature is 22 °C with 350 d frost-free days. The soil is a typical latosolic red soil with a sandy loam texture (16% clay, 32% silt and 52% sand). Table gives the physicochemical properties of the soil at the experimental site and Figure shows meteorological parameters at the research site in 2012 and 2013 (the daily weather data were obtained from Meteorological Bureau of Huidong county of Huizhou city, Guangdong province).
Table 1. Physicochemical characteristics of soils at the experimental site.
The tested rice cultivars were Hefengzhan (HF), Yuejingsimiao (YJ), Qihuazhan (QH), Huangsizhan (HS), Huangruanzhan (HR), Yexianzhan 6 (YX6), Yexianzhan 8 (YX8), Huangxiuzhan (HX), and Yue’erzhan (YE). Table summarizes the physical properties of each rice cultivar. All nine cultivars are conventional rice varieties suitable for both the early and late rice growing season, and are temperature sensitive Indica varieties. All cultivars were manually transplanted in plots of 5 × 2.5 m at 20 × 15 cm spacing from the seeding bed with three repetitions in randomized block design. The dates of seeding, transplanting and harvest (manual) for early rice in 2012 were 1 March, 3 April and 4 July, respectively; for late rice in 2012, 10 July, 15 August and 15 November; for early rice in 2013, 1 March, 7 April and 6 July; and for late rice in 2013, 10 July, 22 August and 24 November, respectively. Fertilization practices applied in this study (Table ) was designed on the basis of local farmers’ habitual practices identified through an investigation conducted around major agricultural regions of Guangdong province in early 2012. Two weeks before transplanting, the field was ploughed by micro-tractor and then flooded, until 1 d before transplanting. Basal fertilizer was broadcast into the field and ploughed to mix it with the soil. Three other fertilization events were conducted at 5 d, 10 d and 16 d after transplanting. In total, 181, 115 and 153 kg ha−1 of nitrogen (urea), P2O5 (calcium superphosphate) and K2O (KCL) with three or two splits were applied to the field. The irrigation regime also followed local famers’ traditional management practices, i.e. flooding two weeks before transplanting, with intermittent irrigation after transplanting until the tillering stage, with midseason drainage (MSD) for 7–10 days followed by rehydration and intermittent irrigation until two weeks before harvest, at which time water was drained until harvest. Other field practices followed local farmers’ conventional management practices.
Table 2. Properties of rice cultivars.
Table 3. Experimental fertilization design (kg ha−1).
2.2. Methane emission and flux measurements
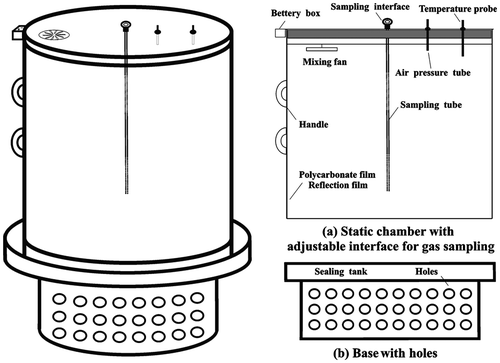
Methane gas fluxes were determined by the closed chamber method described by Qin et al. (Citation2006, Citation2015) for the entire cropping period with measurements conducted once every two days from transplanting until MSD and once every four days after MSD until harvest. The manual transparent closed chamber (1 m high, 0.5 m diameter) with an open bottom was made of polycarbonate (0.5 mm in thickness) with a stainless steel frame (Figure ) (Qin et al. Citation2014). For each plot, one base frame was placed (0.15 m deep into the soil surface) one day before rice transplanting and left in position in the field for observation during the whole season. Holes were drilled on the base under the water tank to ensure water exchange inside and outside the base. Chambers were placed and removed on every sampling day. Water was injected into the water tank to seal the whole system during closure. Four hills of rice plants were included in the base frame. Wooden boardwalks were installed in the rice field prior to all flooding to prevent soil disturbance while sampling. The gas samples were collected during 9:00–11:00 am at 0, 8, 16, 24 and 32 min after the top chamber was covered. This sampling time was based on the diurnal variation pattern of methane emissions from rice fields (Hou et al. Citation2000). Headspace gas samples were obtained with air-tight 30 ml propylene syringes and were immediately pressurized into pre-evacuated 12 ml glass Exetainer® vials (Labco Ltd., Buckinghamshire, UK). A layer of silicone was applied to septa during vial preparation to create a double barrier and prevent contamination with ambient air. The air temperature inside the chamber was auto logged during gas collection using a Hobo data recorder (HOBO Pro-U23003, Onset Inc., USA, 2007), which was equipped into the field before placement of the base frame and remained in position until harvest. The gas sample in the vial was stored for analysis in the laboratory immediately using a gas chromatograph (Agilent 7980A, Agilent Inc., USA, 2007) fitted with a flame ionization detector. The column was packed with a 80–100 mesh porapack Q. Column. Detector and injector temperatures were maintained at 70, 200 and 150 °C, respectively. A purified nitrogen (>99.999%) was used as the carrier gas, hydrogen (>99.999%) as the fuel gas and zero air as the supporting gas with flow rates of 30, 50 and 350 ml min−1, respectively. The standard gas of methane was provided by the China National Institute of Metrology. Concentration of methane was determined from the slope of the mixing ratio change in five samples collected after the chamber was closed. Sample sets were rejected unless they yielded a non-linear regression value of R2 greater than 0.87. Equation (1) was used to calculate the methane fluxes. Daily average flux and standard error of methane were calculated from triplicate plots.
(1)
Where: Fc is methane flux (mg m−2 h−1); dC denotes the difference between the initial and final methane concentrations (10−6 mol mol−1) during an enclosure duration (dt, in h); V is the headspace volume of the chamber (l m³); A represents the bottom area of the chamber (m2); ρ denotes the density of methane at 273 K and 1013 hPa, which is 0.717 g L−1; T is the mean air temperature in chamber during enclosure (°C); P is the air pressure during incubation (hPa), which is taken to be approximately 1013 hPa because the air pressure tube was installed in the chamber to keep the balance of the pressure inside and outside the chamber during sampling (Figure ); and k is a coefficient for dimensional conversion.
CO2 equivalent (CO2 equivalents, CDE, kg CO2-e) of methane emissions was calculated based on the comparative GWP of methane to CO2. Over a 100-year horizon, the GWP of methane is 28 times that of CO2 (IPCC Citation2013). YSME was then calculated by Equation (2).
(2)
Where: YSME is the YSME (kg kg−1 season−1); CDE represents the gross methane emission per season (kg CO2-e season−1); and YIELD denotes the rice grain yield of each cultivar (kg season−1).
Daily average methane flux was calculated using Equation (3) (Fu et al. Citation2012):(3)
Where: F is the daily average methane flux (mg m−2 h−1); subscripts 1 … n represent the number of the discrete daily methane flux; and day of the year (DOY) denotes the calendar day of every year (1–365).
2.3. Rice plant growth parameters, soil properties and environmental factors
During the rice growth season, rice plants were sampled once in every main growth stage (germination to emergence, seedling, tillering, stem elongation, panicle initiation to booting, heading, flowering, milk grain, dough grain and mature grain) in each plot with three repetitions. Plant samples was transferred immediately to a laboratory for height measurement and then put into a drying oven at 105 °C to remove greenness and then heated at 75 °C for 24 h to a constant weight for measurement of root biomass (BIOROOT), culm biomass (BIOCULM) and panicle biomass (BIOPANICLE). The tiller number (TILLER) of each cultivar was counted at the end of the tillering stage. The rice grain yield was measured precisely after harvest using all the plants within the plot except in the marginal area. The harvest index (HI) was calculated as the quotient of the above ground biomass (BIOABOVE) (BIOCULM + BIOPANICLE) divided by BIOPANICLE.
Soil samples were taken from the AP horizon (0–15 depth) once every two weeks from transplanting to harvest with three repetitions using a 5 cm diameter sampling auger, with sample replicates mixed thoroughly. Soil organic matter (SOM), dissolved organic carbon (DOC), total carbon (TC) and the ratio of total carbon and nitrogen (CN) were then analyzed in laboratory conditions. Soil bulk density (BD) was determined using a cutting-ring after harvest in each season.
Chlorophyll content of each rice cultivar (SPAD-502Plus, Konica Minolta Inc., Japan) and soil redox potential (EH) (Ecoscan-pH6, Singapore) were recorded every time the methane gas was sampled with three repetitions. Daily maximum and minimum temperature and daily precipitation of the experimental station from 2012 to 2013 were obtained from the Meteorology Bureau of Boluo county, Huizhou city.
2.4. Data processing and statistical analysis
All the data observed was processed firstly using Microsoft Excel (Version 2010, Microsoft Cooperation, USA) for subsequent analysis. Seasonal mean difference comparison of methane fluxes and rice plant growth parameters as well soil properties among rice cultivars were established by Tukey-Kramer HSD (Honest Significant Difference) (SAS JMP V10.0, SAS Inc., USA) and differences reported at p < 0.05. Regression analysis between methane fluxes and plant as well as soil variables (seasonal average data of each rice cultivar) were carried out using R (R Development Core Team Citation2014, Version 3.1) and correlation coefficient values are reported. Correlation matrix analysis and decision tree analysis was conducted using R package of ‘corrplot’ and ‘rpart’. SigmaPlot (Version 11, Systat Software Inc., USA) and R were used for graph plotting.
3. Results
3.1. Area-scaled methane emission
The overall patterns of methane emission rates were similar among the cultivars in the rice growing seasons of 2012 and 2013 (Figure ). The methane emission rates were lower at the initial vegetative stage, rapidly increased with developing anaerobic soil conditions and plant growth, and peaked at the reproductive stage of the plants. However, methane emission rates and total methane fluxes differed significantly among cultivars (HSD, p < 0.05) during the four growing seasons, except for late rice in 2013. In the early rice growing season of 2012, the highest peak of methane emission rates occurred in the cultivar YX8 (30 April, 30 mg m−2 h−1) and the highest daily average methane flux also occurred in YX8, followed by HX and YX6 (Table ). The cultivar YJ had the lowest daily average methane flux, followed by HS and QH. In the late rice growing season of 2012, different methane emission rates occurred among rice cultivars with the highest peak of methane emission rates and daily average methane flux both emitted by HX (11 September), i.e. 82 mg m−2 h−1 (being the highest daily average flux during the two-year study) and 16 mg m−2 h−1, respectively. The second and third highest daily average methane flux occurred in YJ and HR, respectively. QH had the lowest daily average methane flux, followed by YX8 and HS. During the rice growing season of 2013, a different picture emerged with regard to the seasonal pattern of methane emissions. The duration of the peak was shorter than that in 2012, but the highest peak value of methane emission rates (YJ, 28 April, 63 mg m−2 h−1) was greater than that in early rice growing season of 2012, while the daily average methane flux was dominated by HS, followed by HF and YX6. The lowest daily average methane flux was observed in QH (2.45 mg m−2 h−1) (being the lowest fluxes during the two-year study), followed by YX8 and YE. The seasonal pattern of methane emission rates for the late rice growing season of 2013 was similar with that of early 2013, with the highest peak value of methane emission rates occurring in HS (28 August, 103 mg m−2 h−1), which was also the highest peak value of methane emission rates during the two-year study. On average, HX emitted the highest methane fluxes, followed by HS and HR, and YE transported the lowest methane fluxes, followed by QH and YX8.
Figure 3. Seasonal variation in methane fluxes of different rice cultivars in 2012 and 2013.
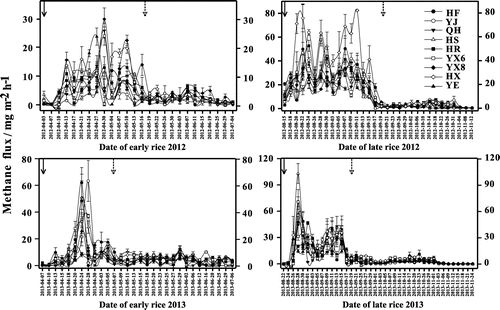
Table 4. Daily average methane flux of different rice cultivars in the rice growing seasons of 2012 and 2013 (mg m−2 h−1).
Generally speaking, compared to the other six cultivars, QH, YE and YX8 had a lower methane emissions from double rice fields, with average methane emission rates during the two-year study of 4.82, 6.00 and 6.01 mg m−2 h−1, respectively. The cultivars HX, HR and HF had the highest methane fluxes, with values of 9.34, 8.01 and 7.25 mg m−2 h−1, respectively. Calculated as CO2 equivalent emissions of methane, the rank order of cultivars was: HX > HR > HF > YX6 > YJ > HS > YE > YX8 > QH. QH had the lowest methane emissions potential with a value of 2440 kg ha−1 CO2-e season−1, followed by YX8 (3085 kg ha−1 CO2-e season−1) and YE (3089 kg ha−1 CO2-e season−1), while the cultivar HX (4788 kg ha−1 CO2-e season−1), HR (4079 kg ha−1 CO2-e season−1) and HF (3698 kg ha−1 CO2-e season−1) had the largest methane emissions potential. The average methane emissions amount of the three low-emission-cultivar (QH, YX8 and YE) was 27% less than the average of the other six cultivars.
3.2. Rice grain yield and YSME
There was seasonal variation in rice grain yield during the two-year study (Figure ). The average grain yield during the four rice growing seasons followed by the sequence: QH > YX8 > YE > HX > YX6 > YJ > HF > HS > HR. Among the nine rice cultivars, QH had the highest average yield with a value of 6777 kg ha−1 season−1, followed by YX8 (6606 kg ha−1 season−1) and YE (6374 kg ha−1 season−1). The lowest rice grain yield was observed in HR (5696 kg ha−1 season−1), followed by HS (6029 kg ha−1 season−1) and HF (6084 kg ha−1 season−1). The average grain yield of the three highest yielding rice cultivars (QH, YX8 and YE) was 8.69% greater than the average of the other six cultivars.
Figure 4. Seasonal accumulated methane emission (kg ha−1) (a); CO2 equivalent emission of methane (b); rice grain yield (c); and YSME (d) of different rice varieties in 2012 and 2013.
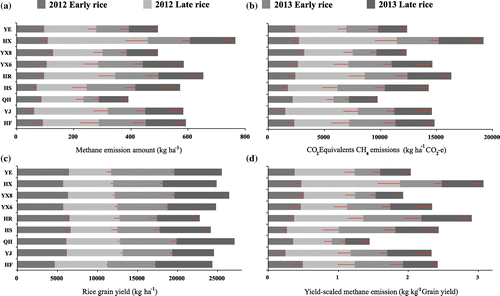
YSME of nine rice cultivars followed the sequence: HX > HR > HS > HF > YJ > YX6 > YE > YX8 > QH (Figure ). YSME of QH (0.36 kg kg−1 yield season−1, average of four rice growing seasons) was the lowest among nine cultivars, followed by YX8 (0.48 kg kg−1 yield season−1) and YE (0.51 kg kg−1 yield season−1). The cultivar HX had the highest YSME (0.77 kg kg−1 yield season−1), followed by HR (0.73 kg kg−1 yield season−1) and HS (0.61 kg kg−1 yield season−1). Thus, the cultivars QH, YX8 and YE had the lower potential YSME, with an average YSME 30% less than the YSME of the other six rice cultivars during the two-year study period. In particular, QH had the lowest potential methane emissions but the highest rice grain yield among the nine rice cultivars.
3.3. Relationship between methane flux and rice cultivar growth parameters and soil characteristics
There was a pronounced relationship between methane fluxes (CH4), YSME and rice cultivar growth parameters as well soil properties during the rice growing seasons of 2012 and 2013 (Figure ). Firstly, both rice grain yield (YIELD) and HI were significantly and negatively correlated with methane fluxes as well YSME (p < 0.05). This suggests a potential strategy of breeding rice cultivars with higher yield but lower methane emission capacity. Furthermore, CH4 was significantly and positively correlated with rice straw biomass (BIOCULM) (p < 0.05) and significantly and negatively correlated with rice BIOPANICLE and plant height (HEIGHT) (p < 0.05), but there was a non-significantly negative relationship between CH4 and rice BIOABOVE, BIOROOT and total biomass (BIOMASS). In contrast, CH4 was significantly and positively correlated with the tiller number (TILLER) and chlorophyll content (SPAD) (p < 0.05). This implies that more tillers and nitrogen nutrition content offer a stronger MTC for rice plants to emit more methane from the rhizosphere to the atmosphere. Moreover, CH4 was extremely significantly and positively correlated with SOM, TC content, DOC and CN ratio (p < 0.01). In addition, CH4 was significantly and positively correlated with soil BD (p < 0.05). This indicates that small values of soil BD permitted more air to penetrate into the soil pores, which enhanced methane oxidation and decreased the portion of methane produced. Finally, positive and negative relationships, respectively, were found between CH4 and water filled pore space and between CH4 and EH. These findings highlight that the anaerobic environment during flooding of the double rice field favored methane production.
Figure 5. Correlation matrix of the relationships among methane fluxes, YSMEs and rice cultivar growth characteristics as well the soil properties. (See text for meaning of abbreviations).
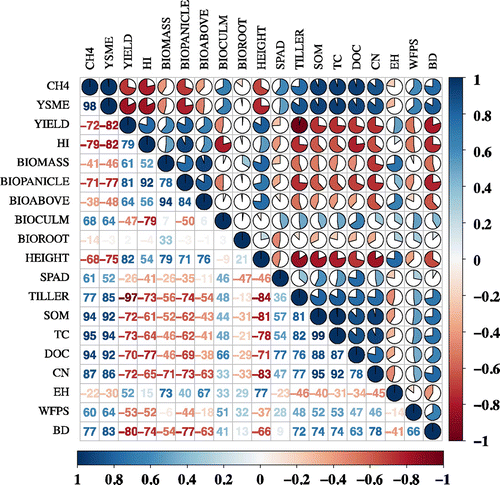
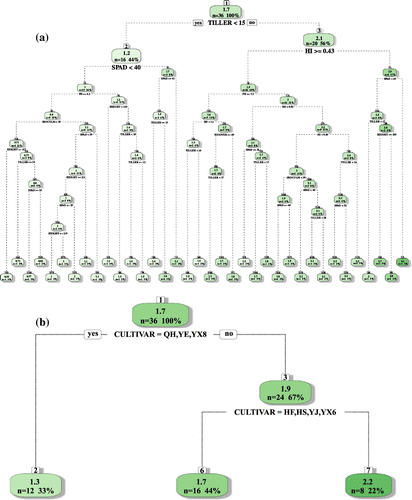
Overall, YSME is significantly correlated to TILLER, HI and other plant growth parameters, and there also is significant difference of YSME among nine rice cultivars. However, this is just unitary linear analysis and should it not be good enough for decision-making by policy-maker. A multivariate DRT analysis of YSME classified by rice plant growth parameters, soil properties and rice cultivar provided a different scenario (Figure ). Among all the rice plant growth parameters and soil properties, TILLER is the first class decision factor for YSME of double rice field of South China. The average value of YSME during the four rice growing seasons is 1.7 kg kg−1 yield season−1, when the TILLER is less than 15, the average value of YSME decreased to 1.2 kg kg−1 yield season−1, while if TILLER is greater than 15, it increased to 2.1 kg kg−1 yield season−1 (Figure (a)). Furthermore, HI and SPAD is the second class decision variable for YSME. When HI ≥ 0.43, average value of YSME is 1.9 kg kg−1 yield season−1, but if HI is less than 0.43, it is 2.9 kg kg−1 yield season−1. For SPAD, which symbolize the assimilation of nitrogen of the rice leaves, when it split into two categories by 40, average value of YSME is 1 and 1.7 kg kg−1 yield season−1, respectively. According to rice cultivar classification, among the nine variants, QH, YX8, and YE is the first class decision variable for YSME, the average value of the three cultivars is 1.3 kg kg−1 yield season−1, whereas the other six cultivars is 1.9 kg kg−1 yield season−1. Additionally, HF and HX have the greatest average value of YSME (2.2 kg kg−1 yield season−1) (Figure (b)).
Figure 6. DRT analysis of YSME classified by (a) rice plant growth parameters and soil properties and (b) rice cultivar.
4. Discussion
4.1. Differences in methane emission among rice cultivars
There is evidence that more than 90% of methane fluxes from paddy soils is mediated by the rice plant via a passive transport mechanism (Jia & Cai Citation2003; Hussain et al. Citation2014). Many researchers have reported the effects of cultivar on methane emission rates. In our study, different methane emissions among nine rice cultivars during four rice growing seasons were observed, with the cultivars QH, YX8 and YE emitting significantly less methane than the other six rice varieties. We also found that methane fluxes were significantly and positively correlated with rice tiller number (Mariko et al. Citation1991; Wang et al. Citation1997) and plant BIOCULM. In contrast, a negative relationship was identified between methane fluxes and HEIGHT, BIOROOTs and BIOPANICLEs, indicating that the number of outlets/channels rather than plant size/biomass itself determines the transport of methane (Aulakh et al. Citation2000, Citation2002) and highlighting the strong MTC of plant culm. The differences in methane diffusion capacities were significantly related to tiller number, because on the one hand, tiller number and methane diffusion capacity become major controlling factors of methane emission rates if the methane source strengths are the same (Wang et al. Citation1997); on the other hand, rice cultivars also differ in their ability to transport oxygen to the rhizosphere (Armstrong Citation1969; Kludze et al. Citation1993; Jia & Cai Citation2003), which effect the oxidation ability of methane. In many previous reports (Sass et al. Citation1991; Neue & Roger Citation1993; Sinha Citation1995; Satpathy et al. Citation1998; Setyanto et al. Citation2004), methane fluxes had a strong linear relationship with the apparent growth characteristics of the rice plants. However, one recent study identified no significant relationship between methane emissions and any plant growth parameters, even though rice grain yield and other plant growth characteristics were significantly different among the different cultivars (Gutierrez et al. Citation2013). This may be because some cultivars appear to allocate more of the products of photosynthesis to root exudation than others (Su et al. Citation2015).
Although not significant, a negative relationship between methane emissions and BIOROOT suggested a strong oxidation ability of the root rhizosphere on produced methane in our experiment. This was consistent with the report of Neue and Roger (Citation1993), who found large differences in methane emissions due to different root oxidizing power among rice cultivars. In fact, root exudates constitute an organic substrate for microbial organisms that could be utilized for methane production and oxidation by methanogens and methanotrophs, respectively (Win et al. Citation2012). Frenzel et al. (Citation1992) reported that 80–90% of the methane produced in the rhizosphere was re-oxidized.
A pronounced positive relationship between methane fluxes and soil organic substrates such as content of SOM, DOC and TC, illustrates a powerful substrate strength of methane production in the methanogenesis of rhizosphere. Wang et al. (Citation1997) reported a difference in methane emission rates among rice cultivars and attributed it mainly to cultivar-controlled differences in methane source strengths. Cultivar influences methane source strength by providing the soil with root exudates and leaf littering. Deposits of organic root exudates, sloughed-off cells, and decaying root debris serve as the major carbon sources for methane production in rice fields (Lu et al. Citation2000a, Citation2000b). We did not study the methane source strengths in this experiment, but our findings still suggest the importance of methanogenesis for methane production. In many conditions, differences in methane emission rates were not proportional to differences in methane source, indicating differences in transport capacity among rice cultivars. Previous studies (Borah & Baruah Citation2015) confirmed this conclusion, finding that differences in methane emissions among different cultivars could be attributed to variation in MTC and substrate producing potentials of each cultivar. As pointed out by Epule et al. (Citation2011), the main vectors behind methane emissions from paddy rice fields are methanogenic bacteria, methane fluxes were correlated positively and negatively with methanogens and methanotrophs abundance, respectively, but not correlated with any apparent plant growth parameters. This also suggests that methane emissions may be directly affected by the substrate-producing potential and gas transport capacity of each cultivar rather than by external plant growth variables (Gutierrez et al. Citation2013). In our study, negative relationships between SOM, DOC, TC and BIOROOT (Figure ) were discovered. This emphasizes that the oxidation ability of roots actually controls the methane emission procedure instead of the methane source. As discussed above, the cultivar-controlled differences in methane source strength, MTC, and root oxidation power suggest opportunities for screening and breeding rice cultivars that give a low methane emission rates (Wang et al. Citation1999).
TILLER, HI and SPAD became the most decisive factors for YSME in a double rice field. Multivariate DRT analysis provided a measurable way to classify the influential variable of YSME. These illustrate the potential way to identify rice cultivars with low YSMEs by their plant growth parameters.
4.2. Yield-scaled methane emission
To compare grain yield and GHG emissions in field studies, many researchers have used yield-scaled GHG as an indicator of the global warming impact of rice production (Mosier et al. Citation2006; Van Groenigen et al. Citation2010; Kim et al. Citation2012, Citation2013). As suggested by Grassini and Cassman (Citation2012), the yield-scaled metric is increasingly used to provide a measure of agronomic efficiency that begins to address both climate change and future food supply concerns. In the current study, we aimed to identify rice cultivars with a high yield potential but lower methane emissions from among nine rice cultivars. Our results indicate that QH, YX8 and YE had the higher yield potential but lower methane emission ability, and thus a lower YSME, our multivariate DRT analysis supported this conclusion, providing a quantitative algorithm to classify the rice cultivars by their YSME, and additionally, offering a decision-making method for policy-maker.
Significant positive relationships between methane fluxes and rice HI and BIOPANICLE was found in our two-year field experiment. This finding agrees with the study of Wang et al. (Citation1997), who found that a newly developed high-yielding cultivar emitted the lowest amount of methane after comparison among three rice cultivars. This suggests that compared to normal rice varieties, the oxidation power of high-yielding cultivars remained high throughout the whole season, and that the high-yielding cultivar has a relatively lower MTC, which is decided by their agronomic characteristics, such as tiller numbers and leaf area (Wang et al. Citation1997). A higher rate of partitioning of photosynthates to the developing panicles and grain accompanied by a higher rate of photosynthesis at the grain filling stage might be the reason for the higher grain yield in varieties with low methane emissions (Das & Baruah Citation2008; Baruah et al. Citation2010).
Our results emphasize that a full accounting of GHG emissions is needed, particularly for rice growing areas such as South China where climate change policies are beginning to support the development of low carbon agriculture by supporting and incentivizing adoption of GHG mitigation practices. Varietal screening is necessary to find cultivars with high yield potential and low methane emissions, and thus a lower YSME potential. The ideal rice cultivars for reducing methane emissions should have a high HI, fewer ineffective tillers, and high root oxidizing power (Wang et al. Citation1997). Consequently, a mitigation measure to reduce methane emissions to the atmosphere would be to cultivate rice varieties that do not have well-developed aerenchymal systems (Wassmann et al. Citation1993; Zou et al. Citation2005). Wang et al. (Citation1999) also pointed out that using rice cultivars as a mitigation option is an easily adopted option because famers may use it without any expensive input such as new types of fertilizer. Similarly, it has been shown using large on-site datasets that high grain yields can be achieved with minimal yield-scaled GHG emissions through improved management of rice cultivars in intensive cereal production systems (Grassini & Cassman Citation2012).
5. Conclusions
A two-year field experiment was conducted in the major rice plantation region of South China, to discover the rice cultivar with low methane emissions and high yield potential. Our research indicates that there were significant differences between methane emission rates among nine rice cultivars. The cultivars of QH, Yexiazhan 8 and YE, especially QH had the highest rice grain yield and lowest YSME. Remarkable relationship between methane emissions with rice plant growth parameters and soil properties also found. Multivariate DRT analysis successfully illustrated a potential way to identify rice varieties for low YSME by decisive parameters of tiller number (<15), HI (>0.43) and nitrogen assimilation of leaves (SPAD < 40). These results suggest that optimal rice cultivar selection may represent an effective way to address both food demand and climate change concerns by improving rice yields while simultaneously minimizing the impact on climate change per unit yield. Rice cultivar optimization represents a key strategy for increasing the economic and environmental sustainability of rice production systems in line with the development of national low carbon development strategies.
Funding
This work was supported by the Non-profit Research Foundation for Agriculture [201103039], the National Basic Research Program of China [2012CB417106] and the National Natural Science Fund of China [41475129].
Acknowledgements
The authors gratefully thank the journal editors and reviewers for their hard work and constructive advices.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adviento-Borbe MA, Pittelkow CM, Anders M, van Kessel C, Hill JE, McClung AM, Six J, Linquist BA. 2013. Optimal fertilizer nitrogen rates and yield-scaled global warming potential in drill seeded rice. J Environ Qual. 42:1623–1634.10.2134/jeq2013.05.0167
- Armstrong W. 1969. Rhizosphere oxidation in rice: an analysis of intervarietal differences in oxygen flux from the roots. Physiol Plant. 22:296–303.10.1111/ppl.1969.22.issue-2
- Aulakh MS, Bodenbender J, Wassmann R, Rennenberg H. 2000. Methane transport capacity of rice plants, II. Variations among different rice cultivars and relationship with morphological characteristics. Nutr Cycling Agroecosyst. 58:367–375.
- Aulakh MS, Wassmann R, Rennenberg H. 2002. Methane transport capacity of twenty-two rice cultivars from five major Asian rice-growing countries. Agric Ecosyst Environ. 91:59–71.10.1016/S0167-8809(01)00260-2
- Baruah KK, Gogoi B, Gogoi P. 2010. Plant physiological and soil characteristics associated with methane and nitrous oxide emission from rice paddy. Physiol Mol Biol Plants. 16:79–91.10.1007/s12298-010-0010-1
- Bodegom PV, Stams F, Mollema L, Boeke S, Leffelaar P. 2001. Methane oxidation and the competition for oxygen in the rice rhizosphere. Appl Environ Microbiol. 67:3568–3597.
- Borah L, Baruah KK. 2015. Physiological and anatomical variations in three rice (Oryza sativa L.) genotypes for transport and emission of methane. Clim Change Environ Sustainability. 3:58–70.
- Butterbach-Bahl K, Papen H, Rennenberg H. 1997. Impact of gas transport through rice cultivars on methane emission from rice paddy fields. Plant Cell Environ. 20:1175–1183.10.1046/j.1365-3040.1997.d01-142.x
- Chen GX, Huang GH, Huang B, Yu KW, Wu J, Xu H. 1997. Nitrous oxide and methane emissions from soil plant systems. Nutr Cycling Agroecosyst. 49:11–15.
- Das K, Baruah KK. 2008. A comparison of growth and photosynthetic characteristics of two improved rice cultivars on methane emission from rainfed agroecosystem of northeast India. Agric Ecosyst Environ. 124:105–113.10.1016/j.agee.2007.09.007
- Epule ET, Peng CH, Mafany NM. 2011. Methane emissions from paddy rice fields: strategies towards achieving a win-win sustainability scenario between rice production and methane emission reduction. J Sustainable Dev. 4:188–196.
- Frenzel P, Rothfuss F, Conrad R. 1992. Oxygen profiles and methane turnover in a flooded rice microcosm. Biol Fert Soils. 14:84–89.10.1007/BF00336255
- Fu ZQ, Huang H, He BL, Xie W, Liao XL. 2007. Correlation between rice plant aerenchyma system and methane emission from paddy field. Acta Agron Sincia. 33:1458–1467.
- Fu ZQ, Huang H, Xie W, He BL. 2009. Effects of high-yielding rice cultivar and cultivation pattern on methane emission from paddy field. Chin J Appl Ecol. 20:3003–3008.
- Fu XQ, Li Y, Su WJ, Shen JL, Xiao RL, Tong CL, Wu JS. 2012. Annual dynamics of N2O emissions from a tea field in southern subtropical China. Plant Soil Environ. 58:373–378.
- Grassini P, Cassman KG. 2012. High-yield maize with large net energy yield and small global warming intensity. Proc Nat Acad Sci. 109:1074–1079.10.1073/pnas.1116364109
- Gutierrez J, Atulba SL, Kim G, Kim PJ. 2014. Importance of rice root oxidation potential as a regulator of CH4 production under waterlogged conditions. Biol Fert Soils. 50:861–868.10.1007/s00374-014-0904-0
- Gutierrez J, Kim SY, Kim PJ. 2013. Effect of rice cultivar on CH4 emissions and productivity in Korean paddy soil. Field Crops Res. 146:16–24.10.1016/j.fcr.2013.03.003
- Herzog T, Baumert KA, Pershing J. 2006. Target-intensity: an analysis of greenhouse gas intensity targets. Washington, DC: World Resources Institute.
- Holzapfel-Pschorn A, Conrad R, Seiler W. 1986. Effects of vegetation on the emission of methane from submerged paddy soil. Plant & Soil. 92(2):223–233.
- Hou AX, Chen GX, Wang ZP, Van Cleemput O, Patrick WH. 2000. Methane and nitrous oxide emissions from a rice field in relation to soil redox and microbiological processes. Soil Sci Soc Am J. 64:2180–2186.10.2136/sssaj2000.6462180x
- Hussain S, Peng S, Fahad S, Khaliq A, Huang J, Cui K, Nie LX. 2014. Rice management interventions to mitigate greenhouse gas emissions: a review. Environ Sci Pollut Res. 22:1–19.
- IPCC. 2013. Summary for Policymakers. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate Change 2013: The physical science basis. Cambridge: Cambridge University Press; 1553 pp.
- IPCC. 2014. Climate Change 2014: mitigation of climate change. Working Group III contribution to the Intergovernmental Panel on Climate Change 5th Assessment Report changes to the underlying scientific/technical assessment. In: Edenhofer O, Pichs-Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K, Adler A, Baum I, Brunner S, Eickemeier P, Kriemann J, Savolainen S, Schlomer C, von Stechow T, Zwickel, Minx JC, editors. Cambridge: Cambridge University Press; 2108 pp.
- Jia ZJ, Cai ZC. 2003. Effects of rice plants on methane emission from paddy fields. Chin J Appl Ecol. 14:2049–2053.
- Kerdchoechuen O. 2005. Methane emission in four rice varieties as related to sugars and organic acids of roots and root exudates and biomass yield. Agric Ecosyst Environ. 108:155–163.10.1016/j.agee.2005.01.004
- Khosa MK, Sidhu BS, Benbi DK. 2010. Effect of organic materials and rice cultivars on methane emission from rice field. J Environ Biol. 31:281–285.
- Kim SY, Gutierrez J, Kim PJ. 2012. Considering winter cover crop selection as green manure to control methane emission during rice cultivation in paddy soil. Agric Ecosyst Environ. 161:130–136.10.1016/j.agee.2012.07.026
- Kim SY, Lee CH, Gutierrez J, Kim PJ. 2013. Contribution of winter cover crop amendments on global warming potential in rice paddy soil during cultivation. Plant Soil. 366:273–286.10.1007/s11104-012-1403-4
- Kludze HK, DeLaune RD, Patrick WH. 1993. Aerenchyma formation and methane and oxygen exchange in rice. Soil Sci Soc Am J. 57:386–391.10.2136/sssaj1993.03615995005700020017x
- Lu YH, Wassmann R, Neue HU, Huang CY. 2000a. Dissolved organic carbon and methane emissions from a rice paddy fertilized with ammonium and nitrate. J Environ Qual. 29:1733–1740.10.2134/jeq2000.00472425002900060002x
- Lu YH, Wassmann R, Neue HU, Huang CY. 2000b. Dynamics of dissolved organic carbon and methane emissions in a flooded rice soil. Soil Sci Soc Am J. 64:2011–2017.10.2136/sssaj2000.6462011x
- Lüke C, Bodrossy L, Lupotto E, Frenzel P. 2011. Methanotrophic bacteria associated to rice roots: the cultivar effect assessed by T-RFLP and microarray analysis. Environ Microbiol Rep. 3:518–525.10.1111/emi4.2011.3.issue-5
- Ma KE, Qui Q, Lu YH. 2010. Microbial mechanism for rice variety control on methane emission from rice field soil. Global Change Biol. 16:3085–3095.
- Maclean JL, Dawe D, Hardy B, Hettel GP. 2002. Rice almanac: source book for the most important economic activity on earth. 3rd ed. Los Baños: International Rice Research Institute.
- Mariko S, Harazono Y, Owa N, Nouchi I. 1991. Methane in flooded soil water and the emission through rice plants to the atmosphere. Environ Exp Bot. 31:343–350.10.1016/0098-8472(91)90059-W
- Mer JL, Roger P. 2001. Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol. 37:25–50.10.1016/S1164-5563(01)01067-6
- Mosier AR, Mohanty SK, Bhadrachalam A, Chakravorti SP. 1990. Evolution of dinitrogen and nitrous oxide from the soil to the atmosphere through rice plants. Biol Fert Soils. 9:61–67.10.1007/BF00335863
- Mosier AR, Halvorson AD, Reule CA, Liu XJ. 2006. Net global warming potential and greenhouse gas intensity in irrigated cropping systems in northeastern Colorado. J Environ Qual. 35:1584–1598.10.2134/jeq2005.0232
- Neue HU, Roger PA. 1993. Rice agriculture: factors controlling emissions. In: Khalil MAK. editor. Atmospheric methane: sources, sinks, and role in global change. Berlin: Springer; p. 254–298.
- Neue HU, Lantin RS, Wassmann R, Aduna JB, Alberto MCR, Andales MJF. 1994. Methane emission from rice soils of the Philippines. In: Minami K, Mosier A, Sass R, editors. CH4 and N2O global emission and controls from ricefields and other agricultural and industrial sources, NIAES series 2. Tokio: Yokendo; p. 55–63.
- People’s Republic of China. 2012. Second National Communication on Climate Change of The People’s Republic of China. Submitted to UNFCCC on 8th November 2012. electronic version at http://unfccc.int/essential_background/library/items/3599.php?rec=j&priref=7666#beg
- Pittelkow CM, Adviento-Borbe MA, Hill JE, Six J, van Kessel C, Linquist BA. 2013. Yield-scaled global warming potential of annual nitrous oxide and methane emissions from continuously flooded rice in response to nitrogen input. Agric Ecosyst Environ. 177:10–20.10.1016/j.agee.2013.05.011
- Pittelkow CM, Adviento-Borbe MA, van Kessel C, Hill JE, Linquist BA. 2014. Optimizing rice yields while minimizing yield-scaled global warming potential. Global Change Biol. 20:1382–1393.10.1111/gcb.2014.20.issue-5
- Qin XB. 2011. Mitigation of greenhouse gas intensity from typical double rice field of Central China [PhD dissertation]. Graduate School of Chinese Academy of Agricultural Sciences. Beijing. p. 128.
- Qin XB, Li YE, Liu KY, Wan YF. 2006. Methane and nitrous oxide emission from paddy field under different fertilization treatments. Trans Chin Soc Agric Eng. 22:143–148 (in Chinese with English abstract).
- Qin XB, Li YE, Wan YF, Liao YL, Fan MR, Gao QZ, Liu S, Ma X. 2014. Effect of tillage and rice residue return on CH4 and N2O emission from double rice field. Trans Chin Soc Agric Eng. 30:216–224 (in Chinese with English abstract).
- Qin XB, Li YE, Wang H, Li JL, Wan YF, Li Y, Liao YL, Fan MR, Zhu JM, Gao QZ, Liu S. 2015. Impact of biochar amendment on carbon emissions intensity in double rice field in South China. Trans Chin Soc Agric Eng. 31:226–234 (in Chinese with English abstract).
- R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. ISBN 3-900051-07-0. Available from: http://www.R-project.org/.
- Raskin I, Kende H. 1985. Mechanism of aeration in rice. Science. 228:322–329.
- Sass RL, Fisher FM, Harcombe PA, Turner FT. 1991. Mitigation of methane emissions from rice fields: possible adverse effects of incorporated rice straw. Global Biogeochem Cycles. 5:275–287.10.1029/91GB01304
- Satpathy SN, Mishra S, Adhya TK, Ramakrishnan B, Rao VR, Sethunathan N. 1998. Cultivar variation in methane efflux from tropical rice. Plant Soil. 202:223–229.10.1023/A:1004385513956
- Schütz H, Holzapfel-Pschorn A, Conrad R, Rennenberg H, Seller W. 1989. A three-year continuous record on the influence of daytime, season, and fertilize treatment on methane emission: rates from an Italian rice paddy field. J Geophys Res. 94:405–416.
- Setyanto P, Rosenani AB, Boer R, Fauziah CI, Khanif MJ. 2004. The effect of rice cultivars on methane emission from irrigated rice field. Indonesian J Agric Sci. 5:20–31.
- Shao KS, Li Z. 1997. Effect of rice cultivars and fertilizer management on methane emission in a rice paddy in Beijing. Nutr Cycling Agroecosyst. 49:139–146.
- Singh S, Kumar S, Jain MC. 1997. Methane emission from two Indian soils planted with different rice cultivars. Biol Fert Soils. 25:285–289.10.1007/s003740050316
- Sinha SK. 1995. Global methane emission from rice paddies: excellent methodology but poor extrapolation. Curr Sci. 68:643–646.
- Su J, Hu C, Yan X, Jin Y, Chen Z, Guan Q, Wang Y, Zhong D, Jansson C, Wang F, Schnürer A, Sun C. 2015. Expression of barley SUSIBA2 transcription factor yields high-starch low-methane rice. Nature. 523:602–606.10.1038/nature14673
- Van Groenigen JW, Velthof GL, Oenema O, Van Groenigen KJ, Van Kessel C. 2010. Towards an agronomic assessment of N2O emissions: a case study for arable crops. Eur J Soil Sci. 61:903–913.10.1111/ejs.2010.61.issue-6
- Wang MX, Li J. 2002. CH4 emission and oxidation in Chinese rice paddies. Nutr Cycling Agroecosyst. 64:43–55.
- Wang B, Neue HU, Samonte HP. 1997. Effect of cultivar difference (‘IR72’, ‘IR65598’ and ‘Dular’) on methane emission. Agric Ecosyst Environ. 62:31–40.10.1016/S0167-8809(96)01115-2
- Wang B, Xu Y, Wang Z, Li Z, Guo Y, Shao K, Chen Z. 1999. Methane emissions from rice fields as affected by organic amendment, water regime, crop establishment, and rice cultivar. Environ Monit Assess. 57:213–228.10.1023/A:1006039231459
- Wassmann R, Aulakh MS. 2000. The role of rice plants in regulating mechanisms of methane missions. Biol Fert Soils. 31:20–29.10.1007/s003740050619
- Wassmann R, Papen H, Rennenberg H. 1993. Methane emission from rice paddies and possible mitigation strategies. Chemosphere. 26:201–217.10.1016/0045-6535(93)90422-2
- Wassmann R, Neue HU, Alberto MCR, Lantin RS, Bueno C, Llenaresas D, Arah JRM, Papen H, Seiler W, Rennenberg H. 1996. Fluxes and pools of methane in wetland rice soils with varying organic inputs. Environ Monit Assess. 42:163–173.10.1007/BF00394048
- Win KT, Nonaka R, Win AT, Sasada Y, Toyota K, Motobayashi T, Hosomi M. 2012. Comparison of methanotrophic bacteria, methane oxidation activity, and methane emission in rice fields fertilized with anaerobically digested slurry between a fodder rice and a normal rice variety. Paddy Water Environ. 10:281–289.10.1007/s10333-011-0279-x
- Xu YC, Wang ZY, Li Z, Wang BJ. 1999. Effect of rice cultivars on methame emission from Beijing rice field. Plant Nutr Fert Sci. 5:93–96 (in Chinese with English abstract).
- Yu J, Wang LL, Yan XJ, Tian YL, Deng AX, Zhang WJ. 2013. Super rice cropping will enhance rice yield and reduce CH4 emission: a case study in Nanjing. Chin Rice Sci. 20:427–433.
- Zheng H, Huang H, Yao L, Liu J, He H, Tang J. 2013. Impacts of rice varieties and management on yield-scaled greenhouse gas emissions from rice fields in China: a meta-analysis. Biogeosci Discuss. 10:19045–19069.10.5194/bgd-10-19045-2013
- Zou JW, Huang Y, Jiang J, Zheng XH, Sass RA. 2005. 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: effects of water regime, crop residue, and fertilizer application. Global Biogeochem Cycles. 19(2):153–174.