Abstract
The 2-min protocol (1 + 1) for the βeta-s.t.a.r. (manufactured by Neogen Corporation, Lansing, MI, USA) was validated at the Technology and Food Science Unit of the Institute for Agricultural and Fisheries Research according to Commission Decision 2002/657/EC. The test was very selective for the group of β-lactam compounds: the only interference found was by clavulanic acid at 2500 µg kg−1 and above. The modified protocol (βeta-s.t.a.r. 1 + 1) detected all β-lactams with a maximum residue limit (MRL) in milk, but not all these compounds were detected at their respective MRL. The detection of cefalexin (detection capability = 6000 µg kg−1; MRL = 100 µg kg−1) and penethamate (detection capability = 80 µg kg−1; MRL = 4 µg kg−1) was especially poor, and also ceftiofur was only detected from 500 µg kg−1 (MRL = 100 µg kg−1). The repeatability of the reader and of the test was very good. The test was very robust: test results were not significantly influenced by small changes in the test protocol, by the milk composition or by the type of milk. The test was also suitable to test the milk of animal species other than cow. Favourable results were obtained in testing monitoring samples, in two national ring trials, and in an international proficiency test. The βeta-s.t.a.r. 1 + 1 is a very fast, simple, and reliable test that could be used at the farm level to prevent tanker milk contamination by β-lactams.
Introduction
Penicillins and cephalosporins belong to the group of β-lactam antibiotics due to their common β-lactam ring structure. Penicillins remain very important in human and veterinary medicine. In 1997, 225 metric tonnes of penicillins were administered to animals in the European Union (European Federation of Animal Health (Fedesa) Citation1998).
Mastitis is the most expensive disease affecting dairy cattle worldwide and, therefore, the single largest cause of antibiotic usage in dairy herds. Bacteria from the genera Staphylococcus and Streptococcus, both Gram-positive, are the most common causal agents of mastitis (Pyörälä Citation1995). β-Lactam antibiotics are the most frequently administered drugs in parenteral and intra-mammary therapy. The drug of choice in many countries is still penicillin, since the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values for the most common mastitis pathogens are very low. When mastitis pathogens show penicillin resistance, a combination of penicillins and clavulanic acid or isoxazolyl penicillins resistant to penicillinase are often used as alternative treatment (Pyörälä Citation1995).
Penicillins all have the same ring structure and are monobasic acids that readily form salts and esters. The penicillin nucleus, 6-aminopenicillanic acid, consists of a fused thiazolidine ring and a β-lactam ring with an amino group at the 6-position. The cephalosporins are semi-synthetic antibacterials derived from cephalosporin C, a natural antibiotic. The active nucleus is very closely related to the penicillin nucleus and consists of a β-lactam ring fused with a six-membered dihydrothiazine ring and having an acetoxymethyl group at position 7. Penicillins and cephalosporins are bactericidal and act by inhibiting synthesis of the bacterial cell wall (Royal Pharmaceutical Society of Great Britain Citation2005).
All antimicrobial drugs administered to cows enter the milk to some degree. Testing for antimicrobial drug residues (i.e., the drug itself or a metabolite) in milk is therefore necessary for ethical, health and technological reasons (Honkanen-Buzalski and Reybroeck Citation1997). The presence of β-lactam residues in milk can have several drawbacks: inhibition of dairy starter cultures used in the production of cheese and yoghurt (Suhren Citation1996; Grunwald Citation2002), possible hypersensitivity reaction by the consumer and contribution to the development of antibiotic resistance.
In the European Union, maximum residue limits (MRLs) were fixed in bovine milk for 16 β-lactam compounds, ranging from 4 to 125 µg kg−1 (European Commission Citation2009; European Union 2010), and in many countries inhibitory substances are screened in routine in farm milk samples as part of the regulatory quality programme. In 2008, of the 1,456,990 farm milk samples in Belgium analysed, 1194 or 0.08% were found to be positive by the milk control stations. In most cases, residues of β-lactam substances were the main reason for bulk tank milk failure. In an identification study performed at Technology and Food Science Unit of the Institute for Agricultural and Fisheries Research (T&V-ILVO) on all positive Flemish farm milk samples leading to penalization in May and June 2003, 79% of the samples contained non-synthetic penicillins, 8% synthetic penicillins, and 4% a combination of β-lactam and non-β-lactam residues. In total, therefore, 91% of the samples contained β-lactams (Reybroeck and Daeseleire Citation2003). As given in the year report of 2005 of Comité du Lait (Walloon region) the percentage of penalizations due to β-lactams was 94.5% in 2003, 90.0% in 2004, and 83.9% in 2005 (Comité du Lait Citation2006). In Germany, β-lactams could, in 95% of cases, be identified in inhibitor-positive milk samples (Kress et al. Citation2007). Penicillin G was still the predominant antibiotic detected (74.6%) during regulatory control, followed by ceftiofur (11%), ampicillin/amoxicillin (6.3%) and isoxazolyl penicillins (3.2%).
It is worth noting that in routine testing mostly microbial inhibitor tests are used, often with Geobacillus stearothermophilus var. calidolactis as the test organism, chosen for its high sensitivity for penicillins (Suhren and Heeschen Citation1996; Reybroeck Citation2004). The consequence of this is that milk is rigorously screened for the presence of β-lactams, while residues of other antibacterial groups are not always detected at their respective MRL. The percentage of β-lactams in the data above is, therefore, an overestimation.
One of the potential issues for an assay for β-lactams in milk is their stability. Internal standard samples consisting of, respectively, penicillin G and cloxacillin spiked in raw milk and stored for 2 months below −18°C did not demonstrate any problems with stability. Storing raw milk in the refrigerator (2–8°C), however, can result in stability problems with β-lactams due to the possible formation of penicillinase by certain milk bacteria (Guay et al. Citation1987).
Since the result of the routine testing of farm milk on antimicrobials by the milk control stations is only known after the milk is processed, in some European countries milk must be checked for the presence of β-lactam residues on entry to the dairy plant or before production. This control is to ensure the technological safety of the milk for the production of fermented dairy products and also protect the consumer, and several rapid screening tests are on the market for this purpose (Neaves Citation1999; Kroll Citation2000; Kroll et al. Citation2000; Food Safety Authority of Ireland Citation2002; Reybroeck Citation2004, Citation2008; Reybroeck and Ooghe Citation2004; Quandt Citation2006; Žvirdauskiené and Šalomskiené Citation2007). Further, non-commercial tests or biosensor and immunosensor-based tests are used in some food laboratories (Gustavsson et al. Citation2002; Cacciatore et al. Citation2004; Knecht et al. Citation2007; Lamar and Petz Citation2007). Results of the testing can be obtained in less than 10 min.
In some countries, rejected milk needs to be destroyed due to very strict legislation, resulting in large costs being incurred in transport, incineration and loss of the milk itself. The dairy industry is therefore interested in testing at the farm before collection of the milk, hence placing more responsibility on the farmer. However, in such a strategy, a short test time is very important due to the number of tests involved.
The βeta-s.t.a.r. (Neogen Corporation, Lansing, MI, USA) is a dipstick receptor assay, which uses a selective receptor linked to gold particles for the detection of β-lactams in milk (Reybroeck Citation2000; Reybroeck and Ooghe Citation2006), that originally involved a 5-min protocol. The βeta-s.t.a.r. 1 + 1 is a faster version of the 5-min βeta-s.t.a.r. protocol using identical reagents, but with two incubation steps of 1 min each giving, overall, a 2-min protocol. The present study describes how a validation of the βeta-s.t.a.r. 1 + 1 was performed at T&V-ILVO according to Commission Decision 2002/657/EC (European Commission Citation2002). The specificity, detection capability and test ruggedness of the assay were demonstrated as meeting the criteria required by the European Commission Decision. Some of the results of this evaluation study were presented in 2008 at the EuroResidue VI Conference on Residues of Veterinary Drugs in Food (Egmond aan Zee, the Netherlands; Reybroeck and Ooghe Citation2008).
Materials and methods
Reagents and standards
Penicillin G (PENNA), amoxicillin (A8523), oxacillin (O10002), cloxacillin (C9393), dicloxacillin (D9016), nafcillin (N3269), cefazolin (C 5020), cephapirin (C8270), and cefoperazone (C4292) were all from Sigma-Aldrich (Bornem, Belgium). Ampicillin (9930212) was from the WHO Collaborating Centre for Chemical Reference Substances (Kungens Kurva, Sweden). Ceftiofur (34001) and cefalexin (33989) were from Riedel-de Haën (Bornem, Belgium). Penethamate (PE-0708004) was from Deltapharma s.a. (Barcelona, Spain); cefquinome (Batch 01-01) from Intervet International GmbH (Unterschleiβheim, Germany); cefacetrile (22020D000) from Novartis Animal Health, Inc. (Basel, Switzerland); cefalonium (2629) from Schering-Plough (Levallois-Perret, France); and clavulanic acid from DSM Anti-Infectives (Delft, the Netherlands).
Comparisons were made for cefazolin (Cat. 1097 603) with reference material from United States Pharmacopeia (Rockville, MD, USA) and for penicillin G (9930226) and cloxacillin (9930261) from the WHO Collaborating Centre for Chemical Reference Substances (Kungens Kurva, Sweden).
Antibiotic standards were dissolved in water except for ceftiofur, cefalonium and for cefazolin (acetonitrile/water, 1:1, v/v). Acetonitrile (01207802) was from Biosolve B.V. (Valkenswaard, the Netherlands). Standard stock solutions of the antibiotic standards of 100 mg l−1 were made in water and kept below 4°C for a maximum of 7 days. Dilutions of 1 and 0.1 mg l−1 were freshly prepared on a daily basis.
The βeta-s.t.a.r-250 kits were from Neogen Corporation. In general, lots TH00616-042405/4 (Exp. 23 November 2005) and lot 051607/2 (Exp. 16 July 2006) were used for the evaluation study. For some parts, e.g. the study of batch-to-batch differences and the stability of the reagents, lot 060409 (Exp. 4 September 2007) and lot 070331 (Exp. 31 March 2008) were also used. The reagents were stored in a cool room at 4 ± 2°C.
The Delvotest SP-NT 5-PACK kits were from DSM-Food Specialties (Delft, the Netherlands). A mixture of raw milk, aseptically collected from four individual cows, was used as blank milk. The cows in mid-lactation were selected on the basis of not being treated with veterinary drugs during the last months and giving milk with a low number of somatic cells (<2 × 105 ml−1). The blank milk was always tested before use with a Delvotest SP-NT 5-PACK.
Material
For the incubation of the glass vials, a dry-block heater Type BS25-230D (Aerne Analytic, Pfaffenhofen, Germany) was used. For the reading of the dipsticks, a reader system (Dipstick Reader, 77 Elektronika Kft, Budapest, Hungary) was used. The reader system was checked daily with a blank calibration strip.
Test procedure and interpretation of the results
For raw milk, no sample pre-treatment was required, while milk powder was reconstituted with distilled water. A total of 200 µl of the milk sample were added to the β-lactam receptor in the glass vial and the mixture was gently swirled after re-closing the glass vial. The homogenous mixture was then incubated for 1 min at 47.5 ± 1°C in the block-heater. β-Lactam antibiotics in the milk form a stable non-active complex with the selective β-lactam receptor. The dipstick of immune-chromatographic medium was placed into the glass vial and incubated at 47.5 ± 1°C for a further 1 min, during which incubation the liquid flowed vertically on the dipstick and passed through the capture zone. The test line captured remaining active receptor for β-lactams; the upper line or control line captured excess reagents. The intensity of the colour that consequently develops at the selective test line and the control line was inversely proportional to the amount of β-lactam residues and could be interpreted both visually and instrumentally.
For instrumental reading the Dipstick Reader was used, which calculates the ratio of colour, based on either the area or the amplitude, at the test line and the control line. During measurement, the control line acts as a reference line. Milk with a ratio >1.00 is free of β-lactams (‘negative’); milk with a ratio ≤1.00 is contaminated (‘positive’).
Due to the very short incubation periods, it is possible that the control line is very weak or virtually absent directly after finishing the test, and where a clear red test line is already present the test can be interpreted as negative even before the control line is fully developed. However, if the control and the test line are both weak, no correct interpretation can be done until proper line development has occurred. In the case where the control line was completely missing, the instrument indicated ‘invalid reading’. This happened a few times during the evaluation period, but the problem was resolved by waiting until the control line developed. Only after the appearance of the control line could a correct interpretation be performed.
Test and reader repeatability
To calculate the repeatability of the Dipstick Reader, negative and positive strips were measured twice. However, in the situation where the colour formation on the dipsticks after the second incubation step was not fully finished, not exactly the same situation was measured twice. Dipsticks were therefore also allowed to become dry and stable, then these dry dipsticks were measured ten times and the standard deviation calculated.
The repeatability of the test was calculated at different ratio levels by analysing and measuring blank and positive milk samples in duplicate.
Test selectivity
The selectivity of the βeta-s.t.a.r. with the classic 5-min protocol test was previously investigated by spiking blank milk with a relatively high concentration (10 × MRL in milk) of a substance belonging to other groups of antibiotics or chemotherapeutics (Reybroeck Citation2000; Reybroeck and Ooghe Citation2004) and testing in duplicate. One substance was chosen from each of the most important groups: oxytetracycline (tetracyclines), sulfadiazine (sulfonamides), enrofloxacin (quinolones), neomycin (aminoglycosides), erythromycin (macrolides), lincomycin (lincosamides), clavulanic acid (β-lactamase inhibitors), colistin (polymyxins), and trimethoprim (diamino pyrimidine derivatives). The forbidden compounds chloramphenicol and dapsone, spiked at 3 and 50 µg kg−1, respectively, were also tested. With the compounds not belonging to the group of β-lactam antibiotics, no interference was observed except in the case of clavulanic acid (Reybroeck Citation2000; Reybroeck and Ooghe Citation2004).
The present study tested 100 blank milk samples free of antibiotics and the minimal concentration of clavulanic acid in milk causing positive results for the βeta-s.t.a.r. 1 + 1. For this study milk spiked with clavulanic acid at different concentrations was tested.
Detection capability
The most important validation parameter is the detection capability (CCβ). This parameter was determined for all β-lactams mentioned in the list of MRLs in milk (European Union 2010). Therefore, starting from the detection capability concentrations of the classic βeta-s.t.a.r., blank milk was spiked with the β-lactams investigated at different concentrations in various ranges in different increments: in the range 1–10 µg kg−1 at 1 µg kg−1 increments; in the range 10–20 µg kg−1 at 2 µg kg−1 increments; in the range 20–50 µg kg−1 at 5 µg kg−1 increments; in the range 50–100 µg kg−1 at 10 µg kg−1 increments; in the range 100–250 µg kg−1 at 25 µg kg−1 increments; in the range 250–500 µg kg−1 at 50 µg kg−1 increments; in the range 500–1000 µg kg−1 at 100 µg kg−1 increments; and in the range >1000–5000 µg kg−1 at 500 µg kg−1 increments. The spiked samples were blind-coded before analysis. The analysis was performed within 4 h after spiking. Each concentration was tested 20 times, in a time period of at least 3 days. Each day a different blank milk was used. For each β-lactam investigated, the CCβ or the lowest concentration giving 19 (low) positive test results on 20 test results was determined, interpreting both visually and with the Dipstick Reader.
Test robustness
Length of incubation
Since incubation time could possibly make the test less robust, other incubation times were tested. The first incubation step was modified to be 45 or 75 s while keeping the second step at 1 min; the second incubation step was modified to be 45 or 75 s while keeping the first step at 1 min; and both incubation steps were modified (45 s each, 75 s each and the combinations of 45 and 75 s). Each situation was tested with four blank milk samples and with four milk samples spiked with one of three β-lactams: penicillin G (4 µg kg−1), ampicillin (6 µg kg−1) or cloxacillin (12 µg kg−1).
Influence of waiting time on reader results
Blank milk samples and samples spiked with one of three β-lactams (penicillin G, 4 µg kg−1; ampicillin, 6 µg kg−1; cloxacillin, 12 µg kg−1) were analysed and the strips were read with the Dipstick Reader directly after the incubation and after 0.5, 1, 3 and 10 min.
Milk influences
Milk quality and composition
The impact of the milk quality (somatic cell count, total bacterial count) and composition (fat and protein content, pH) were tested by comparing the test performance of the βeta-s.t.a.r. 1 + 1 protocol for ten different blank milks with either high somatic cell count (>106 ml−1, 34 samples) or high total bacterial count (>5 × 105 cfu ml−1, 31 samples) and ten different spiked milk samples with a normal and an abnormal composition. Milk of normal and abnormal composition was analysed with and without spiking with one of three β-lactams (penicillin G, 4 µg kg−1; ampicillin, 6 µg kg−1; cloxacillin, 12 µg kg−1). For each different milk type the average, the highest and the lowest ratios were calculated.
Milk samples with a high number of somatic cells were selected at the milk control station based on Fossomatic 5000 (Foss, Hillerød, Denmark) measurements. Milk samples with a high total bacterial count were obtained by keeping normal milk samples for 4–6 h at room temperature. The final bacterial count was determined by performing a spiral plate count (Eddy Jet, IUL sa, Barcelona, Spain) on plate count agar plates after 3 days of incubation at 30°C. Milk samples with a low fat content were obtained by removal of the fat layer by centrifugation (3050 g, 10 min, at 5°C). Milk samples with a high fat content were obtained by addition of cream (50% fat, 6 g) to milk (60 ml). The final fat content was measured by infrared with a MilcoScan 4000 (FOSS, Hillerød, Denmark). Milk samples with a low (<2.5 g 100 ml−1) and a high protein content (>4 g 100 ml−1) were natural milk samples with extreme protein content that were selected at the milk control station based on infrared spectroscopic results (MilcoScan 4000). To gain samples with an abnormal pH, normal milk was initially adjusted to pH 6.0 and 7.5 with 1 M HCl or 1 M NaOH, respectively, then the pH was further adjusted with the addition of either 0.1 M HCl or 0.1 M NaOH.
Type of milk and animal species
UHT milk, sterilized milk, reconstituted milk powder, thawed milk, goats’ milk, ewes’ milk and mares’ milk were also tested to determine if the βeta-s.t.a.r. 1 + 1 was a suitable test for these types of milk. Ten different samples of each milk type were tested, with the exception that a higher number of blank samples were tested for goats’ milk (29 samples), ewes’ milk (31 samples) and mares’ milk (30 samples).
Test for false-positive/false-negative results
A total of 117 farm milk samples, 65 truck milk samples, 32 consumer milk samples, and 18 milk powders were analysed with βeta-s.t.a.r. 1 + 1 as part of a monitoring programme. The same samples were also tested by Delvotest SP-NT (DSM Food Specialties, Delft, the Netherlands), Bacillus cereus-test (Suhren and Heeschen Citation1993), Escherichia coli-test (Suhren Citation1997), and Charm MRL Beta-lactam Test (Charm Sciences, Inc., Lawrence, MA, USA).
To test the rate of false-negative results, 82 incurred milk samples originating from 27 individual cows treated with a veterinary drug containing penicillin G and neomycin were analysed with the βeta-s.t.a.r. 1 + 1 and with other microbiological and β-lactam receptor screening tests. Sampling started at the end of the withholding period. The exact concentration of penicillin G present in the milk samples was determined by liquid chromatography-tandem mass spectrometry (LC/MS-MS) in an external laboratory.
Reagent influence (batch differences)
To study the differences of different batches of reagents, blank and spiked milk samples were analysed at the same time with two different batches of βeta-s.t.a.r. reagents (Lot 70405, Exp. 5 April 2008; and Lot 70213, Exp. 13 February 2008) and Lot 70205 (Exp. 5 February 2008). Besides spiking with penicillin G (4 µg kg−1), ampicillin (6 µg kg−1) or cloxacillin (12 µg kg−1), 20 milk samples were also spiked with 28 µg kg−1 cephapirin to obtain ratios close to the cut-off value of 1.00. In the area around the cut-off, any change in intensity of the test line can be quickly noted.
The stability of reagents during shelf-life was also checked. Blank and spiked milk samples were tested with reagents of Lot 70405 shortly after the production date and just 1 week before the expiry date.
Inter-laboratory testing
Twice a year T&V-ILVO organizes a national ring trial for the Belgian dairy industry regarding the detection of residues of antibiotics in milk by microbiological and rapid tests. In the two ring trials of 2007 the βeta-s.t.a.r. 1 + 1 procedure was included.
T&V-ILVO participated with the βeta-s.t.a.r. 1 + 1 in the international proficiency study for the analysis of β-lactam residues in raw milk, organized in 2007 by AFFSA Fougères, Community Reference Laboratory for antimicrobial residues in food of animal origin.
Daily control samples
During the study, blank and control samples spiked separately with penicillin G (4 µg kg−1), ampicillin (6 µg kg−1) and cloxacillin (12 µg kg−1) were analysed daily.
Results and discussion
Test and reader repeatability
All repeatability results are shown in . The repeatability of the Dipstick Reader was very good; very low standard deviations of repeatability (s r) were obtained. The more positive the samples, the better the repeatability. It is worth noting that, in general, with wet dipsticks a slightly lower ratio was obtained for the second reading due to further colour formation on the strips as they dried.
Table 1. Repeatability of the reader (dry and wet dipsticks) and repeatability of the βeta-s.t.a.r. 1 + 1 test at different ratios.
The repeatability of the test was also very good; very low s r-values were again obtained. It is noteworthy that the same s r-values for the test repeatability and reader repeatability were obtained: this indicates that the test is very robust and that for replicates of a sample the same binding, flow, and colour formation are essentially obtained. The repeatability for positive samples is also better than that for blank samples and is not influenced by the ratio level.
Test selectivity
All 100 blank milk samples tested negative. To test the minimum concentration of clavulanic acid in milk that could cause positive results for the βeta-s.t.a.r. 1 + 1, milk spiked with clavulanic acid at different concentrations was tested, and interference by clavulanic acid was only obtained at 2500 µg kg−1 and above. Therefore, the βeta-s.t.a.r. 1 + 1 is very selective for the detection of β-lactams. Interference by the β-lactamase inhibitor clavulanic acid in the βeta-s.t.a.r. 1 + 1 protocol could be expected since this molecule has a β-lactam structure resembling that of the penicillin nucleus, except that the fused thiazolidine ring of the penicillins is replaced by an oxazolidine ring (Royal Pharmaceutical Society of Great Britain Citation2005).
Within the β-lactam group the test is not specific for any particular β-lactam, but non-synthetic penicillins and the group of synthetic penicillins and cephalosporins could be differentiated after pre-treatment of the milk with penase (data not shown).
Detection capability
A summary of the detection capabilities of the βeta-s.t.a.r. 1 + 1 is given in . Not all the β-lactam compounds were detected at their respective MRL. The detection of cefalexin and penethamate was poor, and also ceftiofur was only detected from 500 µg kg−1. It should be noted, however, that from a practical perspective, the high detection capability of 80 µg kg−1 for penethamate in relation to the MRL (4 µg kg−1) is of no significance since penethamate is not stable in milk and is rapidly and completely hydrolysed to penicillin G and diethylaminoethanol. At 37°C and at pH 7.3 (reflecting the physiological conditions of cows), the half-life of penethamate in aqueous solution is 23 min. In tissue homogenates at 32°C, 50% of the penethamate has been shown to be hydrolysed within 2 h and 100% by 20 h (The European Agency for the Evaluation of Medicinal Products Citation2000).
Table 2. Detection capabilities in raw cows’ milk of the βeta-s.t.a.r. 1+1 instrumental reading with a cut-off ratio of 1.00a in comparison to the βeta-s.t.a.r.
Since the analysis was performed not just immediately, but within 4 h after spiking, a negative influence of the protein binding effect on the test capabilities could not be excluded. When performing the βeta-s.t.a.r. 1 + 1 protocol instead of the classic 3 + 2-min protocol (Reybroeck and Ooghe Citation2004), the test generally lost some capability resulting in a reduced number of compounds detectable at the MRL level.
Test robustness
Length of incubation
Performing the βeta-s.t.a.r. 1 + 1 protocol with the different incubation times tested had no significant impact on the ratios obtained for blank milk (data not shown). With a longer first incubation period, slightly lower ratios were obtained with positive milk samples, while a shorter first incubation step resulted in slightly higher ratios (data not shown). The effect was limited for milk spiked with penicillin G (4 µg kg−1) and cloxacillin (12 µg kg−1), but more visible for ampicillin (6 µg kg−1). This indicated that the time needed for a quantitative binding of the receptor to ampicillin is longer in comparison with binding to penicillin G or cloxacillin. The data indicated that for the first incubation a minimum of 1 min is needed to be respected. Even when the second incubation period differed from the standard protocol, correct and acceptable results were obtained, proving that, within the limits tested, strict adherence to timing for the second incubation was not a critical point.
Influence of waiting time on reader results
If the reading of the dipsticks after incubation was delayed, the ratios decreased, giving a tendency to more positive results. Nevertheless, all blank milk samples remained clearly negative with 2.25 as the lowest ratio obtained for a delay of 10 min after incubation before reading. Therefore, delaying the reading does not cause incorrect results but does improve the detection capability.
Milk quality and composition
With respect to testing the impact of the milk quality and composition (somatic cell count, total bacterial count, fat and protein content and pH), the mean, the highest ratio, and the lowest ratio value for each milk type are given in , and .
Figure 1. Ratios for normal and abnormal blank milk (![]()
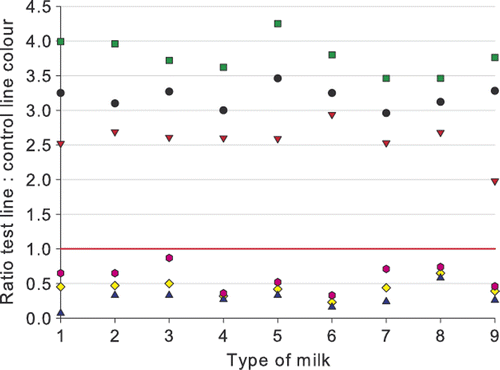
Figure 2. Ratios for normal and abnormal milks containing 6 µg kg−1 ampicillin (![]()
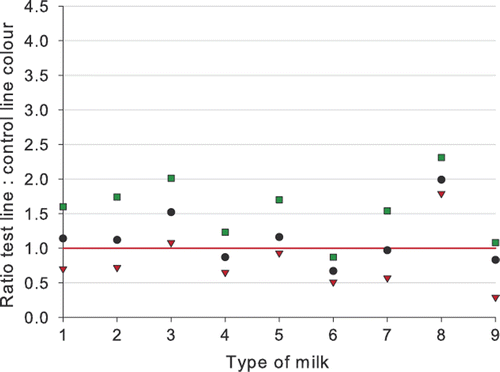
Figure 3. Ratios for normal and abnormal milks containing 4 µg kg−1 penicillin G (![]()
The milk quality and composition had no influence on the performance of the βeta-s.t.a.r. 1 + 1 when testing blank milk: all blank milk samples were clearly negative with ratios all above 2.5, except for milk with a high pH for which the lowest ratio was 1.98. With positive milk samples spiked with penicillin G or cloxacillin, only small effects with abnormal milk quality or composition were noticed. In all spiked samples the detection of the β-lactam was never completely hampered. In milk with a low pH (6.0), sensitivity of the test was decreased, and was most pronounced for milk with 6 µg kg−1 ampicillin. Further, the detection capability of the test was slightly diminished when testing milk with a high bacterial load, which could be explained by the possible production of penicillinase by certain bacteria. In such a case penicillin G and ampicillin would be expected to be more quickly cleaved than cloxacillin.
It should be noted that the best detection was obtained in milk with low protein content, which could be the result of decreased binding of the antibiotics to protein material. However, in large volumes of commingled milk, such extreme values of composition will not occur; for instance, while a high pH milk can occur in individual cow milk due to damage of the blood/milk barrier by subclinical mastitis, it is unlikely that an entire bulk collection of milk will be affected. Further, it must also be recognized that the test is qualitative rather than quantitative and is used only to discriminate between β-lactam residue-free milk and milk containing such residues. In general, the test is very robust and not severely influenced by the milk composition.
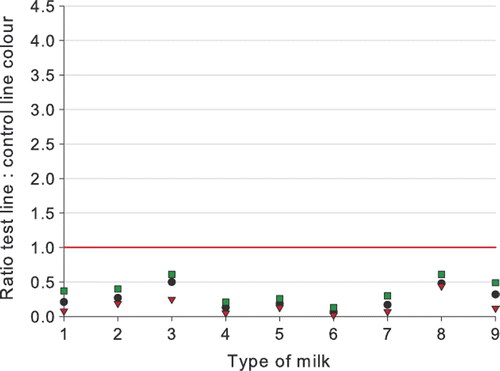
Type of milk and animal species
The results of the testing of UHT milk, sterilized milk, reconstituted milk powder, thawed milk, goats’ milk, ewes’ milk, and mares’ milk are presented in , and . No significant differences were noticed in testing different types of milk. All blank milk samples tested negative (all ratios > 1.0), although for one out of 29 blank goats’ milk and for one out of 31 blank ewes’ milk samples a ratio below 2.0 was obtained. This may have been caused by an abnormal flow of the milk on the dipstick.
Figure 4. Ratios for blank milk (![]()
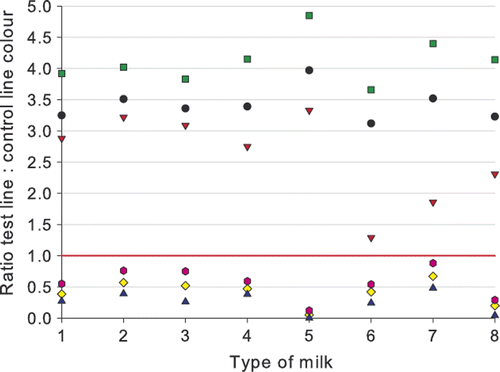
Figure 5. Ratios for different milks containing 6 µg kg−1 ampicillin (![]()
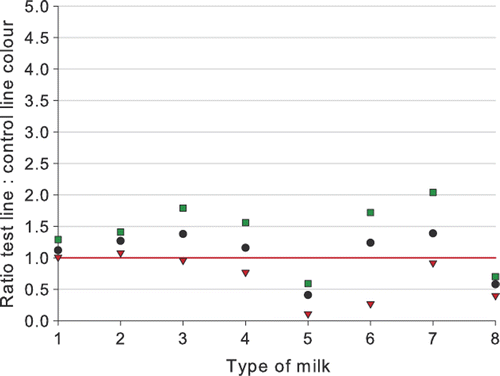
Figure 6. Ratios for different milks containing 4 µg kg−1 penicillin G (![]()
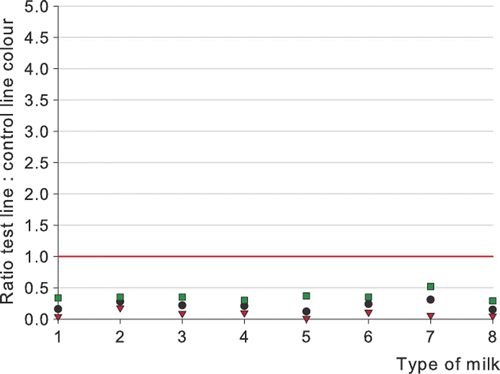
Cloxacillin (12 µg kg−1) and penicillin G (4 µg kg−1) were always detected in quite a uniform way in the different milk types and in the milk from animal species different from the cow. The detection of 6 µg kg−1 ampicillin gives a higher variation in detection and ratios. The level of detection of ampicillin in sterilized milk, in reconstituted milk powder, in goats’ milk, and for a lesser extent in ewes’ milk would be higher than 7 µg kg−1 as determined in cows’ milk (). It is worth noting that the thawed milk samples were tested with reagents of Lot 70405, while the other milk types were tested with Lot 70213. Lot 70405 was more sensitive, and the difference in detection capability for ampicillin is especially notable. The biggest variation in ratios was noticed for the detection of ampicillin in goats’ milk. Of all milk types spiked with ampicillin and tested with reagents of Lot 70213, the best detection was obtained for mares’ milk. The difference in detection for different milk types is smaller than the difference in test capability by the use of different batches of reagents.
The βeta-s.t.a.r. 1 + 1 is therefore not only valid as a raw cows’ milk test, but also can be used to test UHT milk, sterilized milk, reconstituted milk powder or thawed milk, and milks of non-bovine species (goat, ewe, mare). However, for UHT milk, sterilized milk, reconstituted milk powder or thawed milk the use of the classic 3 + 2 version with better detection capabilities for some compounds is recommended.
Test for false-positive/false-negative results
No false-positive or false-negative results were obtained when testing farm milk, truck milk, consumption milk samples, and milk powders as part of a monitoring programme. For testing the rate of false-negative results, incurred milk samples originating from individual cows treated with a veterinary drug, containing penicillin G and neomycin, were analysed with the βeta-s.t.a.r. 1 + 1. All 59 milk samples with a penicillin G content ≤2.4 µg kg−1) tested negative on βeta-s.t.a.r. 1 + 1; all 23 incurred milk samples with a penicillin G content ≥2.5 µg kg−1 were testing positive, except for the sample containing 2.7 µg kg−1 penicillin G. These data confirm that the detection capability for penicillin G in spiked milk (3 µg kg−1; ) is also valid for the detection of penicillin G in incurred milk samples.
Reagent influence (batch differences)
A summary of the results of the testing of spiked milk samples with two different batches of βeta-s.t.a.r. reagents is given in . Differences in test capability were found between batch Lot 70405 and Lots 70213 and 70205, the former giving lower ratios for the spiked milk samples than Lots 70213 and 70205. The difference was most pronounced for the milk samples spiked with 6 µg kg−1 ampicillin and 28 µg kg−1 cephapirin. Note that the detection capability of ampicillin and cephapirin is 7 and 28 µg kg−1, respectively (). Blank milk gave the same ratios with all the different batch lots tested.
Table 3. Ratios obtained when testing the same positive and negative milk samples with βeta-s.t.a.r. reagents from different batches.
The stability of reagents during shelf-life was also checked. Blank and spiked standards were tested with reagents of Lot 70405 shortly after the production date and 1 week before the expiry date. In general, comparable results were obtained, confirming the stability of the reagents over the recommended shelf-life (means were: blank = 3.23, penicillin G at 4 µg kg−1 = 0.03, ampicillin at 6 µg kg−1 = 0.27, and cloxacillin at 12 µg kg−1 = 0.32).
Interlaboratory testing
Twice a year T&V-ILVO organizes a national ring trial for the Belgian dairy industry regarding the detection of residues of antibiotics in milk by microbiological and rapid tests. In the two ring trials of 2007, the βeta-s.t.a.r. 1 + 1 was also included as a rapid test.
The results for the βeta-s.t.a.r. 1 + 1 in both ring trials were excellent: no false-positive results were obtained and the spiked milk samples gave the expected positive results with the exception that the sample spiked with penicillin at 3 µg kg−1 tested negative (ratio = 1.281, test capability = 3 µg kg−1). The sample spiked with penicillin at 4 µg kg−1 gave a clear positive (ratio = 0.033). Of all the samples that tested positive with the classic (3+2) βeta-s.t.a.r. 3 + 2 protocol, only three samples tested negative with the rapid βeta-s.t.a.r. 1 + 1 protocol, i.e., samples spiked with 4 µg kg−1 ampicillin (test capability = 7 µg kg−1), 20 µg kg−1 cefquinome (test capability = 28 µg kg−1) and 3 µg kg−1 penicillin G (test capability = 3 µg kg−1). Details of the results are given in separate reports (Ooghe and Reybroeck Citation2007a, Citation2007b).
In the international proficiency study organized by AFSSA Fougères, six blind coded milk samples were distributed among the participating laboratories. The blank milk and the five spiked milk samples, respectively, containing cefquinome at 50 µg kg−1, cloxacillin at 40 µg kg−1, cefalonium at 20 µg kg−1, and penicillin G at 6 µg kg−1 (in duplicate), were all correctly analysed by βeta-s.t.a.r. 1 + 1 at T&V-ILVO (Community Reference Laboratory for Antimicrobial Residues in Food from Animal Origin Citation2008).
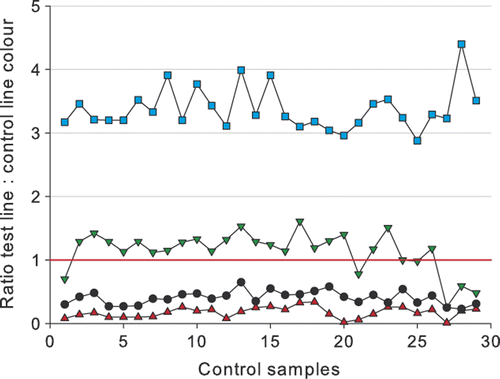
Daily control samples
During the study, blank and control samples spiked with 4 µg kg−1 penicillin G, 6 µg kg−1 ampicillin or 12 µg kg−1 cloxacillin were analysed daily. The results are shown in . Over 29 working days the control samples gave very constant ratios. It should be noted that tests for days 1–26 used reagent Lot 70213, while from day 27 onwards Lot 70405 was used, which was significantly better at detecting ampicillin. For the entire period, the following average ratios were obtained: blank milk = 3.38 ± 0.34, milk spiked with 4 µg kg−1 penicillin G = 0.17 ± 0.08, milk spiked with 6 µg kg−1 ampicillin = 0.14 ± 0.31, and milk spiked with 12 µg kg−1 cloxacillin = 0.40 ± 0.10. Since most of the time the 6 µg kg−1 ampicillin control sample was giving negative results, its value for quality control could be discussed. However, small differences in test capability of the reagents can be detected by the use of milk samples spiked with ampicillin. This compound is therefore used by Neogen Corporation in their quality control for product release.
Conclusions
With a total test time of 2 min, the βeta-s.t.a.r. 1 + 1 is, presently, the fastest single test on the market for the detection of β-lactam residues in milk meeting the criteria required by Commission Decision (EC) No. 2002/657. The short test time, the very easy test protocol and the possibility of visual interpretation of the test enables the use of the test at the farm before collection. Shortening of the test protocol from 5 min to 2 min influences the test capability for some β-lactam compounds, but does not challenge test robustness. Further, the use of βeta-s.t.a.r. 1 + 1 at the farm level instead of using the classic 5-min βeta-s.t.a.r. test at the entrance of the dairy plant would resolve the issue of dilution ‘disguising’ contaminated milk (since milk is bulked from an average of ten farms) and would lead to stronger on-farm practices. On-farm checking would also reduce costs for the destruction of large volumes of β-lactam-contaminated milk, since again it would be at the individual farm level, rather than bulked milk from several farms. On the other hand, testing at the farm means a larger number of determinations, higher costs for reagents and more work for the truck driver. It is the task of those responsible for milk collection to make a balance of pros and cons and to calculate the price differences between the two strategies. If time is not the crucial factor (dairy entrance control) or no further dilution of the milk is expected, the classic 5-min βeta-s.t.a.r. protocol could still be preferred to obtain the best test sensitivity. The βeta-s.t.a.r. 1 + 1 protocol is not only suitable for raw cows’ milk, but also could be used to test milk of species other than the cow (goat, ewe, mare).
Acknowledgements
The authors appreciate the valuable work performed by Veroniek de Paepe (T&V-ILVO) and Jorien Lambrecht (KaHo Sint-Lieven, Sint-Niklaas, Belgium) and thank Neogen Corporation for kindly providing βeta-s.t.a.r. test reagents, Tim Coolbear (Fronterra Co-operative Group Limited, Palmerston North, New Zealand) for correction of the manuscript, MelkControleCentrum Vlaanderen for providing part of the raw cows’ milk samples with a special composition or quality and for the MilcoScan 4000 and Fossomatic 5000 measurements, Siegrid de Baere (University of Gent) for the incurred milk samples used for testing of false-negative results and for the determination of penicillin G content of these incurred samples.
References
- Cacciatore , G , Petz , M , Rachid , S , Hakenbeck , R and Bergwerff , AA . 2004 . Development of an optical biosensor test for residues of β-lactam antibiotics in milk using the penicillin-binding protein PBP 2× . Anal Chim Acta , 520 : 105 – 115 .
- Comité du Lait . 2006 . Rapport des activités 2005 , Battice, , Belgium : Comité du Lait .
- Community Reference Laboratory for Antimicrobial Residues in Food from Animal Origin. 2008. Proficiency study for the analysis of beta-lactam residues in raw milk. November 2007–March 2008. Final report. Fougères (France): Community Reference Laboratory for Antimicrobial Residues in Food from Animal Origin, AFSSA Fougères.
- European Commission. 2002. Commission Decision (EC) No. 2002/657 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Comm. L221:8–36.
- European Commission. 2009. Council Regulation (EC) No. 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No. 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No. 726/2004 of the European Parliament and of the Council laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Off J Eur Union. L152:11–22.
- European Commission. 2010. Commission Regulation (EU) No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union. L15:1–72.
- European Federation of Animal Health (Fedesa) . 1998 . Survey of antimicrobial usage in animal health in the EU , Brussels, , Belgium : Fedesa .
- Food Safety Authority of Ireland . 2002 . Rapid test kits for the detection of antibiotics and sulphonamides in milk , Dublin (Ireland) : Food Safety Authority of Ireland .
- Grunwald L. 2002. Untersuchungen zur Analytik und zum Einfluss technologischer Prozesse auf Penicillinrückstände in Milch [PhD thesis]. [Wuppertal (Germany)]: Bergische Universität Wuppertal.
- Guay , R , Cardinal , P , Bourassa , C and Brassard , N . 1987 . Decrease of penicillin G residue incidence in milk: a fact or an artefact? . Int J Food Microbiol , 4 : 187 – 196 .
- Gustavsson , E , Bjurling , P and Sternesjö , Å . 2002 . Biosensor analysis of penicillin G in milk based on the inhibition of carboxypeptidase activity . Anal Chim Acta , 468 : 153 – 159 .
- Honkanen-Buzalski , T and Reybroeck , W . 1997 . Monograph on residues and contaminants in milk and milk products , Brussels, , Belgium : International Dairy Federation. . Chapter 4, Antimicrobials; 28–34
- Knecht , BG , Strasser , A , Dietrich , R , Märtlbauer , E , Niessner , R and Weller , MG . 2007 . Automated microarray system for the simultaneous detection of antibiotics in milk . Anal Chem , 73 : 646 – 654 .
- Kress , C , Seidler , C , Kerp , B , Schneider , E and Usleber , E . 2007 . Experiences with an identification and quantification program for inhibitor-positive milk samples . Anal Chim Acta , 586 : 275 – 279 .
- Kroll S. 2000. Zur Eignung von Schnelltestverfahren zum Rückstandsnachweis von Beta-laktam-Antibiotika in Milch. [PhD thesis]. [Munich (Germany)]: Ludwig-Maximilians-Universität.
- Kroll S, Usleber E, Zaadhof K-J, Schneider E, Märtlbauer E. 2000. Evaluation of commercial rapid tests for β-lactam antibiotics in raw milk. Paper presented at: Euroresidue IV Conference on Residues of Veterinary Drugs in Food; 8–10 May 2000; Veldhoven, the Netherlands.
- Lamar , J and Petz , M . 2007 . Development of a receptor-based microplate assay for the detection of beta-lactam antibiotics in different food matrices . Anal Chim Acta , 586 : 296 – 303 .
- Neaves P. 1999. Monitoring antibiotics in milk–the changing world of test methods. Paper presented at: British Mastitis Conference; 13 October 1999; Stoneleigh, UK.
- Ooghe , S and Reybroeck , W . 2007a . Report: Ringonderzoek antibiotica 24 mei 2007: microbiologische testen en sneltesten , Melle, , Belgium : T&V-ILVO .
- Ooghe , S and Reybroeck , W . 2007b . Report: Ringonderzoek antibiotica 18 oktober 2007: microbiologische testen en sneltesten , Melle, , Belgium : T&V-ILVO .
- Pyörälä , S . 1995 . The bovine udder and mastitis , Helsinki, , Finland : Faculty of Veterinary Medicine, University of Helsinki. . Chapter 2.3., Staphylococcal and streptococcal mastitis; 143–148
- Quandt B. 2006. Identifizierung von antimikrobiellen Rückständen in Milch mittels Schnelltestsystemen. [PhD thesis]. [Munich (Germany)]: Ludwig-Maximilians-Universität.
- Reybroeck W. 2000. Evaluation of the βeta-s.t.a.r. for the detection of β-lactam antibiotics. Paper presented at: 2nd International FoodSENSE Workshop; 30 March–2 April 2000; Zeven, Germany.
- Reybroeck , W . 2004 . Résidus d’antibiotiques dans le lait. Utilisation des kits de dépistage des inhibiteurs . Le Point Vétérinaire , 242 : 52 – 57 .
- Reybroeck , W . 2008 . The use of microbiological, immunological and receptor tests for monitoring of residues of antimicrobials in milk: the Belgian approach . Bull IDF , 428 : 13 – 16 .
- Reybroeck , W and Daeseleire , E . 2003 . Invloed bewaring op antibioticaresiduen in melk. Heranalyse positieve stalen en identificatie van remstoffen verantwoordelijk voor een ongunstige resultaat bij de officiële kwaliteitsbepaling van melk. Resultaten , Melle, , Belgium : T&V-ILVO .
- Reybroeck W, Ooghe S. 2004. Rapid screening for residues of antibiotics in milk at the factory. Paper presented at: IDF/FAO International Symposium on Dairy Safety and Hygiene. A Farm-to-Table Approach for Emerging and Developed Dairy Countries; 2–5 March 2003; Cape Town, South Africa.
- Reybroeck , W and Ooghe , S . 2006 . Gebruik van sneltesten als bevestigingstest bij de opsporing van bacteriegroeiremmende stoffen in melk in het kader van de officiële kwaliteitsbepaling van rauwe melk , Rapport voor het Wetenschappelijk Comité van het FAVV. Melle, , Belgium : T&V-ILVO .
- Reybroeck W, Ooghe S. 2008. Validation of the βeta-s.t.a.r. 1 + 1 for fast screening of raw milk on the presence of β-lactam antibiotics. Paper presented at: Euroresidue VI Conference on Residues of Veterinary Drugs in Food; 19–21 May 2008; Egmond aan Zee, the Netherlands.
- Royal Pharmaceutical Society of Great Britain . 2005 . Martindale: The complete drug reference , London (UK) : Royal Pharmaceutical Society of Great Britain, Pharmaceutical Press .
- Suhren , G . 1996 . Influence of residues of antimicrobials in milk on commercially applied starter cultures–model trials . Kieler Milchwirtschaftliche Forschungsberichte , 48 : 131 – 149 .
- Suhren G. 1997. Microbiologischer Hemmstofftest mit Escherichia coli zum nachweis von Chinolon-Rückständen in Milch. Paper presented at: 38. Arbeitstagung des Deutsches veterinairmedizinische Gesellschaft Lebensmittelhygiene, 29 September–2 October 1997; Garmisch Partenkirchen, Germany.
- Suhren , G and Heeschen , W . 1993 . Detection of tetracyclines in milk by a Bacillus cereus microtitre test with indicator . Milchwissenschaft , 48 : 259 – 263 .
- Suhren , G and Heeschen , W . 1996 . Detection of inhibitors in milk by microbial tests . A review Food/Nahrung , 40 : 1 – 7 .
- The European Agency for the Evaluation of Medicinal Products . 2000 . Committee for Veterinary Medicinal Products: Penethamate (summary report) , London (UK) : Veterinary Medicines Evaluation Unit, The European Agency for the Evaluation of Medicinal Products .
- Žvirdauskiené , R and Šalomskiené , J . 2007 . An evaluation of different microbial and rapid tests for determining inhibitors in milk . Food Contr , 18 : 541 – 547 .