Abstract
For the analysis of blue–green algal food supplements for cylindrospermopsin (CYN), a C18 solid-phase extraction column and a polygraphitized carbon solid-phase extraction column in series was an effective procedure for the clean-up of extracts. Determination of CYN was by liquid chromatography with ultraviolet light detection. At extract spiking levels of CYN equivalent to 25–500 µg g−1, blue–green algal supplement recoveries were in the range 70–90%. CYN was not detected in ten samples of food supplements and one chocolate product, all containing blue–green algae. The limit of detection for the method was 16 µg g−1, and the limit of quantification was 52 µg g−1.
Introduction
Cylindrospermopsin (CYN) was first obtained from Cylindrospermopsis raciborskii isolated from a reservoir in Australia where the water had caused human hepatotoxicity (Byth Citation1980; Bourke et al. Citation1983; Hawkins et al. Citation1985; Ohtani et al. Citation1992; Griffiths and Saker Citation2003; Falconer and Humpage Citation2006). CYN has been found to be genotoxic in in vitro systems and carcinogenic in mice (Falconer and Humpage Citation2006). It is now known to be a metabolite of several other freshwater cyanobacteria belonging to the genera Anabaena, Umezakia, Raphidiopsis, and Aphanizomenon (Falconer and Humpage Citation2006; Spoof et al. Citation2006) as well as more recently Lyngbya (Seifert et al. Citation2007). The related compounds deoxy-CYN and 7-epi-CYN have also been isolated as co-metabolites (Norris et al. Citation2001; Banker et al. Citation2000; Li et al. Citation2001a, Citation2001b; Seifert et al. Citation2007). Of particular interest is the formation of CYN by the species Aphanizomenon flos-aquae isolated from German lakes (Preuβel et al. 2006; Fastner et al. Citation2007) and which is harvested from natural blooms in Klamath Lake (Oregon, USA) to be marketed as a food supplement (Carmichael et al. Citation2000). Spoof et al. (Citation2006) reviewed levels of CYN in cyanobacteria; 2.3–6.6 mg g−1 of CYN have been found in lyophilized culture material of A. flos-aquae (Preuβel et al. 2006). More recently, 3.44–9.33 mg CYN g−1 was determined in freeze-dried A. ovalisporum and Cylindrospermopsis raciborskii (Yilmaz et al. Citation2008). It was therefore of interest to analyse algal supplements for CYN.
Procedures for the detection and determination of CYN isolated from water and cyanobacteria include enzyme-linked immunosorbent assay (ELISA) (Bláhová et al. Citation2009), liquid chromatography (LC) with ultraviolet (UV) detection (Harada et al. Citation1994; Li et al. Citation2001a, Citation2001b; Welker et al. Citation2002; Kubo et al. Citation2005; Spoof et al. Citation2006; Kokociński et al. 2009; Wormer et al. Citation2009), LC-mass spectrometry (MS) (Kubo et al. Citation2005), LC-MS/MS (Eaglesham et al. Citation1999; Li et al. Citation2001a, Citation2001b; Kikuchi et al. Citation2007; Bláhová et al. Citation2009; Gallo et al. Citation2009; Kokociński et al. 2009), hydrophilic interaction LC-MS (Dell’Aversano et al. Citation2004), and capillary electrophoresis (Vasas et al. Citation2004). CYN is not retained by C18 solid-phase extraction (SPE) adsorbents, but graphite columns do retain it, so they have been used for clean-up of water, usually in series with C18 SPE (Norris et al. Citation2001; Metcalf et al. Citation2002; Metcalf and Codd Citation2005; Wormer et al. Citation2009). An anion-exchange column was used by Kikuchi et al. (Citation2007), styrene polymer and anion exchange cartridges in series by Kubo et al. (Citation2005), and C18 and HP-20 polymer resin columns by Harada et al. (Citation1994) for analysis of algal cells.
There is no method previously reported for analysis of blue–green algal (BGA) food supplements for CYN. We have adapted a method that incorporates both a combined ‘in-series’ SPE system with a C18 column connected to a polygraphitized carbon (PGC) column and LC-UV. This method uses LC-UV rather than more expensive LC-MS and should be useful for screening these products for CYN.
Materials and methods
Extraction
Samples of BGA products with different brand names and from five manufacturers were purchased through the Internet. Their ingredient composition was variable and not all were 100% BGA. They included tablets, powder, capsules, food bars, and one sample of chocolates, which were ground with a coffee grinder and mixed. The BGA in most samples was stated to be A. flos-aquae or came from Klamath Lake, which would be this species (Carmichael et al. Citation2000). For each sample container, the complete contents were processed, and a representative subsample was taken for extraction. NRC-CRM-CYN stock solution (12.6 µg ml−1) was purchased from the Institute for Marine Biosciences (National Research Council, Halifax, NS, Canada). CYN working solutions with different concentrations were prepared by dilution of the stock solution with water. Methanol was LC grade. Formic acid and trifluoroacetic acid (TFA) were of analytical grade. The aqueous extraction solution was 5% formic acid. Water was doubly deionized. SPE columns were C18 (500 mg/3 ml, Supelco LC-18; Oakville, ON, Canada) and polygraphitized carbon (PGC) (HyperSep PGC, 100 mg/1 ml; Thermo Scientific, Waltham, MA, USA).
BGA products (0.4 g) were weighed into 15 ml polyethylene centrifuge tubes, and 6 ml of 5.0% aqueous formic acid added. The mixture was homogenized for 3 min using ultrasonication (Sonic Dismembrator Model 100; Fisher Scientific, Pittsburgh, PA, USA). After centrifuging at 11,000 rpm for 10 min (Eppendorf Centrifuge 5804R with F-34-6-38 rotor, Mississauga, ON, Canada), the supernatant was collected and transferred to a 15 ml glass graduated test tube. Another 3 ml of extraction solvent were added to the residue in the centrifuge tube, vortex mixed and centrifuged. The supernatants were combined and made up to a final volume of 10 ml with water. The crude extracts were stored in a refrigerator for repeat use if necessary.
Clean-up
Clean-up of an aliquot of the crude extract was performed by a combined ‘in-series’ SPE system with the C18 column connected to the PGC column. The SPE columns were conditioned with 10 ml methanol containing 0.1% (v/v) TFA, followed by 10 ml of water. A total of 2 ml of water were added to 0.25 ml of the crude extract of BGA (0.01 g of BGA powder equivalent) and the solution loaded onto the combined SPE system. A reservoir was attached to the top of the C18 column. The solution was passed through the columns at a flow rate of approximately 1 ml min−1. Hydrophobic compounds in the solution were removed by the C18 column. The combined system was washed with 9 ml of water. The columns were not allowed to go dry at any point. CYN eluted from the C18 column was adsorbed by the PGC column. After washing the columns with water, the PGC column was removed from the first (C18) column and eluted with 4 × 4 ml fractions of 0.1% TFA in methanol. On analysing each fraction, no CYN was found in the first two fractions (8 ml of eluted solvent), but it started to elute in the third fraction and was completely eluted after the fourth fraction. The 16 ml were collected in a glass container. A 4 ml aliquot of the eluate was evaporated under nitrogen. The residue was resuspended in 0.4 ml of water for LC-diode array detection (DAD) analyses. (As an alternative, we propose that the total eluate could be evaporated to dryness on a rotary evaporator with a water bath set at 40°C.)
Liquid chromatography (LC)
LC of CYN was carried out on an Agilent 1100 system which included a quaternary pump, autosampler, on-line vacuum degasser, and diode array detector (Agilent Technologies Canada, Mississauga, ON, Canada) with a 2.1 × 250 mm Genesis® AQ column (Grace Jones, 120 Å, 4.0 µm; Chromatographic Specialties, Brockville, ON, Canada) and attached guard C18 column. The column temperature was 35°C. The LC gradient consisted of two mobile phases: A, 5 mM ammonium acetate in water; and B, 5 mM ammonium acetate in methanol, used under the following conditions: 5–60% B for 10 min, then held at 60% B for the next 5 min; returned to 5% B over 0.5 min, then held for another 14.5 min with 5% B before the next injection. The injection volume was 20 µl, and the flow rate was 0.2 ml min−1. The detection wavelength was 262 nm. To make a calibration curve, CYN standard was added to a cleaned extract of Klamath BGA then diluted with the cleaned extract to make a series of concentrations. Calibration curves in the range 6.0–63 ng injected were linear (R 2 ≥ 0.9980). The LOD was about 2.0 ng CYN injected (signal-to-noise ratio = 3 : 1) and the LOQ was 6.5 ng CYN injected (signal-to-noise ratio = 10 : 1) in BGA supplements.
Method performance was checked by spiking an extract of Klamath BGA in which no CYN could be detected with different levels of CYN (i.e., a certain volume of CYN stock solution spiked in 0.25 ml BGA extract and 2 ml of water added, then the solution loaded onto the combined SPE system). The spiking levels of 25–500 µg g−1 (ppm) were calculated based on the 0.4 g of product which was extracted. Spikes were carried out in triplicate at 126 µg g−1 (i.e., 100 µl of CYN standard spiked in 0.25 ml BGA extract); other spiked experiments (19.8–400 µl of CYN standard spiked in 0.25 ml BGA extract) were single at 25, 100, 252 and 504 µg g−1. Recoveries (%) were calculated by the equation:
Results and discussion
Recoveries for SPE clean-up were in the range 70–90% for CYN and the standard deviation (SD) for the triplicate spike was acceptable ().
Table 1. SPE clean-up recoveries of CYN from blue–green algal food supplement extract.a
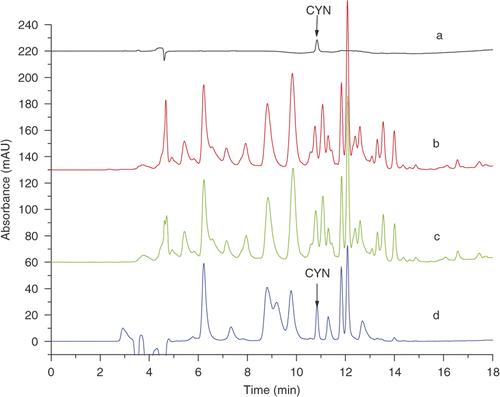
The C18 plus PGC double-column clean-up procedure provided an effective, reasonably accurate, and useful method for screening CYN in BGA food supplements by LC-UV. For other SPE columns – mixed ion exchange polymers (Oasis MCX; Waters), weak anion exchange (Oasis WAX; Waters) and NH2 – the breakthrough from these columns either during application of the extract solution or during the subsequent wash was unacceptably high. Different types of LC columns with dimension of 4.6 × 150 mm, such as Supelcosil LC-18, C18 Phenomenex Ultracarb column, and Zorbax C18, were also tried; however, even with clean-up by the double columns, co-eluted compounds affected the detection of CYN at 262 nm. Therefore, a longer column, Genesis AQ 120 Å, 2.1 × 250 mm, 4.0 µm, was selected for the LC-UV method development. Typical chromatograms are shown in . As shown in , the chromatograms indicate that quantitative analysis of CYN in crude BGA is very difficult due to the interference of co-eluted compounds close to CYN at R t = 10.85 min. In contrast, the chromatogram in indicates most of the interferences close to CYN were efficiently removed after clean-up by the double columns. These results showed that this clean-up was effective for the determination of CYN in complex BGA matrices.
Figure 1. LC-UV chromatograms of (a) CYN analytical standard, 10.5 ng injected; (b) crude BGA extract, 0.125 mg matrix-equivalent injected; (c) crude BGA extract spiked with CYN at 126 µg g−1, 0.125 mg matrix-equivalent injected; and (d) crude BGA extract spiked with CYN at 126 µg g−1, followed by C18 plus PGC clean-up, 0.125 mg matrix-equivalent injected.
We analysed ten algal food supplements and one sample of chocolates containing BGA, all of which were found to be free of CYN. The LOD of the overall method was 16 µg g−1 of food supplement, with LOQ = 52 µg g−1. Although we do not know the concentrations of algae in the supplements, 16 µg g−1 is a useful LOD as CYN can be present at mg g−1 concentrations in dried cyanobacteria, e.g. up to 6.6 mg g−1 dry weight in A. flos-aquae (Preuβel et al. 2006; Yilmaz et al. Citation2008).
The clean-up procedure using LC-UV for detection rather than the more expensive LC-MS should be useful for screening algal food supplements for CYN.
Acknowledgements
The authors are grateful to the Office of the Chief Scientist, Health Canada, for a Fellowship awarded to H.L.
References
- Banker , R , Teltsch , B , Sukenik , A and Carmeli , S . 2000 . 7-Epicylindrospermopsin, a toxic minor metabolite of the cyanobacterium Aphanizomenon ovalisporum from Lake Kinneret . Israel J Nat Prod , 63 : 387 – 389 .
- Bláhová , L , Oravec , M , Maršálek , B , Šejnohová , L , Šimek , Z and Bláha , L . 2009 . The first occurrence of the cyanobacterial alkaloid toxin cylindrospermopsin in the Czech Republic as determined by immunochemical and LC/MS methods . Toxicon , 53 : 519 – 524 .
- Bourke , ATC , Hawes , RB , Neilson , A and Stallman , ND . 1983 . An outbreak of hepato-enteritis (the Palm Island mystery disease) possibly caused by algal intoxication . Toxicon , 21 ( Suppl. 3 ) : 45 – 48 .
- Byth , S . 1980 . Palm Island mystery disease . Med J Australia , 2 : 40 – 42 .
- Carmichael , WW , Drapeau , C and Anderson , DM . 2000 . Harvesting of Aphanizomenon flos-aquae Ralfs ex Born. and Flah. var. flos-aquae (Cyanobacteria) from Klamath Lake for human dietary use . J Appl Phycol , 126 : 585 – 595 .
- Dell’Aversano , C , Eaglesham , GK and Quilliam , MA . 2004 . Analysis of cyanobacterial toxins by hydrophilic interaction liquid chromatography-mass spectrometry . J Chromatogr A , 1028 : 155 – 164 .
- Eaglesham , GK , Norris , RL , Shaw , GR , Smith , MJ , Chiswell , RK , Davis , BC , Neville , GR , Seawright , AA and Moore , MR . 1999 . Use of HPLC–MS/MS to monitor cylindrospermopsin, a blue–green algal toxin, for public health purposes . Environ Toxicol , 14 : 151 – 154 .
- Falconer , IR and Humpage , AR . 2006 . Cyanobacterial (blue–green algal) toxins in water supplies: cylindrospermopsins . Environ Toxicol , 21 : 299 – 304 .
- Fastner , J , Rücker , J , Stüken , A , Preuβel , K , Nixdorf , B , Chorus , I , Köhler , A and Wiedner , C . 2007 . Occurrence of the cyanobacterial toxin cylindrospermopsin in northeast Germany . Environ Toxicol , 22 : 26 – 32 .
- Gallo , P , Fabbrocino , S , Cerulo , MG , Ferranti , P , Bruno , M and Serpe , L . 2009 . Determination of cylindrospermopsin in freshwaters and fish tissue by liquid chromatography coupled to electrospray ion trap mass spectrometry . Rapid Commun Mass Spectrom , 23 : 3279 – 3284 .
- Griffiths , DJ and Saker , ML . 2003 . The Palm Island mystery disease 20 years on: a review of research on the cyanotoxin cylindrospermopsin . Environ Toxicol , 18 : 78 – 93 .
- Harada , K , Ohtani , I , Iwamoto , K , Suzuki , M , Watanabe , MF , Watanabe , M and Terao , K . 1994 . Isolation of cylindrospermopsin from a cyanobacterium Umezakia natans and its screening method . Toxicon , 32 : 73 – 84 .
- Hawkins , PR , Runnegar , MTC , Jackson , ARB and Falconer , IR . 1985 . Severe hepatotoxicity caused by the tropical cyanobacterium (blue–green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir . Appl Environ Microbiol , 50 : 1292 – 1295 .
- Kikuchi , S , Kubo , T and Kaya , K . 2007 . Cylindrospermopsin determination using 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES) as the internal standard . Anal Chim Acta , 583 : 124 – 127 .
- Kokociński , M , Dziga , D , Spoof , L , Stefaniak , K , Jurczak , T , Mankiewicz-Boczek , J and Meriluoto , J . 2009 . First report of the cyanobacterial toxin cylindrospermopsin in the shallow, eutrophic lakes of western Poland . Chemosphere , 74 : 669 – 675 .
- Kubo , T , Sano , T , Hosoya , K , Tanaka , N and Kaya , K . 2005 . A new simply and effective fractionation method for cylindrospermopsin analyses . Toxicon , 46 : 104 – 107 .
- Li , R , Carmichael , WW , Brittain , S , Eaglesham , G K , Shaw , GR , Liu , Y and Watanabe , MM . 2001a . First report of the cyanotoxins cylindrospermopsin and deoxycylindrospermopsin from Raphidiopsis curvata (Cyanobacteria) . J Phycol , 37 : 1121 – 1126 .
- Li , R , Carmichael , WW , Brittain , S , Eaglesham , GK , Shaw , GR , Mahakhant , A , Noparatnaraporn , N , Yongmanitchai , W , Kaya , K and Watanabe , MM . 2001b . Isolation and identification of the cyanotoxin cylindrospermopsin and deoxy-cylindrospermopsin from a Thailand strain of Cylindrospermopsis raciborskii (Cyanobacteria) . Toxicon , 39 : 973 – 980 .
- Metcalf , JS , Beattie , KA , Saker , ML and Codd , GA . 2002 . Effects of organic solvents on the high performance liquid chromatographic analysis of the cyanobacterial toxin cylindrospermopsin and its recovery from environmental eutrophic waters by solid phase extraction . FEMS Microbiol Lett , 216 : 159 – 164 .
- Metcalf , JS and Codd , GA . 2005 . “ Standard operating procedures for cylindrospermopsin ” . In TOXIC: Cyanobacterial monitoring and cyanotoxin analysis , Edited by: Meriluoto , J and Codd , GA . 121 – 136 . Turku (Finland) : Åbo Akademi University Press .
- Norris , RLG , Eaglesham , GK , Shaw , GR , Senogles , P , Chiswell , RK , Smith , MJ , Davis , BC , Seawright , AA and Moore , MR . 2001 . Extraction and purification of the zwitterions cylindrospermopsin and deoxycylindrospermopsin from Cylindrospermopsis raciborskii . Environ Toxicol , 16 : 391 – 396 .
- Ohtani , I , Moore , RE and Runnegar , MTC . 1992 . Cylindrospermopsin: a potent hepatotoxin from the blue–green alga Cylindrospermopsis raciborskii . J Amer Chem Soc , 114 : 7941 – 7942 .
- Preuβel , K , Stüken , A , Wiedner , C , Chorus , I and Fastner , J . 2006 . First report on cylindrospermopsin producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes . Toxicon , 47 : 156 – 162 .
- Seifert , M , McGregor , G , Eaglesham , G , Wickramasinghe , W and Shaw , G . 2007 . First evidence for the production of cylindrospermopsin and deoxy-cylindrospermopsin by the freshwater benthic cyanobacterium, Lyngbya wollei (Farlow ex Gornont) Speziale and Dyck . Harmful Algae , 6 : 73 – 80 .
- Spoof , L , Berg , KA , Rapala , J , Lahti , K , Lepistö , L , Metcalf , JS , Codd , GA and Meriluoto , J . 2006 . First observation of cylindrospermopsin in Anabaena lapponica isolated from the boreal environment (Finland) . Environ Toxicol , 21 : 552 – 560 .
- Vasas , G , Gáspár , A , Páger , C , Surányi , G , Máthé , C , Hamvas , MM and Borbely , G . 2004 . Analysis of cyanobacterial toxins (anatoxin-a, cylindrospermopsin, microcystin-LR) by capillary electrophoresis . Electrophoresis , 25 : 108 – 115 .
- Welker , M , Bickel , H and Fastner , J . 2002 . HPLC-PDA detection of cylindrospermopsin – opportunities and limits . Water Res , 36 : 4659 – 4663 .
- Wormer , L , Carrasco , D , Cirés , S and Quesada , A . 2009 . Advances in solid phase extraction of the cyanobacterial toxin cylindrospermopsin . Limnol Oceanogr Meth , 7 : 568 – 575 .
- Yilmaz , M , Phlips , EJ , Szabo , NJ and Badylak , S . 2008 . A comparative study of Florida strains of Cylindrospermopsis and Aphanizomenon for cylindrospermopsin production . Toxicon. , 51 : 130 – 139 . erratum. Toxicon. 2008 52:594–595