Abstract
A sensitive and selective isotope dilution ion chromatography/tandem mass spectrometry (ID IC-MS/MS) method was developed and validated for the determination of perchlorate in infant formula. The perchlorate was extracted from infant formula by using 20 ml of methanol and 5 ml of 1% acetic acid. All samples were spiked with 18O4 isotope-labelled perchlorate internal standard prior to extraction. After purification on a graphitised carbon solid-phase extraction column, the extracts were injected into an ion chromatography system equipped with an Ionpac AS20 column for separation of perchlorate from other anions. The presence of perchlorate in samples was quantified by isotope dilution mass spectrometry. Analysis of both perchlorate and its isotope-labelled internal standard was carried out on a Waters Quattro Ultima triple quadrupole mass spectrometer operating in a multiple reaction monitoring (MRM) negative ionisation mode. The method was validated for linearity and range, accuracy, precision, sensitivity, and matrix effects. The limit of quantification (LOQ) was 0.4 µg l−1 for liquid infant formula and 0.95 µg kg−1 for powdered infant formula. The recovery ranged from 94% to 110% with an average of 98%. This method was used to analyse 39 infant formula, and perchlorate concentrations ranging from <LOQ to 13.5 µg l−1.
Introduction
Perchlorate is an environmental contaminant that occurs through both anthropogenic processes and natural sources. It can originate from the use of perchlorate salts in military and industrial products such as solid rocket fuels, munitions, explosives and fireworks, road flares, air bag inflation systems, and some fertilisers (Susala et al. Citation1999; Motzer Citation2001; Dasgupta et al. Citation2006). It also can be found in places near potash deposits and in arid regions (Rao et al. Citation2007). Exposure to perchlorate is associated with potential health effects including disruption of the thyroid function by competitively inhibiting iodide transport (Urbansky Citation2002; Kirk Citation2006). Thyroid hormones are responsible for regulating metabolic and developmental function and are critical for normal foetal and neonatal development. Consequently, pregnant women and their foetuses, infants, and people with iodine deficiency or with thyroid dysfunction are particularly at risk to perchlorate exposure.
Dairy products have been associated with human exposure to perchlorate (Rice et al. Citation2007), and infants, in particular, can be exposed to perchlorate through bovine milk, human breast-milk (Gindberg et al. Citation2007; Rice et al. Citation2007), and infant formula (Schier et al. Citation2010). Kirk et al. (Citation2003, Citation2005) reported the presence of perchlorate in all 47 dairy milk samples collected from 11 states and in 35 of 36 human milk samples collected from 18 states in United States. The maximum values of perchlorate in dairy and human milk were 11 and 92 µg l−1, with means of 2.0 and 10.5 µg l−1, respectively (Kirk et al. Citation2005). Since the initial report by Kirk et al. (Citation2003), perchlorate has been detected in dairy milk and human milk samples collected nationally and internationally. In two later studies conducted by the same group, perchlorate was detected in human milk at levels ranging from 0.5 to 40 µg l−1 (n = 110) (Kirk et al. Citation2007) and from 0.01 to 48 µg l−1 (n = 457) (Dasgupta et al. Citation2008). Dyke et al. (Citation2007) found the perchlorate in dairy milk samples collected from 48 different locations in Japan (9.4 ± 2.7 µg l−1, n = 54). Pearce et al. (Citation2006) detected perchlorate in all 49 human milk samples with a mean of 33 ± 77 µg l−1 in US women, ranging from 1.3 to 411 µg l−1. Shi et al. (Citation2007) reported perchlorate concentrations ranging from 0.69 to 7.62 µg l−1 in 17 milk samples collected from Beijing, China. In 2005, the US Food and Drug Administration (USFDA) launched a study to determine perchlorate levels in total diet study (TDS) samples, and a mean of 5.8 µg l−1 of perchlorate was reported in 125 dairy milk samples (Murray et al. Citation2008). The recent discovery of perchlorate in infant formula samples by the US Center of Disease Control (CDC) gave rise to an intense public concern regarding infant exposure to perchlorate (Schier et al. Citation2010).
A variety of analytical methods have been developed for the determination of perchlorate. However, ion chromatography (IC) coupled with conductivity cell detection (CD) is the most common approach (Anderson and Wu Citation2002), and it is the first method approved by the US Environmental Protection Agency (USEPA) for the determination of perchlorate in drinking water (USEPA Citation1999). Although USEPA Method 314.0 is widely used for the determination of trace levels of perchlorate in water, the method has some challenges for the analysis of perchlorate in more complex matrices, such as a higher likelihood of false-positive results and a lack of selectivity (Yu et al. Citation2006). In order to determine trace levels of perchlorate in complex matrices, IC was coupled to tandem mass spectrometry (MS/MS), taking advantage of the high selectivity and sensitivity of mass spectrometry operated in multiple reactions monitoring (MRM) mode. Accordingly, the USEPA approved another method based on IC-MS or IC-MS/MS for the determination of perchlorate in drinking water (USEPA Citation2005). IC-MS/MS was demonstrated to yield limits of detection (LOD) of 5–25 ng l−1 when determining perchlorate in a variety of matrices, including water, urine, amniotic fluid, wine and food (El Aribi et al. Citation2006; Krynitsky et al. Citation2006; Snyder et al. Citation2006). Several methods based on IC-MS or IC-MS/MS were developed to determine perchlorate in dairy milk and milk (Kirk et al. Citation2005; Dyke et al. Citation2006; Sanchez et al. Citation2008). Though determination of perchlorate in infant formula has been reported by two recent studies (Pearce et al. Citation2006; Schier et al. Citation2010), there is no systematic study on the development and validation of a highly sensitive and selective method for determination of perchlorate in infant formula.
In order to estimate the possible human exposure to perchlorate through the consumption of food in Canada, Health Canada began to investigate the occurrence of perchlorate in fruits and vegetables in 2005, and a quantitative ID IC-MS/MS method was developed and validated (Wang et al. Citation2009). As a continuous study of perchlorate surveillance, the first objective of the present work was to develop an ID IC-MS/MS method enabling accurate identification and quantification of perchlorate in infant formula. A new ID IC-MS/MS method was developed, with a new sample preparation scheme, including sample preparation, clean-up and chromatographic separation. The method was validated by assessing its linearity and range, accuracy, precision, LOD, LOQ and matrix effects for determination of perchlorate in infant formula. Although the determination of perchlorate in infant formula is particularly important because of its potential health impact on infants and children, there is no such study available for Canadians. Therefore, the second objective of this study was to determine the occurrence and concentration of perchlorate in 39 infant formula samples collected from Ottawa, Ontario, Canada.
Materials and methods
Reagents and standards
Acetonitrile and methanol (Ominisolv grade) were purchased from EMD Chem., Inc. (Gibbstown, NJ, USA). Acetic acid (ACS reagent grade) was obtained from Sigma (St Louis, MO, USA). Isotope-labelled sodium perchlorate (Cl18O4, isotope purity >90 atom%) was purchased from Icon Stable Isotopes (Mt. Marion, NY, USA). Sodium perchlorate (NaClO4, >98.0%) as sodium perchlorate monohydrate was purchased from Fluka (Buchs, Switzerland). Deionised water prepared using a Barnstead Diamond nano-pure-grade water purification system (Barnstead Intern., Dubuque, IA, USA) was used to prepare all the solutions.
Sample collection and preparation
Infant formula samples (n = 39), including powdered and liquid formula, were purchased from different retail food outlets in Ottawa from August to October 2008. Products were purchased on the basis of their immediate availability. Both milk- and soy-based formula were selected for analysis.
A total of 5 g of each sample were transferred into 50 ml polypropylene centrifuge tubes. To each sample, 45 µl of 4.0 mg l−1 18O4-labelled perchlorate were added as an internal standard to correct for matrix effects on measured signals. After adding 20 ml of methanol and 5 ml of 1% acetic acid, the centrifuge tubes were capped and shaken on a tumbler for 10 min. Sample tubes were then centrifuged at 6000 rpm for 20 min at ambient temperature. The supernatant was cleaned on a graphitised carbon solid-phase extraction (SPE) column. The Supelclean ENVI-Carb SPE column (Supelco, Bellefonte, PA, USA) was conditioned with 5 ml of methanol, followed by 5 ml of 1% acetic acid. After drying the column for approximately 30 s under a vacuum of about 20 mm Hg, a 6 ml aliquot of supernatant was driven through the dried cartridge at approximately two to three drops per second under the vacuum. The eluent was collected, and an aliquot of the eluent was then filtered through a 0.20 µm Acrodisc polyethersulfone (PES) syringe filter (Pall Life Sciences, NY, USA) into a 1.5 ml autosampler vial. The filtered samples were stored at 4°C until analysis. For quality-control purposes, reagent blanks and spiked matrix samples containing 20–50 µl of 0.1 mg l−1 native perchlorate were analysed for each batch. One duplicate sample was analysed for each batch of 12 samples.
ID IC-MS/MS system
A Dionex ICS2000 (Dionex, Sunnyvale, CA, USA) system coupled to a Waters Quattro Ultima triple quadrupole mass spectrometer (Manchester, UK) with Z-spray electrospray (ESI) interface was used for perchlorate detection and quantification. The IC system was a compact mode consisting of an AS50 autosampler and an analytical column (AS20, 2 × 250 mm; Dionex). A guard column (AG20, 2 × 50 mm; Dionex) was installed before the analytical column. The mobile phase containing 55 mM KOH was generated by an ICS2000 reagent-free system from a KOH eluent generator cartridge. An ASRS (ASRS 300, 2 mm; Dionex)-regenerating suppressor membrane device was connected between the IC and MS/MS to remove the KOH from the eluent. The injection volume was 100 µl. A six-port Rheodyne model MX9900-000 (Rheodyne, Rohnert Park, CA, USA) divert valve was used to divert the eluent to waste for the first 6 min of each chromatographic run (total of 20 min). The operational parameters of ESI MS/MS were as follows: polarity, negative-ion mode; capillary voltage, −2.5 kV; cone voltage, 40 V; source temperature, 140°C; desolvation temperature, 350°C; cone gas flow, 200 l h−1; desolvation gas flow, 600 l h−1; ion energy for quadrupole 1, 0.5 eV; and ion energy for quadrupole 2, 1.5 eV.
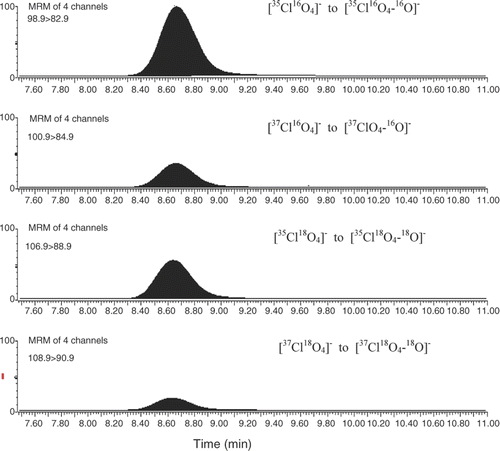
The presence of perchlorate in samples was quantified by isotope dilution mass spectrometry. Four transitions were monitored under an MRM-negative ionisation mode: m/z 98.9 → 82.9 and 100.9 → 84.9 for [35Cl16O4]− to [35Cl16O4–16O]− and [37Cl16O4]− to [37Cl16O4–16O]−, respectively; m/z 106.9 → 88.9 and 108.9 → 90.9 for [35Cl18O4]− to [35Cl18O4–18O]− and [37Cl18O4]− to [37Cl18O4–18O]−, respectively.
Method validation
The validation of the method was carried out by determination and assessment of the following performance parameters: linearity and range, accuracy, precision, LOD, LOQ, and matrix effects. Linearity and range were determined by linear regression analysis of the calibration curve with 1/x-axis weighting. The calibration curve was constructed by plotting the response factors (RF) of calibration standard solutions versus perchlorate concentrations. Calibration standard solutions were prepared at seven concentration levels (0.1, 1.0, 2.0, 5.0, 10.0, 25.0, 50 µg l−1
), each containing 6 µg l−1 of the 18O4-labelled internal standard. RF is the response ratio between the quantitation ion of the native perchlorate and the internal standard. For each standard solution, RF was calculated according to:
After linearity and range were determined from the calibration curve, the perchlorate concentration in samples was calculated using:
The accuracy was determined at three perchlorate concentration levels by calculating the percentage recovery of perchlorate spiked in infant formula. Precision was calculated based on duplicate analyses of spiked samples at three perchlorate concentration levels that were analysed in the same day (repeatability) and on different days (reproducibility). LOD and LOQ were determined based on a signal-to-noise requirement of 3:1 and 9:1, respectively. Matrix effects were examined according to the approach proposed by Matuszewski et al. (Citation2003). Briefly, the matrix effects were evaluated by comparing the MS/MS response (peak areas or peak heights) of a spiked analyte in sample matrix at any given concentration with the MS/MS response of the same analyte present in the reagent blank.
Data analysis
QuanLynx (Version 4.0; Waters Corp., Milford, MA, USA) was used to analyse mass spectrometry data. Concentrations were expressed as mean ± standard deviation (SD).
Results and discussion
Sample preparation
The determination of perchlorate in infant formula is complicated by the concurrent presence of large amounts of fats, proteins, carbohydrates and other salts. Schier et al. (Citation2010) used cold ethanol to precipitate the milk proteins. After centrifugation, the supernatant was transferred in a new tube and dried by nitrogen evaporation. The dried pellet was then redissolved in water and cleaned by a C-18 cartridge. In another infant formula study, Pearce et al. (Citation2006) analysed the infant formula samples directly (Valentn-Blasini et al. Citation2005). In our study, to enable accurate identification and quantification of trace levels of perchlorate, we utilised three approaches during sample preparation to mitigate the matrix effects, including extraction, SPE clean-up and the use of a stable isotope-labelled internal standard.
Ideally, only perchlorate would be completely extracted from infant formula, while other components of the matrix would be excluded from the extract. The extraction solution was selected to consist of 4 vols of organic solvent and 1 vol. of 1% acetic acid. The role of organic solvent is to precipitate proteins and the role of 1% acetic acid is to dissolve perchlorate (Krynitsky et al. Citation2004, Citation2006). We compared differences in extraction efficiency between 20 ml acetonitrile + 5 ml 1% acetic acid (solution A) and 20 ml methanol + 5 ml 1% acetic acid (solution B). A total of 30 µl of 1.00 mg l−1 perchlorate and 45 µl of 4.00 mg l−1 18O4-labelled perchlorate were spiked into two aliquots of one infant formula sample. These two fortified aliquots were then extracted by using solution A and B, respectively. Because perchlorate and 18O4-labelled perchlorate were spiked into formula samples before extraction, the two extracts were called pre-extraction spikes. Additionally, another two aliquots of the same infant formula sample were first extracted with the two different extract solutions, and then the same amounts of perchlorate and 18O4-labelled perchlorate were spiked into the two extracts, and these two extracts were considered post-extraction spikes. Perchlorate and 18O4-labelled perchlorate peak areas after duplicate analyses of these four spike samples by IC-MS/MS are listed in . Absolute recovery, defined as:
Table 1. Comparison of differences in extraction efficiency between solution A and B.
A graphitised carbon SPE column was used further to remove proteins, lipids, and carbohydrates from the supernatant after extraction and centrifugation at 6000 rpm. Compared with reversed-phase SPE columns, graphitised carbon columns have a strong affinity not only for hydrophobic substances such as lipids and proteins, but also for hydrophilic substances such as carbohydrates (Hennion Citation2000; Forgacs Citation2002). Therefore, it is able to adsorb proteins, lipids, and carbohydrates onto the graphitised carbon support and still elute the perchlorate, when an aliquot of supernatant is passed through the column.
The addition of an isotope-labelled perchlorate internal standard (InStd) prior to extraction also aided in compensating for matrix effects. Because the InStd and analyte are chemically equivalent, they present the same behaviour during sample preparation and are affected in the same way by sample preparation conditions. This is confirmed in , which demonstrates that the InStd and native perchlorate presented equivalent performance for extraction efficiency despite the use of different extraction solutions. Another advantage of the use of InStd is that possible variation in sample volume during sample preparation steps will have little or no influence on the final determination results.
ID IC-MS/MS performance
Ion-exchange chromatography systems are frequently employed to separate perchlorate from other anions, since the perchlorate ion is negatively charged. In the present method we chose an Ionpac AS20 hydroxide selective anion-exchange column as the separation column because it has a high capacity and is highly compatible with the reagent-free ion chromatography (RFIC) technology for automatic eluent generation. Analysis of both perchlorate and its InStd was performed using a triple quadrupole mass spectrometer operating in the MRM negative ion mode. shows the typical MRM mass chromatograms obtained from the perchlorate analysis. Four transitions from both 35Cl and 37Cl containing species were monitored: m/z 98.9 → 82.9 and 100.9 → 84.9 for [35Cl16O4]− to [35ClO4–16O]− and [37Cl16O4]− to [37ClO4–16O], respectively; m/z 106.9 → 88.9 and 108.9 → 90.9 for [35Cl18O4]− to [35Cl18O4–18O]− and [37Cl18O4]− to [37Cl18O4–18O]−, respectively. Transitions from 35Cl containing species were monitored to quantify the levels of perchlorate, while transitions from 37Cl containing species were monitored to improve the selectivity of the analysis. For quality-control purposes, the peak width of native ion pairs must be the same as the peak width of the internal standard ion pair.
Method validation
Linearity and range
A concentration range from the LOQ to 50 ng l−1 was selected for the linear range. The upper range was arbitrarily decided and samples containing perchlorate beyond the calibration range would be diluted and reanalysed. The correlation coefficient (r 2) obtained from linear regression analysis was calculated for each batch of samples. r 2 ranged from 0.9998 to 1.00 (n = 8), representing a good linearity within this range and effective quantification of the method.
Accuracy and precision
No certified reference material (CRM) was available for analysis of perchlorate in infant formula samples. Therefore, the accuracy and precision tests were conducted by using fortified samples. A preliminary screening of samples was first performed to select an infant formula with a relatively low level of perchlorate to be used as the blank sample. Three replicates of this sample were analysed to determine the background concentration of perchlorate. This background concentration of perchlorate was subtracted in the recovery calculations. Test samples were fortified with native perchlorate at 0.4, 10, 50 µg l−1 for liquid formula and 2, 10 and 50 µg l−1 for powdered infant formula. Four replicates at each level were analysed. The results of the study are summarised in , representing excellent recovery over the range of fortified concentrations and excellent precision among the replicate analyses. The accuracy and precision study demonstrated that this method is suitable for the accurate determination of the perchlorate. Evaluation of accuracy and precision was also conducted during real sample analysis. Eleven matrix-fortified samples were analysed during real sample analysis in different batches, and the recovery ranging from 94% to 110% was obtained with a mean of 98%. The precision, expressed as relative standard deviation (RSD) for those intraday analyses, was 5.88%.
Table 2. Accuracy and precision of the method.
Limit of quantitation
LOD is often defined as the concentration giving a signal equal to three times the baseline noise, and LOQ is similarly defined as the concentration giving a signal equal to nine times the baseline noise. The peak-to-peak baseline noise was measured using baseline signals adjacent to the retention time of analyte peak. LOQs were calculated as 0.40 µg l−1 and 0.95 µg g−1 for liquid infant formula and powered infant formula, respectively.
Matrix effect
The matrix effect, expressed as matrix recovery, was examined by comparing the MS/MS response (peak areas or peak heights) of perchlorate in post-extraction spike with the MS/MS response of the same concentration of perchlorate in the reagent blank (blank spike). Duplicate analyses were conducted, and the matrix recoveries as shown in suggested that the matrix effect of infant formula is quite low after the sample preparation steps. Our experiments of other baby food products indicated more matrix effects (data not shown); however, those matrix effects can be further corrected by the use of isotope dilution in IC-MS/MS analysis.
Application of the method
A total of 39 infant formula samples were purchased from local supermarkets and analysed for perchlorate levels using the developed method. The results are summarised in . Results were presented in two ways: perchlorate concentration (ng g−1) in samples as sold and perchlorate concentration (µg l−1) in serving solutions prepared according to the manufacturer's recommendation. Perchlorate concentrations in serving solution were used to compare with other reported perchlorate levels in infant formula. Perchlorate was detected in 37 of 39 of the tested formula samples with concentrations ranging from <LOQ to 13.5 µg l−1. The perchlorate concentrations in nine soy-based samples ranged from 0.21 to 1.27 µg l−1, and the mean was 0.52 ± 0.37 µg l−1. The perchlorate concentrations in 30 milk-based samples ranged from <LOQ to 13.5 µg l−1, and the mean was 2.64 ± 3.07 µg l−1. Soy-based infant formula had relatively lower perchlorate levels than milk-based infant formula, which is comparable with the results of Schier et al. (Citation2010).
Table 3. Summary of perchlorate concentrations in 39 infant formula samples.
The percentile distribution of perchlorate concentrations in all infant formula samples is shown in . Approximately 40% of the samples had perchlorate levels equal to or less than 1 µg l−1, and 90% of the samples had perchlorate levels less than 3.64 µg l−1. However, there were three samples containing relatively high concentration of perchlorate at the levels of 13.5, 8.17, and 11.5 µg l−1. It was noticed that these three samples were all for toddler use and labelled as high in iron and calcium, and also had various nutritional supplements, which suggested that the addition of those ingredients may have introduced additional perchlorate into the infant formula or may have induced severe matrix effects that could lead to inaccurate results. A standard addition experiment was conducted to verify further the perchlorate concentrations of these samples. These three samples and two other samples with lower levels of perchlorate were each spiked with native perchlorate at 0, 5, 10, 15 and 20 µg l−1. These results are shown in . Apparently, all perchlorate levels obtained by the standard addition method were within 20% deviation from the corresponding levels obtained by isotope dilution method, which suggested that the high levels of perchlorate in these three samples were likely due to the addition of more perchlorate containing ingredients into the toddler formula.
Table 4. Percentile distribution of perchlorate concentrations (µg l−1) in the 39 infant formula samples.
Table 5. Comparison in quantifications of perchlorate between the standard addition (SA) and isotope dilution methods.
Conclusion
An ID IC-MS/MS method was developed and validated for the accurate identification and quantification of perchlorate in infant formula. The method was optimised and evaluated for sample preparation, instrument analysis and method performance. The combination of extraction, SPE purification and the use of 18O4-labelled internal standard greatly minimised the matrix effects on the determination of perchlorate, and the use of tandem mass spectrometry in the MRM mode further improved the sensitivity and selectivity of the method. The determination of perchlorate in infant formula demonstrated the applicability of the method to sample analysis. Further work will be conducted to apply this method on other food samples.
Acknowledgments
The authors would like to thank Dr Sheryl Tittlemier and Dr Xu-liang Cao (Food Research Division, Health Canada) for providing infant formula information and samples; as well as Dr Adam Becalski, Dr Thea Rawn and Dr Rudolf Krska for providing helpful comments to the manuscript.
References
- Anderson , TA and Wu , TH . 2002 . Extraction, clean-up, and analysis of the perchlorate anion in tissue samples . Bull Environ Contam Toxicol , 68 : 684 – 691 .
- Dasgupta , PK , Jason , VD , Kirk , AB and Jackson , WA . 2006 . Perchlorate in the United States . Analysis of relative source contributions to the food chain. Environ Sci Technol , 40 : 6608 – 6614 .
- Dasgupta , PK , Kirk , AB , Dyke , JV and Ohira , S . 2008 . Intake of iodine and perchlorate and excretion in human milk . Environ Sci Technol , 42 : 8115 – 8121 .
- Dyke , JV , Ito , K , Obitsu , T , Hisamatsu , Y , Dasgupta , PK and Blount , BC . 2007 . Perchlorate in dairy milk, comparison of Japan versus the United States . Environ Sci Technol , 41 : 88 – 92 .
- Dyke , JV , Kirk , AB , Martinelango , PK and Dasgupta , PK . 2006 . Sample processing method for the determination of perchlorate in milk . Anal Chim Acta , 567 : 73 – 78 .
- El Aribi , H , Le Blanc , YJC , Antosen , S and Sakuma , T . 2006 . Analysis of perchlorate in foods and beverages by ion chromatography coupled with tandem mass spectrometry (IC-ESI-MS/MS) . Anal Chim Acta , 567 : 39 – 47 .
- Forgacs , E . 2002 . Retention characteristics and practical applications of carbon sorbents . J Chrom A , 975 : 229 – 243 .
- Gindberg , GL , Hattis , DB , Zoeller , TR and Rice , DC . 2007 . Evaluation of the U.S. EPA/OSWER preliminary remediation goal for perchlorate in groundwater: focus on exposure to nursing infants . Environ Health Perspect , 115 : 361 – 369 .
- Hennion , M-C . 2000 . Graphitized carbons for solid-phase extraction . J Chrom A , 885 : 73 – 95 .
- Kirk , AB . 2006 . Environmental perchlorate: why it matters . Anal Chim Acta , 567 : 4 – 12 .
- Kirk , AB , Dyke , JV , Martin , CF and Dasgupta , PK . 2007 . Temporal patterns in perchlorate, thiocyanate, and iodide excretion in human milk . Environ Health Perspect , 115 : 182 – 186 .
- Kirk , AB , Martinelango , K , Dutta , A , Tian , K , Smith , EE and Dasgupta , PK . 2005 . Perchlorate and iodide in dairy and breast milk . Environ Sci Technol , 39 : 2011 – 2017 .
- Kirk , AB , Smith , EE , Tian , K , Anderson , TA and Dasgupta , PK . 2003 . Perchlorate in milk . Environ Sci Technol , 37 : 4979 – 4981 .
- Krynitsky , AJ , Niemann , RA and Nortrup , DA . 2004 . Determination of perchlorate anion in foods by ion chromatography-tandem mass spectrometry . Anal Chem , 76 : 5518 – 5522 .
- Krynitsky , AJ , Niemann , RA , Williams , AD and Hopper , ML . 2006 . Streamlined sample preparation procedure for determination of perchlorate anion in foods by ion chromatography-tandem mass spectrometry . Anal Chim Acta , 567 : 94 – 99 .
- Matuszewski , BK , Constanzer , ML and Chavez Eng , CM . 2003 . Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS . Anal Chem , 75 : 3019 – 3030 .
- Motzer , WE . 2001 . Perchlorate: problems, detection, and solutions . Environ Forensics , 2 : 301 – 311 .
- Murray , CW , Egan , SK , Kim , H , Beru , N and Bolger , PM . 2008 . US Food and Drug Administration's total diet study: dietary intake of perchlorate and iodine . J Expos Sci Environ Epidem , 962 : 1 – 10 .
- Pearce , EN , Leung , AM , Blount , BC , Bazrafshan , HR , He , X , Pino , S , Valentin-Blasini , L and Braverman , LE . 2006 . Breast milk iodine and perchlorate concentrations in lactating Boston-area women . J Clin Endocrin Metabol , 92 : 1673 – 1677 .
- Rao , B , Anderson , TA , Orris , GJ , Rainwater , KA , Rajagopalan , S , Sandvig , RM , Sganlon , BR , Stonestrom , DA , Walvoord , MA and Jackson , WA . 2007 . Widespread natural perchlorate in unsaturated zones of the southwest United States . Environ Sci Technol , 41 : 4522 – 4528 .
- Rice , CP , Baldwin , VIR , Abbott , LC , Hapeman , CJ , Capuco , AV , Le , A , Bialek-Kalinsk , K , Bannerman , DD , Hare , W Paape , MJ . 2007 . Predicting perchlorate exposure in milk from concentration in dairy feed . J Agric Food Chem , 55 : 8806 – 8813 .
- Sanchez , CA , Blount , BC , Valentin-Blasini , LV , Lesch , SM and Krieger , RI . 2008 . Perchlorate in the feed-dairy continuum of the southwestern United States . J Agric Food Chem , 56 : 5443 – 5450 .
- Schier , JG , Wolkin , AF , Valentin-Blasini , L , Belson , MG , Kieszak , SM , Rubin , CS and Blount , BC . 2010 . Perchlorate exposure from infant formula and comparisons with the perchlorate reference dose . J Exp Sci Environ Epidem , 20 : 281 – 287 .
- Shi , Y , Zhang , P , Wang , Y , Shi , J , Cai , Y , Mou , S and Jiang , G . 2007 . Perchlorate in sewage sludge, rice, bottled water and milk collected from different areas in China . Environ Intern , 33 : 955 – 962 .
- Snyder , SA , Pleus , RC , Vanderford , BJ and Holady , JC . 2006 . Perchlorate and chlorate in dietary supplements and flavour enhancing ingredients . Anal Chim Acta , 567 : 26 – 32 .
- Susala , S , Collette , TW , Garrison , AW , Wolfe , NL and McCutcheon , SC . 1999 . Perchlorate identification in fertilizers . Environ Sci Technol , 33 : 3469 – 3472 .
- Urbansky , ET . 2002 . Perchlorate as an environmental contaminant . Environ Sci Pollut Res , 9 : 187 – 192 .
- US Environmental Protection Agency (USEPA). 1999. Method 314.0 determination of perchlorate in drinking water using ion chromatography. Revision 1.0. Cincinnati (OH): USEPA
- US Environmental Protection Agency (USEPA). 2005. Method 331.0 determination of perchlorate in drinking water by liquid chromatography electrospray ionization mass spectrometry. Revision 1.0. Cincinnati (OH): USEPA
- Valentn-Blasini , L , Mauldin , JP , Maple , D and Blount , BC . 2005 . Analysis of perchlorate in human urine using ion chromatography and electrospray tandem mass spectrometry . Anal Chem , 77 : 2475 – 2481 .
- Wang , Z , Forsyth , D , Lau , PY , Pelletier , L , Bronson , R and Gartner , D . 2009 . Estimated dietary exposure of Canadians to perchlorate through the consumption of fruits and vegetables available in Ottawa markets . J Agric Food Chem , 57 : 9250 – 9255 .
- Yu , L , Cheng , Q , Canas , J , Valentin-Blasini , L , Blount , BC and Anderson , T . 2006 . Challenges in determining perchlorate in biological tissues and fluids: implications for characterizing perchlorate exposure . Anal Chim Acta , 567 : 66 – 72 .