Abstract
Acrylamide concentrations in prune products – baby strained prunes (range = 75–265 µg kg−1), baby apple/prune juice (33–61 µg kg−1), prune juice (186–916 µg kg−1) and prunes (58–332 µg kg−1) – on the Canadian market were determined. The formation of acrylamide in a simulated plum juice was also investigated under ‘drying conditions’ in an open vessel at temperatures <100°C for 24 h and under ‘wet conditions’ in a closed vessel at a temperature of 120°C for 1 h. Acrylamide was produced in a simulated plum juice under ‘drying conditions’ in amounts comparable with those found in prunes and prune juices. Acrylamide was not produced in simulated plum juice under ‘wet conditions’ in a closed vessel at temperature of 120°C for 1 h, but under the same condition an authentic prune juice doubled its acrylamide concentration. Formation of acrylamide in prune products was attributed to the presence of asparagine and sugars in the starting materials.
Introduction
When acrylamide was detected in foods for human consumption (Tareke et al. Citation2000, Citation2002; Rosen and Hellenas Citation2002) initial and subsequent investigations concluded that relatively high temperatures are necessary for its formation in foods. The minimum temperature required for the formation of acrylamide was often quoted as being about 120°C. However, instances of acrylamide formation at low temperatures, such as its artefactual formation during Soxhlet extraction of potato chips in methanol at 65°C (Pedersen and Olsson Citation2003; Grob et al. Citation2004; DeVries and Post Citation2004; Tanaka et al. Citation2004) and subsequent findings of the presence of acrylamide in products such as dried fruits and prune juices (Amrein et al. Citation2007; United States Food and Drug Administration Citation2009), has not led to further significant investigation into the formation of acrylamide at lower temperatures. The levels of acrylamide in prune juice reported in the United States ranged from 138 to 268 µg kg−1 and in pitted prunes themselves from 31 to 87 µg kg−1. Even higher values were reported for a particular variety of German dried pear. Consumption of a glass (250 ml) of prune juice at a level of 250 µg kg−1 would provide a 62.5 µg intake of acrylamide. This intake may be considered substantial if consumed frequently when compared with the average intake of acrylamide, estimated in many countries to be in the range of 0.4–0.5 µg kg−1 bw day−1 (Mills et al. Citation2009; CIAA Citation2009). Such an intake, for frequent consumers of prune juice, might be comparable with the intake of acrylamide from French fries, the commodity thought to make the greatest contribution to the acrylamide burden in foods for the general population.
For a successful reduction/mitigation strategy, knowledge of current levels of acrylamide in foods is required (European Commission Citation2007). Therefore, we have determined levels of acrylamide in prunes and prune juice (as part of a wider survey), including both adult and baby products, on the Canadian market. The products were purchased mostly in one locality (Ottawa).
We also conducted a model study where a simulated plum juice was heated at elevated temperatures (85–120°C) mimicking conditions that might be used during the production of prunes and during the bottling of prune juice.
A previously developed LC-MS/MS method was employed for the analysis of acrylamide (Becalski et al. Citation2004). This method was initially developed for the determination of acrylamide in fried potato products, but it was also used for analysis of other foods, including prune juice.
Materials and methods
Chemicals
Dichloromethane (pesticide grade) and methanol (HPLC grade) were obtained from EM Science (Gibbstown, NJ, USA). Water was obtained from a purification system (Millipore, Milli-Q Gradient A10). Acrylamide, 99.8%, was obtained from Fluka (Oakville, ON, Canada) and the 13C3 (98%)-labelled standard of acrylamide (CLM-813) was from Cambridge Isotope Laboratories (Andover, MA, USA). All other reagents were of analytical grade. All stock acrylamide solutions and calibration solutions were prepared in water. Working quantities of standards were stored at 4°C while the stock solutions were kept frozen below –10°C.
Foods
Retail packages of prunes and prune juice were purchased from local outlets. Entire packages were homogenised, if required, in a blender and subsamples, 2–4 g, were analysed. Most of the products were sampled in replicate to ascertain both within-lot and lot-to-lot variability.
Method description
Samples were analysed by an isotope dilution (13C3) acrylamide method. Prune samples were homogenised with water; other liquid samples were used as is. Water extracts were partitioned with dichloromethane to remove non-polar interferences, filtered through a 5 kDa centrifuge filter, cleaned on a HLB Oasis polymeric and Accucat mixed-mode anion and cation exchange SPE columns, and analysed by LC-MS/MS operating in ESI+ mode (Becalski et al. Citation2004).
Quality control/result verification
FAPAS test control material (bread matrix) #T3021 (from the Food and Environment Research Agency, Sand Hutton, York, UK) was run along with the samples. Analyses of FAPAS #T3021 material (assigned a value of 862 ng g−1) gave a mean acrylamide concentration of 840 ng g−1 with a standard deviation of 54 (n = 46) and RSD of 6.4%.
Two subsamples of prune juice were spiked with native acrylamide at levels of 50 and 250 ng g−1. Analyses of replicates (n = 3) of prune juice spiked with acrylamide at 50 ng g−1 gave an average recovery rate of 122%, while analyses of replicates (n = 3) of prune juice spiked with acrylamide at 250 ng g−1 gave an average recovery rate of 97%. Replicates (n = 8) of prune juice were analysed for an average concentration of 240 ng g−1 with a standard deviation of 6.0 and RSD of 2.5%.
Model reactions
General ‘drying’ procedure
A simulated plum juice (1.3–1.5 ml, equivalent to 1 g of plums), with a composition shown in a , was added to a 50 ml Teflon centrifuge tube (Oak Ridge type, Nalgene) and placed (without a cap) in a laboratory oven (forced air) at 85 or 95°C for 24 h. Afterwards the sample was diluted with water, spiked with 13C3 acrylamide, purified on the SPE columns and analysed by LC-MS/MS.
Table 1. Composition of the simulated plum juice with seven components.
General ‘wet’ procedure
A simulated plum juice (1.3–1.5 ml, equivalent to 1 g of plums) was added to a custom-made 4 ml glass pressure vessel (with cap) and placed in a heating block at 120°C for 1 h or at 95°C for 24 h. Afterwards the sample was diluted with water, spiked with 13C3 acrylamide, purified on SPE columns and analysed by LC-MS/MS.
In some experiments commercial prune juice already containing 188 µg kg−1 of acrylamide was used.
We conducted several experiments in replicates and the precision of these experiments is listed. We run other reactions only in duplicate as the differences between duplicates were less than 15%. Average concentrations of acrylamide per 1 g of a simulated juice are given.
Results
Survey of prune products
Retail food samples were collected in 2009; they included 24 baby foods and 18 other prune products. To capture variability between different products, the samples were chosen to encompass a variety of sources and processes.
There are significant differences in acrylamide concentration between different brands; however, lot-to-lot variations were relatively minor for prune products (). For example, in the strained prune baby food category, brand A contains, on average, more than three times the amount of acrylamide found in brand B. A similar observation applies to the differences in acrylamide concentrations between brands E and F of the prune nectar products tested. The presence of high levels (approximately 900 ppb) of acrylamide in one organic brand of prune nectar is rather surprising. However, it should be noted that a limited number of samples of pitted prunes (both organic and non-organic) and organic nectar were analysed. The same brand of organic prune juice (brand ‘E’, the only organic variety brand we were able to source from the market) was purchased again about 10 months later and found to contain acrylamide at 500 ng g−1. Levels of acrylamide in other products were generally similar to the levels found in other surveys.
Table 2. Concentrations of acrylamide (ng g−1) in prune products sampled from the Canadian retail market.
Model studies
For the model study a simulated plum juice was prepared according to the published data on the composition of plums. This simulated juice contained four sugars, two acids and asparagine. The amino acid asparagine is the most abundant free amino acid in plums (Stacewicz-Sapuntzakis et al. Citation2001; Dikeman et al. Citation2004). The composition of the simulated plum juice is listed in . Some samples also contained pectin and microcrystalline cellulose at, respectively, 7.5 and 2.5 mg per 1 g equivalent of the simulated juice to make them resemble more closely the natural product.
Prunes are produced industrially by dehydration of plums at temperatures of 85–90°C for 18 h. Prune juice is produced by boiling the prunes in water until soluble solid content reaches approximately 18% and some processes might also include pasteurisation. Conditions used in the model study were chosen to be similar to those used in industrial processes (Stacewicz-Sapuntzakis et al. Citation2001).
No formation of acrylamide was observed in any experiments when asparagine was omitted from the solutions or when asparagine was heated on its own.
Heating of 1 g equivalent of simulated plum juice (seven components listed in ) at 85°C for 24 h under ‘drying’ conditions, in an open vessel, produced 15 µg kg−1 of acrylamide. A similar reaction run at 95°C for 24 h produced an average of 94 µg kg−1 (SD = 5, n = 6) of acrylamide. The inclusion of pectin and cellulose did not markedly change the concentration of acrylamide, i.e. 106 µg kg−1 was produced. However, when malic and quinic acids were omitted from the simulated juice (at 95°C for 24 h) the acrylamide concentration increased to 670 µg kg−1 (SD = 166, n = 4). When only asparagine and fructose were present in the simulated juice, the acrylamide concentration increased further to 1740 µg kg−1 (SD = 95, n = 4).
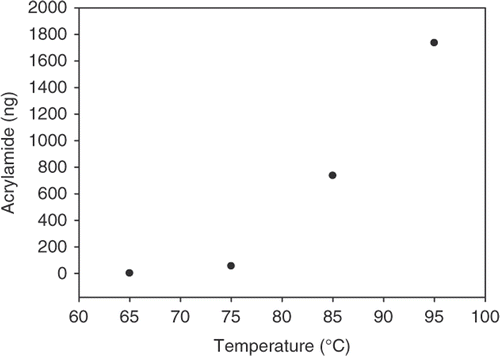
The reaction with asparagine and fructose at the levels present in the simulated juice was further studied at temperatures of 85, 75 and 65°C for 24 h (drying condition, open vessel). The yield of acrylamide decreased sharply with the decrease in temperature, i.e. at 65°C only trace amounts of acrylamide were detected ().
Figure 1. Yield of acrylamide in a reaction between fructose (50 mg) and asparagine (1.5 mg) at 95°C for 24 h in an open vessel.
When an actual prune juice sample already containing 188 µg kg−1 of acrylamide was heated at 95°C for 24 h (drying condition, open vessel), the concentration of acrylamide increased to 438 µg kg−1. When the same juice was heated at 95°C for 24 h (drying condition, open vessel) with an additional couple of milligrams of asparagine, the acrylamide concentration further increased to 684 µg kg−1. Heating of 1 g equivalent of the simulated plum juice (seven components; ) at 95°C for 24 h (wet condition, closed vessel) produced only 11 µg kg−1 of acrylamide.
When the same reaction was run under the ‘wet’ condition (closed vessel) at 120°C, but only for 1 h no formation of acrylamide was observed. However, when prune juice already containing 188 µg kg−1 of acrylamide was heated under the ‘wet’ condition (closed vessel) at 120°C for 1 h, the concentration of acrylamide increased to 375 µg kg−1. When the same juice was heated as above, but with an additional couple of milligrams of asparagine, the acrylamide concentration further increased to 423 µg kg−1.
Discussion
The results clearly indicate that substantial amounts of acrylamide can be generated at temperatures lower than 100°C under conditions that resemble the drying of foods, such as plums. Acrylamide in prunes and prune juice very likely originates from asparagine which is present in the starting material, i.e. plums. The results may also explain the higher concentration of acrylamide found in prune juice compared with the concentration found in prunes. It is probable that prolonged boiling and/or pasteurisation are responsible for the observed increase in acrylamide. If the juice is allowed to become ‘dry’ (e.g., on the sides of the vessel used to boil the prune juice), an increase in acrylamide concentration might occur.
The increased activity of fructose towards generation of acrylamide as compared with the mixture of four sugars is probably linked to the water activity of the mixture. When sorbitol and sucrose are present in a simulated plum juice they might act as humefactants.
The first reaction – formation of an imine – in the cascade of reactions leading to the formation of acrylamide (Stadler Citation2006) is generating water. Since this reaction is reversible, removal of water from the reaction mixture would facilitate imine formation. It is thus plausible that a facile formation of acrylamide under the condition of Soxhlet extraction is related to the relatively anhydrous environment present in the reaction vessel which favours imine formation.
There could be several reasons why the prune juice appears to be more ‘active’ in generating the acrylamide than the simulated juice. Firstly, the pH of the prune juice employed in this investigation was in the 3.8–3.9 range, while the pH of the simulated juice was 3.3. A lower pH could retard the reaction through inhibition of imine formation. When two acids were omitted from the simulated juice, the yield of acrylamide increased more than six-fold. Secondly, the prune juice would likely contain products of the Maillard reaction from the drying process of the plums, namely hydroxyaldehydes, which are known to generate acrylamide at greater yield than hexoses (Mills et al. Citation2009).
Conclusions
Acrylamide could form in asparagine-containing food products if these products are dehydrated even at temperatures lower than 100°C for a prolonged period. While acrylamide formation is not desirable, it is well known that the Maillard reaction, in addition to forming compounds which could be deleterious, e.g. acrylamide, also produces potentially beneficial compounds, e.g. those with antioxidative properties (Lee and Shibamoto Citation2002). This is true especially for prune products (Donovan et al. Citation1998; Piga et al. Citation2003).
The results indicated that prune products vary considerably in the amount of acrylamide, perhaps due to differences in recipes and processing/manufacturing conditions or variation in asparagine content in the raw fruit.
Minimisation of acrylamide formation through the modification of conditions under which prune juice/prunes are prepared, while retaining the beneficial properties of this food group, should be encouraged.
Acknowledgements
The authors would like to thank C. Hilts and K. Hislop (Health Canada) for helpful comments and suggestions on the manuscript.
References
- Amrein , TM , Andres , L , Escher , F and Amado , R . 2007 . Occurrence of acrylamide in selected foods and mitigation options . Food Addit Contam A , 24 : 13 – 25 .
- Becalski , A , Lau , BP-Y , Lewis , D , Seaman , SW , Hayward , S , Sahagian , M , Ramesh , M and Leclerc , Y . 2004 . Acrylamide in French fries: influence of free amino acids and sugars . J Agric Food Chem , 52 : 3801 – 3806 .
- CIAA. 2009. The CIAA acrylamide ‘Toolbox’ Rev. 12. Electronic citation. Available from: http://ec.europa.eu/food/food/chemicalsafety/contaminants/ciaa_acrylamide_toolbox.pdf/
- DeVries JW, Post BE. 2004. Comment on ‘Soxhlet extraction of acrylamide from potato chips’ by J.R. Pedersen and J.O. Olsson, Analyst, 2003, 128, 332. Analyst 129:93–95
- Dikeman , CL , Bauer , LL and Fahey , GC JR . 2004 . Carbohydrate composition of selected plum/prune preparations . J Agric Food Chem , 52 : 853 – 859 .
- Donovan , JL , Meyer , AS and Waterhouse , AL . 1998 . Phenolic composition and antioxidant activity of prunes and prune juice (Prunus domestica) . J Agric Food Chem , 46 : 1247 – 1252 .
- European Commission. 2007. Commission recommendation on the monitoring of acrylamide levels in food. Off J Eur Union L 123/33. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:123:0033:0040:EN:PDF/
- Grob K, Biedermann M, Hoenicke K, Gatermann R. 2004. Comment on ‘Soxhlet extraction of acrylamide from potato chips’ by J.R. Pedersen and J.O. Olsson, Analyst, 2003, 128, 332. Analyst 129:92
- Lee , K-G and Shibamoto , T . 2002 . Toxicology and antioxidant activities of non-enzymatic browning reaction products: review . Food Rev Int , 18 : 151 – 175 .
- Mills , C , Mottram , DS and Wedzicha , BL . 2009 . “ Acrylamide ” . In Process-induced food toxicants. Occurrence, formation, mitigation and health risks , Edited by: Stadler , RH and Lineback , DR . 23 – 50 . Hoboken (NJ) : Wiley .
- Pedersen , JR and Olsson , JO . 2003 . Soxhlet extraction of acrylamide from potato chips . Analyst , 128 : 332 – 334 .
- Piga , A , Del Caro , A and Corda , G . 2003 . From plums to prunes: influence of drying parameters on polyphenols and antioxidant activity . J Agric Food Chem , 51 : 3675 – 3681 .
- Rosen , J and Hellenas , K-E . 2002 . Analysis of acrylamide in cooked foods by liquid chromatography tandem mass spectrometry . Analyst , 127 : 880 – 882 .
- Stacewicz-Sapuntzakis , M , Bowen , PE , Hussain , EA , Damayanti-Wood , BI and Farnsworth , N . 2001 . Chemical composition and potential health effects of prunes: a functional food? . Crit Rev Food Sci Nutr , 41 : 251 – 286 .
- Stadler , RH . 2006 . “ The formation of acrylamide in cereal products and coffee ” . In Acrylamide and other hazardous compounds in heat-treated foods , Edited by: Skog , K and Alexander , J . 23 – 40 . Boca Raton (FL) : CRC Press .
- Tanaka M, Yoneda Y, Terada Y, Endo E, Yamada T. 2004. Comment on ‘Soxhlet extraction of acrylamide from potato chips’ by J.R. Pedersen and J.O. Olsson, Analyst, 2003, 128, 332. Analyst 129:96–98
- Tareke , E , Rydberg , P , Karlsson , P , Eriksson , S and Tornqvist , M . 2000 . Acrylamide: a cooking carcinogen? . Chem Res Toxicol , 13 : 517 – 522 .
- Tareke , E , Rydberg , P , Karlsson , P , Eriksson , S and Tornqvist , M . 2002 . Analysis of acrylamide, a carcinogen formed in heated foodstuffs . J Agric Food Chem , 50 : 4998 – 5006 .
- United States Food and Drug Administration. 2009. Survey data on acrylamide in food: individual food products. Electronic citation. Available from: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Acrylamide/ucm053549.htm/