Abstract
Chicken eggs categorised as conventional, omega-3 enriched, free range and organic were collected at grading stations in three regions of Canada between 2005 and 2006. Free run eggs, which were only available for collection from two regions, were also sampled during this time frame. Egg yolks from each of these egg types (n = 162) were analysed to determine brominated flame retardant levels, specifically polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane (HBCD). PBDEs were detected in 100% of the 162 samples tested, while HBCD was observed in 85% of the egg yolks. Total PBDE concentrations in egg yolks ranged from 0.018 to 20.9 ng g−1 lipid (median = 3.03 ng g−1 lipid), with PBDE 209 identified as being the major contributor to ΣPBDE concentrations. In addition to PBDE 209, PBDE 99, 47, 100, 183 and 153 were important contributors to ΣPBDE concentrations. Total HBCD concentrations ranged from below the limit of detection to a maximum concentration of 71.9 ng g−1 lipid (median = 0.053 ng g−1 lipid). The α-isomer was the dominant contributor to ΣHBCD levels in Canadian egg yolks and was the most frequently detected HBCD isomer. ΣPBDE levels exhibited large differences in variability between combinations of region and type. ΣHBCD concentrations were not significantly different among regions, although differences were observed between the different types of egg yolks analysed in the present study.
Introduction
The research associated with brominated flame retardants (BFRs) has largely focused on the polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane (HBCD), in part because they are added to consumer products, rather than covalently bound to the products as is the case for tetrabromobisphenol-A (TBBPA) (de Wit Citation2002). As a result, they are subject to leaching during production and application processes, to volatilisation and leaching during use, and to loss following product disposal (Voorspoels et al. Citation2003). PBDEs and HBCD are detected in a wide variety of environmental compartments and biological tissues. North American concentrations are higher than observed in Europe with elevated levels being related to differences in registration status over time (Vorkamp et al. Citation2011). Human exposure to these compounds via ingestion of food, in addition to oral and dermal intake of dust and soil have been reported in the literature (Roosens et al. Citation2010). Exposure to BFRs is of concern because they are associated with negative health effects in experimental animals, including neurotoxic and reproductive effects, impacts on the thyroid system and liver enzyme activity (de Wit Citation2002; Birnbaum and Staskal Citation2004; Abdallah et al. Citation2008; Ema et al. Citation2008; Öberg et al. Citation2010).
PBDEs and HBCDs are amenable to analysis using existing sample preparation methodologies applied to other persistent organic pollutants (POPs) because of their similar physical–chemical properties. The PBDEs are bromine substituted aromatic ethers, structurally related to the polychlorinated biphenyls (PCBs) and polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs). The numbering system for individual PBDE congeners follows that of the PCBs. In contrast to PCBs, only a limited number of individual congeners are present in commercial PBDE mixtures (Birnbaum and Staskal Citation2004). HBCDs are not aromatic, but are rather cyclic aliphatic hydrocarbons (Covaci et al. Citation2006).
Foods of animal origin are generally thought to be the greatest source of exposure to humans of highly persistent, bioaccumulative compounds and, therefore, PBDEs and HBCDs have been studied to determine levels of these contaminants in a wide range of foods (Huwe et al. Citation2002; Domingo Citation2004; Huwe and Larsen Citation2005; Darnerud et al. Citation2006; Gómara et al. Citation2006; Voorspoels et al. Citation2007). Market basket studies, in addition to commodity-based studies, have been performed to determine dietary exposure to the BFRs. Eggs are an important commodity in Canada with the average Canadian consuming three to four eggs each week (Canadian Egg Marketing Agency Citation2007). They also are considered to be a good indicator of ambient POP levels (Windal et al. Citation2009). POPs in free range eggs (eggs from chickens allowed free access to outdoors) and eggs laid by home-raised chickens have been reported to have elevated levels relative to those produced commercially in some European countries (Schoeters and Hoogenboom Citation2006; de Vries et al. Citation2006; van Overmeire et al. Citation2006). Recently, a Belgian study reported elevated PCDD/F levels in home-produced eggs relative to commercially produced eggs (van Overmeire et al. Citation2009; Waegeneers et al. Citation2009). In contrast, PBDE and HBCD levels in the home-produced eggs were similar to levels reported in other regions of Europe and North America (Covaci et al. Citation2009).
Eggs from three discrete regions of Canada were collected to establish whether POP levels in Canadian organic eggs were higher than concentrations in eggs from other production types, similar to what has been observed in Europe. Commercial eggs from the four major production types (conventional, omega-3 enriched, free range, organic) in Canada were collected. When available, free-run eggs also were collected. Egg categories vary in Canada according to production type, with conventional eggs being produced by chickens raised in cages. Free range chickens are provided with access to the outdoors, while free run chickens have freedom to move around in the barn and are not restricted to cages. Organic chickens must be raised following certified organic production, allowed access to the outdoors and fed organic feed. Omega-3-enriched eggs are produced by chickens fed a diet enriched in omega-3 fatty acids.
Materials and methods
Sample collection
A minimum of ten large chicken eggs belonging to each of the four main categories of eggs on the Canadian market (i.e., conventional, omega-3 enriched, free range, and organic) were collected from separate producers in British Columbia (BC), Quebec and the Maritime provinces, representing, western, central and eastern Canada, respectively in 2005/06. Eggs identified as free run were only collected from Quebec and the Maritimes due to a lack in availability from BC. Egg collection was performed by Canadian Food Inspection Agency (CFIA) inspectors at grading stations. Upon collection, samples were shipped to the lab and frozen at −18°C until ready for analysis.
Reagents
HPLC-grade sulphuric acid as well as HPLC/GC residue-grade acetone, dichloromethane (DCM), hexane and toluene were purchased from EMD (Ottawa, ON, Canada). Distilled water was prepared using a Barnstead Nanopure Diamond water system (Thermo, Waltham, MA, USA). Anhydrous sodium sulphate (Na2SO4) was obtained from Fisher Scientific (Ottawa, ON, Canada). Silica gel (60–200 mesh) was obtained from J.T. Baker (Ottawa, ON, Canada), Florisil (60–100 mesh) and celite 545 were obtained from Fisher Scientific (Ottawa, ON, Canada). Carbopack C (60–80 mesh) was obtained from Supleco (Bellefonte, PA, USA). Analytical standards of analytes (12C12), surrogates (13C12) and performance standards (12C12 and 2H18) were purchased from Wellington Laboratories (Guelph, ON, Canada) and Cambridge Isotope Laboratories (Andover, MA, USA), as available.
Sample preparation
A reagent blank and an internal laboratory QC subsample of homogenised butter from a sample that has been analysed many times, was included with each set of samples analysed. Eggs were thawed at room temperature and yolks were separated from whites. The first eggs to be tested (n = 4) were analysed separately (whites and yolks) to determine whether POPs were present in whites as well as the yolks. Following confirmation that POP levels in whites were below the limit of detection (n = 4), the lipid-rich yolks were retained for extraction and analysis. Individual yolks (12–36 g) were weighed into Erlenmeyer flasks and surrogate standards (500 pg 13C12-labelled PBDE 15, 28, 47, 99, 100, 153, 154, 183, 200 and 209; and 10 ng 13C12-labelled α, β- and γ-HBCD) were added. The samples were homogenised for 1 min with 80 ml acetone–hexane (2:1) using a Polytron®, the phases were allowed to separate and the hexane layer was transferred to a 250 ml separatory funnel. The samples were re-homogenised for 1 min with 40 ml hexane, and after phase separation the hexane layer was combined with the first hexane fraction. Deionised water (15 ml) was added to each separatory funnel and slowly mixed. The water phase was then removed and the hexane fraction was collected in a round-bottom flask after passing through a funnel filled with approximately 30 g anhydrous sodium sulphate and the volume was reduced to 100 ml. A subsample (5%) was retained for gravimetric lipid determination and the remaining extract was concentrated to allow for transfer of the extract and rinsing of the flask into a 10 ml volumetric flask.
Because egg yolks are known to contain cholesterol, the raw extracts were taken to a volume of 10 ml in hexane and added to silica gel (2 g) columns, pre-conditioned with 10 ml hexane. Extracts were eluted with 4 ml hexane to remove cholesterol, diluted to a volume of 80 ml and were further cleaned up through sequential washing in a 250 ml separatory funnel with eight fresh 20 ml volumes of concentrated sulphuric acid; 20 ml deionised water; 20 ml 1% KOH and a final rinse with 20 ml deionised water. The organic extracts were then dried on a bed of anhydrous Na2SO4 and collected in a round-bottom flask and reduced in volume to approximately 1 ml.
A 2 g acidic silica gel column (75 g silica gel: 50 g concentrated sulphuric acid) conditioned with 10 ml hexane, and a 100% activated Florisil column (1.5 g), conditioned with 10 ml DCM and 10 ml hexane, were set up in series. The extracts were added to the top of the silica column and 20 ml hexane was used to elute the column, the silica gel column was then removed and a second 20 ml aliquot of hexane was added to the Florisil column. This hexane fraction, containing PBDE 209, was removed and 60 ml DCM was added to the top of the Florisil column to elute the remaining PBDEs and HBCD. The extracts were evaporated to 1 ml under vacuum. A final clean-up step was performed on the DCM fraction using a 0.4 g 18% Carbopack C: Celite column (conditioned with 3 ml toluene and 1 ml 1:1 DCM: hexane) where the analytes were eluted with 1 ml (1:1) hexane: DCM, evaporated to dryness and reconstituted with 10 µl of a 20 pg µl−1 mixture of the performance standard containing PCB 200, 209 and decachlorodiphenyl ether in toluene and placed into v-vials for gas chromatographic-high resolution mass spectrometric analysis. Once the PBDE analyses were completed, samples were concentrated just to dryness using a gentle stream of nitrogen and reconstituted with 5 ng deuterated C12 2H18Br6 HBCD performance standard in 100 µl methanol–water (80:20) in preparation for the liquid chromatographic-mass spectrometric analysis.
The hexane fraction, containing PBDE 209, was evaporated to dryness and re-constituted in 10 µl of 20 pg µl−1 decabromodiphenyl ether and sent for GC-MS analysis.
Analysis
A Micromass Autospec Ultima high-resolution mass spectrometer (Waters Corporation) coupled to an Agilent 6890 gas chromatograph was used in the PBDE analyses. A 15 m J&W DB-5MS, 0.25 mm i.d., 0.1 µm film thickness column was used and 1 µl of each sample was introduced via a cool on-column injector that was set to track the oven temperature. The initial temperature was set to 80°C and held for 1 min, followed by an increase in temperature to 225°C at a rate of 32°C min−1. The temperature was held at 225°C for 1 min and increased at a rate of 3°C min−1 to 230°C and held for 8 min. The final increase was set at a rate of 40°C min−1 to 295°C. The head pressure initially was 28 kPa and held for 2 min, followed by an increase at a rate of 7 kPa min−1 to 83 kPa and held for 15 min with a final increase to 173 kPa at a rate of 69 kPa min−1. Helium was used as the carrier gas and the mass spectrometer was operating in EI positive-ion mode at 50 eV, the trap current was 650 µA and the source temperature was 250°C. The resolution for all analytes was approximately 8000 (10% valley definition).
HBCD was quantified using an Acquity UPLC coupled to a Quattro Premier XE triple quadrupole MS/MS (Waters) with electrospray ionisation in the negative ion detection mode using a 2.1 × 100 mm, Hypersil Gold C18, 1.9 µm column (Thermo Scientific). Water (mobile phase A) and acetonitrile–methanol (2:1) (mobile phase B) were used in separation of the HBCD isomers and the gradient was as follows: 40% mobile phase B for 1 min, 40–80% mobile phase B by 4 min, 80–90% B by 13 min, then returned to 40% B over 0.5 min where it remained until 18 min. The flow rate was maintained at 0.175 ml min−1 and the column temperature was maintained at 15°C to resolve completely the deuterated HBCD analogues from the 13C-labelled and native analogues. The capillary and cone voltage were −3.5 kV and 20 V, respectively. The source temperature and desolvation temperature were 140°C and 400°C, respectively. Cone gas flow and desolvation gas flow were 47 and 947 l h−1, respectively. Argon was the collision gas set at 5 × 10−3 mbar and resolution was established at 90% valley at base for both quadrupole analysers. Transitions monitored for native HBCD, 13C HBCD isomers and deuterated HBCD were 639 → 79, 641 → 79, 641 → 81 and 643 → 81; 651 → 79, 653 → 79, 653 → 81 and 655 → 81; and 656 → 79, 658 → 79, 658 → 81 and 660 → 81, respectively. Dwell times were set to 5 ms.
Statistical analysis
Analytes below the limit of detection were reported as zero for statistical analysis. Statistical analysis of PBDEs and HBCD levels was performed using SigmaStat for Windows 3.11. Because of the lack of homogeneity of variance for the PBDEs, however, it was necessary to analyse the data using non-parametric techniques, and because of the interaction between region and type, it was necessary to assess the effect of each factor within the levels of the other factor. The non-parametric Kruskal–Wallis test was used to assess the overall significance of each factor (e.g., region of collection, production type). The results were sorted by the mean rank score and contingent groups with no significant differences in score were found.
Results and discussion
Quality assurance/quality control
PBDE amounts that were observed in the reagent blanks analysed with each set of samples were subtracted from the samples in the corresponding set, prior to determination of levels in individual egg yolk samples. Generally, only PBDE 47, 99 and 209 were detected in the reagent blanks. The reagent blanks tested for HBCD levels were consistently observed to have non-detected or negligible levels of all three HBCD analogues.
Average surrogate recoveries in egg yolks ranged from 22% to 70%, for PBDE 209 and PBDE 100, respectively, while average, recoveries for α-HBCD, β-HBCD, γ-HBCD were 36%, 15% and 17%, respectively. Because the method used was optimised for polychlorinated dibenzo-p-dioxins, dibenzofurans (PCDD/Fs) and polychlorinated biphenyls (PCBs), lower recoveries observed for these analytes was not unexpected. PCDD/F and PCB concentrations will be reported elsewhere. Reported concentrations were recovery corrected.
The average instrumental limit of detection for the PBDEs, determined based on a signal-to-noise ratio of (S/N) 3:1, ranged from 0.045 pg (0.001 ng g−1 lipid) to 13.9 pg (0.228 ng g−1 lipid) for PBDE 15 and PBDE 209, respectively. All samples were blank subtracted for all congeners, although only PBDE 47, 99 and 209 were consistently observed in the reagent blank samples. The average instrumental limit of detection was 5.4 pg (0.010 ng g−1 lipid), 3.2 pg (0.006 ng g−1 lipid) and 2.8 pg (0.006 ng g−1 lipid) for α-, β-, and γ-HBCD, respectively, based on 15 µl injection volumes. HBCD was not observed in any reagent blank samples.
The internal quality assurance system employed in the laboratory was confirmed through the successful participation in the Norwegian Institute of Public Health international interlaboratory study, where several classes of POPs, including PBDEs and HBCD in various foods including eggs are examined (Haug and Becher Citation2006; Haug et al. Citation2008). PBDE and HBCD levels observed in the in-house internal quality control sample also were found to range within the expected values.
PBDE levels and profiles
PBDE and HBCD levels were below the limit of detection in the egg whites (n = 4) analysed during confirmation activities to ensure that the correct egg fraction would be the focus of investigation in the present study. Although there has been discussion related to whether PBDE 209 would be associated with lipid rich yolk or present in egg white, in the present study PBDE 209 was not observed in any of the egg whites tested but present above the average limit of detection in 66% of the egg yolks analysed (). PBDE 209 was the dominant contributor to ΣPBDE concentrations, when present. The other congeners contributing to ΣPBDE levels in egg yolks were 99 > 47 > 100 > 183 and 153. The detection frequency of these congeners ranged from 96% (PBDE 47 and 99) to 99% (PBDE 100). The very high contribution of PBDE 209 to ΣPBDE levels is consistent with reports obtained from other studies (Gómara et al. Citation2006; Covaci et al. Citation2009).
Figure 1. Traces for [M-2Br] ion (m/z 799.333) corresponding to PBDE 209 in (a) egg white and (b) egg yolk from a conventionally produced egg.
![Figure 1. Traces for [M-2Br] ion (m/z 799.333) corresponding to PBDE 209 in (a) egg white and (b) egg yolk from a conventionally produced egg.](/cms/asset/19408f25-83e9-48ce-a3b3-c52329ab000d/tfac_a_545443_o_f0001g.gif)
PBDEs were detected in all egg yolks (n = 162) analysed in the present study. ΣPBDE concentrations ranged from 0.018 ng g−1 lipid to 20.9 ng g−1 lipid, the maximum and minimum concentration were both observed in yolks from free run eggs, although the low concentration was observed in a yolk collected in Quebec while the yolk from an Atlantic free run egg had the highest observed ΣPBDE concentrations (). The mean and median ΣPBDE concentrations based on all egg yolks analysed were 5.49 ng g−1 lipid and 3.03 ng g−1 lipid, respectively.
Table 1. Blank-corrected PBDE concentration range (median) in Canadian egg yolks (ng g−1 lipid).
The PBDE levels in eggs exhibited large differences in variability between various combinations of region and type, and the effects of region and type were not additive (i.e., there was evidence of an interaction) (). No one type of yolk from BC had significantly different ΣPBDE levels than yolks from another type. Conventional and free run yolks from the Maritimes, however, had higher ΣPBDE concentrations than other egg types from that region (p < 0.05) and free range yolks had lower ΣPBDE concentrations (p < 0.05). In eggs collected from Quebec, ΣPBDE concentrations were significantly highest in omega-3-enriched yolks (p < 0.05). There was no evidence of an effect of region on levels for free run eggs.
HBCD levels and profiles
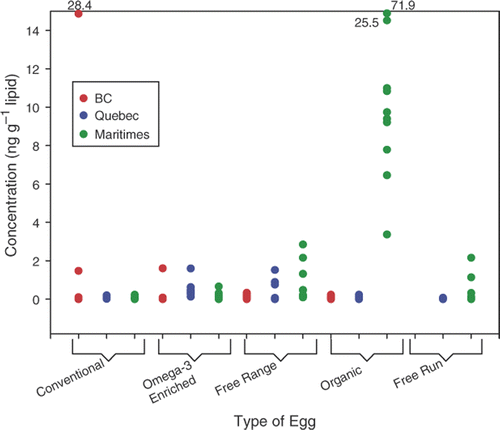
HBCD was detected in 85% of the egg yolks analysed in the present study. Concentrations ranged from below the limit of detection to 71.9 ng g−1 lipid, although most yolks (n = 142) had ΣHBCD concentrations below 1 ng g−1 lipid (). In addition to being the most frequently detected HBCD isomer (83%), α-HBCD was the predominant isomer, contributing 87% to ΣHBCD concentrations in Canadian egg yolks, while β- and γ-contributed less than 1% and 13%, respectively. The β- and γ-isomers were detected in 9% and 27% of the egg yolks analysed in the present study.
Table 2. HBCD concentration range (median) in Canadian egg yolks (ng g−1 lipid).
HBCD concentrations were not significantly different among regions of collection. Concentrations observed in organic egg yolks collected from the Maritimes, however, were significantly higher than those observed in yolks collected from other production types (p < 0.001) ().
Comparison of BFR levels with other studies
The inclusion of PBDE 209 does have a major impact on the ΣPBDE levels observed in food samples, including eggs (Covaci et al. Citation2009, Fernandes et al. Citation2009). The variability between programs does somewhat limit the comparison of levels obtained by different researchers. Additionally, most researchers analyse the whole egg, rather than yolks only, however, a comparison of results on a lipid weight basis does allow for comparison of PBDE levels among different research groups.
The ΣPBDE concentrations observed in the present study are within the range reported in the literature for eggs (). The range in ΣPBDE concentrations observed in Canadian egg yolks is greater than reported by most researchers (), although a larger number of individual egg yolks were measured than is generally reported. The highest concentration observed in the present study (20.9 ng g−1 lipid) was in a commercially produced free run egg yolk collected from the Maritimes, but was below the maximum level reported by Covaci et al. (Citation2009) (39.3 ng g−1 lipid) in eggs from home produced chickens that were fed kitchen waste.
Table 3. ΣPBDE concentrations (ng g−1 lipid) in literature studies relative to levels observed in the present work.
Although HBCD has been reported at concentrations from below the limit of detection to levels in excess of 5000 ng g−1 lipid in eggs of predatory bird species (Lindberg et al. Citation2004; Jaspers et al. Citation2005; Gauthier et al. Citation2007; Janák et al. Citation2008), reported levels of HBCD in chicken eggs or egg yolks are rare in the literature (Driffield et al. Citation2008; Covaci et al. Citation2009). HBCD can be measured as a total using gas chromatography-mass spectrometry, however, isomer separation can only be achieved using liquid chromatography-mass spectrometry (Janák et al. Citation2008). Covaci et al. (Citation2009) reported ΣHBCD levels ranging from 0.06 to 23.9 ng g−1 lipid in Belgian home-produced eggs, which is below the maximum observed in an organic egg yolk analysed in the present study (71.9 ng g−1 lipid). Driffield et al. (Citation2008) reported HBCD levels below the limit of detection in eggs (0.19, 0.078 and 0.110 ng g−1 fresh weight, for α-HBCD, β-HBCD, γ-HBCD, respectively) collected as part of the UK Total Diet Study in 2004, where the number of eggs sampled was limited in number. Despite the high maximum concentration observed in yolks in the present study, the mean (1.39 ng g−1 lipid) and median (0.052 ng g−1 lipid) ΣHBCD concentrations were similar to the levels reported by others.
No statistical (r 2 = 0.012) relationship between ΣPBDE concentrations and ΣHBCD concentrations were observed in the yolks analysed as part of the present study.
Conclusions
The brominated flame retardants PBDEs and HBCD are present in Canadian egg yolks at levels similar to those observed throughout the world. The inclusion of PBDE 209 in routine analysis of foods has only recently been performed; however, this congener has a large contribution to ΣPBDE concentrations. HBCD is generally present in Canadian egg yolks at detectable levels with α-HBCD being the most frequently detected isomer, with the others (β- and γ-HBCD) observed infrequently. Currently, Canada has no established guidelines for PBDE and HBCD residues in foods.
Acknowledgements
The authors thank the Inspectors from the Canadian Food Inspection Agency (CFIA) who collected eggs for inclusion in this study.
References
- Abdallah , MA-E , Harrad , S , Ibarra , C , Diamond , M , Melymuk , L , Robson , M and Covaci , A . 2008 . Hexabromocyclododecanes in indoor dust from Canada, the United Kingdom and the United States . Environ Sci Technol , 42 : 459 – 464 .
- Birnbaum , LS and Staskal , DF . 2004 . Brominated flame retardants: cause for concern? . Environ Health Perspect , 112 : 9 – 17 .
- Bocio , A , Llobet , JM , Domingo , JL , Corbella , J , Teixidó , A and Casas , C . 2003 . Polybrominated diphenyl ethers (PBDEs) in foodstuffs: human exposure through the diet . J Agric Food Chem , 51 : 3191 – 3195 .
- Canadian Egg Marketing Agency. 2007. The Canadian egg industry fact sheet. Ottawa (ON): Canadian Egg Marketing Agency; [cited 2010 Mar 4]. Available from: http://www.eggs.ca/Files/Resource/Canada%20Egg%20Industry%20Fact%20Sheet.pdf/
- Covaci , A , Gerecke , AC , Law , RJ , Voorspoels , S , Kohler , M , Heeb , NV , Leslie , H , Allchin , CR and deBoer , J . 2006 . Hexabromocyclododecanes (HBCDs) in the environment and humans: a review . Environ Sci Technol , 40 : 3679 – 3688 .
- Covaci , A , Roosens , L , Dirtu , AC , Waegeneers , N , van Overmeire , I , Neels , H and Goeyens , L . 2009 . Brominated flame retardants in Belgian home-produced eggs: levels and contamination sources . Sci Tot Environ , 407 : 4387 – 4396 .
- Darnerud , PO , Atuma , S , Aune , M , Bjerselius , R , Glynn , A , Grawé , KP and Becker , W . 2006 . Dietary intake estimations of organohalogen contaminants (dioxins, PCB, PBDE and chlorinated pesticides, e.g., DDT) based on Swedish market basket data . Food Chem Toxicol , 4 : 1597 – 1606 .
- de Vries , M , Kwakkel , RP and Kijlstra , A . 2006 . Dioxins in organic eggs: a review . NJAS-Wagen J Life Sc , 54 : 207 – 221 .
- de Wit , CA . 2002 . An overview of brominated flame retardants in the environment . Chemosphere , 46 : 583 – 624 .
- Domingo , JL . 2004 . Human exposure to polybrominated diphenyl ethers through the diet . J Chromatogr A , 1054 : 321 – 326 .
- Driffield , M , Harmer , N , Bradley , E , Fernandes , AR , Rose , M , Mortimer , D and Dicks , P . 2008 . Determination of brominated flame retardants in food by LC-MS/MS: diastereoisomer-specific hexabromocyclododecane and tetrabromobisphenol A . Food Addit Contam A , 25 : 895 – 903 .
- Ema , M , Fujii , S , Hirata-Koizuma , M and Matsumoto , M . 2008 . Two generation reproductive toxicity study of the flame retardant hexabromocyclododecane in rats . Reprod Toxicol , 25 : 335 – 351 .
- Fernandes , AR , Tlustos , C , Smith , F , Carr , M , Petch , R and Rose , M . 2009 . Polybrominated diphenyl ethers (PBDEs) and brominated dioxins (PBDD/Fs) in Irish food of animal origin . Food Addit Contam B , 2 : 86 – 94 .
- Gauthier , LT , Hebert , CE , Weseloh , DVC and Letcher , RJ . 2007 . Current-use flame retardants in the eggs of herring gulls (Larus argentatus) from the Laurentian Great Lakes . Environ Sci Technol , 41 : 4561 – 4567 .
- Gómara , B , Herrero , L and González , MJ . 2006 . Survey of polybrominated diphenyl ether levels in Spanish commercial foodstuffs . Environ Sci Technol , 40 : 7541 – 7547 .
- Haug , LS and Becher , G . 2006 . Interlaboratory comparison on dioxins in food 2006. Seventh round of an interlaboratory study , Nydalen (Norway) : Norwegian Institute of Public Health .
- Haug , LS , Thomsen , C , Liane , VH and Becher , G . 2008 . Comparison of GC and LC determinations of hexabromocyclododecane in biological samples – results from two interlaboratory comparison studies . Chemosphere , 71 : 1087 – 1092 .
- Huwe , JK and Larsen , GL . 2005 . Polychlorinated dioxins, furans, and biphenyls, and polybrominated diphenyl ethers in a U.S. meat market basket and estimates of dietary intake . Envrion Sci Technol , 39 : 5606 – 5611 .
- Huwe , JK , Lorentzsen , M , Thuresson , K and Bergman , Å . 2002 . Analysis of mono- to deca-brominated diphenyl ethers in chickens at the part per billion level . Chemosphere , 46 : 635 – 640 .
- Janák , K , Sellström , U , Johansson , A-K , Becher , G , de Wit , CA , Lindberg , P and Helander , B . 2008 . Enantiomer-specific accumulation of hexabromocyclododecane in eggs of predatory birds . Chemosphere , 73 : S193 – S200 .
- Jaspers , V , Covaci , A , Maervoet , J , Dauwe , T , Voorspoels , S , Schepens , P and Eens , M . 2005 . Brominated flame retardants and organochlorine pollutants in eggs of little owls (Athene notura) from Belgium . Environ Poll , 136 : 81 – 88 .
- Kiviranta , H , Ovaskainen , M-L and Vartianen , T . 2004 . Market basket study on dietary intake of PCDD/Fs, PCBs, and PBDEs in Finland. 2004. . Environ Int , 30 : 923 – 932 .
- Lindberg , P , Sellström , U , Häggberg , L and de Wit , CA . 2004 . Higher brominated diphenyl ethers and hexabromocyclododecane found in eggs of Peregrine falcons (Falco peregrinus) breeding in Sweden . Environ Sci Technol , 38 : 93 – 96 .
- Öberg , M , Westerholm , E , Fattore , E , Stern , N , Hanberg , A , Hagland , P , Wiberg , K , Bergendorff , A and Häkansson , H . 2010 . Toxicity of Bromkal 70-5DE, a technical mixture of polybrominated diphenyl ethers, following 28 d of oral exposure in rats and impact of analysed impurities . Chemosphere , 80 : 137 – 143 .
- Roosens , L , Cornelis , C , D’Hollander , W , Bervoets , L , Reynders , H , van Campenhout , K , van den Heuvel , R , Neels , H and Covaci , A . 2010 . Exposure of the Flemish population to brominated flame retardants: model and risk assessment . Environ Intern , 36 : 368 – 376 .
- Schecter , A , Päpke , O , Harris , TR , Tung , KC , Musumba , A , Olson , J and Birnbaum , L . 2006 . Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex . Environ Health Perspect , 114 : 1515 – 1520 .
- Schoeters , G and Hoogenboom , R . 2006 . Contamination of free-range chicken eggs with dioxins and dioxin-like polychlorinated biphenyls . Mol Nutr Food Res , 50 : 908 – 914 .
- Tritscher , A , Stadler , R , Scanlan , F , Collingro , C and Päpke , O . 2003 . Determination of polybrominated diphenylethers in samples of raw cows’ milk, fish and egg . Organohalogen Compounds , 61 : 131 – 134 .
- van Overmeire , I , Pussemier , L , Hanot , V , de Temmerman , L , Hoenig , M and Goeyens , L . 2006 . Chemical contamination of free-range eggs from Belgium . Food Addit Contam A , 23 : 1109 – 1122 .
- van Overmeire , I , Waegeneers , N , Sioen , I , Bilau , M , de Henauw , S , Goeyens , L , Pussemier , L and Eppe , G . 2009 . PCDD/Fs and dioxin-like PCBs in home-produced eggs from Belgium: levels, contamination sources and health risks . Sci Tot Environ , 407 : 4419 – 4429 .
- Voorspoels , S , Covaci , A , Neels , H and Schepens , P . 2007 . Dietary PBDE intake: a market-basket study in Belgium . Environ Intern , 33 : 93 – 97 .
- Voorspoels , S , Covaci , A and Schepens , P . 2003 . Polybrominated diphenyl ethers in marine species from the Belgian North Sea and the Western Scheldt Estuary: Levels, profiles and distribution . Environ. Sci. Technol , 37 : 4348 – 4357 .
- Vorkamp , K , Thomsen , M , Frederiksen , M , Pedersen , M and Knudsen , LE . 2011 . Polybrominated diphenyl ethers (PBDEs) in the indoor environment and associations with prenatal exposure . Environ Intern. , 37 : 1 – 10 .
- Waegeneers , N , de Steur , H , de Temmerman , L , van Steenwinkel , S , Gellynck , X and Viaene , J . 2009 . Transfer of soil contaminants to home-produced eggs and preventative measures to reduce contamination . Sci Tot Environ , 407 : 4438 – 4446 .
- Windal , I , Hanot , V , Marchi , J , Huysmans , G , van Overmeire , I , Waegeneers , N and Goeyens , L . 2009 . PCB and organochlorine pesticides in home-produced egg in Belgium . Sci Tot Environ , 407 : 4430 – 4437 .