Abstract
Migration experiments with small sheets cut out from ovenable PET trays were performed in two-sided contact with 3% acetic acid as food simulant at various temperatures. The fraction of diffusible antimony (Sb) was estimated to be 62% in the PET sample under study. Apparent diffusion coefficients of Sb in PET trays were determined experimentally. Measurement of migration between 20 and 150°C yielded a linear Arrhenius plot over a wide temperature range from which the activation energy (E a) of 188 ± 36 kJ mol−1 and the pre-exponential factor (D 0) of 3.6 × 1014 cm2 s−1 were determined for diffusing Sb species. E a was similar to previously reported values for PET bottles obtained with a different experimental approach. E a and D 0 were applied as model parameters in migration modelling software for predicting the Sb transfer in real food. Ready meals intended for preparation in a baking oven were heated in the PET trays under study and the actual Sb migration into the food phase was measured by isotope dilution ICP-MS. It was shown that the predictive modelling reproduces correctly experimental data.
Introduction
Packed food, as consumed, may contain different substances which are undesirable. Migration of substances into foodstuffs is a subject of increasing interest and an important aspect of food packaging. Advances in food packaging technology have made available a wide variety of convenience foods, which can be taken directly from the freezer to the microwave or conventional oven. The polymer of choice used for these products is polyethylene terephthalate (PET) due to its excellent thermal stability, both in the deep freezer and in the oven. It will not thermally deform below 220°C, and accordingly PET trays are used for cooking or reheating of food. In addition, PET serves as bottle material for a variety of products, especially carbonated beverages, bottled water and fruit juices.
Polymers often contain contaminants as a result of their synthesis or manufacturing procedure. PET is manufactured by polycondensation of ethylene glycol and terephthalic acid in the presence of antimony trioxide (Sb2O3) as catalyst (Duh Citation2002). Residual molecular antimony (Sb) catalyst materials can migrate into food or water and be a potential contaminant from PET packaging materials. Sb was established as catalyst of choice because it has some favourable properties, e.g. it gives bright, shiny polymers. There are two other main catalysts for PET: germanium oxide and titanium compounds (Thiele Citation2001). Due to the increasing importance of PET in food packaging, an understanding of the migration properties of its residual compounds is important.
The possible migration of Sb into food is of great concern due to its toxicity. A toxicological similarity between arsenic and Sb exists (Gebel Citation1997). Oestrogenic activity of Sb(III) may impair the reproductive function in adults (Choe et al. Citation2003). In addition, Sb trioxide is classified as possibly carcinogenic to humans (Group 2B) by the International Agency for Research on Cancer (IARC) (Citation1989). In a recent study, elemental Sb showed genotoxicity in chromosomal aberration tests in cultured mammalian cells (Asakura et al. Citation2009). However, due to lack of in vivo studies genotoxicity in humans cannot be determined at this time.
In recent years, some examples of contamination of mineral water, drinks and foodstuffs have been reported with PET. Several studies have examined the leaching of Sb from PET bottles into mineral water or juices (BAG Citation2005; Rusz Hansen & Pergantis Citation2006; Shotyk et al. Citation2006; Westerhoff et al. Citation2008). However, even after a long storage time the levels in water were well below the limits commonly specified for waters, i.e. 5 µg l−1 for natural mineral water set by European Commission Directive Citation2003/40 (European Commission Citation2003) and the WHO (Citation2003) drinking water guideline value of 20 µg l−1 (Keresztes et al. Citation2009). In contrast, at high temperatures much larger quantities of Sb migrate from ovenable PET trays for ready meals into food (Haldimann et al. Citation2007). With regard to the TDI of 6 µg kg−1 body weight day−1 for Sb established by the WHO, even the most contaminated PET trays did not pose a risk to health (WHO Citation2003). On the other hand, the European Commission (Citation2005) set a specific migration limit (SML) of 40 µg kg−1 for Sb. In some ready meals prepared in PET trays that were exposed to high temperatures, the Sb concentration found in food exceeded the SML (Haldimann et al. Citation2007). In fact, the European Food Safety Authority (EFSA) (Citation2004) acknowledged in its Scientific Opinion that the SML might be exceeded at high temperature. Therefore, testing for Sb migration has some utility in checking for compliance with regulations (SML).
Even though a number of studies on migration from PET have been conducted, there are few investigations on the determination of specific mass transfer characteristics (Begley et al. Citation2005; Welle & Franz Citation2011). In general, PET shows low levels of global migration (Castle et al. Citation2004). PET belongs to the class of engineering polymers which are excellent barrier materials with small diffusion coefficients (Bharadwaj & Boyd Citation1999). In semi-crystalline PET, diffusion is strongly influenced by its crystallinity fraction and morphology. Due to the low diffusivity of most migrants in PET, the determination of diffusion coefficients requires long-term experiments. To examine the extent of Sb migration from PET tray packaging materials, 3% acetic acid was used as food simulant. Preliminary results were already presented at the 4th International Symposium on Food Packaging (Alt et al. Citation2008).
The main objective of the present work is to report experimental Sb migration data, i.e. concentration of Sb in food simulant and food as a result of diffusion from the PET polymer interface. Diffusion coefficients were calculated at various temperatures and the Arrhenius parameters activation energy and pre-exponential factor were determined. These parameters served as input for migration modelling, which was performed using a software package (AKTS Citation2012).
For this purpose, experiments were carried out in an autoclave batch reactor over a wide temperature range with diluted acetic acid as food simulant. The increase of the Sb concentrations in the simulant and its content in the PET were measured by ICP-MS. In addition, the PET content was verified by X-ray fluorescence spectroscopy (XRF).
Food simulating liquids can affect the migration from PET polymers. Non-polar food stimulants for high-temperature applications are not suitable for Sb migration tests, e.g. Sb was not detected in olive oil (Ashby Citation1988; Fordham et al. Citation1995). On the other hand, polar food simulants may interact with PET (Begley et al. Citation2005). In response to this situation, actual food that reflects real conditions without the drawbacks of simulants was used for the validation of the migration modelling.
Materials and methods
Chemicals and standards
An isotopic Sb(III,V)-oxide (Sb2O4) standard with 98.6% enriched 123Sb (1.4% 121Sb) was obtained from Chemotrade (Düsseldorf, Germany). A total of 126.0 mg Sb2O4 was dissolved to a final total Sb concentration of 1.0 mg ml−1 in 15% HCl. From this solution the concentration for the ICP-MS isotope spike was made up. Sb(III) oxide puriss. p.a.Fluka (Sigma-Aldrich, Buchs, Switzerland) was used for the XRF standards. Nitric acid 65% Suprapur®(Merck, Darmstadt, Germany) was used for the mineralisation of food and PET. Additionally, hydrofluoric acid 40% Suprapur® (Merck) was added to the mineralisation of PET. Demineralised water with a specific conductivity of 18 MΩ cm−1 was produced in a cartridge system (Purelab ultra, ELGA LabWater, Celle, Germany). Polyethylene (ERM®-EC680k, Institute for Reference Materials and Measurements, Geel, Belgium) and white cabbage (BCR 679) were used as reference materials for quality control measuring.
PET tray sample
New empty PET (Etimex E1231, Etimex Primary Packaging, Dietenheim, Germany) trays which are produced for ovenable ready-to-eat lasagne were obtained from a food producer (Bischofszell Food, Bischofszell, Switzerland). The thermoformed tray was a multilayer sheet made of crystallised PET (CPET) covered by a layer of amorphous PET (APET). The overall thickness of the tray, 0.031 ± 0.004 cm (n = 6), was determined by analysis of electron microscopy images (Hitachi TM-1000, Hitachi, Krefeld, Germany). The inner side of the tray was coated with a thin APET as a sealing layer against the covering foil. According to the tray manufacturer layer thickness is only about 15 µm. The density of 1.35 g cm−3 was determined with a density kit mounted on an analytical balance (Mettler-Toledo, Greifensee, Switzerland). The form of the PET tray looked like a truncated pyramid (upside down) with a height of 3.8 cm, an internal base area of 156.8 cm2 (14 × 11.2 cm) and a top area of 91.3 cm2 (11 × 8.3 cm). Thus, a volume of 466 cm3 was obtained, and it was verified by weighing a fill of demineralised water. The corresponding maximal contact surface was 260 cm2. The glass transition temperature was measured by differential scanning calorimetry (DSC 1, Mettler-Toledo) and fell in the range between 74.5 to 76.3°C. The apparent degree of crystallinity of this particular PET tray was measured from DSC spectra and amounted to 27%. There was no DSC response that could be attributed to the amorphous layer.
Mineralisation of PET for ICP-MS measurement
Microwave-assisted pressure digestion of PET was carried out in an autoclave (MLS Ultraclave III, MLS, Leutkirch, Germany). Small PET slabs of 20–40 mg were cut out from trays and directly weighed into perfluoralkoxy (PFA) polymer tubes (10 ml). A total of 2.0 ml HNO3 65% (Merck, Suprapur®) and 0.2 ml HF 40% (Merck, Suprapur®) were added. The reactor was initially pressurised with nitrogen from the laboratory gas supply at 55 bar by a compressor (Junior II, Bauer, Munich, Germany). The digestion was initialised by gradually increasing the temperature to 220°C. The temperature level was then maintained for 120 min at 220°C. The process was terminated by cooling. After releasing pressure at room temperature, the reactor chamber was vented for some 30 min. Subsequently, the clear and colourless mineralised solutions were transferred into 50 ml polypropylene (PP) tubes (Sarstedt, Sevelen, Switzerland), and diluted to volume with pure water. For measurements, the sample solution was prepared with 50 µl of the diluted mineralised solution in 4950 µl HNO3 2.5%. Subsequently, the solutions were measured by sector-field ICP-MS (Element2, Thermo Fisher Scientific, Bremen, Germany) with external calibration and rhodium as internal standard. Because a certified reference material with PET properties was not available, the ICP-MS method was validated measuring a low-density polyethylene (LDPE, ERM-EC680k) with a certified Sb content of 10.1 ± 1.6 mg kg−1. PE differs from PET with respect to the mineralisation reaction and final composition of the sample solution. The measured Sb concentration of 10.4 ± 0.14 mg kg−1 (n = 4) fell into the 95% confidence interval of the certified value, thereby indicating accuracy and precision of the Sb measurements in a polymer matrix.
Determination of Sb in PET by XRF
A PET foil containing only 8 mg kg−1 Sb was used to prepare matrix-matched XRF standards. The standards were ground with a mixer mill (MM 301, Retsch, Haan, Germany). The insulated steel jars in which cryogenic grinding was carried out were cooled in liquid nitrogen. The embrittled PET was subsequently milled for 7 min. The resulting fine polymer powder was sieved to provide a grain size fraction below 0.5 mm. The appropriate quantities of Sb (as Sb2O3) were weighed directly to the low Sb PET in a microbalance (Mettler, UMT2) and distributed homogenously in the polymer matrix by mixing in the same mill at ambient temperature. A total of 5 g quantities of the mixtures were then pelletised in an automatic hydraulic press (Atlas auto press, Specac, Orpington, Kent, UK) at applied load of 25 t. Pellets of 25 mm diameter and 9.5 mm thickness were obtained, which exhibited mechanical stability with flat, hard and shiny surfaces. Subsequently, the pellets were used to calibrate the XRF measurement of Sb in PET at levels of 8, 100, 200, 300 and 400 mg kg−1. The Sb content of the PET under study was measured with energy dispersive XRF (PANalytical, Almelo, The Netherlands). The instrument was equipped with an Sc-W X-ray tube, operated at 600 W. The tube irradiated secondary targets, which acted as an X-ray source. Accordingly, radiation coming from the target was used to excite the samples. To measure Sb, an aluminium oxide (Al2O3) target that scattered tube radiation was inserted. In this way, Sb-Kα1 and Sb-Kα2 peak counts at 26.4 and 26.1 keV, respectively, were recorded during 200 s. A linear calibration curve was obtained measuring both sides of the pellets and averaging the counts for each level.
Determination of Sb migration into 3% acetic acid
Identical PET sheets, 0.31 mm thick, 60 mm in length and 30 mm in height, were used for the migration tests. The slices were cut out from original PET trays using scissors and a cardboard cutter. The slices were cleaned in a supersonic bath with demineralised water for 1 min. After drying, the slices were weighed; the average mass was 761 ± 42.7 mg. A single sample slab was placed vertically in the a quartz cell with a volume of 130 ml. A total of 89 ml 3% acetic acid was poured into the quartz cell, thereby the liquid level rose above the PET slab. The cells were covered with Teflon caps to prevent evaporation and then placed in a holder, which was mounted in the same autoclave used for the digestion of PET (MLS Ultraclave III). In the autoclave chamber the cells are immersed in water, which takes up microwave energy and ensures homogenous temperature distribution in all simulant solutions. The temperature of the solutions was controlled according to pre-assigned temperatures. The maximum microwave power of 1 kW was used to keep the temperature ramp short. Migration tests were made at 20, 30, 45, 60, 75, 90, 105, 120, 135 and 150°C. Each isothermal test lasted for 1, 5 and 24 h, or in some cases 46 h. In fact, all times are approximate because the cool-down ramps were neglected. Initially, the chamber was pressurised with nitrogen to 50 bar or up to 90 bar for the long tests, respectively (≥24 h). The high pressure is necessary to prevent boiling of the acetic acid. The pressure effect on the diffusion of molecules such as Sb glycolate can be neglected, assuming it does not change drastically between 1 and 50 bar (Fischer Citation1997). Moreover, the effect of pressure on the density of PET is very small (Eslami & Müller-Plathe Citation2009). At low temperatures of 20 and 30°C, the same diffusivity cells were thermostat controlled in a warming cupboard (Thermocenter, Renggli Salvis Lab, Rotkreuz, Switzerland) at atmospheric pressure. After cooling and releasing the pressure, the PET slabs were removed immediately. Aliquot parts of the migration solution were diluted to 50 ml with 2.5% nitric acid and measured by ICP-MS. The external calibration standards were matrix-matched with acetic acid and rhodium was used as internal standard. The detection limit of this method for Sb in migration solutions was 0.04 µg l−1.
Sb migration into food samples
Ready meals intended for oven heating were obtained from local supermarkets. The food samples were homogenised in a laboratory mixer (Grindomix GM200, Retsch) to ensure random and even distribution of any irregularities in the food matrix. The PET trays under study were equipped with two Pt-100 temperature sensors soldered to a metallic sheet (5 × 2 × 1.3 mm) and encapsulated by electrically insulating plastic (Innovative Sensor technology). The sensors were placed on the bottom of the tray at opposite corners and immersed in the mixed food. The filling height was measured and contact surface and volume were calculated for each food mix. Some of the PET trays were filled to the brim. According to the quantity of food the surface/volume ratios varied between 0.6 and 0.7. The food samples in the PET trays were heated for 30 min in a thermostatic heating cupboard (Thermocenter, Renggli Salvis Lab, Rotkreuz, Switzerland) at 200°C, while the temperature in the food was recorded every 3 s with a temperature-measuring device (Ecolog TP4-L, Elpro-Buchs, Buchs, Switzerland). In preliminary measurements, both thermometers showed nearly identical readings, indicating a homogeneous temperature distribution in the tray. After heating, the food samples were allowed to cool for some time before being removed from the PET tray. The samples were weighed before and after heating. Again, the samples were homogenised in the laboratory mixer to obtain a uniform distribution of the migrated Sb in the food.
Mineralisation of food samples
A total of 0.2 g of the freeze-dried food samples was digested with 2 ml nitric acid, 10 mol l−1, in the same microwave autoclave used for PET mineralisation. To each sample, a volume of 150 µl of a 37.5 ng ml−1 123Sb-spike (tracer) solution was added for the subsequent isotope dilution analysis by ICP-MS. The reactor was initially pressurised with nitrogen at 55 bar. The digestion temperature was maintained at 250°C during 45 min, whereby the pressure increased to 150 bar. At the completion of the reaction, the reactor was cooled to 50°C and the pressure was gradually released. The reactor chamber was opened and the processed samples were vented to remove NOx and then diluted to 10 ml with demineralised water.
Isotope dilution analysis of food
A Thermo Fisher Scientific (Bremen, Germany) sector-field ICP-MS Element 2 instrument was equipped with a mini-cyclonic glass spray Chamber (Twinnabar, Glass-Expansion, West Melbourne, Australia) and a concentric glass nebuliser (Sea Spray, Glass-Expansion, West Melbourne, Australia), which was operated in an argon sample gas flow range of 0.96–1.0 l min−1. The auxiliary gas flow was 0.77–0.88 l min−1. Optimisation of the instrument was performed with 1 ng ml−1 indium to obtain maximum signals and best stability. Low resolution (Δm/m at 10% height) at approximately 300 was used. The ICP-MS was operated under the “E-scan” mode, i.e. the magnetic field was kept constant while the acceleration lenses voltage was changed. Although this approach is the most efficient for isotope ratio measurements, it can be a source of mass discrimination effects. For the present study, however, it did not have much of an impact on the measurements since only the two Sb isotopes (123Sb, 121Sb) were involved for the sample measurement. The experimentally measured 121Sb/123Sb ratio of a standard Sb solution was 1.31 ± 0.017, which agreed with the theoretical ratio of 1.34 for natural Sb. The detector dead time was 25 ns. The Sb isotopes were monitored 10 ms for each measurement channel. Twenty samples per peak were recorded. The resulting measuring time for this particular segment was 0.25 s. The scans were repeated 12 times, which amounted to a total measuring time of 73 s. The take-up and wash times were 2 and 3 min, respectively. 121Sb/123Sb ratios of the mineralised sample solutions spiked with 123Sb tracer were directly measured and the Sb concentration was calculated with the Element 2 isotope dilution software.
A white cabbage sample (BCR-679) with a certified Sb concentration of 20.6 ± 2.6 µg kg−1 was used to test the accuracy of the isotope dilution analysis for the ICP-MS measurement of Sb in food. The measured Sb concentration of 21.2 ± 2.2 µg kg−1 fell in to the 95% confidence interval of the certified value. Cabbage was not an ideal food matrix because the samples measured in this study consisted mainly of pasta, meat or fish. However, the behaviour of the two Sb isotopes is very similar across the sample introduction system and ICP-MS instrument, and thus various matrix effects attributed to food are compensated.
Determination of apparent diffusion coefficients
Assessment of migration contaminants from food packaging was based on Fick's Second Law, which relates the time rate of concentration change to the second derivative of the concentration gradient through the diffusion coefficient (Crank Citation1956). The migration of a readily soluble migrant from the two sides of the thin plane polymer sheet contacting a large volume of food or simulant solvent can be described in a simplified way:
Determination of activation energy
Diffusion of Sb in the PET polymer is a thermally activated process, with diffusion coefficients D that depend on temperature, as described by the Arrhenius equation:
Statistical analysis
The statistic software SAS 9.2 was used to estimate the Arrhenius parameters D o and E a with confidence intervals from linear regression analysis (Equation (Equation2)). In contrast, non-linear estimation normally includes a broad variety of statistical procedures. We performed non-linear least squares, which is analogous to ordinary least squares. It minimises squared deviations of the dependent variable data values from values estimated by the function at the same independent variable data points (Equation (Equation3)). The system is solved numerically in an iterative process by the least squares Gauss–Newton method using Systat 13 software. The starting values had to be set close to the previously estimated values of D 0 and E a (from Equation (Equation2)); otherwise, the algorithm failed to converge. A paired t-test was carried out to compare the difference between measured and simulated Sb quantities.
Model calculations
The SML software release 4.5.3 (AKTS Citation2012) was used to predict the amount of Sb that migrates from the PET tray to the food. It employs finite element analysis (FEA) to solve migration problems where an analytical solution of the Fickian equation is not possible. This numerical analysis is based on a system of nodes which construct a grid or mesh. The grid-point distribution is chosen with variable step lengths (Roduit et al. Citation2005). Among other models the software comprises the Arrhenius equation for temperature dependence, for which the experimentally determined parameters D o and E a were required as inputs. The packaging specification was defined as the surface-to-volume ratio based on the actual contact surface and the volume of the food. The PET was treated as a one-layer polymer of average properties, i.e. density, thickness and initial Sb concentration were set as measured. The Sb partition coefficient was arbitrarily set to 1. The program can calculate the migration based on customised temperature profiles, which allows use of experimentally measured temperatures in the food matrix. The software uses a non-linear regression algorithm by Levenberg–Marquardt.
Results and discussion
Measurements of Sb in polymer and food matrices
The total concentration of Sb in the PET under study was concurrently determined by ICP-MS and energy-dispersive XRF. Both methods required different sample preparation techniques, i.e. nitric acid mineralisation and direct measurement of solid PET. ICP-MS and XRF measurements yielded comparable Sb concentrations of 290 ± 5.7 and 318 ± 18.1 mg kg−1, respectively. Taking into consideration that XRF suffers from matrix effects, the agreement between the two methods was satisfactory; the difference was statistically not significant (p = 0.1). The sample PET tray had a normal level of residual Sb when compared with the previously reported results (Haldimann et al. Citation2007). In addition, the CRMs were used for quality control purposes.
Diffusion coefficients of Sb in PET
After being exposed to acidic solution at high temperatures, the PET sheets did not show any visual damage or decomposition at the outer surface. Acetic acid dissolved the migrants readily, even though at temperatures above 135°C fine white powder residues were observed, which most likely originate from partial hydrolytic degradation or concomitant migration compounds of the PET, e.g. terephthalic acid. The residues were isolated and its mass fractions were less than 5%. The powders were analysed by XRF analysis and no Sb, titanium or other inorganic constituents were detected. Moreover, the infrared spectrum (IR) obtained nearly coincided with a reference spectrum of terephthalic acid. According to the library assignment, the residue also contained isophthalic acid.
From the series of experimental data for migration level in relation to time, the parameters of the linear regression model were calculated for each temperature. An example plot of the diffusion experimental data and fitted curves by using Equation (Equation1) for three cases is shown in . It depicts the experimental migration progress of Sb to acetic acid as function of the square root of time. The plot of Mt /M 0 versus t 0.5 showed a linear relationship, which confirms the validity of Fick's law to describe the diffusion process of Sb in PET. From the slope of the curves, the apparent diffusion coefficients (D Fick) were determined. Poor correlation was observed at temperatures lower than 90°C. This may be due to inconsistent diffusion of Sb at low temperatures during short migration times associated with higher experimental errors observed at low concentrations. For this reason, diffusion coefficients (D) were derived from repeated measurements of 24-h migration tests. The calculated apparent diffusion coefficient values are summarised in .
Figure 1. Selected kinetics of migrated Sb fractions (m t) from a PET tray into 3% acetic acid versus the square root of time at 150°C (•), 135°C (▴) and 105°C (♦). The PET tray contains 290 mg kg−1 Sb, of which 62% is available for diffusion, i.e. m 0/m t = 1. The value >1 is attributed to experimental errors.
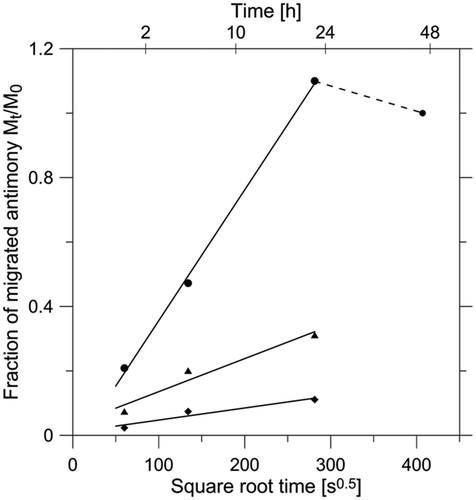
Table 1. Comparison of the Arrhenius parameters obtained from a series of isothermal migration measurements of Sb from PET sheets into 3% acetic acid with 95% confidence intervals (CI).
Sb mass balance
The equilibrium Sb concentration in the Etimex E1231 PET polymer and that in the contacting phase were determined at 150°C. The highest amount of a Sb that can migrate to food simulant was estimated as illustrated in . It is assumed that after a contact time of 24 h, most of the diffusible Sb the PET migrated to the 3% acetic acid phase and did not increase any further after a contact time of 48 h. Accordingly, the fraction of diffusible Sb was about 62% ().
Polycondensation catalysis by Sb compounds (Sb2O3) is homogeneous, i.e. the catalyst is soluble in the reaction medium. The reaction of an applied Sb compound with ethylene glycol leads to the formation of a glycolate complex, which is the pre-catalyst (Biros et al. Citation2002; Duh Citation2002; El-Toufaili et al. Citation2006). Thus, the predominant diffusible species are monodentate glycolate (−Sb−OCH2CH2OH) and chelate ligand (−OCH2CH2O−) Sb complexes. In contrast, the portion of diffusible inorganic Sb is probably very small.
In general, glycolate ligand derivatives of Sb are sensitive to hydrolysis (Maerov Citation1979). Thus, reactivity of the migrant in the food phase may prevent back diffusion of Sb into the PET. Sb compounds of this glycolate type are believed to be the active catalyst component in the condensation polymerisation to PET (Aharoni Citation1998). In contrast, a large Sb fraction of approximately 38% is not available for diffusion, i.e. Sb is either bound to the polymer chains or trapped within the PET crystalline zones and thus immobilised in the polymer matrix. In absence of any losses, the general mass balance gives the total amount of Sb present in the PET sheet (M Total) that is equal to the sum of the amount migrating into the acetic acid phase (M HAc) plus the amount remaining in the PET (M R). This is valid at any instance:
Equation (Equation4) was verified at two different temperatures. The relative recoveries of the remaining Sb in the PET slabs after 24-h migration at 120 and 135°C were 95% and 86%, respectively. In other words, the recovered Sb from the sheets was approximately complementary to the migrated fractions ().
Experimental determination of Ea and D0 under isothermal conditions
In , the experimentally measured diffusion coefficients are plotted as a conventional Arrhenius graph and summarised in . Equation (Equation2) yields consistent Arrhenius parameters. However, deviation from linearity can be observed, which indicates non-Arrhenius behaviour. The Arrhenius plot of the experimental points in apparently shows an upward curvature in the temperature domain below the glass transition temperature (T g). In contrast, the data suggest a linear relationship above T g. The temperature dependence of the diffusion coefficient behaves in an Arrhenius fashion. The obtained average activation energy was approximately 188 kJ mol−1, and did not change significantly with additional isothermal measurements down to 45°C. High values of the coefficient of determination (R 2 > 0.99) were obtained for all cases over the temperature range from 45 to 150°C. In a strict sense, at the temperature of 150°C the ratio Mt /M 0 exceeded 0.5 and application of Equation (Equation1) adds to the margin of error. However, the regression line fits all the points above 45°C, which indicates absence of a significant deviation. The variations of the pre-exponential factor (D 0) estimates from the Arrhenius plot become greater due to the reduced number of degrees of freedom. The confidence intervals were large, attributed to the temperature dependence for extrapolation of ln(D 0). In a statistical sense, the extrapolation error must be large, because the curves are fitted in one temperature domain and used for prediction in another.
Figure 2. Temperature dependence (Arrhenius plot) of the apparent diffusion coefficient of Sb in PET. The dotted line refers to the data of Welle and Franz (Citation2011). T g denotes the glass transition temperature range.
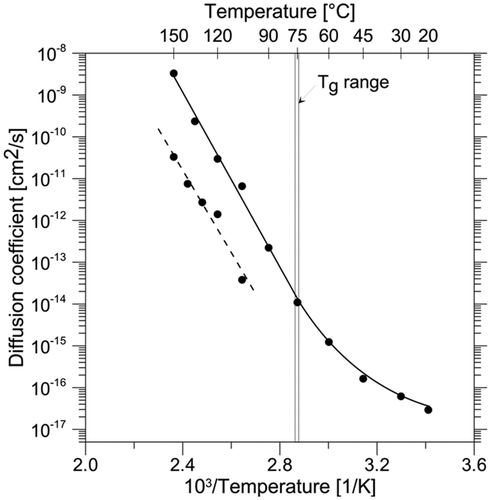
When all the diffusion coefficients obtained at temperatures below 45°C are included, the linear regression fit to the Arrhenius plot is considerably weakened, which was indicated by a lower coefficient of determination (R 2 < 0.98). The activation energy (E a) was therefore determined by linear regression analysis of a number of consecutive temperature steps that yielded an R 2 ≥ 0.99, which are all the points preceding unambiguous deviation from linear Arrhenius plot. The non-linear behaviour of the Arrhenius relationship is difficult to understand. Normally, below T g the decreased mobility of APET chains should hinder the diffusion of migrants and a downward curvature of the plot is expected. On the contrary, temperature seems to have a limited effect on the diffusion and an upward curvature of the Arrhenius plot related to lower E a values was observed. Since activation energy is specific to the type of compound, i.e. molecular weight and size, this finding could indicate that the composition of the migrating Sb fraction is different in the low-temperature domain.
Alternatively, all the data (n = 10) were reanalysed by non-linear regression analysis using Equation (Equation3). Linear regression provided somewhat biased results depending on the number of data points (), whereas non-linear regression analysis does not suffer from the same problem. The E a (±95% CI) and D 0 values of 187 ± 0.08 kJ mol−1 and 4 × 1014 cm2 s−1, respectively, were almost identical to those obtained by linear regression with five data points. The 95% confidence interval of E a was considerably smaller, which demonstrated that more precise estimates can be obtained from all data instead of selected data points in the linear range. The comparison shows that linear regression is in principle not inferior to non-linear regression; however, the latter is robust and not susceptible to deviation from Arrhenius behaviour at low temperatures.
Comparison with literature data
Comparing the results with those found in the literature is instructive. If the diffusion of an Sb compound proceeded not disturbed by experimental conditions in identically structured PET, the parameters D 0 and E a would have unique values. Hence, all diffusion data fall onto the same line of an Arrhenius plot and the scatter in the data is due to experimental error. Consequently, another PET material will have a different pre-exponential factor or a different activation energy, i.e. the bottle PET data depicted in lie in a different region of the Arrhenius plot (Welle & Franz Citation2011). The apparent diffusion coefficients of Sb in were higher than previously reported experimental data from bottle PET (Welle & Franz Citation2011). As illustrated in , the linear part of the Arrhenius plot runs parallel to the data found by Welle and Franz (Citation2011). Thus, the activations energies are essentially the same, whereas the pre-exponential factors differ.
Factors controlling migration are the nature of the Sb migrants and the morphology of the PET. Concerning migrants, molecular weight and shape, i.e. the chemical structure of Sb glycolate complexes, is of particular importance. Diffusion coefficients in PET are apparently more sensitive to the molecular weight of the diffusing species than in polyolefins (Pennarun et al. Citation2004a). Therefore, the molecular weight distribution of the diffusible Sb–glycol complexes is a key factor.
Furthermore, an influence of the PET morphology on the diffusion coefficients can be expected. Semi-crystalline polymers such as PET contain separate amorphous and crystalline morphological regions. Diffusants cannot penetrate crystalline phases of PET polymers readily and take the path of least resistance through amorphous regions (Whitehead Citation1977). However, the properties of amorphous regions depend also on crystallites, e.g. the mobility of polymer chains can be restricted by cross-linking of crystallites. Accordingly, crystallinity in combination with orientation can explain differences in diffusion coefficients among polymers (Dudler & Muinos Citation1996). The degree of crystallinity in the bottle PET investigated by Welle and Franz (Citation2011) was 43% and was considerably higher than that in the PET under study (27%). Strain induced crystallisation is a result of the stretch blow moulding process used to make PET bottles. In contrast to the non-oriented crystalline structures of thermoformed trays, this manufacturing process results in bottles with highly oriented crystallites, which could possibly explain the differences in the diffusion coefficients.
Another prominent difference between ovenware and bottle PET is the content of titanium pigment, which is primarily used to alter the appearance. In addition, it may suppress strain-induced crystallisation (Taniguchi & Cakmak Citation2004). The titanium content of 0.50% ± 0.01% was measured by ICP-MS. Titanium dioxide (TiO2) is a mineral that exhibits sorption properties. In particular, TiO2 nanoparticles have a large adsorption capacity for arsenic, which is an element with similar chemical properties to Sb (Sun et al. Citation2009). In bulk PET, the TiO2 particles are normally present in sub-micron size and possess large surfaces (Taniguchi & Cakmak Citation2004), and thus they may be effective in delaying Sb diffusion. In the PET under study, it is however unlikely that the presence of TiO2 is responsible for the differing apparent diffusion coefficients shown in . The presence of TiO2 does not impede diffusion of Sb because it proceeds faster in the tray PET when compared with bottle PET.
Experimental and simulated Sb migration into real food
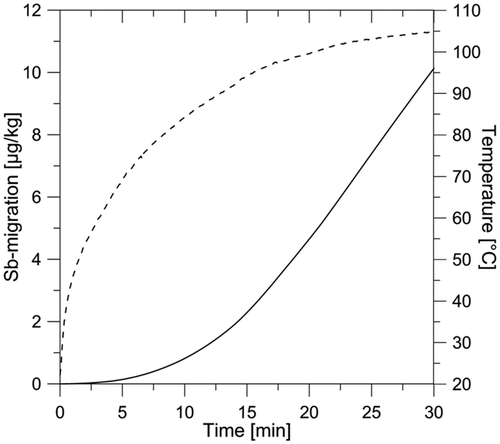
Simulated Sb contents were obtained using the SML software AKTS, run with experimental Arrhenius parameters as input: E a = 188 kJ mol−1 and D 0 = 3 × 1014 cm2 s−1. Even though the thin sealing lamination was the actual food contact phase, the PET tray was treated as a monolayer structure. These values were selected among the data reported in , because above 90°C (T g) diffusion coefficients (D Fick) were additionally derived from the slope of the relation Mt /M 0 versus t 0.5 and thus better established. In the absence of any specific data, the partition coefficient (K p) was taken to be 1, meaning that the Sb migrant is readily soluble in the food phase. It was assumed that food and simulant did not interact with the PET tray material, i.e. did not penetrate into the polymer phase. For this reason, the diffusion coefficient of the Sb migrant was considered to be constant and independent of time. The software used for the simulation of migration accounts for the progressive increase of diffusion coefficients with temperature. In each batch of food, the migrated quantity of Sb was measured and the corresponding temperature profile recorded during the heating process. The temperature profile measured in a certain food depends on its overall heat capacity. illustrates an example of the simulated migration process with the measured food temperature while heating in the oven.
Figure 3. The solid line displays the simulated progressive migration of Sb into food (cannelloni). The increase in concentration represents Sb that was migrated into food as a result of heating, i.e. the difference between total and background Sb. For comparison, the dashed curve represents the respective temperature measured in the actual food matrix.
The purpose of this study was to obtain quantitative and reproducible data on Sb migration, and therefore mixing and grinding of semi-liquid foods was necessary to ensure maximum contact between the phases. The result of the practical migration experiments are listed in . The Sb concentrations in the raw food products were clearly above background levels because they depended on previous contact with PET polymers (Haldimann et al. Citation2007). A three- to six-fold increase of the Sb concentration in food was observed as a result of heating in the oven.
Table 2. Increase of Sb concentration in ready meals after cooking in a conventional oven. Comparison between experimentally measured and simulated Sb migrating to food.
The results indicate there is no significant difference (p = 0.1) between measured and simulated Sb quantities, i.e. migration modelling successfully fitted the experimental Sb data and made it possible to predict the migrated quantities with satisfactory accuracy. Furthermore, this agreement demonstrated that the sealing layer has no barrier properties towards Sb compounds from the parent PET. The simulated values depend on Equation (Equation1), which represents a case of negligible mass transfer resistance on the side of the food, i.e. the migration process is controlled by diffusion in PET. However, progressive drying of the food during the heating process may narrow down the actual contact surface by formation of tiny hollow spaces and cracks. Moreover, experience has shown that the correct measurement of the temperature was a key factor in the success of the experiments.
The absolute worst-case migration would be the concentration of Sb in the food if all migrants were transferred from the polymer, which was not the case for the PET under study. However, the migrated Sb in the study samples certainly exceeded the quantities that can be expected in food after preparing it for consumption. In this study, some of the PET trays were filled to the brim with food, which is not normally the case in retail packages. In addition, the pre-mixing of the food facilitated migration of Sb into the food phase. Even though the measured Sb concentrations were not normalised with respect to the surface/volume ratios, it is evident that the SML of 40 µg kg−1 was not exceeded in any of the food samples. In fact, the major part of the ready-to-use food products on sale are intended for heating in a microwave oven. In microwave foods the temperature approaches 100°C in a few minutes and the cooking times are shorter. However, temperature monitoring of food in a microwave field is experimentally very difficult. In tray-shaped products microwave energy concentrates in the corners, giving rise to hot spots (Campanone & Zaritzky Citation2005). Hence, the non-uniform temperature profile in the food would make it difficult to simulate accurately the Sb migration with the SML software. In any case, migration of Sb from PET trays into food irradiated by microwaves is inferior to that heated in conventional baking ovens (Haldimann et al. Citation2007).
Conclusion
This study reports the migration properties of Sb from PET to 3% acetic acid and real food for a food-contact material, which represents the bulk of polymers currently used in ovenable ready meal packaging. The specific Arrhenius parameters needed in migration modelling were derived. Migration modelling based on Fick's law gave a prediction of the Sb migrant concentration under defined contact conditions between food simulant and PET. A very satisfactory agreement between model results and experimentally measured food data was obtained, demonstrating the validity of the model and its inputs, i.e. E a, D 0 and temperature profiles for a monolayer structure.
Metals and semimetals such as Sb are not included in the current European Union legislation for the estimation of their migration levels by recognised diffusion models (European Commission Citation2002). However, with respect to regulatory guidelines, migration modelling can be used as a tool for orientation purposes. In industrial practice, simulations can be performed to study the effect of the thickness, temperature and product volumes on the Sb concentration in food, saving time and reducing the number of laboratory analysis. Furthermore, this kind of migration modelling might also be useful for estimating human exposure to ensure a consumer safety margin.
References
- Aharoni , SM . 1998 . The cause of the grey discoloration of PET prepared by the use of antimony-catalysts . Polym Eng Sci , 38 : 1039 – 1047 .
- AKTS. 2012. AKTS-SML software [Internet]. Available from: http://www.akts.com/sml-diffusion-migration-multilayer-packaging/download-diffusion-prediction-software.html. Siders: AKTS
- Alt A, Haldimann M, Dudler V. 2008. Diffusion coefficient of antimony catalysts in polyethylene terephthalate (PET) materials. Poster presented at: 4th International Symposium on Food Packaging – Scientific Developments Supporting Safety and Quality; 2008 Nov 19; Prague, Czech Republic
- Asakura , K , Satoh , H , Chiba , M , Okamoto , M , Serizawa , K , Nakano , M and Omae , K . 2009 . Genotoxicity studies of heavy metals: lead, bismuth, indium, silver and antimony . J Occupat Health , 51 : 498 – 512 .
- Ashby , R . 1988 . Migration from polyethylene terephthalate under all conditions of use . Food Addit Contam , 5 ( sup 001 ) : 485 – 492 .
- BAG . 2005 . Antimon in Mineralwasser . Beurteilung des Gesundheitsrisikos Bull , 44 : 796 – 797 .
- Begley , T , Castle , L , Feigenbaum , A , Franz , R , Hinrichs , K , Lickly , T , Mercea , P , Milana , M , O’Brien , A Rebre , S . 2005 . Evaluation of migration models that might be used in support of regulations for food-contact plastics . Food Addit Contam , 22 : 73 – 90 .
- Bharadwaj , RK and Boyd , RH . 1999 . Small molecule penetrant diffusion in aromatic polyesters: a molecular dynamics simulation study . Polymer , 40 : 4229 – 4236 .
- Biros , SM , Bridgewater , BM , Villeges-Estrada , A , Tanski , JM and Parkin , G . 2002 . Antimony ethylene glycolate and catecholate compounds: structural characterization of polyesterification catalysts . Inorgan Chem , 41 : 4051 – 4057 .
- Brauner , N and Shacham , M . 1997 . Statistical analysis of linear and nonlinear correlation of the Arrhenius equation constants . Chem Eng Process Process Intensif , 36 : 243 – 249 .
- Campanone , LA and Zaritzky , NE . 2005 . Mathematical analysis of microwave heating process . J Food Eng , 69 : 359 – 368 .
- Castle , L , Macarthur , R , Mead , EM and Read , WA . 2004 . Measurement uncertainty associated with overall migration testing . Food Addit Contam , 21 : 256 – 264 .
- Choe , SY , Kim , SJ , Kim , HG , Lee , JH , Choi , Y , Lee , H and Kim , Y . 2003 . Evaluation of estrogenicity of major heavy metals . Sci Total Env , 312 : 15 – 21 .
- Crank , J . 1956 . Diffusion in an plane sheet. In: The mathematics of diffusion , 42 – 61 . Chap. 4. Oxford : Oxford University Press; .
- Dudler , V and Muinos , C . 1996 . Diffusion of benzotriazoles in polypropylene: influence of polymer morphology and stabilizer structure . ACS Adv Chem Ser , 249 : 441 – 454 .
- Duh , B . 2002 . Effect of antimony catalyst on solid-state polycondensation of poly(ethylene terephthalate) . Polymer , 43 : 3147 – 3154 .
- El-Toufaili , FA , Feix , G and Reichert , KH . 2006 . Mechanistic investigations of antimony-catalyzed polycondensation in the synthesis of poly(ethylene terephthalate) . J Polym Sci A: Polym Chem , 44 : 1049 – 1059 .
- Eslami , H and Müller-Plathe , F . 2009 . Structure and mobility of poly(ethylene terephthalate): a molecular dynamics simulation study . Macromolecules , 42 : 8241 – 8250 .
- European Commission . 2002 . Commission Directive 2002/72/EC of 6 August 2002 relating to plastic materials and articles intended to come into contact with foodstuffs . Off J Eur Union. L , 39 : 1 – 42 .
- European Commission . 2003 . Commission Directive 2003/40/EC of 16 May 2003 establishing the list, concentration limits and labelling requirements for the constituents of natural mineral waters and the conditions for using ozone-enriched air for the treatment of natural mineral waters and spring waters . Off J Eur Union , L 126 : 34 – 39 .
- European Commission . 2005 . Commission Directive 2005/79/EC of 18 November 2005 amending Directive 2002/72/EC relating to plastic materials and articles intended to come into contact with food . Off J Eur Union , L 302 : 35 – 45 .
- European Food Safety Authority (EFSA) . 2004 . Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to a 2nd list of substances for food contact materials . EFSA J , 24 : 1 – 13 .
- Fischer , T . 1997 . Study on different parameters influencing the additive-polymer compatibility [dissertation] , Montanuniversität : University of Leoben, Institute of Materials Science and Testing of Plastics .
- Fordham , PJ , Gramshaw , JW , Crews , HM and Castle , L . 1995 . Element residues in food contact plastics and their migration into food simulants, measured by inductively coupled plasma-mass spectrometry . Food Addit Contam , 12 : 651 – 669 .
- Gebel , T . 1997 . Arsenic and antimony: comparative approach on mechanistic toxicology . Chemico-Biol Interact , 107 : 131 – 144 .
- Haldimann , M , Blanc , A and Dudler , V . 2007 . Exposure to antimony from polyethylene terephthalate (PET) trays used in ready-to-eat meals . Food Addit Contam , 24 : 860 – 868 .
- International Agency for Research on Cancer (IARC). 1989. Some organic solvents, resin monomers and related compounds, pigments and occupational exposures in paint manufacture and painting. 47:291. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans
- Keresztes , S , Tatar , E , Mihucz , VG , Virag , I , Majdik , C and Zaray , G . 2009 . Leaching of antimony from polyethylene terephthalate (PET) bottles into mineral water . Sci Total Env , 407 : 4731 – 4735 .
- Maerov , SB . 1979 . Influence of antimony catalysts with hydroxyethoxy ligands on polyester polymerization . J Polym Sci Polym Chem Ed , 17 : 4033 – 4040 .
- Pennarun , PY , Dole , P and Feigenbaum , A . 2004a . Functional barriers in PET recycled bottles. Part I. Determination of diffusion coefficients in bioriented PET with and without contact with food simulants . J Appl Polym Sci , 92 : 2845 – 2858 .
- Pennarun , PY , Dole , P and Feigenbaum , A . 2004b . Overestimated diffusion coefficients for the prediction of worst case migration from PET: application to recycled PET and to functional barriers assessment . Packag Technol Sci , 17 : 307 – 320 .
- Roduit , B , Borgeat , CH , Cavin , S , Fragnière , C and Dudler , V . 2005 . Application of finite element analysis (FEA) for the simulation of release of additives from multilayer polymeric packaging structures . Food Addit Contam , 22 : 945 – 955 .
- Rusz Hansen , H and Pergantis , SA . 2006 . Detection of antimony species in citrus juices and drinking water stored in PET containers . J Anal At Spectrom , 21 : 731 – 733 .
- Shotyk , W , Krachler , M and Chen , B . 2006 . Contamination of Canadian and European bottled waters with antimony from PET containers . J Environ Monit , 8 : 288 – 292 .
- Sun , H , Zhang , X , Zhang , Z , Chen , Y and Crittenden , JC . 2009 . Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite . Environ Poll , 157 : 1165 – 1170 .
- Taniguchi , A and Cakmak , M . 2004 . The suppression of strain induced crystallization in PET through sub micron TiO2 particle incorporation . Polymer , 45 : 6647 – 6654 .
- Thiele , UK . 2001 . The current status of catalysis and catalyst development for the industrial process of poly(ethylene terephthalate) polycondensation . Int J Polymeric Mat , 50 : 387 – 394 .
- Vergnaud , JM . 1991 . Liquid transport processes in polymeric materials: modeling and industrial applications , 1 – 17 . Englewood Cliffs , NJ : Prentice Hall. Chapter 1, Diffusion in a plane sheet; .
- Welle , F and Franz , R . 2011 . Migration of antimony from PET bottles into beverages: determination of the activation energy of diffusion and migration modelling compared with literature data . Food Addit Contam A , 28 : 115 – 126 .
- Westerhoff , P , Prapaipong , P , Shock , E and Hillaireau , A . 2008 . Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water . Water Res , 42 : 551 – 556 .
- Whitehead , BD . 1977 . The crystallization and drying of polyethylene terephthalate (PET) . Ind Eng Chem Process Des Dev , 16 : 341 – 346 .
- World Health Organization (WHO) . 2003 . Antimony in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. WHO/SDE/WSH/03.04/74 , Geneva : WHO .