Abstract
Aflatoxins contaminate approximately 25% of agricultural products worldwide. They can cause liver failure and liver cancer. Kenya has experienced multiple aflatoxicosis outbreaks in recent years, often resulting in fatalities. However, the full extent of aflatoxin exposure in Kenya has been unknown. Our objective was to quantify aflatoxin exposure across Kenya. We analysed aflatoxin levels in serum specimens from the 2007 Kenya AIDS Indicator Survey – a nationally representative, cross-sectional serosurvey. KAIS collected 15,853 blood specimens. Of the 3180 human immunodeficiency virus-negative specimens with ≥1 mL sera, we randomly selected 600 specimens stratified by province and sex. We analysed serum specimens for aflatoxin albumin adducts by using isotope dilution MS/MS to quantify aflatoxin B1-lysine, and normalised with serum albumin. Aflatoxin concentrations were then compared by demographic, socioeconomic and geographic characteristics. We detected serum aflatoxin B1-lysine in 78% of serum specimens (range = <LOD–211 pg/mg albumin, median = 1.78 pg/mg albumin). Aflatoxin exposure did not vary by sex, age group, marital status, religion or socioeconomic characteristics. Aflatoxin exposure varied by province (p < 0.05); it was highest in Eastern (median = 7.87 pg/mg albumin) and Coast (median = 3.70 pg/mg albumin) provinces and lowest in Nyanza (median = <LOD) and Rift Valley (median = 0.70 pg/mg albumin) provinces. Our findings suggest that aflatoxin exposure is a public health problem throughout Kenya, and it could be substantially impacting human health. Wide-scale, evidence-based interventions are urgently needed to decrease exposure and subsequent health effects.
Introduction
Aflatoxins are fungal toxins derived from some strains of Aspergillus flavus. They contaminate an estimated one-quarter of agricultural products worldwide, with maize, cereals and groundnuts being the most susceptible (Williams et al. Citation2004; Wild & Gong Citation2010). Although aflatoxins are ubiquitous, their growth appears to be encouraged by factors such as drought, high humidity, insect infestation and sub-optimal harvesting, drying and crop storage practices. These factors, in addition to a lack of consistently enforced aflatoxin regulatory limits, cause people in developing countries to be more adversely affected by aflatoxin exposure (Williams et al. Citation2004; Wild & Gong Citation2010).
Aflatoxins have a variety of hepatotoxic and carcinogenic characteristics. Chronic exposure has been linked to hepatocellular carcinoma (Ross et al. Citation1992), stunted growth (Khlangwiset et al. Citation2011) and impaired immunity (Jiang et al. Citation2005). Acute exposure (i.e., aflatoxicosis) can lead to jaundice, vomiting, abdominal pain and liver failure, with documented fatality rates as high as 40% (Centers for Disease Control and Prevention Citation2004; Wild & Gong Citation2010).
Aflatoxin exposure has been well documented in Kenya; the first reported aflatoxicosis outbreak occurred in 1981 (Ngindu et al. Citation1982). Multiple aflatoxicosis outbreaks have been documented since 2004, resulting in nearly 500 acute illnesses and 200 deaths (Centers for Disease Control and Prevention Citation2004; Azziz-Baumgartner et al. Citation2005). Most reported aflatoxicosis outbreaks have occurred among people living in rural, subsistence farming communities in Kenya’s Eastern province and were usually associated with consuming homegrown maize (Azziz-Baumgartner et al. Citation2005; Mwanda et al. Citation2005; Daniel et al. Citation2011).
Like most countries, Kenya has no national aflatoxicosis surveillance. Thus, it is unknown whether aflatoxicosis outbreaks and aflatoxin exposure are truly limited to a part of the Eastern province. It is also not known whether aflatoxin exposure varies by demographic, socioeconomic or ecologic factors. This information is critical for determining the total burden of disease attributed to aflatoxin exposure and for targeting public health interventions. Thus, we conducted a cross-sectional serological and epidemiologic survey to assess aflatoxin exposure throughout Kenya and to compare aflatoxin exposure by demographic, socioeconomic and geographic characteristics.
Materials and methods
Sampling
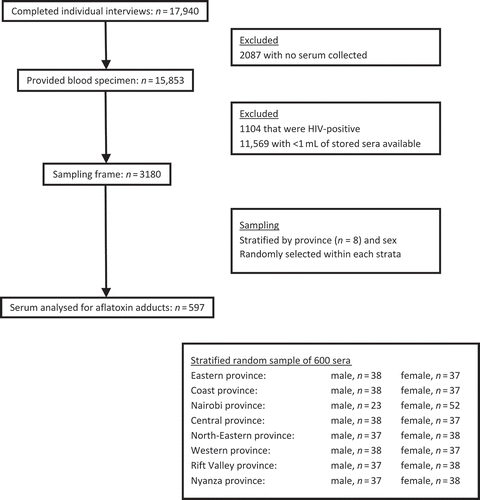
We analysed aflatoxin levels in serum specimens that were originally collected during the 2007 Kenya AIDS Indicator Survey (KAIS) (Kaiser et al. Citation2011). KAIS was a nationally representative, cross-sectional survey designed primarily to study the prevalence and knowledge, attitudes and beliefs regarding human immunodeficiency virus (HIV) and sexually transmitted infections in Kenya. The 2007 KAIS completed 17,940 individual interviews at 9691 households throughout Kenya. Blood specimens were provided by 15,853 individuals. Of these 15,853 blood specimens, 3180 met our inclusion criteria, which required that the sample be HIV-negative and contain ≥1 mL of sera (Figure ).
We had a target sample size of 600 serum specimens for this aflatoxin exposure assessment, based on what was logistically and financially feasible. We stratified the 3180 available specimens by Kenya’s eight provinces (Nyanza, Western, Rift Valley, Central, Nairobi, Eastern, North Eastern and Coast) and sex and randomly selected an approximately equal number of specimens from each of the 16 possible combinations of province and sex (n = 37 or 38). The exception was Nairobi, where only 23 male serum specimens met the inclusion criteria and thus we subsequently sampled 52 female serum specimens from Nairobi.
Of the 600 selected serum specimens, 3 specimens were of insufficient quantity/quality for laboratory analysis and 2 specimens were mislabelled, preventing us from being able to link them to demographics data. Thus, non-stratified analyses (which did not require linkage) include 597 participants; stratified analyses (which required linkage) include 595 participants.
Serum specimens were linked to household- and individual-level questionnaires that were also collected as part of the KAIS. All participants were aged 15–64 years. The questionnaires captured demographics, socioeconomic status and general health status (i.e., had they been sick in the past week, had they sought health care in the past 3 months and had anyone in their household sought health care in the past 4 weeks). Data were collected during August–November of 2007.
The 600 serum specimens selected for the aflatoxin assessment were similar in sex and age to the full KAIS sample of 15,853 specimens. There was some geographical variation. The parent KAIS selected participants from provinces with probability proportionate to size, whereas we selected an equal number of serum specimens from each province. Kenya’s North-Eastern province is less populated than the rest of the country. Thus, the North-Eastern province accounted for only 5% of all KAIS participants but 12% of the serum specimens for this aflatoxin assessment.
Laboratory analysis
During initial collection, blood samples were transported to the National Reference Laboratory in Nairobi, Kenya, for HIV testing and remnant serum was stored at −70°C for future testing. HIV test results were returned to participants. The 600 serum specimens included in this study were shipped on dry ice to the Centers for Disease Control and Prevention (CDC) National Center for Environmental Health Division of Laboratory Sciences (DLS, Atlanta, Georgia, USA) for analysis. CDC DLS analysed serum specimens for aflatoxin B1 albumin adduct, which consisted of two measurements: (1) analysis of aflatoxin B1-lysine (AFB1‐lys) by using LC-MS/MS (McCoy et al. Citation2005) and (2) albumin measurement. To allow the release of AFB1-lys from albumin, protein in serum specimens was digested in the presence of stable‐isotopically labelled internal standard (2H4‐AFB1‐lys) in 4 hours at 37°C by the use of a commercially available mixture of proteinases (Pronase™). AFB1‐lys and 2H4‐AFB1‐lys were then extracted by the use of mixed‐mode anion exchange reversed-phase solid phase extraction (SPE). Each SPE eluate was evaporated, reconstituted in the mobile phase and injected onto a reversed-phase C18 column. AFB1-lys was chromatographically separated from other compounds by using gradient mobile phase. Both AFB1-lys and 2H4‐AFB1‐lys were detected with positive electrospray inonization in the selective reaction monitoring mode by using tandem quadrupole mass spectrometry (McCoy et al. Citation2005). Quantitation was based on peak area ratios interpolated against a seven‐point aqueous linear calibration curve with 1/x weighting. The calibration range for serum AFB1‐lys was 0.025–10 ng/mL. Specimens with serum AFB1‐lys values of >10 ng/mL were diluted and repeated for confirmation. There are no established health-related thresholds for serum AFB1-lys concentrations. The LOD for AFB1-lys was 0.02 ng/mL. CDC DLS also analysed serum albumin on the Hitachi Modular P clinical analyzer (Indianapolis, IN, USA) by using the Roche® colorimetric assay (Indianapolis, IN, USA). The LOD for albumin was 0.2 g/dL. Human serum albumin – and subsequently albumin-corrected serum AFB‐lys – has a half-life of approximately 20 days in vivo (Gan et al. Citation1988; Skipper & Tannenbaum Citation1990). Thus, the detection of AFB1-lys in this assay suggests a likelihood of exposure to aflatoxin within the previous 1–2 months.
Statistical analysis
We analysed data by using SAS version 9.3. We assigned AFB1-lys concentrations <LOD (0.02 ng/mL) a value equal to the LOD divided by the square root of 2. We normalised serum AFB1-lys concentrations to serum albumin concentrations and present aflatoxin adduct levels in units of pg/mg (McCoy et al. Citation2005). We calculated geometric means (GMs) by taking the antilog of the mean of natural log-transformed values. Because natural log-transformed values were not normally distributed, we compared aflatoxin concentrations between groups by using the Kruskal-Wallis and Wilcoxon Mann-Whitney tests, and we used the Pearson chi-square test to compare categorical variables. Because this investigation was hypothesis-generating, we did not adjust for multiple comparisons. We also conducted stepwise ordinal logistic regression to determine which factors were most predictive of elevated aflatoxin exposure. For this regression, aflatoxin exposure was divided into four groups, consisting of persons with exposure <LOD, and the remainder divided into exposure tertiles. We considered all variables with p < 0.10 in dichotomous analyses for inclusion.
We also compared the aflatoxin exposure levels seen here to those in other countries. However, aflatoxin exposure levels calculated by different laboratories by using different assays are not directly comparable. To correctly interpret aflatoxin data across studies, we applied a method conversion factor. Such factors have been computed previously as follows: radioimmunoassay LC-MS/MS ≈ radioimmunoassay ÷ 32); enzyme-linked immunosorbent assay (LC-MS/MS ≈ enzyme-linked immunosorbent assay ÷ 3.3); and HPLC using fluorometric detection (LC-MS/MS ≈ HPLC using fluorometric detection ÷ 0.71) (Wang et al. Citation1996; McCoy et al. Citation2008). Because of potential assay fluctuations over time, these formulas may not always capture the appropriate correction factor at the specific time of use; nonetheless, they provide a general idea of the relationship between assays.
Ethics statement
Participants provided informed oral consent for interviews, blood draws and storage of blood for future testing of unspecified pathogens. Survey participants were informed that future test results would not be returned to them. The survey protocol was approved by the Ethics Review Committee of the Kenya Medical Research Institute and the Institutional Review Board of the US CDC.
Results
Aflatoxin exposure
AFB1-lys adducts were detected in 78% of serum specimens (range = <LOD–9.52 ng/mL; median = 4.5 ng/mL). Albumin-corrected serum AFB1-lys levels ranged from <LOD to 211 pg/mg, with a median of 1.78 pg/mg.
Aflatoxin exposure was ubiquitous by sex, age, marital status and religion (Table ). Aflatoxin exposure was fairly universal across several socioeconomic characteristics, including highest level of education and employment status (Table ). There was some variation by wealth (p < 0.05); however, there was no linear trend, and median AFB1-lys levels were not statistically different between participants in the highest (2.06 pg/mg albumin) and lowest (1.53 pg/mg albumin) wealth quintiles. There was also some variation by occupation (p < 0.05); exposure was highest among participants who worked in the craft/trades (median = 4.35 pg/mg albumin) and elementary (e.g., street vendors, farm hands, and construction/manufacturing labourers; median = 3.04 pg/mg albumin) occupations.
Table 1. AFB1-lys levels (pg/mg albumin) by demographic characteristics, Kenya 2007.
Table 2. AFB1-lys levels (pg/mg albumin) by socioeconomic characteristics, Kenya 2007.
Geographic location
Aflatoxin exposure varied by geographic location (Table ). Serum specimens from the Eastern province had the highest exposure: the median aflatoxin adduct level (7.87 pg/mg albumin) was twofold higher than the median aflatoxin adduct level for the next highest province – the Coast province (3.70 pg/mg albumin; p < 0.01). Median aflatoxin levels were much lower in the other six provinces: Nairobi (2.44 pg/mg albumin), Central (2.33 pg/mg albumin), North-Eastern (1.40 pg/mg albumin), Western (1.28 pg/mg albumin), Rift Valley (0.70 pg/mg albumin) and Nyanza (<LOD). There was also considerable variation within provinces (Figure ). Out of Kenya’s eight provinces, four provinces (Central, Coast, North-Eastern and Western) had one district where aflatoxin was concentrated, with statistically higher levels than other districts in the same province.
Figure 2. Map of AFB1-lys levels (pg/mg albumin) by district, Kenya 2007. AFB1-lys, aflatoxin B1-lysine.
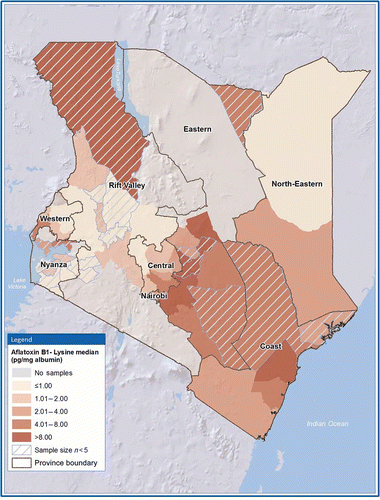
Table 3. AFB1-lys levels (pg/mg albumin) by geographic characteristics, Kenya 2007.
Aflatoxin levels were higher in urban (median = 2.23 pg/mg albumin) than in rural participants (median = 1.49 pg/mg albumin; p < 0.05). Aflatoxin exposure by sex differed in Central (for men, median = 3.80 pg/mg albumin and for women, median = 1.48 pg/mg albumin; p < 0.05) and Rift Valley provinces (for men, median = <LOD and for women, median = 0.95 pg/mg albumin; p < 0.05).
The final multivariate ordinal logistic regression model indicated that province and occupation were most closely related to aflatoxin exposure; all other characteristics were not statistically significant after this adjustment (data not shown).
Health status
The three health-related variables captured by the KAIS were all related to aflatoxin exposure: participants who reported that they were sick in the past week had higher aflatoxin adduct levels (median = 2.29 pg/mg albumin) than did those who reported not being sick (median = 1.67 pg/mg albumin, p < 0.01); participants seeking health care outside the home in the past 3 months had higher aflatoxin adduct levels (median = 2.67 pg/mg albumin) than did those not seeking health care outside the home (median = 1.59 pg/mg albumin, p < 0.01) and participants living in a household in which someone (including possibly themselves) sought outpatient care during the past 4 weeks had higher aflatoxin adduct levels (median = 3.06 pg/mg albumin) than did households with no one seeking outpatient care (median = 1.61 pg/mg albumin, p < 0.01). No further information was available regarding signs and symptoms from these events.
Discussion
We found widespread aflatoxin exposure across Kenya. Over three-quarters of serum specimens had evidence of recent exposure, and this exposure persisted across the spectrum of age, sex and socioeconomic status. This widespread exposure could negatively impact health throughout Kenya. Research suggests that chronic aflatoxin exposure at the levels seen here could stunt growth (Gong et al. Citation2004; Khlangwiset et al. Citation2011) and impair immunity (Jiang et al. Citation2005). Furthermore, aflatoxin is a carcinogen, and cumulative exposure to any amount increases the risk of hepatocellular carcinoma (Wang et al. Citation1996; Williams et al. Citation2004; Wu et al. Citation2009).
During aflatoxicosis outbreaks in Kenya in 2004, 2005 and 2010, GM aflatoxin levels among patients with potential liver dysfunction ranged from 120 to 1200 pg/mg albumin (Azziz-Baumgartner et al. Citation2005). Although most participants in our study had much lower levels, six participants (1%) had aflatoxin adduct levels above 120 pg/mg albumin. This is particularly notable for three reasons. Firstly, this survey was conducted in 2007 – the first year since 2004 when no aflatoxicosis outbreaks were reported. Secondly, maize samples collected from Kenya’s Eastern province had lower aflatoxin levels in 2007 (84% of maize samples were below 20 parts per billion) than in 2006 (only 48% of maize samples were below 20 parts per billion; unpublished data from authors). Thirdly, this survey sampled participants systematically, without regard to symptoms or expected exposure. The fact that we found extensive aflatoxin exposure during a relatively low-risk year suggests that even during optimal times, aflatoxin remains a persistent health threat in Kenya.
We found higher aflatoxin levels among persons who reported recently being sick or recently seeking health care. We do not know the details or the timing of the illness, and thus we cannot definitively link increased aflatoxin exposure to illness. It is possible that there is no causal association. However, the fact that all three of the health-related questions were associated with higher aflatoxin levels suggests a possible link. One possibility is that aflatoxin was causing liver damage or suppressing the immune system. Or, perhaps there was confounding between aflatoxin exposure, sickness and an unmeasured third variable such as diet, food security or physiological factors. We still know relatively little about the health effects of aflatoxin exposure, particularly the threshold at which physiologic changes begin to occur, and thus more research in this area is needed.
This study reinforces building evidence that aflatoxin is a problem in many African countries. After applying the appropriate method conversion factors as discussed in the “Materials and methods” section, aflatoxin exposure levels from this study (GM = 2.0 pg/mg albumin) appear to be slightly lower than levels reported previously from some other African countries. For example, Ethiopia (GM = 1 pg/mg albumin; unpublished data from authors), Gambia (published GM = 22–57 pg/mg albumin; LC-MS/MS-normalised GM = 7–80 pg/mg albumin) (Allen et al. Citation1992; Turner et al. Citation2000, Citation2007), Ghana (published GM = 406–455 pg/mg albumin; LC-MS/MS-normalised GM = 13–14 pg/mg albumin) (Jiang et al. Citation2005; Jolly et al. Citation2006), Benin and Togo (published GM = 33 pg/mg albumin; LC-MS/MS-normalised GM = 10 pg/mg albumin) (Gong et al. Citation2002, Citation2003) and Guinea (published GM = 9 pg/mg albumin; LC-MS/MS-normalised GM = 3 pg/mg albumin) (Turner, Sylla, Kuang, et al. 2005). It is important to note that because our study was a countrywide assessment, we selected specimens for analysis without regard to expected exposure. In contrast, most of the other studies cited here focussed on answering specific health-related research questions. Thus, they often targeted specific geographic regions and/or persons with expected higher aflatoxin exposure.
There is a large disparity in aflatoxin exposure between developed and developing countries. The largest population-based aflatoxin exposure assessment to date – and the only other national, baseline assessment that is directly comparable to our results – was conducted in the United States (Centers for Disease Control and Prevention Citation2012). Here, researchers measured AFB1-lys concentrations in 2104 serum samples from the 1999–2000 National Health and Nutrition Examination Survey. Using laboratory methods similar to those used here, they detected exposure in only 1.3% of the participants. The highest level found in the United States was 0.20 ng/mL – an order of magnitude lower than the median (4.5 ng/mL) reported here.
This serosurvey did not collect food samples. Maize is presumably the largest source of aflatoxin exposure in Kenya because it is the staple crop (Export Processing Zones Authority Citation2005) and it is very susceptible to aflatoxin contamination (Basappa Citation2009). Previous research from Benin and Togo found maize to be the primary risk factor for aflatoxin exposure, contributing more to elevated aflatoxin biomarker levels than do groundnuts (Egal et al. Citation2005). Kenya had a maize aflatoxin regulatory limit of 20 parts per billion in 2007; however, this can be difficult to enforce. Small-scale farmers produce three-fourths of Kenyan maize (Export Processing Zones Authority Citation2005). Small-scale farmers sometimes sell their maize to market vendors (Lewis et al. Citation2005); this can allow contaminated maize to enter the market distribution system and find its way to commercial millers, where maize is milled, packaged and distributed throughout the country. This may be one reason why we found aflatoxin exposure in urban areas, where persons would presumably be purchasing their maize rather than growing it themselves.
These data have some limitations. We selected serum specimens in an attempt to provide an indication of exposure throughout Kenya. However, they are not a statistically derived sample and thus we cannot make national estimates. Limited data were available on aflatoxin-related health outcomes or on risk factors for aflatoxin exposure, because the original survey was designed to study HIV. Finally, only one-fifth of participants providing a blood specimen for the KAIS were eligible for inclusion in the aflatoxin analysis. It is unlikely that aflatoxin exposure was related to the volume of available serum; however, it is possible that the aflatoxin level may have been related to HIV status. Evidence suggests that aflatoxin levels are higher among persons with HIV (Obuseh et al. Citation2011); thus, our results may underestimate the true burden of aflatoxin in Kenya.
Conclusions
Our data demonstrate an urgent need to implement evidence-based interventions in Kenya to decrease aflatoxin exposure and subsequently avert adverse health effects. We found that aflatoxin distribution was not homogenous across province. Thus, these data should be used to target interventions to specific regions of high exposure. Various interventions have demonstrated effectiveness in controlled studies. These include planting resistant cultivars (Hell et al. Citation2008), spraying atoxigenic strains of Aspergillus flavus on maize to crowd out toxigenic strains (Cotty et al. Citation2007; Yin et al. Citation2008) and developing strategies to assist small-scale farmers with appropriate drying and storage techniques (Turner, Sylla, Gong, et al. 2005; Hell et al. Citation2008). However, research demonstrating the effectiveness of these and other interventions in real life and in various populations is limited. Resources are needed to implement and evaluate these interventions in field studies in Kenya to determine their effectiveness in reducing human aflatoxin biomarkers; then, successful interventions can be scaled-up.
We documented exposure across Kenya, including in regions with no prior evidence of exposure and no documented aflatoxicosis outbreaks. Thus, this raises the question as to whether similar exposures may be occurring in neighbouring countries with a similar lack of previous exposure history. Similar serosurveys in other African countries that rely on aflatoxin-susceptible crops such as maize and groundnuts are warranted. With planning across public health sectors, other countries may also be able to reduce costs of such assessments by leveraging nationally representative serosurveys, such as the HIV prevalence survey that was leveraged here.
Acknowledgements
This study was funded through the United States President’s Emergency Plan for AIDS Relief and the Centers for Disease Control and Prevention (CDC), Department of Health and Human Services. We thank the following organisations for their assistance in completing this research: the Government of Kenya, Kenya Ministry of Public Health and Sanitation, Kenya National HIV Reference Laboratory, CDC Global Disease Detection Division and the CDC Division of Global HIV/AIDS within CDC-Kenya.
Declaration of interest: The sponsor of the study had some role in study design and data collection; they had no role in data analysis, data interpretation, or manuscript preparation. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. We declare that we have no conflicts of interest.
Notes
Mamo Umuro Abudo, Willis Akhwale, and Shahnaaz K. Sharif waive their assertion of copyright but not their right to be named as authors of the paper.
References
- Allen SJ, Wild CP, Wheeler JG, Riley EM, Montesano R, Bennett S, Whittle HC, Hall AJ, Greenwood BM. 1992. Aflatoxin exposure, malaria and hepatitis B infection in rural Gambian children. Trans R Soc Trop Med Hyg. 86:426–430.
- Azziz-Baumgartner E, Lindblade K, Gieseker K, Rogers HS, Kieszak S, Njapau H, Schleicher R, McCoy LF, Misore A, DeCock K, et al. 2005. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ Health Perspect. 113:1779–1783.
- Basappa SC. 2009. Aflatoxins: formations, analysis, and control. Oxford: Alpha Science International, Ltd.
- Centers for Disease Control and Prevention. 2004. Outbreak of aflatoxin poisoning – eastern and central provinces, Kenya, January–July 2004. MMWR Morb Mortal Wkly Rep. 53:790–793.
- Centers for Disease Control and Prevention. 2012. National Health and Nutrition Examination Survey: aflatoxin B1-lysine concentration in serum (SSAFB_A). [Internet]. [cited 2012 Oct 17]. Available from: http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/SSAFB_A.htm.
- Cotty PJ, Antilla L, Wakelyn PJ. 2007. Competitive exclusion of aflatoxin producers: farmer driven research and development. In: C. Vincent, N. Goettel, and G. Lazarovitis, editors, Biological control: a global perspective. Wallingford: CAB International; p. 241–253.
- Daniel JH, Lewis LW, Redwood YA, Kieszak S, Breiman RF, Flanders WD, Bell C, Mwihia J, Ogana G, Likimani S, et al. 2011. Comprehensive assessment of maize aflatoxin levels in Eastern Kenya, 2005–2007. Environ Health Perspect. 119:1794–1799.
- Egal S, Hounsa A, Gong YY, Turner PC, Wild CP, Hall AJ, Hell K, Cardwell KF. 2005. Dietary exposure to aflatoxin from maize and groundnut in young children from Benin and Togo, West Africa. Int J Food Microbiol. 104:215–224.
- Export Processing Zones Authority. 2005. Grain Production in Kenya: 2005. [Internet]. [cited 2012 Oct 25]. Available from: http://www.epzakenya.com/UserFiles/files/GrainReport.pdf
- Gan LS, Skipper PL, Peng XC, Groopman JD, Chen JS, Wogan GN, Tannenbaum SR. 1988. Serum albumin adducts in the molecular epidemiology of aflatoxin carcinogenesis: correlation with aflatoxin B1 intake and urinary excretion of aflatoxin M1. Carcinogenesis. 9:1323–1325.
- Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, Wild CP. 2002. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. BMJ. 325:20–21.
- Gong YY, Egal S, Hounsa A, Turner PC, Hall AJ, Cardwell KF, Wild CP. 2003. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: the critical role of weaning. Int J Epidemiol. 32:556–562.
- Gong Y, Hounsa A, Egal S, Turner PC, Sutcliffe AE, Hall AJ, Cardwell K, Wild CP. 2004. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ Health Perspect. 112:1334–1338.
- Hell K, Fandohan P, Bandyopadhyay R, Kiewnick S, Sikora R, Cotty P. 2008. Pre- and post-harvest management of aflatoxin in maize: an African perspective. In: J. Leslie, R. Bandyopadhyay and A. Visconti, editors, Mycotoxins: detection methods, management, public health, and agricultural trade. Wallingford: CAB International; p. 219–229.
- Jiang Y, Jolly PE, Ellis WO, Wang JS, Phillips TD, Williams JH. 2005. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. Int Immunol. 17:807–814.
- Jolly P, Jiang Y, Ellis W, Awuah R, Nnedu O, Phillips T, Wang JS, Afriyie-Gyawu E, Tang L, Person S, et al. 2006. Determinants of aflatoxin levels in Ghanaians: sociodemographic factors, knowledge of aflatoxin and food handling and consumption practices. Int J Hyg Environ Health. 209:345–358.
- Kaiser R, Bunnell R, Hightower A, Kim AA, Cherutich P, Mwangi M, Oluoch T, Dadabhai S, Mureithi P, Mugo N, et al. 2011. Factors associated with HIV infection in married or cohabitating couples in Kenya: results from a nationally representative study. PLoS One. 6:e17842.
- Khlangwiset P, Shephard GS, Wu F. 2011. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 41:740–755.
- Lewis L, Onsongo M, Njapau H, Schurz-Rogers H, Luber G, Kieszak S, Nyamongo J, Backer L, Dahiye AM, Misore A, et al. 2005. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ Health Perspect. 113:1763–1767.
- McCoy LF, Scholl PF, Schleicher RL, Groopman JD, Powers CD, Pfeiffer CM. 2005. Analysis of aflatoxin B1-lysine adduct in serum using isotope-dilution liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 19:2203–2210.
- McCoy LF, Scholl PF, Sutcliffe AE, Kieszak SM, Powers CD, Rogers HS, Gong YY, Groopman JD, Wild CP, Schleicher RL. 2008. Human aflatoxin albumin adducts quantitatively compared by ELISA, HPLC with fluorescence detection, and HPLC with isotope dilution mass spectrometry. Cancer Epidemiol Biomarkers Prev. 17:1653–1657.
- Mwanda OW, Otieno CF, Omonge E. 2005. Acute aflatoxicosis: case report. East Afr Med J. 82:320–324.
- Ngindu A, Johnson BK, Kenya PR, Ngira JA, Ocheng DM, Nandwa H, Omondi TN, Jansen AJ, Ngare W, Kaviti JN, et al. 1982. Outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya. Lancet. 1:1346–1348.
- Obuseh FA, Jolly PE, Kulczycki A, Ehiri J, Waterbor J, Desmond RA, Preko PO, Jiang Y, Piyathilake CJ. 2011. Aflatoxin levels, plasma vitamins A and E concentrations, and their association with HIV and hepatitis B virus infections in Ghanaians: a cross-sectional study. J Int AIDS Soc. 14:53.
- Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. 1992. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 339:943–946.
- Skipper PL, Tannenbaum SR. 1990. Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis. 11:507–518.
- Turner PC, Collinson AC, Cheung YB, Gong Y, Hall AJ, Prentice AM, Wild CP. 2007. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int J Epidemiol. 36:1119–1125.
- Turner PC, Mendy M, Whittle H, Fortuin M, Hall AJ, Wild CP. 2000. Hepatitis B infection and aflatoxin biomarker levels in Gambian children. Trop Med Int Health. 5:837–841.
- Turner PC, Sylla A, Gong YY, Diallo MS, Sutcliffe AE, Hall AJ, Wild CP. 2005. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: a community-based intervention study. Lancet. 365:1950–1956.
- Turner PC, Sylla A, Kuang SY, Marchant CL, Diallo MS, Hall AJ, Groopman JD, Wild CP. 2005. Absence of TP53 codon 249 mutations in young Guinean children with high aflatoxin exposure. Cancer Epidemiol Biomarkers Prev. 14:2053–2055.
- Wang LY, Hatch M, Chen CJ, Levin B, You SL, Lu SN, Wu MH, Wu WP, Wang LW, Wang Q, et al. 1996. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int J Cancer. 67:620–625.
- Wild CP, Gong YY. 2010. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 31:71–82.
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. 2004. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 80:1106–1122.
- Wu HC, Wang Q, Yang HI, Ahsan H, Tsai WY, Wang LY, Chen SY, Chen CJ, Santella RM. 2009. Aflatoxin B1 exposure, hepatitis B virus infection, and hepatocellular carcinoma in Taiwan. Cancer Epidemiol Biomarkers Prev. 18:846–853.
- Yin YN, Yan LY, Jiang JH, Ma ZH. 2008. Biological control of aflatoxin contamination of crops. J Zhejiang Univ Sci B. 9:787–792.