ABSTRACT
Bisphenol F (BPF) was found in mustard up to a concentration of around 8 mg kg−1. Contamination of the raw products or caused by the packaging could be ruled out. Also, the fact that only the 4,4ʹ-isomer of BPF was detected spoke against contamination from epoxy resin or other sources where technical BPF is used. Only mild mustard made of the seeds of Sinapis alba contained BPF. In all probability BPF is a reaction product from the breakdown of the glucosinolate glucosinalbin with 4-hydroxybenzyl alcohol as an important intermediate. Hot mustard made only from brown mustard seeds (Brassica juncea) or black mustard seeds (Brassica nigra) contained no BPF. BPF is structurally very similar to bisphenol A and has a similar weak estrogenic activity. The consumption of a portion of 20 g of mustard can lead to an intake of 100–200 µg of BPF. According to a preliminary risk assessment, the risk of BPF in mustard for the health of consumers is considered to be low, but available toxicological data are insufficient for a conclusive evaluation. It is a new and surprising finding that BPF is a natural food ingredient and that this is the main uptake route. This insight sheds new light on the risk linked to the family of bisphenols.
Graphical Abstract
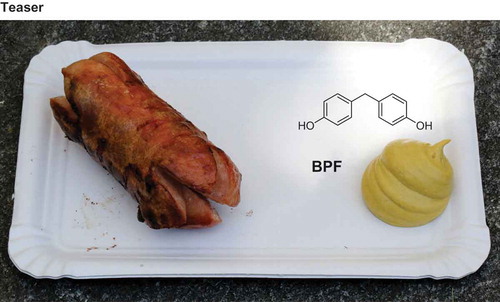
Introduction
Mustard is one of the most popular and widely used condiments worldwide. In Europe, it is frequently used as condiment for sausages and cold meats, and is also often an ingredient of salad dressings. It is very common to consume 10–20 g of mustard during a meal. The average daily consumption in Switzerland is estimated to be in the range of 1–2 g based on statistics of production, import and export. Dietary habits vary in European countries, but probably there are regions where consumption of mustard is even higher than in Switzerland.
The main ingredient of mustard is mustard seed. For the commercial production of mustard three types of seed are used: white or yellow mustard (Sinapis alba L.), brown or Indian mustard (Brassica juncea (L.) Czern.) or black mustard (Brassica nigra (L.) K. Koch) (Ebermann & Elmadfa Citation2008). The tastes of mustard range from sweet to spicy, and the grade of pungency is largely determined by the seed types used for preparation. Mustards prepared from white mustard seeds usually have a less pungent flavour than mustards made from brown or black mustard seeds.
The typical pungent taste and flavour is primarily caused by isothiocyanates, also known as mustard oils. They are breakdown products of glucosinolates, natural ingredients of mustard seeds, and are formed in a reaction catalysed by the enzyme myrosinase after tissue injury. The mustard species used for the preparation of mustard differ in glucosinolate composition: black and brown mustards contain primarily sinigrin, which is converted to allyl isothiocyanate responsible for the more pungent taste of these seeds compared with white mustard. The relatively mild white mustard contains glucosinalbin instead, which is mainly converted to 4-hydroxybenzyl isothiocyanate and may further react to 4-hydroxybenzyl alcohol (4-HBA) and thiocyanate (Kawakishi et al. Citation1967; Fenwick et al. Citation1983; Buskov et al. Citation2000; Agerbirk et al. Citation2008; Popova & Morra Citation2014).
A Swiss producer of mustard previously informed the Swiss Federal Food Safety and Veterinary Office (FSVO) that quality controls showed bisphenol F (BPF) in its products and that only products containing white mustard were affected. BPF belongs to the bisphenols, a group of chemical compounds with two hydroxyphenyl functionalities, and most often of the diphenylmethane type. The best known representative of this group of compounds is bisphenol A (BPA), a high production-volume chemical used in the production of polycarbonate plastic and several types of resins. BPA has been one of the most controversially discussed substances in the last few years. It was evaluated several times by different regulatory bodies and the most recent comprehensive evaluation was performed by EFSA (Citation2015). The heated debates mainly focused on possible hormonal effects of this compound. Similar effects have been ascribed also to BPF.
The goals of the present study were therefore to determine the BPF content in market samples of mustard, to elucidate the origin of the substance (anthropogenic or natural), to obtain some insight into the mechanism of its formation, and to perform a preliminary risk assessment. Some important aspects of this work were made available to the consumer on our homepage (FSVO Citation2015).
Material and methods
Chemicals and reagents
All LC solvents were obtained in LC-MS grade (Chromasolv®) from Sigma-Aldrich (Buchs, Switzerland). All other solvents were at least per analysis grade and also from Sigma-Aldrich or Merck Millipore (Darmstadt, Germany). Ultrapure water was obtained from an Elga Purelab ultra-water purification system (Labtec Services, Villmergen, Switzerland). 2,2ʹ-Bisphenol F (CASRN 2467–02-9, Aldrich B4,680–8), 4,4ʹ-bisphenol F (CASRN 620–92-8, Aldrich B47006), 4,4ʹ-bisphenol A (CASRN 80–05-7, Fluka 14939), 4-hydroxybenzyl alcohol (CASRN 623–05-2, Aldrich W398705) and vanillin (4-hydroxy-3-methoxybenzaldehyde, CASRN 121–33-5, Fluka 94750) were from Sigma-Aldrich. 2,4ʹ-Bisphenol F (CASRN 2467–03-0, ABCR AB141845) was from ABCR (Karlsruhe, Germany). 4,4ʹ-Bisphenol A-D6 (propane-D6, unlabelled CASRN 80–05-7, labelled CASRN 86,588–58-1, CIL DLM-2775; BPA-D6) and phenol-D5 (ring-D5, unlabelled CASRN 108–95-2, labelled CASRN 4165–62-2, CIL DLM-695) were from Cambridge Isotope Laboratories (Tewksbury, MA, USA). 4-Hydroxy-3-methoxybenzyl alcohol (vanillyl alcohol, CASRN 498–00-0, Merck 841197) 4-hydroxybenzaldehyde (CASRN 123–08-0, Merck 804536) and ascorbic acid (CASRN 50–81-7, Merck 100468) were from Merck Millipore. Formaldehyde 37% (VWR 437533 W) was from VWR International (Leuven, Belgium). Zeolite β, ammonium (Alfa Aesar 45874) was from Alfa Aesar (Karlsruhe, Germany). 4,4ʹ-Bisphenol F-D8 (ring labelled, unlabelled CASRN 620–92-8) was synthesised according to Jana et al. (Citation2005). The compound still contained 2,4ʹ-BPF and some 2,2ʹ-BPF, but was of sufficient purity for use as an internal standard. For details, see the supplemental data online.
Mustard seeds were purchased locally from different spice traders and also from seed companies.
Sampling for a market survey of mustard
Most samples were bought in retail outlets in the region of Bern, Switzerland. Care was taken that the most common types of mustard of the leading retailers and discounters were included. On the other hand, it was tried to include as many different types of mustard as readily available.
Analytical method for a market survey of mustard
Sample extraction
A total of 2 g of mustard was diluted with 10 ml of a solution of 50% methanol. A total of 180 µl of internal standard solution (51.1 µg BPA-D6 ml−1) was added and shaken vigorously for 30 s followed by 1.5 min of vortex mixing. After centrifugation for 10 min at 4500 rpm and 20°C, an aliquot of the supernatant was transferred and used for analysis.
LC-MS/MS method
The chromatographic system consisted of a Shimadzu UFLC binary gradient system (Shimadzu, Reinach, Switzerland) with pumps LC-30AD, vacuum degasser DGU-20A, thermostated column compartment CTO-20 and autosampler SIL-30A. Separations were performed on a Kinetex XB-C18, 100A (100 × 2.1 mm, 1.7 µm particle size) with a precolumn (Phenomenex, Torrance, CA, USA). The injection volume was 1.0 µl; the column temperature was maintained at 50°C. A gradient programme was used starting with 50% methanol in water and ramped linearly over the course of 3 min to 95% methanol, held for 1 min at this condition, then ramped back within 0.1 min and re-equilibrated for 2.9 min at 50% methanol. The flow rate was 0.3 ml min−1. Under these conditions the retention times of 4,4ʹ-BPF, 2,4ʹ-BPF, 4,4ʹ-BPA-D6 and 2,2ʹ-BPF were 2.0, 2.2, 2.7 and 2.7 min, respectively. MS/MS analysis was carried out on an API 5000 system (AB Sciex, Framingham, CT, USA) equipped with a turbo ion-spray source (ESI). The following instrumental settings were used: source temperature, 600°C; curtain gas, 31; collision gas, 7; gas-1, 50; gas-2, 70; ionspray, −4500 V. Measurements were carried out using MRM in negative mode. Dwell time was 100 ms for all transitions. The following transitions were monitored ([M – H]‒ underlined, quantifier bold, qualifier plain): BPA (227 → 211/133/93); BPA-D6 (233 → 138/215); 4,4ʹ-BPF (199 → 105/123); 2,4ʹ-BPF (199 → 93); 2,2ʹ-BPF (199 → 93). For quantitation, matrix-matched calibration was used. Standard calibration samples were prepared by spiking internal standard and different concentrations of 4,4ʹ-BPF to a mustard sample containing 0.4 mg 4,4ʹ-BPF kg−1. By addition of 3.8, 7.8 and 11.6 mg 4,4ʹ-BPF kg−1, a four-point calibration curve over the range of 0.4–12.0 mg kg−1 mustard was achieved. The repeatability of the method was evaluated by double analysis of a certain mustard sample during 11 series over a time span of approximately 6 weeks. The mustard sample had a mean concentration of 3.9 mg kg−1 4,4ʹ-BPF and the RSD of the 11 measurements was 18.2%. The LOQ for 4,4ʹ-BPF in mustard was 0.03 mg kg−1 (S/N ≥ 10, all MRMs). The LOD for 4,4ʹ-BPF, 2,4ʹ-BPF, 2,2ʹ-BPF and BPA was 0.01, 0.003, 0.001 and 0.01 mg kg−1, respectively (S/N = 3). The LOQ and LOD were evaluated by the addition of respective standards to a sample of brown mustard with no detectable bisphenols. Recovery was tested for each of the 61 mustard samples by spiking with 2 mg 4,4ʹ-BPF kg−1. The median recovery was 91% with a range from 42% to 154%.
Method for the detection of BPF by high-resolution mass spectrometry (HRMS)
Sample preparation
To 5 g of commercial or self-prepared mustard 4.5 ml of methanol, 0.5 ml of aqueous hydrochloric acid solution (1 M) and of 50 µl of internal standard (BPA-D6, 502 ng µl−1) were added and homogenised by a Polytron mixer. To 5 g of mustard seed powder 8 ml of methanol, 1 ml of water, 1 ml of aqueous hydrochloric acid solution (1 M) and 100 µl of internal standard (BPA-D6, 502 ng µl−1) were added, homogenised by a Polytron mixer and allowed to swell for 2 h. After centrifugation (10 min at 4500 rpm and 15°C) 1 ml of supernatant was transferred into a solution of 9 ml water with 1 ml of hydrochloric acid solution (1 M). The resulting 11 ml solution were transferred to a Gilson (Mettmenstetten, Switzerland) Aspec XL for automated sample cleanup. Extraction was performed on 3 ml Oasis HLB (WAT094226) cartridges from Waters (Milford, MA, USA). The SPE columns were preconditioned with 3 ml of ethyl acetate, followed by 3 ml of water. Then the columns were loaded with 10 ml of sample solution. After washing with 3 ml of water and drying under a flow of nitrogen for 15 min (for LC-HRMS) and 60 min (for GC-HRMS), respectively, the analytes were eluted with 5 ml of ethyl acetate and evaporated to 0.25 ml (for LC-HRMS) and 0.5 ml (for GC-HRMS), respectively, under a flow of nitrogen at 40°C. For analysis with GC-HRMS the extended drying time of the SPE columns was necessary to avoid residual water amounts that otherwise disturbed chromatographic performance. For LC-HRMS the samples were further diluted by addition of 0.25 ml of water prior to injection.
GC-HRMS method and LC-HRMS method
See the supplemental data online.
Method for the detection of BPF in bamboo shoots and in certain mustard samples using BPF-D8 as an internal standard
See the supplemental data online.
Self-prepared mustard samples
In order to investigate the parameters that lead to BPF formation, mustards were prepared under different conditions and the subsequent BPF content was measured. The following process was common for all mustards: mustard seed is ground in a mortar (10 g), water (30 ml), acid (0 or 5 ml acetic acid, 12%) and supplementary ingredients added (0–1 g each), the mixture homogenised, heated at 40°C for 3 h, then heated at 60°C for 15 min and the paste allowed to rest for 48 h before analysis at RT. The following parameters were applied: use of white, black or brown seeds; addition of acid, or not; addition of ingredients like sugar, salt and curcuma, or not; heating after blending to 40°C for 3 h, or not.
Investigations on the formation of 4,4ʹ-BPF from 4-hydroxybenyzl alcohol
The reactions were carried out as described for the reaction of indole-3-carbinol to 3,3ʹ-diindolylmethane (De Kruif et al. Citation1991; Chang et al. Citation1999). 4-HBA, 4-hydroxybenzaldehyde, vanillyl alcohol and vanillin were used in an amount from about 5 to 25 mg and diluted in an aqueous solution of 5 ml hydrochloric acid (1 M) corresponding to a concentration range of about 8–40 mM. The compounds were used alone or in mixtures of two different compounds. In most cases the compounds were first dissolved in one of the following organic solvents: dimethyl sulfoxide, methanol or ethanol. The solution was stirred with a magnetic stirrer for 30 min at RT. An aliquot was diluted 1000-fold in eluent before analysis by LC-MS.
Results and discussion
Analytical methods
The analytical methods used were optimised for certainty in the qualitative result and therefore three different analytical approaches were applied: LC-MS/MS, LC-HRMS and GC-HRMS. At the beginning of the survey the deuterium-labelled BPF was not yet synthesised. All used methods gave consistent results and also the quantitative values corresponded to the expected range. Mustard proved to be a difficult matrix regarding precision. As mentioned above, the RSD for repeatability over the whole period of the market survey was 18%.
Self-prepared mustard samples and the proposed reaction mechanism
Self-prepared mustard samples
Measurements of reagent blank at frequent intervals showed no contamination problem. Also the fact that only the 4,4ʹ-isomer of BPF was detected in positive samples argued against contamination from technical sources as in this case the detection of all three isomers would have been expected. No other isomer was detected, while spiking experiments revealed that their detection sensitivity was comparable or even better than for 4,4ʹ-BPF.
No formation of BPF was observed when processing brown mustard seeds (Brassica juncea (L.) Czern.) or black mustard seeds (Brassica nigra (L.) K. Koch). It turned out that only white seeds (Sinapis alba L.) produced BPF, that acid was necessary for BPF formation, that the addition of ingredients like sugar, salt or curcuma had no influence on BPF content, and that heating after blending accelerated BPF formation. The prepared mustards had a BPF content of up to 4.1 mg kg−1.
In conclusion, in order to produce BPF, the formulation must contain white mustard seed (i.e., glucosinalbin) and be treated with acid (organic or inorganic; see below). Elevated temperature catalyses the faster formation of BPF. The found parameters, which influence BPF formation, are in agreement with the information that was submitted by the mustard manufacturer to the FSVO.
Proposed reaction mechanism
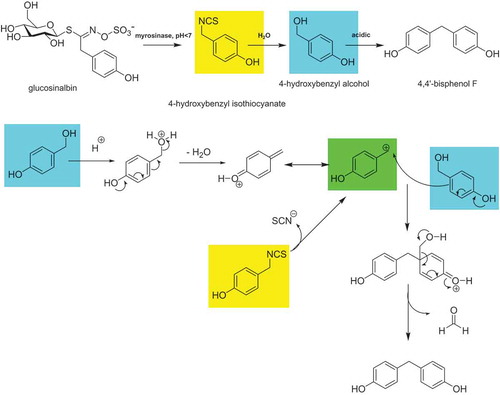
In a subsequent investigation, the proposed formation mechanism in analogy to the reactions of indole glucosinolates according to Pedras and Hossain (Citation2011) was examined. Briefly, formulations were made with different acids, concurring intermediates and ascorbic acid. Independent from whether organic (acetic acid) or inorganic (hydrochloric acid) acids were used for mustard preparation, BPF was detectable in the mustards. In accordance with Pedras and Hossain, the addition of ascorbic acid reduced the BPF formation significantly and the corresponding ascorbigen of 4-HBA was detected by HRMS. Additionally, it turned out that the addition of vanillyl alcohol in excess led to the formation of mixed dimers, less BPF and further reaction products (see the supplemental data online). On the other hand, the addition of benzyl alcohol or 4-hydroxybenzoic acid had no effect on BPF formation. In commercial mustard samples 2,4-bis(4-hydroxybenzyl)phenol () was also detected (see the supplemental data online).
In the experiments in an aqueous solution of hydrochloric acid (1 M) immediate formation of BPF was observed when 4-HBA was the reactant. Also 2,4-bis(4-hydroxybenzyl)phenol () was formed (see the supplemental data online).
All these observations indicate that 4-HBA might be the relevant intermediate. The mechanism depicted in might be the principal one. Unequivocal proof can only be obtained with further experiments with labelled compounds.
Figure 2. (colour online) Proposed reaction mechanism for the formation of 4,4ʹ-BPF. The cleavage of the glucosinolate glucosinalbin is catalysed by the enzyme myrosinase and leads primarily to the formation of the corresponding isothiocyanate. 4-Hydroxybenzyl isothiocyanate is hydrolysed to 4-HBA. In a further reaction, 4,4ʹ-BPF is formed. The carbocation can be formed from the isothiocyanate by loss of thiocyanate or from the 4-hydroxybenzyl alcohol by protonation and loss of water. A further 4-hydroxybenzyl alcohol molecule reacts with the carbocation.
Comparison with well-known reactions of other glucosinolate breakdown products
A similar dimerisation and trimerisation reaction is well known for an analogous glucosinolate breakdown product. Indole-3-carbinol is a breakdown product of glucobrassicin which readily reacts to 3,3ʹ-diindolylmethane and also to 2-(indol-3-ylmethyl)-3,3ʹ-diindolylmethane. The reaction can also take place in the acidic environment of the stomach (De Kruif et al. Citation1991; Chang et al. Citation1999). Also for 4-HBA it is conceivable that it will react to 4,4ʹ-BPF in the stomach. Therefore, it may be possible that products containing no BPF but only 4-HBA may also lead to an intrinsic formation and uptake of 4,4ʹ-BPF.
Other known and suggested natural sources of 4,4ʹ-BPF
It is not common knowledge that BPF is a natural compound, but BPF was described as a natural compound by Yi-Ming et al. (Citation1993) in the dried rhizome of the orchid Galeola faberi. They extracted 7.2 mg kg−1 4,4ʹ-BPF, 5.4 mg kg−1 2,4-bis(4-hydroxybenzyl)phenol and also 3.6 mg kg−1 of 4-HBA. If BPF is regarded as a dimer of 4-HBA, 2,4-bis(4-hydroxybenzyl)phenol would be the corresponding trimer ( and ). Noda et al. (Citation1995) described 4,4ʹ-BPF (50 mg kg−1), 2,4-bis(4-hydroxybenzyl)phenol (16 mg kg−1) and 4-HBA (500 mg kg−1) in the tubers of the orchid Gastrodia elata. For BPF they referred to Taguchi et al. (Citation1981), but the latter did not mention BPF in their paper on the constituents of G. elata. G. elata is mentioned as having been used in traditional Chinese medicine, and 4-HBA and/or its glucoside and 4-hydroxybenzaldehyde are important ingredients (Taguchi et al. Citation1981). Also the glucoside of 4,4ʹ-BPF was detected in G. elata (Zhang et al. Citation2013) and 4,4ʹ-BPF and 4-HBA were also found in the orchid Coeloglossum viride (Huang et al. Citation2004).
4-HBA is relatively widespread in the plant kingdom. It is biochemically related to 4-hydroxybenzoic acid and to the amino acid tyrosine. For instance, it is described in embryogenic cells of carrots and in pumpkin seeds. But absolute amounts are usually quite small. There exists another biochemical pathway that is somehow related to the pathway from tyrosine to glucosinalbin leading to another important secondary plant product. Starting also from tyrosine, it leads to the cyanogenic glucoside dhurrin and to its stereoisomer taxiphyllin (Chen & Andreasson Citation2001). Dhurrin occurs in sorghum, but usually in quite low concentrations. Bamboo shoots may contain high concentrations of cyanogenic glycosides in the range of 1000 mg kg−1 of hydrogen cyanide, corresponding to a content of 11,000 mg kg−1 of taxiphyllin (Singhal et al. Citation2013). The cleavage of glucose from taxiphyllin leads to 4-hydroxymandelonitrile and further reaction to 4-hydroxybenzaldehyde and probably also to 4-HBA. Thus, under suitable conditions the formation of BPF, according to the lower part of , may also occur in bamboo shoots.
In fresh bamboo shoots no BPF was detectable even after treatment with diluted acetic acid. But in Europe fresh bamboo shoots are not readily available and we might have obtained a sample containing a very low amount of cyanogenic glycosides. In the preserves (two different canned products and one in a plastic pouch) where the use of acid was indicated on the label small amounts of BPF were detectable: about 0.05 mg kg−1 in the sprouts and about 0.02 mg kg−1 in the liquid medium (covering liquid). In two preserves where no addition of acid was indicated the concentration of BPF was below the LOD. Also the samples of bamboo shoots like the mustard samples contained only the 4,4ʹ-isomer of BPF.
Market survey on mustard
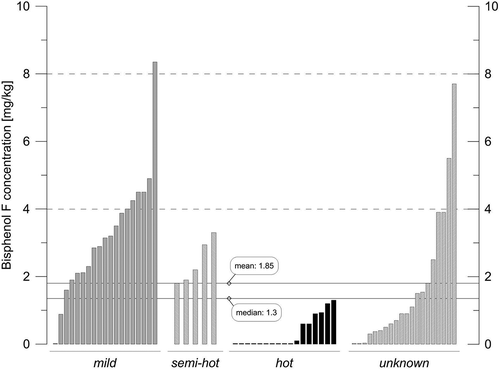
Sixty-one commercial samples of mustard were analysed. All products were produced in Europe. Usually it is not indicated on the label which cultivar of mustard seed was used for production, but often there are some indications regarding pungency, such as hot mustard, semi-hot mustard, mild mustard or Dijon-style mustard. Usually Dijon-style mustard contains no white mustard, but the use of the term is not regulated. As white mustard is the mildest type, highest concentrations were expected in mild mustards. The results of the survey are depicted in . As expected, the hot mustards showed the lowest contents and the mild mustards the highest ones. The semi-hot mustards and mustards of unknown pungency were in between. The sample in the class ‘mild’ that contained no BPF was indicated on the label as a Dijon-style mustard as well as mild. The product seemed to be a kind of diluted mustard preparation. Quite high concentrations of BPF were detected. Only the 4,4ʹ-isomer of BPF was detected. The mean of all samples (n = 61) was 1.85 mg kg−1, the median 1.30 mg kg−1 and the highest value 8.35 mg kg−1. For the calculation of the mean and median values, the 13 values below the LOD of 0.01 mg kg−1 were set to zero. There was no sample with a value between the LOD and the LOQ. One sample of the class ‘unknown’ was at the LOQ of 0.03 mg kg−1. The mean and median values of all mild mustards (n = 19) were 3.20 and 3.15 mg kg−1 respectively. Also in this case the sample below the LOD was set to zero.
Figure 3. Results of the market survey on mustard. The products were classified according to information on the label regarding pungency. Dijon-style mustards, without further information regarding pungency, were included in the class ‘hot’. BPF was detected in 48 out of 61 samples. For visibility, values below the LOD were assigned a small value. For further details, see the text.
Intake of BPF through mustard consumption
Mustard is often used as a condiment for sausages and cold meats and is also often an ingredient of salad dressings. The average daily consumption in Switzerland is estimated to be in the range of 1–2 g based on statistics of production, import and export. Single servings of mustard sold in Switzerland contain 12 g. Information on typical portion sizes of mustard are available from a pilot study on food consumption in Switzerland: the mean single portion size was 8.12 g, the median 4.53 g and the largest single portion was 65 g. The maximum amount of mustard consumed per day was 88 g. It is very common to consume 10–20 g of mustard along with a sausage in Switzerland.
It is assumed that brand and product loyalty of the consumer might be quite common regarding mustard. There are many mustard products containing BPF in the range of 2–4 mg kg−1. If one assumes a consumption of 10 or 20 g of mustard and calculates with a content of 3 mg kg−1, this leads to an intake of 30–60 µg of BPF, respectively. The consumption of 20 g of mustard with a content of 8 mg kg−1 leads to an intake of 160 µg. There may be a minority of consumers who eat mustard on a daily basis, but probably the majority use it only one or two times a week for direct consumption. Daily exposure to lower amounts through the consumption of further processed foods such as salad dressings or meat marinade may be more common.
Preliminary risk assessment for BPF intake through mustard consumption
A more detailed risk assessment and discussion of some new toxicokinetic data for BPF in humans together with a more detailed comparison with BPA will be published elsewhere.
Short discussion of published data
BPA has been one of the most controversially discussed substances in the last few years. It was evaluated several times by different regulatory bodies and the most recent comprehensive evaluation was performed by EFSA (Citation2015). The intense debates mainly revolved around possible hormonal effects, but the provisional TDI that EFSA derived is primarily based on a relative kidney weight increase in male mice of the F0 generation and not on hormonal effects. EFSA used a benchmark dose approach and the human equivalent dose to derive the temporary TDI of 4 µg kg−1 body weight (bw) day–1. It is undisputed that BPA is toxic to liver and kidney in rat and mouse at high doses. Available toxicological data for BPF are much less extensive and were summarised by USEPA (Citation2014). Neither a chronic study nor a study with prenatal exposure is available on BPF. The most relevant sub-acute study is from Higashihara et al. (Citation2007). In this 28-day repeated-dose toxicity rat study it was tried to determine the endocrine-mediated properties of BPF. Doses of 0, 20, 100 or 500 mg kg−1 bw day–1 were applied by gavage. At higher doses the main effect of BPF was concluded to be liver toxicity based on clinical biochemical parameters and liver weight, but without histopathological changes. The NOAEL for BPF was under 20 mg kg−1 bw day–1 since decreased bw, decreased serum total cholesterol, glucose and albumin values were observed in female rats at this dose level. At doses of 20 mg kg−1 bw day–1 or higher, the thyroxin level in serum of female rats was increased. At 500 mg kg−1 bw day–1 the relative testes weights were increased. The weak estrogenic activity of BPA and BPF was already described in 1936 based on findings in a uterotrophic assay (Dodds & Lawson Citation1936). The NOAEL for a uterotrophic effect of BPF in immature rats was determined by Stroheker et al. (Citation2003) at 50 mg kg−1 bw day–1. In contrast BPA showed no effect in the same test up to a dose of 200 mg kg−1 bw day–1. Two recent reviews concluded that the hormonal activity of BPF is quite similar to that of BPA (Eladak et al. Citation2015; Rochester & Bolden Citation2015). Newer in vitro tests show similar binding affinities for BPA and BPF to estrogen receptors. With respect to steroidogenesis and androgenic properties, there might be some minor differences between BPF and BPA (Rosenmai et al. Citation2014; Goldinger et al. Citation2015).
BPA is used in the production of polycarbonate and epoxy resins. Epoxy resins containing BPA (BPA diglycidyl ether) are also used in can coatings for food and beverages. France has recently banned the use of BPA in food-contact materials. BPF is used to a lesser extent. The primary use is in epoxy resins also mainly as diglycidyl ether. Novolak resins are usually mixtures of molecules with different polymerisation degree. In epoxy resins, BPF is present in the form of its three isomers: 2,2ʹ-BPF, 2,4ʹ-BPF and 4,4ʹ-BPF (Pilato Citation2010). The use of BPF for food-contact materials is restricted in the European Union. According to Commission Regulation No. Citation1895/2005, BPF-containing resins are only allowed for food contact in ‘heavy-duty coatings’ for containers or storage tanks having a capacity greater than 10 000 litres and for pipelines belonging to them.
Derivation of a tolerable range of intake
Based on the LOAEL of 20 mg kg−1 bw day–1 from the sub-acute oral study in rats (Higashihara et al. Citation2007) and aiming for a margin of exposure of at least 1800, a daily intake 0.67 mg of BPF may be tolerable for a person of 60 kg bw. The margin of exposure of 1800 consists of the default uncertainty factor of 100 to take account of inter-species and intra-subject variability in toxicokinetics and toxicodynamics, an additional factor of 3 for using a LOAEL instead of an NOAEL and a factor of 6 for using a sub-acute study instead of a chronic study. But this is a very rudimentary approach and one should also consider the available comprehensive knowledge on toxic effects of the structurally related BPA. A total of 0.67 mg of BPF corresponds to the amount present in 80 g of mustard containing 8.35 mg kg−1 or 362 g containing 1.85 mg kg−1, the maximum and mean, respectively, measured in our study. Therefore, probably most of the consumers will be in the tolerable range of intake.
Discussion of monitoring data of BPF in the literature
Our findings can explain many of the peculiarities found in the literature concerning the detection of BPF in relation to BPA. As BPA is much more used in food packaging and in products of daily use, one would expect a much higher exposure to BPA compared with BPF. But that is not exactly what is found. Zhou et al. (Citation2014), for instance, analysed 100 urine samples of adult volunteers for BPA and BPF and other compounds. Detection frequencies were 95% and 55% and the median concentrations were 0.72 and 0.08 ng ml−1, respectively, which is in agreement with expectations. However, the highest values were 37.7 and 212 ng ml−1 for BPA and BPF, respectively. This means the maximum value for BPF was more than five times higher than that for BPA. Or in other words, the variance of the distribution of the concentrations of BPF is much higher. This could easily be explained by the probable distribution of mustard consumption. Liao and Kannan (Citation2013) studied bisphenols in foods sold in the United States. Detection frequencies and median values were mostly much lower for BPF compared with BPA, but the highest value in all samples was 1.1 mg kg−1 BPF in a mustard dressing. The mustard fits in very well. Also in the groups ‘fish and seafood’ and ‘meat and meat products’ the maximum value for BPF is around two to three times higher than the maximum value for BPA. Also here mustard might explain the findings as it is often used as a condiment in products of this category. Liao and Kannan (Citation2014) analysed foods from nine Chinese cities. Here detection frequency, median and mean values are much higher for BPA than for BPF. Only the highest value in the group ‘vegetables’, which is also the highest value of all products, was 0.62 mg BPF kg−1. Interestingly, this product was a can containing bamboo shoots. As explained above, this may not be an accidental finding but could be caused by the reaction of breakdown products of the cyanogenic glycoside taxiphyllin. Lee et al. (Citation2015) studied bisphenols in sludge of wastewater treatment plants (WWTPs) in South Korea. They analysed sludge from domestic, mixed and industrial WWTPs. Interestingly the findings for BPA and BPF were quite different. BPA concentrations were lowest in domestic, higher in mixed and highest in industrial WWTPs, whereas for BPF the concentrations were lowest in industrial and much higher in mixed and domestic WWTPs. Also this finding would be in accordance with the assumption that foodstuffs are the main source of BPF.
Conclusions
This study shows that BPF is naturally formed during the production of mustard in mg kg−1 amounts using seeds of white mustard. Also, the CVUA Stuttgart (Citation2015), an official food control laboratory in southern Germany, found similar BPF concentrations in mustard samples. BPF is a breakdown product of the glucosinolate glucosinalbin and fairly certainly 4-HBA is an important intermediate. Mustard is the main source for the human intake of BPF at least in Europe, perhaps worldwide. It is conceivable that further relevant natural sources exist. For instance, preserves of bamboo shoots with medium to high contents of the cyanogenic glycoside taxiphyllin may also be a relevant source. Further natural sources with low contents and, therefore, low importance probably exist.
More than 20 years ago BPF was discovered as a natural compound in the rhizome or tuber of certain species of the Orchidaceae family, particularly in G. elata, but this fact did not receive any attention.
The risk of BPF to human health was assessed in a preliminary evaluation and led to the conclusion that it is probably of low concern. However, it has to be kept in mind that toxicity tests with chronic or prenatal exposure are missing. Mustard is a food allergen, but otherwise we are not aware of any study connecting mustard consumption with an increased risk for human health.
Our unexpected and surprising findings may shed a new light on the risk linked to the family of bisphenols and in particular to the controversially discussed BPA. BPF is structurally very similar to BPA and has similar hormonal effects and now we realise that BPF has been consumed over centuries in a popular condiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
Supplementary Material
Download PDF (149.5 KB)Acknowledgement
The authors acknowledge the detailed information submitted by a Swiss mustard producer that led to the start of this project. They had already shown that BPF was formed only when white mustard seeds were used for mustard production. The authors also thank Dr Roger Marti, Haute école d’ingénierie et d’architecture Fribourg, for valuable discussions concerning the reaction mechanism; as well as Dr Vincent Dudler from our office for valuable comments.
ORCID
Otmar Zoller ![]() http://orcid.org/0000-0003-0956-7604
http://orcid.org/0000-0003-0956-7604
References
- Agerbirk N, Warwick SI, Hansen PR, Olsen CE. 2008. Sinapis phylogeny and evolution of glucosinolates and specific nitrile degrading enzymes. Phytochemistry. 69:2937–2949.
- Buskov S, Hasselstrøm J, Olsen CE, Sørensen H, Sørensen JC, Sørensen S. 2000. Supercritical fluid chromatography as a method of analysis for the determination of 4-hydroxybenzylglucosinolate degradation products. J Biochem Biophys Methods. 43:157–174.
- Chang YC, Riby J, Chang GH, Peng BC, Firestone G, Bjeldanes LF. 1999. Cytostatic and antiestrogenic effects of 2-(indol-3-ylmethyl)-3,3′-diindolylmethane, a major in vivo product of dietary indole-3-carbinol. Biochem Pharmacol. 58:825–834.
- Chen S, Andreasson E. 2001. Update on glucosinolate metabolism and transport. Plant Physiol Biochem. 39:743–758.
- Commission Regulation (EC) No. 1895/2005. Commission regulation (EC) no 1895/2005 of 18 November 2005 on the restriction of use of certain epoxy derivatives in materials and articles intended to come into contact with food. Off J Eur Union L. 302:28–32.
- CVUA Stuttgart. 2015. Bisphenol F in mustard - how does this bisphenol A-similar substance end up in this spice? [Internet]. Aug 24. [cited 2015 Sept 05]. Available from: http://www.cvuas.de/pub/beitrag.asp?subid=1&Thema_ID=3&ID=2138&lang=EN&Pdf=No
- De Kruif CA, Marsman JW, Venekamp JC, Falke HE, Noordhoek J, Blaauboer BJ, Wortelboer HM. 1991. Structure elucidation of acid reaction products of indole-3-carbinol: detection in vivo and enzyme induction in vitro. Chem Biol Interact. 80:303–315.
- Dodds EC, Lawson W. 1936. Synthetic œstrogenic agents without the phenanthrene nucleus. Nature. 137:996.
- Ebermann R, Elmadfa I. 2008. Lehrbuch Lebensmittelchemie und Ernährung. [ Textbook food chemistry and nutrition]. Wien: Springer.
- EFSA. 2015. Scientific opinion on the risks to public health related to the presence of bisphenol A in foodstuffs: executive summary. Part I – exposure assessment. PART II – toxicological assessment and risk characterisation. Efsa J. 13:3978.
- Eladak S, Grisin T, Moison D, Guerquin MJ, N’Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R. 2015. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 103:11–21.
- Fenwick GR, Heaney RK, Mullin WJ. 1983. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 18:123–201.
- FSVO (Federal Food Safety and Veterinary Office). 2015. Bisphenol F in Senf [Bisphenol F in mustard] [Internet]. May 20. [cited 2015 Jun 05]. Available from: http://www.blv.admin.ch/themen/04678/04711/06141/
- Goldinger DM, Demierre AL, Zoller O, Rupp H, Reinhard H, Magnin R, Becker TW, Bourqui-Pittet M. 2015. Endocrine activity of alternatives to BPA found in thermal paper in Switzerland. Regul Toxicol Pharmacol. 71:453–462.
- Higashihara N, Shiraishi K, Miyata K, Oshima Y, Minobe Y, Yamasaki K. 2007. Subacute oral toxicity study of bisphenol F based on the draft protocol for the ‘Enhanced OECD test guideline no. 407’. Arch Toxicol. 81:825–832.
- Huang SY, Li GQ, Shi JG, Mo SY. 2004. Chemical constituents of the rhizomes of Coeloglossum viride var. bracteatum. J Asian Nat Prod Res. 6:49–61.
- Jana SK, Okamoto T, Kugita T, Namba S. 2005. Selective synthesis of bisphenol F catalyzed by microporous H-beta zeolite. Appl Catal A-Gen. 288:80–85.
- Kawakishi S, Namiki M, Watanabe H, Muramatsu K. 1967. Studies on the decomposition of sinalbin. Part II. The decomposition products of sinalbin and their degradation pathways. Agr Biol Chem. 31:823–830.
- Lee S, Liao C, Song G-J, Ra K, Kannan K, Moon H-B. 2015. Emission of bisphenol analogues including bisphenol A and bisphenol F from wastewater treatment plants in Korea. Chemosphere. 119:1000–1006.
- Liao C, Kannan K. 2013. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 61:4655–4662.
- Liao C, Kannan K. 2014. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 31:319–329.
- Noda N, Kobayashi Y, Miyahara K, Fukahori S. 1995. 2,4-Bis(4-hydroxybenzyl) phenol from Gastrodia elata. Phytochemistry. 39:1247–1248.
- Pedras MS, Hossain S. 2011. Interaction of cruciferous phytoanticipins with plant fungal pathogens: indole glucosinolates are not metabolized but the corresponding desulfo-derivatives and nitriles are. Phytochemistry. 72:2308–2316.
- Pilato L. 2010. Resin chemistry. In: Pilato L, editor. Phenolic resins: a century of progress. Heidelberg: Springer; p. 41–91.
- Popova IE, Morra MJ. 2014. Simultaneous quantification of sinigrin, sinalbin, and anionic glucosinolate hydrolysis products in Brassica juncea and Sinapis alba seed extracts using ion chromatography. J Agric Food Chem. 62:10687–10693.
- Rochester JR, Bolden AL. 2015. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect. 123:643–650.
- Rosenmai AK, Dybdahl M, Pedersen M, Alice Van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, Vinggaard AM. 2014. Are structural analogues to bisphenol a safe alternatives? Toxicol Sci. 139:35–47.
- Singhal P, Bal LM, Satya S, Sudhakar P, Naik SN. 2013. Bamboo shoots: a novel source of nutrition and medicine. Crit Rev Food Sci Nutr. 53:517–534.
- Stroheker T, Chagnon MC, Pinnert MF, Berges R, Canivenc-Lavier MC. 2003. Estrogenic effects of food wrap packaging xenoestrogens and flavonoids in female Wistar rats: a comparative study. Reprod Toxicol. 17:421–432.
- Taguchi H, Yosioka I, Yamasaki K, Kim IH. 1981. Studies on the constituents of Gastrodia elata Blume. Chem Pharm Bull. 29:55–62.
- USEPA. 2014. Bisphenol A alternatives in thermal paper. Final report. Jan. [ cited 2015 May 25]. Available from: http://www2.epa.gov/sites/production/files/2014-05/documents/bpa_final.pdf
- Yi-Ming L, Zhuo-Lun Z, Yong-Fu H. 1993. New phenolic derivatives from Galeola faberi. Planta Med. 59:363–365.
- Zhang ZC, Su G, Li J, Wu H, Xie XD. 2013. Two new neuroprotective phenolic compounds from Gastrodia elata. J Asian Nat Prod Res. 15:619–623.
- Zhou X, Kramer JP, Calafat AM, Ye X. 2014. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 944:152–156.