ABSTRACT
Fusarium head blight is one of the most important cereal diseases worldwide. Cereals differ in terms of the main occurring Fusarium species and the infection is influenced by various factors, such as weather and cropping measures. Little is known about Fusarium species in barley in Switzerland, hence harvest samples from growers were collected in 2013 and 2014, along with information on respective cropping factors. The incidence of different Fusarium species was obtained by using a seed health test and mycotoxins were quantified by LC-MS/MS. With these techniques, the most dominant species, F. graminearum, and the most prominent mycotoxin, deoxynivalenol (DON), were identified. Between the three main Swiss cropping systems, Organic, Extenso and Proof of ecological performance, we observed differences with the lowest incidence and toxin accumulation in organically cultivated barley. Hence, we hypothesise that this finding was based on an array of growing techniques within a given cropping system. We observed that barley samples from fields with maize as previous crop had a substantially higher F. graminearum incidence and elevated DON accumulation compared with other previous crops. Furthermore, the use of reduced tillage led to a higher disease incidence and toxin content compared with samples from ploughed fields. Further factors increasing Fusarium infection were high nitrogen fertilisation as well as the application of fungicides and growth regulators. Results from the current study can be used to develop optimised cropping systems that reduce the risks of mycotoxin contamination.
Introduction
Fusarium head blight (FHB) is one of the most noxious diseases in cereals and is caused by a complex of different Fusarium species (Parry et al. Citation1995); with F. graminearum (Schwabe; teleomorph Gibberella zeae Schwein, (Petch)) being the predominant species in wheat in Switzerland and worldwide (Parry et al. Citation1995; Vogelgsang et al. Citation2011). FHB-causing species are known to produce different mycotoxins, amongst others trichothecenes (deoxynivalenol (DON), nivalenol, T-2 toxin and HT-2 toxin) or the mycoestrogen zearalenone which threaten human and animal health (Desjardins Citation2006). In 2006, the European Commission set maximum limits of 1250 µg kg–1 DON and 100 µg kg–1 zearalenone for unprocessed cereals for human consumption and established guidance values for feedstuff (European Commission Citation2006), which were adopted in Swiss legislation.
Although frequently isolated from symptomatic ears together with Fusarium species, Microdochium nivale/M. majus ((Fries) Samuel and Hallet; teleomorph Monographella nivalis (Schaffnit) Müller) is a non-toxigenic FHB-causing species (Nielsen et al. Citation2014). The species complex responsible for FHB in Switzerland was studied in wheat (Triticum aestivum L.) and maize (Zea mays L.) (Dorn et al. Citation2009; Vogelgsang et al. Citation2009), but only little is known about the occurrence of Fusarium species in Swiss barley (Hordeum vulgare L.).
Barley is grown either as fodder (winter barley), for malt and beer production (spring barley) as well as for baking and cooking purposes. Infections with Fusarium species and thus accumulation of mycotoxins is harmful in all uses. Fodder barley contaminated with deoxynivalenol can result in feed refusal, diarrhoea and vomiting in farm animals (D’Mello et al. Citation1999). In addition, although not acutely toxic, the estrogenic effect of zearalenone can severely disrupt the reproductive system of animals (D’Mello et al. Citation1999). The contamination of barley for human consumption may also cause problems, since mycotoxins frequently remain in the final product (Malachova et al. Citation2012).
There are several approaches to reduce the risk of Fusarium mycotoxins in cereals: cropping measures (Blandino et al. Citation2012), biocontrol with microorganisms (Schisler et al. Citation2002) and fungicides (Haidukowski et al. Citation2012). However, no single approach but an integrated pest management can sufficiently reduce the risk of Fusarium infections.
Switzerland has three production systems that differ in terms of production methods and intensity. (1) The ÖLN system (Ökologischer Leistungs-Nachweis = proof of ecological performance (PEP)) is the minimum standard for an environmentally friendly agriculture to obtain direct payments. Some of the most important requirements are: balanced use of fertilisers, strict crop rotation, appropriate measures for soil protection and selective application of plant protection products. (2) The Extenso system renounces additionally the use of growth regulators, fungicides, insecticides and synthetic stimulators of natural defence mechanisms. (3) The Organic system completely prohibits the use of synthetic plant protection products and mineral fertilisers. The cultivation of barley is possible in all three systems and the modification of various factors within the respective cropping system, e.g., choice of variety, tillage, crop rotation, fertilisation and many more can influence the diseases pressure, especially for Fusarium species (Bernhoft et al. Citation2012).
Therefore, the objective of this two-year monitoring was to gain fundamental knowledge about the occurrence of Fusarium species and their associated mycotoxin spectrum in commercial barley samples in Switzerland and to determine and quantify the most influencing cropping techniques to reduce FHB infection.
Materials and methods
Collection of samples and identification of Fusarium species
With support of the cantonal plant protection officers, several hundred farmers were addressed to send a sample of their barley harvest together with the respective agronomic data (see Table S1 in the supplemental data online). These data were, for example: type of cropping system, variety, previous crop, pre-previous crop and tillage.
In 2013, 280 samples from 18 cantons, and in 2014, 160 samples from 17 cantons were obtained. Samples were collected after harvest directly from the combine, from trailers leaving the field or from the silo. To ensure a representative sample, all participating growers received written instructions (see in the supplemental data online) on how to take 10 subsamples and mix these to a sample of approximately 1 kg. The growers sent their samples in plastic bags within 1 day to Agroscope, Zurich-Reckenholz, along with a completed questionnaire on agronomic data pertaining to the particular field sample. Upon receipt, the samples (ø 12% relative humidity) were transferred into paper bags and stored at 10°C until further processing.
In order to obtain representative subsamples, the total sample was processed using a riffle divider (Schieritz & Hauenstein AG, Arlesheim, Switzerland). Fusarium species incidence (per cent infection) was determined (approximately 6 g equivalent to 100 grains), using an seed health test as described by Vogelgsang et al. (Citation2008), and 150 g of the respective subsamples were taken for mycotoxin analysis. The different Fusarium species were identified according to Leslie and Summerell (Citation2006). Samples of 150 g each were ground with a grain mill (Cyclotec 1093 sample mill, Foss Tecator, Höganäs, Sweden), using a 1 mm screen and the flour was stored at –20°C until analysis.
Mycotoxin analysis
All ground samples were analysed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) for the content of type A and B trichothecenes: nivalenol (NIV), DON, fusarenon-X (FUS-X), acetyldeoxynivalenol (AcDON), neosolaniol (NEO), diacetoxyscirpenol (DAS), T-2 (T-2), HT-2 (HT-2) and for zearalenone (ZEA). A reference sample of naturally DON-contaminated barley (Trilogy, Washington, MO, USA) was included in each run as method control.
For mycotoxin extraction, 10 g of flour were suspended in 40 ml extraction solution (acetonitrile/acetone/Milli-Q® water (50:25:25)) (acetonitrile and acetone from Scharlau Multisolvent®, Sentmenat, Spain; Milli-Q® water was produced with a Milli-Q® gradient A10 water purification system from Millipore, Bedford, MA, USA) and shaken for 90 min at 175 rpm on a rotary shaker. The suspension was filtered through a Whatman® 595 ½ 150 mm filter (GE Healthcare UK Ltd, Buckinghamshire, Little Chalfont, UK).
Extract cleaning was performed by using 3 ml SPE tubes, fitted with 20 µm frits (Biotage, Uppsala, Sweden) at the bottom. A mixture of 0.15 mg Alox:Celite (50:50; Alox activated 6 h by 400°C) was weighed into the tube and closed with an additional frit. The cartridges mounted on a Visiprep Solid Phase Extraction Vacuum Manifold (12-port model) (Supelco, Bellefonte, PA, USA) were washed with 2 ml of the extraction solution. Extracts were filtered through the cartridges and thereafter the solid phase was washed with 2 ml of extraction solution. The remaining solvent in the tubes was drawn into 5 ml conical reaction vessels (Supelco) under vacuum.
The eluate was concentrated by a gentle airstream at maximal 50°C. The concentrated solvent (approximately 400 µl) was then transferred into a 1.5 ml HPLC-Vial by a Pasteur pipette. A total of 400 µl water–methanol (90:10) was added into the collection vial, vortexed and combined in the HPLC vial which was then volumetrically adjusted with water–methanol (90:10) to a volume of 1 ml.
LC-MS/MS was performed with a Varian 1200L LC-MS System (Varian Inc., Walnut Creek, CA, USA). Separation of the trichothecenes was performed by using a Polaris Amide C18-A column (3 µm, 50 × 2.0 mm; Varian) and a guard column (SecurityGuardTM Cartridge C18 4 × 2.0 mm, Phenomenex Inc., Torrance, CA, USA). Eluent A consists of Milli-Q® water/methanol (95/5 v/v) and eluent B of Milli-Q® water/methanol (5/95 v/v). Both eluents contained 5 mM ammonium acetate. The mobile phase flow rate was 0.25 ml min–1. Detection of the trichothecenes was performed with the APCI interface using negative and positive modes and ions. The interface parameters and gradient elution of the LC-MS/MS are shown in .
Table 1. Interface parameters and gradient elution of the LC-MS/MS in positive and negative mode used for mycotoxin analysis.
The method was validated by recovery experiments of differently spiked samples (200 and 2500 µg kg–1), respectively. The recovery range for each trichothecene was between 85 and 120%. The limit of quantification (LOQ) was determined as 10 times the baseline noise. The limit of detection (LOD) was determined as three times the baseline noise. The ranges of LOQ and LOD are shown in . The LOQ and LOD vary because the samples were measured in different runs.
Table 2. Limits of quantification (LOQ) and limits of detection (LOD).
Statistical analysis
Statistical analysis was conducted with ‘Rʼ version 3.1.0 (R Core Team Citation2015) and ‘R Studioʼ version 0.98.994 (R Studio Team Citation2015). The package ‘agricolaeʼ (de Mendiburu Citation2015) was used to calculate Pearson’s correlation, the package ‘userfriendlyscienceʼ (Peters Citation2016) for the Games–Howell post-hoc test, and the package ‘multcompʼ (Hothorn et al. Citation2008) for the Tukey–Kramer post-hoc test. Modell selection was done by the aid of the packages ‘Muminʼ (Barton Citation2016) and ‘nlmeʼ (Pinheiro et al. Citation2016).
Homogeneity of variance and normality of residuals were checked graphically using plots of fitted values versus the root of the standardised residuals and normal Q-Q plot, respectively. To meet homogeneity of variance and normal distribution of residuals, incidence of F. graminearum data and DON concentrations were arcsine (square root) and log transformed, respectively. Mycotoxin concentrations below the respective LOQ or LOD were calculated as LOQ/2 or LOD/2, respectively.
To determine influencing factors on F. graminearum incidence and DON concentration, a multiple linear regression model was employed using stepwise backward/forward regression. The obtained regression models were evaluated by using the Akaike information criterion (AIC). All factors were checked for multicollinearity using a correlation matrix, factors with a correlation coefficient > 0.3 were removed from the model. The model with the greatest explanation rate was used for further analysis.
Significant influencing factors were further analysed with the Games–Howell test, whereas interactions of the factors previous crop × additional chopping, previous crop × tillage and previous crop × pre-previous crop were analysed with the Tukey–Kramer test. Data analysis was performed with a probability value of 5% (α = 0.05). The correlation between the DON concentration and the incidence of F. graminearum were calculated using Pearson’s correlation coefficient (r) on transformed data (see above).
The co-existence of species of the FHB complex and the respective mycotoxins was explored using a principal component analysis (PCA) on the correlation matrix of 14 variables. These variables were incidence of different Fusarium species (%) of F. graminearum, F. avenaceum, F. poae, F. spp., Microdochium spp. and mycotoxin content (µg kg–1) of DON, NIV and ZEA. For figures and tables, untransformed data were used. Figures were created with ‘Rʼ version 3.1.0 (R Core Team Citation2015) and ‘R Studioʼ version 0.98.994 (R Studio Team Citation2015) and Microsoft® Excel 2013.
Results
Due to the overall low occurrence of Fusarium species and mycotoxins, the analysis on potential cropping factors was done only for the main occurring species/mycotoxin, F. graminearum and DON, and pooled over both years, since the influencing factors were the same in both years. In order to obtain meaningful sample sizes, cropping factors were grouped as follows: barley varieties were Caravan, Cassia, Franziska, Fridericus, Landi, Meridian, Quench, Semper, Zoom or Other (12 different varieties with each fewer than 15 samples); previous crops were cereals (barley, oats, spelt, emmer, triticale, wheat), maize, pasture, canola or other (12 different crops with each fewer than 15 samples); pre-previous crops were cereals (barley, oats, spelt, emmer, triticale, wheat), maize, pasture, canola, sugar beet or other (16 different crops with each fewer than 10 samples); additional chopping was yes or no; tillage was plough or reduced tillage (including direct sowing); fungicide ingredient were triazoles + strobilurins, strobilurins only, triazoles only or no fungicide; applied nitrogen (N) in kg ha–1 were 1 = 1–50 kg N ha–1, 2 = 51–100 kg N ha–1, 3 = 101–150 kg N ha–1, 4 = 151–200 kg N ha–1 or 5 > 200 kg N ha–1. If the N amount kg ha–1 was not indicated, it was calculated according to averaged values for farmyard manure.
Fusarium species spectrum in Swiss barley samples from 2013 and 2014
Overall, nine different Fusarium species were detected in Swiss barley samples. These were, in descending order, F. graminearum (FG), F. avenaceum (Fries, Saccardo; teleomorph Gibberella avenacea, Cook), F. poae (Peck, Wollenweber; no teleomorph known), F. culmorum (Smith, Saccardo; no teleomorph known), F. crookwellense (Burgess, Nelson & Toussoun; no teleomorph known), F. dimerum (Penzig; no teleomorph known), F. equiseti (Corda, Saccardo; teleomorph: Gibberella intricans Wollenweber), F. tricinctum (Corda, Saccardo; teleomorph: Gibberella tricincta El-Gholl, McRitchie, Schoulties & Ridings) and F. sporotrichioides (Sherbakoff; no teleomorph known).
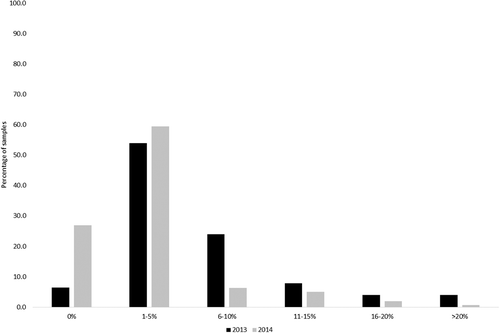
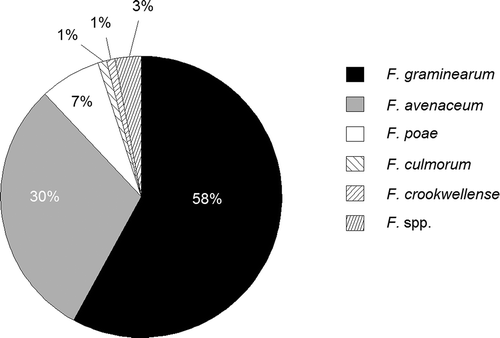
Fusarium graminearum was the predominant species in 2013 (62% of all detected Fusarium species) and 2014 (45%), whereas F. avenaceum was the second most occurring species with 29% and 33%, respectively. Fusarium poae was the third most frequent species with 4% and 17%, respectively. Other Fusarium species occurred with 1% or lower (). The non-toxigenic species Microdochium nivale/M. majus were the most occurring FHB-causing species in both years (74%, 69%). However, due to their inability to produce mycotoxins, the corresponding data were not included in . The samples with the highest occurrence of FG in 2013 and 2014 showed infection rates of 53% and 25%, respectively.
Figure 1. Ratio of different species from all detected Fusarium species in Swiss barley samples collected in 2013 and 2014; F. spp.: Fusarium species with an incidence of < 1%; number of samples = 440.
However, the average incidence of the three predominant Fusarium species was relatively low (< 5%) in both years (), but with a higher infection rate in 2013 (3.8%) compared with 2014 (2.4%) ().
Table 3. Mean incidence of F. graminearum (FG), F. avenaceum (FA) and F. poae (FP) in Swiss barley samples collected in 2013 and 2014.
Fusarium mycotoxins in Swiss barley samples from 2013 and 2014
The mycotoxin quantification via LC-MS/MS revealed DON as the predominant mycotoxin in 2013 and 2014 with an average of 235 and 47 µg kg–1, respectively. The contamination with DON and T-2/HT-2 was higher in 2013, whereas the contamination with NIV and ZEA was higher in 2014 (). The European Union maximum limit for human consumption (European Commission Citation2006) of DON of 1250 µg kg–1 for unprocessed barley was only exceeded in nine samples (3%) in 2013 and in one sample (< 1%) in 2014. The highest measured DON contents were 4860 µg kg–1 in 2013 and 1725 µg kg–1 in 2014. The maximum limit of 100 µg kg–1 ZEA in unprocessed cereals was exceeded only in 2014 by three samples (2%).
Table 4. Deoxynivalenol (DON), nivalenol (NIV), zearalenone (ZEA) and T-2/HT-2 toxins in Swiss barley samples collected in 2013 and 2014 described by mean, 95th confidence interval, maximum detected value and number of samples with mycotoxins below the limit of detection (LOD).
The toxins T-2/HT-2 were detected in 16 (6%) samples in 2013 and in 10 (6%) samples in 2014. The proposed indicative limit of 200 µg kg–1 in unprocessed barley (European Commission Citation2006) was exceeded only in 2013 in two (< 1%) samples.
Although the contamination with mycotoxins was low in both years, a significant positive correlation (r = 0.72, p < 0.001) was found between DON content and FG incidence. In addition, a significant positive relationship between F. poae incidence and nivalenol content (r = 0.60, p < 0.001) was observed.
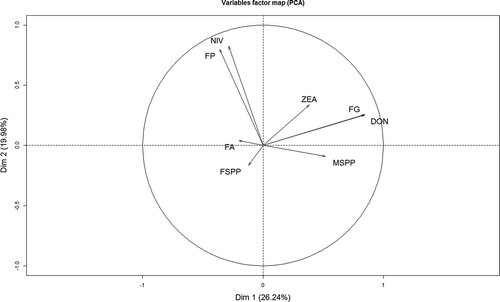
Fusarium species incidence and mycotoxin content were plotted as a biplot () to show the distribution of the samples in the two most descriptive dimensions of the descriptive factors and the variables (species and mycotoxins) projected onto these two axes. On the x- and y-axes, factors 1 and 2 describe 26.2% and 19.9% of the variability, respectively. There was a strong positive relationship between F. poae and NIV and between FG, DON and ZEA. In addition, there was also a positive relationship between FG and Microdochium spp., while F. avenaceum showed a positive relationship with F. spp., except F. poae and FG. In contrast, there was a strong negative relationship between Microdochium spp. and F. poae and between FG and F. avenaceum.
Figure 3. Biplot of the principal component analysis of Fusarium species incidence (%) with F. graminearum (FG), F. avenaceum (FA), F. poae (FP), F. spp. (FSPP), Microdochium spp. (MSPP) as well as the mycotoxin content (µg kg–1) with deoxynivalenol (DON), nivalenol (NIV) and zearalenone (ZEA) in Swiss barley samples collected in 2013 and 2014, FSPP: F. culmorum, F. crookwellense, F. equiseti, F. tricinctum, F. dimerum and F. sporotrichioides, n = 440.
Cropping system
In both years, differences were found between the three main cropping systems in Switzerland: Organic (n = 42), Extenso (n = 173) and PEP (n = 225). Due to multicollinearity, this factor was removed from the model and thus no statistical analysis was done. In 2013 and 2014, the samples from the PEP system showed a higher mean incidence of FG (5.4%, 4.1%) and a higher mean contamination with DON (324.4, 78.3 µg kg–1), respectively, compared with the other cropping systems. Samples from the organic system had the lowest mean FG incidence (0.6%, 0.4%) and contamination with DON (21.8, 26.0 µg kg–1), respectively.
Variety
Differences between the grown barley varieties were observed. Due to multicollinearity, this factor was also removed from the model and thus no statistical analysis was performed. In winter varieties, the mean FG incidence (3.1%) and DON content (179.5 µg kg–1) were higher compared with spring varieties (0.8%; 39.1 µg kg–1). For example, the spring variety Quench showed the lowest incidence (0.6%) and DON content (30.0 µg kg–1), whereas the winter varieties Meridian and Zoom showed the highest FG incidences (5.3%, 3.7%) and DON contents (300.0 µg kg–1).
Crop rotation and tillage
The cultivation of maize before barley resulted in a significantly higher (p < 0.001) mean incidence of FG (7.3%) and DON content (447.7 µg kg–1) compared with other previous crops except canola. From the Organic, Extenso and PEP cropping systems, maize as a previous crop was grown at 14%, 8% and 20%, respectively. The lowest average FG incidence (0.8%) and DON content (31.1 µg kg–1) was noticed when the previous crop was pasture (). From the Organic, Extenso and PEP cropping systems, pasture as a previous crop was grown at 36%, 6% and 1%, respectively. A reduction ranging from 48% to 89% FG incidence and from 53% to 93% DON content was obtained when maize was not the previous crop ().
Table 5. Effect of previous crop on F. graminearum (FG) incidence and deoxynivalenol (DON) content in Swiss barley samples, collected in 2013 and 2014.
Overall, samples from fields with ploughed soils showed significantly (p < 0.001) less FG incidence and DON content compared with samples from reduced tillage fields regardless of the previous crop (). The reduction of FG incidence and DON content by ploughing was 47% and 29%, respectively. In fact, 95% of the organic farmers ploughed the soil before growing barley in contrast to 64% (Extenso) and 58% (PEP). Furthermore, samples from ploughed fields showed significantly lower FG incidence if the previous crop was a cereal (p = 0.046) or maize (p < 0.001) compared with samples from reduced tillage fields with the same previous crops.
Table 6. Effect of tillage and the interaction of previous crop × tillage on F. graminearum (FG) incidence and deoxynivalenol (DON) content in Swiss barley samples collected in 2013 and 2014.
Maize as a previous crop in combination with reduced tillage showed the highest mean incidence of FG (9.8%) and DON content (448.5 µg kg–1) (). All organic farmers ploughed the soil after growing maize, compared with 12% of the Extenso and 53% of the PEP farmers.
A significantly (p < 0.001) lower DON content was only observed in samples from ploughed fields with previous crop cereals compared with samples from cereal fields with reduced tillage (). A total of 96% of the organic, 71% of the Extenso and 65% of the PEP farmers ploughed their fields after growing cereals.
Additional chopping of previous crop residues showed also an effect on FG incidence and DON content. Chopping maize residues significantly reduced mean FG incidence (p = 0.013) and DON content (p = 0.017) compared with non-chopped maize residues. The resulting reduction with previous crop maize was 53% in terms of incidence and 42% in terms of DON content. This effect was not observed in any of the other previous crops (data not shown). This additional treatment was carried out by 83% of the organic farmers, compared with 57% of the Extenso and 25% of the PEP farmers.
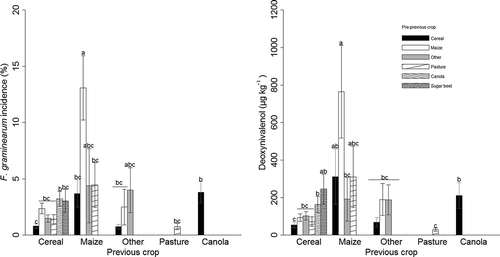
The crop rotation two years before barley showed a higher FG incidence when maize was grown after maize and this combination was significantly different (p < 0.001) from all other crop rotations except maize–other and other–other. With respect to DON content, the difference was not significant from samples with the rotations cereal–sugar beet, maize–cereal and maize–other (). Cultivating maize after maize before barley was, however, a rare rotation with none of the organic farmers, only 2% of the Extenso and 8% of the PEP farmers. Samples from crop rotations with two years cereals or two-year pasture in a row before barley had the lowest average FG incidence and DON content (). Since some crop rotation sequences occurred only rarely, combinations with fewer than five samples (16 samples) were excluded from the statistical analysis, leading to plotted results of 424 samples.
Figure 4. Effect of the previous two crops on F. graminearum incidence (%) and deoxynivalenol content (µg kg–1) in Swiss barley samples, collected in 2013 and 2014, n = 424. Error bars represent the standard error of mean, means with the same letters are not significantly different according to a Tukey-Kramer test for interactions at α = 0.05. Meanings in cropping factors are as explained in the main text.
Fertilisation, fungicides and growth regulators
Samples with a nitrogen fertilisation of more than 100 kg N ha–1 had on average a higher FG incidence and DON content compared with lower nitrogen regimens. The highest DON contamination (318 µg kg–1) was measured in samples with a fertilisation of > 200 kg N ha–1 ().
Table 7. Effect of nitrogen fertilisation on F. graminearum (FG) incidence and deoxynivalenol (DON) content in Swiss barley samples collected in 2013 and 2014.
The highest FG incidence (5.6%) and DON content (342.2 µg kg–1) were observed in samples, where organic in combination with mineral fertilisers, were applied and the lowest (0.4%; 35.9 µg kg–1) if only organic fertilisers were applied (data not shown). Samples without additional nitrogen fertilisation (32 samples) were excluded from the analysis. We suppose that the farmers forgot to add this information in the questionnaire, since a commercial barley production without fertilisation is impractical.
Application of fungicides and growth regulators is not permitted for organic and Extenso farmers, while more than 90% of the PEP farmers used both. Fungicide use resulted in a significantly higher FG incidence (p < 0.001) and DON content (p < 0.001), irrespective of the active ingredient(s). The majority was applied during growth stages 31/32, 37/39 and 49/51 in 2013 and during 31/32, 39 and 45 in 2014. The combination of fungicides belonging to the group of triazoles and strobilurins led to the highest mean FG incidence (6.2%) and DON content (333.8 µg kg–1). The lowest mean incidence (1.4%) and DON content (83.2 µg kg–1) was observed when no fungicide was applied (). The reduction without application of fungicides ranged from 61% to 77% (FG incidence) and from 61% to 75% (DON content) depending on the fungicide.
Table 8. Effect of fungicide group on F. graminearum (FG) incidence and deoxynivalenol (DON) content in Swiss barley samples collected in 2013 and 2014.
Due to multicollinearity, no statistical analysis was done for the factor growth regulator. The use of growth regulators resulted in a higher average FG incidence (4.6%) compared with no use of growth regulators (1.5%). The same effect was observed for the mean DON contamination (252.8 versus 88.0 µg kg–1) (data not shown). The reduction was 67% in terms of FG incidence and 65% in terms of DON content.
Discussion
In the current study, we investigated the FHB-causing species complex and the respective mycotoxins in farm samples with natural infection from Switzerland. The non-toxigenic species Microdochium majus/M. nivale were by far the dominant species isolated from barley, whereas FG and F. avenaceum were in both years the dominant toxin producing species.
The high frequency of Microdochium species in barley was also observed in other European countries, such as the UK (Nielsen et al. Citation2014).
In the UK, Nielsen et al. (Citation2014) detected lower amounts of FG DNA in malting barley, but determined F. avenaceum, F. poae, F. tricinctum and F. langsethiae (Torp and Nierenberg, no teleomorph known) as dominating species. In a French survey on barley (2000–2002), employing microscopic identification, F. graminearum was classified as second or third while F. poae or F. avenaceum were the predominant species, depending on the year (Ioos et al. Citation2004).
Hence, the species spectrum in the above mentioned studies are overall in agreement with our findings. However, differences between molecular and microscopic identification and quantification methods exist and may have partially affected the obtained results in terms of the predominant species. Furthermore, environmental and climate conditions have a strong influence on the Fusarium species spectrum (Osborne & Stein Citation2007) and thus differences between the geographical regions within Europe can be expected.
DON was the predominant mycotoxin followed by NIV, T-2/HT-2 and ZEA. In accordance with our results, Nathanail et al. (Citation2015) detected DON in Finnish barley samples most frequently followed by NIV, T-2/HT-2 and ZEA. Similar to our studies, they only found low amounts of T-2/HT-2, which is produced amongst others by F. langsethiae (Torp & Nirenberg Citation2004). The low occurrence of T-2/HT-2 in our samples without identifying F. langsethiae could be due to the agar plate method, which might not be suited to recover this species from grains since it grows slowly and might easily be overgrown (Torp & Nirenberg Citation2004). However, in an extensive study using a great number of F. langsethiae, F. poae and F. sporotrichioides isolates, Thrane et al. (Citation2004) observed that some F. poae strains are also able to produce T-2/HT-2, although the majority was produced by F. langsethiae.
The higher NIV content in 2014 correlated with the higher occurrence of F. poae, which is a known NIV producer (Desjardins Citation2006). This is in line with Yli-Mattila et al. (Citation2008), who described a correlation between F. poae DNA and NIV content in barley and reported that F. poae seems to be the most important NIV producer. Although some isolates of FG and F. culmorum are known to produce NIV (cited in Desjardins Citation2006), we did not observe a significant correlation between these species and the NIV content.
Since not all farmers indicated the flowering date, the relation between weather conditions and Fusarium species infection and toxin contamination remains unclear. Furthermore, due to the overall low occurrence/contamination, no influence of the weather during calculated flowering periods (based on the sowing date and the geographic origin) at the different sites was detected. Also, most barley varieties are flowering while still in the boot stage and thus the ear is sheltered by the flag leaf which may prevent an infection.
A central aspect of this study was to elucidate the effect of agronomic factors within the different cropping systems. Differences between the varieties were observed, and, overall, winter varieties showed a greater FG incidence and a higher DON content. Only four varieties (Caravan, Cassia, Meridian and Semper) were grown in all three production systems. Generally, these varieties showed a lower FG occurrence and DON content in Organic farming systems. However, since the sample size was rather low, caution has to be taken in interpreting the results. Furthermore, the variety Quench showed the lowest FG incidence and DON content, but was only cultivated by organic farmers. Hence, lower infection rates and smaller amounts of DON could have been due to other factors such as different crop rotation or tillage. In a survey in Norway, Bernhoft et al. (Citation2012) found statistically significant differences between commonly grown varieties in terms of mycotoxins and Fusarium occurrence and observed less occurrence in organically produced cereals. For this reason, we also investigated barley samples from Agroscope variety experiments, since these were all grown under the same agricultural practices. However, no differences were found, probably due to the even lower average incidence of FG (0–6%) and no detection of DON (data not shown). In a five-year monitoring (2007–2011) on UK malting barley with different varieties, Nielsen et al. (Citation2014) found only one variety that had a significantly lower Fusarium DNA content compared with all other varieties. Thus, further studies are needed to elucidate the susceptibility of different barley varieties.
The reason for higher FG incidence and DON contamination in winter varieties compared with spring varieties remains unclear. Although it seems possible that the longer cultivation period of winter varieties provides more time for fungal development and toxin production, we found no positive correlation between vegetation duration and FG incidence or DON content. Likewise, Xue et al. (Citation2004) stated no significant effect of harvesting date on DON concentration in wheat, but observed a higher FG incidence in delayed harvest samples, which could be explained by more colonisation time and a wider infection window.
The current study has demonstrated that maize as a previous crop contributes to a higher FG infection and DON content. This finding is not unexpected since FG can infect maize and survive saprophytically on these residues and thus can serve as an inoculum in the next growing season (Osborne & Stein Citation2007). Similar results were obtained in other field studies on FHB in wheat and barley (Dill-Macky & Jones Citation2000). The cultivation of 2 years maize before barley resulted in the highest FG incidence and DON content. We assume that this led to an increased disease pressure and favoured the infection conditions.
Fusarium graminearum can also survive on canola residues (Fernandez Citation2007), and hence it was not surprising that in samples from fields with previous crop canola, a higher FG incidence and DON contamination was detected compared with other previous crops.
Ploughing of residues from the previous crops revealed a lower occurrence of FG and DON. Ploughing buries potentially infected crop material and the decaying by microorganisms is favoured (Pereyra & Dill-Macky Citation2008). The greatest effect of reduced tillage was observed in combination with the previous crops cereals and maize, which are both hosts for FHB-causing species, as shown in barley by Fernandez et al. (Citation2007).
The reduction of FG incidence and DON content through additional chopping of residues from the previous crop maize was also observed in a five-year field experiment by Vogelgsang et al. (Citation2011). The treatments with a field shredder and other mulching equipment reduced disease symptoms, disease incidence and DON content in wheat. Most probably, the decomposition was accelerated, leading to reduced survival of the fungal inoculum. However, in a microcosm study by Vestergaard et al. (Citation2001), no effect on decomposition was observed when maize leaves were finely ground. We hypothesise that the resulting surface enlargement could also lead to space and nutrition competition between FG and other microorganisms and thus reduce the inoculum potential. Moreover, we assume that earthworms can use chopped residues more efficiently and thus increase the reduction of crop debris. This effect was shown in field studies by Wolfarth et al. (Citation2011) with wheat straw. Here, earthworms reduced the amount of Fusarium-infected straw, which was a more attractive food compared with the control.
As the application of fungicides during anthesis in barley is currently not permitted in Switzerland, we assume that fungicides were not used to control Fusarium species. The use of fungicides before anthesis may have reduced or eliminated the incidence of other fungi on the plant surface. As a result, the potential for the colonisation on the plants or crop debris could have been higher and may have resulted in an easier spreading and infection which in consequence led to a higher FG incidence and DON content.
Triazole fungicides are known to be effective against FG and F. culmorum, whereas strobilurins are more effective against M. majus/M. nivale in wheat, as reported by Simpson et al. (Citation2001). They observed a lower occurrence of FG when triazoles were applied and a lower occurrence of Microdochium species when strobilurins were applied. In the last years, however, resistance of Microdochium species against strobilurins was observed in barley (Nielsen et al. Citation2013).
The majority of fungicide studies were carried out with wheat and observed a reduction of FHB and mostly of DON after the application of strobilurins, triazoles or a mixture of both (Blandino et al. Citation2006; Haidukowski et al. Citation2012). A direct comparison with these results is difficult to achieve, since in our survey, not all farmers indicated the time of fungicide application and the anthesis period.
A higher FG incidence and DON content was detected when growth regulators were applied. The ears of shortened plants are closer to the ground and thus an infection through ejected ascospores is favoured (Maji & Imolehin Citation2002). The modified crop characteristic was described by Bernhoft et al. (Citation2012), who assumed that plants become bushier, which enhances the humidity and the spreading of Fusarium species. They also stated that higher nitrogen fertilisation favours lodging due to heavier ears, which is known to increase infections. Additionally, depending on the time of application and the dose, the plant is set under abnormal stress, which makes it in general more susceptible to plant diseases.
With increasing nitrogen fertilisation (> 100 kg ha–1), FG incidence and DON content was up to twice as high compared with lower doses, irrespective if it was applied as organic or chemical fertiliser. Lemmens et al. (Citation2004) observed an increase of visual disease symptoms and DON content with increasing nitrogen rate in wheat and hold the changed microclimate or longer flowering periods responsible for that finding. In contrast, nitrogen deficiency may render plants vulnerable to disease and, in fact, Yang et al. (Citation2010) concluded that FG infection and mycotoxin levels were more severe in barley plants with low nitrogen fertilisation. Our results indicate that following the current fertilisation recommendations for barley in Switzerland (110 kg N ha–1 for winter barley and 90 kg N ha–1 for spring barley), the risk of a FHB infection can be reduced. However, the data must be interpreted with caution, since the organic fertilisation was calculated using averaged values and data about Nmin were not inquired. Furthermore, we observed that the organic farmers fertilised less nitrogen compared with Extenso and PEP farmers and thus other factors (tillage, previous crop) could have a bigger influence on FG incidence and DON content.
Conclusions and outlook
Our two-year barley monitoring clearly demonstrated that the occurrence of Fusarium species and their respective mycotoxins is affected by several cropping factors. Frequently, not only one but rather the combination of different factors has to be considered, since maize as the previous crop together with reduced tillage led to the highest infections and DON contents. Thus, the entire cropping system should be taken into account to retrieve factors influencing the respective Fusarium species. The fact that we observed a lower occurrence and contamination in organically cultivated barley compared with samples from Extenso or PEP was probably due to cropping techniques that reduce the inoculum potential, such as a wider crop rotation, ploughing, no use of fungicides or growth regulators and less nitrogen fertilisation.
Since the overall disease pressure in 2013 and 2014 was low, further monitoring of barley is imperative to confirm our findings or to observe potential changes in the Fusarium species spectrum and their respective mycotoxins. Studies that investigate the differences between the flowering of barley and wheat in terms of infection and epidemiology should be conducted to increase knowledge about the infection process. Results from the current study together with further monitoring data can be used to develop cropping systems and disseminate recommendations that allow farmers to produce cereals with low risks of mycotoxin contamination.
Supplemental Data. Instructions for sub sampling
Download MS Word (12.4 KB)Supplemental Data. Questionnaire
Download MS Word (14.7 KB)Acknowledgments
The authors thank the Swiss cantonal plant protection officers who provided addresses of barley growers; and all participating growers for kindly providing samples of their harvest and for completing the questionnaires. Excellent technical support was provided by Irene Bänziger, Eveline Jenny and Andreas Kägi.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Barton K. 2016. MuMIn: multi-model inference [Internet]. R package version 1.15.6. [cited 2016 Aug 18]. Available at https://cran.r-project.org/web/packages/MuMIn/index.html
- Bernhoft A, Torp M, Clasen PE, Løes AK, Kristoffersen AB. 2012. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit Contam A. 29:1129–1140.
- Blandino M, Haidukowski M, Pascale M, Plizzari L, Scudellari D, Reyneri A. 2012. Integrated strategies for the control of Fusarium head blight and deoxynivalenol contamination in winter wheat. Field Crops Res. 133:139–149.
- Blandino M, Minelli L, Reyneri A. 2006. Strategies for the chemical control of Fusarium head blight: effect on yield, alveographic parameters and deoxynivalenol contamination in winter wheat grain. Eur J Agron. 25:193–201.
- D’Mello JPF, Placinta CM, Macdonald AMC. 1999. Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity. Anim Feed Sci Technol. 80:183–205.
- de Mendiburu F. 2015. agricolae: statistical procedures for agricultural research [Internet]. R package version 1.2-4. [cited 2016 Aug 18]. Available at https://cran.r-project.org/web/packages/agricolae/index.html
- Desjardins AE. 2006. Fusarium mycotoxins – chemistry, genetics, and biology. St. Paul (MN): APS Press.
- Dill-Macky R, Jones RK. 2000. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Dis. 84:71–76.
- Dorn B, Forrer HR, Schürch S, Vogelgsang S. 2009. Fusarium species complex on maize in Switzerland: occurrence, prevalence, impact and mycotoxins in commercial hybrids under natural infection. Eur J Plant Pathol. 125:51–61.
- European Commission. 2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuff. Official Journal of the European Union, L 364, 99:5–24.
- Fernandez MR. 2007. Fusarium populations in roots of oilseed and pulse crops grown in eastern Saskatchewan. Can J Plant Sci. 87:945–952.
- Fernandez MR, Zentner RP, DePauw RM, Gehl D, Stevenson FC. 2007. Impacts of crop production factors on Fusarium head blight in barley in eastern Saskatchewan. Crop Sci. 47:1574–1584.
- Haidukowski M, Visconti A, Perrone G, Vanadia S, Pancaldi D, Covarelli L, Balestrazzi R, Pascale M. 2012. Effect of prothioconazole-based fungicides on Fusarium head blight, grain yield and deoxynivalenol accumulation in wheat under field conditions. Phytopathol Mediterr. 51:236–246.
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom J. 50:346–363.
- Ioos R, Belhadj A, Menez M. 2004. Occurrence and distribution of Microdochium nivale and Fusarium species isolated from barley, durum and soft wheat grains in France from 2000 to 2002. Mycopathologia. 158:351–362.
- Lemmens M, Haim K, Lew H, Ruckenbauer P. 2004. The effect of nitrogen fertilization on Fusarium head blight development and deoxynivalenol contamination in wheat. J Phytopathol. 152:1–8.
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Ames, IA, USA: Blackwell Publishing.
- Maji E, Imolehin E. 2002. Influence of plant growth regulators and nitrogen on Fusarium head blight of wheat (Triticum aestivum L. Var. Kalysona). Acta Agron Hung. 50:19–25.
- Malachova A, Varga E, Schwartz H, Krska R, Berthiller F. 2012. Development, validation and application of an LC-MS/MS based method for the determination of deoxynivalenol and its conjugates in different types of beer. World Mycotoxin J. 5:261–270.
- Nathanail AV, Syvähuoko J, Malachová A, Jestoi M, Varga E, Michlmayr H, Adam G, Sieviläinen E, Berthiller F, Peltonen K. 2015. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal Bioanal Chem. 407:4745–4755.
- Nielsen L, Cook D, Edwards S, Ray R. 2014. The prevalence and impact of Fusarium head blight pathogens and mycotoxins on malting barley quality in UK. Int J Food Microbiol. 179:38–49.
- Nielsen L, Justesen A, Jensen J, Jørgensen L. 2013. Microdochium nivale and Microdochium majus in seed samples of Danish small grain cereals. Crop Protect. 43:192–200.
- Osborne LE, Stein JM. 2007. Epidemiology of Fusarium head blight on small-grain cereals. Int J Food Microbiol. 119:103–108.
- Parry DW, Jenkinson P, Mcleod L. 1995. Fusarium ear blight (scab) in small-grain cereals – a review. Plant Pathol. 44:207–238.
- Pereyra SA, Dill-Macky R. 2008. Colonization of the residues of diverse plant species by Gibberella zeae and their contribution to Fusarium head blight inoculum. Plant Dis. 92:800–807.
- Peters G-J. 2016. userfriendlyscience: quantitaitve analysis made accessible [Internet]. R package version 0.4-1. [cited 2016 Aug 18]. Available at https://cran.r-project.org/web/packages/userfriendlyscience/index.html
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. 2016. {nlme}: linear and nonlinear mixed effects models. R package version 3.1-128.
- R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- R Studio Team. 2015. RStudio: integrated development for R. Boston, MA, USA: RStudio, Inc.
- Schisler DA, Khan NI, Boehm MJ. 2002. Biological control of Fusarium head blight of wheat and deoxynivalenol levels in grain via use of microbial antagonists. Adv Exp Med Biol. 504:53–69.
- Simpson DR, Weston GE, Turner JA, Jennings P, Nicholson P. 2001. Differential control of head blight pathogens of wheat by fungicides and consequences for mycotoxin contamination of grain. Eur J Plant Pathol. 107:421–431.
- Thrane U, Adler A, Clasen PE, Galvano F, Langseth W, Logrieco A, Nielsen KF, Ritieni A. 2004. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Int J Food Microbiol. 95:257–266.
- Torp M, Nirenberg HI. 2004. Fusarium langsethiae sp. nov. on cereals in Europe. Int J Food Microbiol. 95:247–256.
- Vestergaard P, Rønn R, Christensen S. 2001. Reduced particle size of plant material does not stimulate decomposition, but affects the microbivorous microfauna. Soil Biol Biochem. 33:1805–1810.
- Vogelgsang S, Hecker A, Musa T, Dorn B, Forrer HR. 2011. On-farm experiments over 5 years in a grain maize/winter wheat rotation: effect of maize residue treatments on Fusarium graminearum infection and deoxynivalenol contamination in wheat. Mycotoxin Res. 27:81–96.
- Vogelgsang S, Jenny E, Hecker A, Bänziger I, Forrer HR. 2009. Fusaria and mycotoxins in wheat – monitoring of harvest samples from grower’s fields [in German]. Agrarforschung. 16:238–242.
- Vogelgsang S, Sulyok M, Hecker A, Jenny E, Krska R, Schuhmacher R, Forrer HR. 2008. Toxigenicity and pathogenicity of Fusarium poae and Fusarium avenaceum on wheat. Eur J Plant Pathol. 122:265–276.
- Wolfarth F, Schrader S, Oldenburg E, Weinert J, Brunotte J. 2011. Earthworms promote the reduction of Fusarium biomass and deoxynivalenol content in wheat straw under field conditions. Soil Biol Biochem. 43:1858–1865.
- Xue A, Frégeau-Reid J, Rowsell J, Babcock C, Hoekstra G, Sparry E. 2004. Effect of harvesting time on incidence of seed-borne Fusarium spp. in spring wheat in eastern Ontario. Can J Plant Sci. 84:757–763.
- Yang F, Jensen JD, Spliid NH, Svensson B, Jacobsen S, Jørgensen LN, Jørgensen HJL, Collinge DB, Finnie C. 2010. Investigation of the effect of nitrogen on severity of Fusarium Head Blight in barley. J Proteomics. 73:743–752.
- Yli-Mattila T, Paavanen-Huhtala S, Jestoi M, Parikka P, Hietaniemi V, Gagkaeva T, Sarlin T, Haikara A, Laaksonen S, Rizzo A. 2008. Real-time PCR detection and quantification of Fusarium poae, F. graminearum, F. sporotrichioides and F. langsethiae in cereal grains in Finland and Russia. Arch Phytopathol Plant Protect. 41:243–260.