ABSTRACT
This review describes the European Union and the US regulations applicable to food colours. Despite the different regulatory frameworks, the overall approach is similar, based on well-established risk-assessment procedures and risk-management measures. However, differences impacting free movement of goods can be found in the details and implementation of regulations. Using additives approved only in the US or in the EU implies that producers aiming to export need to adjust their product composition to the export market. Failure to comply may give rise to claims of adulteration, misbranding or non-compliance and rejection at the border or recall from the market. A careful comparison of the level of protection provided by the two sets of regulations, the criteria of good manufacturing practice (GMP) inspections and the certification requirements could be key to aligning the rules and to negotiating mutual recognition agreements. This review provides an extensive overview of the similarities and differences in regulating food colours in the EU and the US.
Introduction
Colour is an important part of consumers’ perception of food and a significant factor determining its taste (Johnson & Clydesdale Citation1982; Clydesdale Citation1991; Frick Citation2003; Burrows Citation2009; Spence Citation2016). Therefore, the food industry uses natural colours or synthetic dyes to make processed food more attractive to consumers. Colour is added to compensate for colour loss due to processing or storage or to balance out variation in natural colour (Frick Citation2003; Stich Citation2016). Colour is also added to products with no inherent colour, such as sugar-based candies and soft drinks to make them appealing to consumers and to match their expectations.
Before the 19th century food was mostly prepared at home. Only occasionally and by wealthy people, colorants obtained from animals, vegetables or minerals were used as food decoration (Frick Citation2003; Burrows Citation2009). After the Industrial Revolution, food was increasingly processed at large scale and new technologies including preservation altered the natural appearance of foods (Downham & Collins Citation2000; Griffiths Citation2005). Therefore, inexpensive and stable synthetic and mineral dyes with high tinctorial strengths and bright shades were excessively applied to a wide variety of foods. Some of these colorants such as copper sulphate, mercury sulphide, red lead, lead chromate and indigo have toxic properties. To restrict their abuse in the United States (US), a list of approved food colours was published in 1906 (US Food and Drug Act) (Burrows Citation2009). In 1960, the Color Additive Amendment incorporated the Delaney Clause prohibiting additives that induce cancer in humans or animals (Sanchez Citation2015). In the UK, several colorants were prohibited in 1923 and, in 1957, a legally binding list of permitted colours was established (Burrows Citation2009). A joint expert committee on food additives (JECFA), administered jointly by the FAO and WHO, was established as early as 1956, and has since provided an extensive review of 1500 substances, including food colours, setting the standards for safety assessment globally. Also the International Programme on Chemical Safety (IPCS) of the WHO assesses the health impact of chemicals in food.
At present, colours are probably the most rigorously regulated additives in food all over the world. However, despite global cooperation and harmonisation efforts, the rules vary from country to country. The international guidelines are generally recognised but are not necessarily translated into the national (and regional) frameworks of regulations, standards and procedures designed to ensure food safety. As trade is becoming more global, sourcing from across continents, regional and national regulations may become trade barriers that increase the transaction costs, more specifically the regulatory compliance costs, leading to economically negative effects. Beyond the economic aspects, technical barriers may impede using globally all available foods through free trade to reduce hunger and poverty (Magnuson et al. Citation2013).
Technical barriers to exporting agricultural products and foods especially from developing countries have been widely studied (e.g., Sheldon Citation2009; Ederington & Ruta Citation2016). More recently, as regulatory transparency and harmonisation have become a topic in trade agreement negotiations between developed countries like the Transatlantic Trade and Investment Partnership (TTIP) between the European Union (EU) and the US, some studies comparing EU and US food legislation have been published and overviews of specific aspects of both can be found in the literature (Magnuson et al. Citation2013; Baumgartner & Uebe Citation2014; Wilhelms Citation2014; Scotter Citation2015; Stich Citation2016). The objective of this review is to focus on regulation of colour additives, contrasting food colour regulations in the EU and US, to highlight the most important differences, to elucidate underlying issues and to suggest ways to increase regulatory coherence.
Food colours in the legal framework of the EU and US
Both in the EU and the US only approved colours can be used in foods. The respective rules are, however, embedded in two very different legal frameworks. As a result, the definition of a food colour, the requirements for approval, the approved colours and their specifications, restrictions for their use and responsibilities for rule-making and monitoring compliance are different. The main rules regulating colours in food in the EU and US are listed in .
Table 1. Overview of primary EU and US regulations on colour additives.
EU legal framework
In the EU, food colours are regulated as food additives under a comprehensive set of regulations for food improvement agents. Regulation (EC) No. 1331/2008 (EC Citation2008a) sets out a common authorisation procedure, and Regulation (EC) No. 1333/2008 (EC Citation2008c) on food additives and its amendment, Regulation (EC) No. 1129/2011 (EC Citation2011), include the rules for food colours. The annexes of the Regulation (EC) No. 1333/2008 contain food categories and a positive list of colours permitted in the EU including maximum quantities and instructions for use. Regulation (EU) No. 231/2012 (EC Citation2012) lays down the specifications for food additives listed in Annexes II and III to Regulation (EC) No. 1333/2008.
As a food additive, a food colour is a substance not normally consumed as a food or a characteristic ingredient of food that can be used to add or restore colour. The colours are artificial dyes or natural constituents of foods and natural sources. Also extracts derived from natural sources containing pigments selectively enriched relative to the nutritive or aromatic constituents are defined as food colours (EC Citation2013; Reinhart Citation2014). Substances considered as food, e.g., fruit or vegetable concentrate and saffron used because of their colouring properties, are defined as colouring foods which do not fall within the scope of the food additives regulation. They should be used in accordance with the rules of the Regulation (EC) No. 178/2002, i.e., the general food law (EC Citation2002) and other applicable rules, e.g., Regulation (EU) No. 1332/2008 (EC Citation2008b) for enzymes, Directive 2009/32/EC (EC Citation2009) for solvent extraction and Regulation (EC) No. 1881/2006 (EC Citation2006) for contaminants. In addition, an extract from a plant or animal source should comply with the pesticide residue legislation and contaminants such as naturally occurring toxic substances should not be concentrated to amounts of toxicological concern during extraction. If the colouring food was not or only negligibly available on the European market before 1997, it also needs to be evaluated under Regulation (EC) No. 258/97 on novel foods (EC Citation1997) and from the end 2017 onwards under the new Regulation (EU) No. 2015/2283 (EC Citation2015) before placing on the market.
US legal framework
In the US, the federal colour additive regulation is based mainly on the US Federal Food, Drug and Cosmetic Act (FFDCA), and its amendments namely the Food Additive and the Color Additive Amendment, the Food Safety and Modernization Act (FSMA) of 2012, the Fair Packaging and Labeling Act (FPLA) of 1966 and the Public Health Security and Bioterrorism Preparedness and Response Act (Bioterrorism Act) of 2002 (Barrows et al. Citation2003; Northcutt & Parisi Citation2013). The statutes pertaining to food colours are primarily enforced by the USFDA, an agency of the Department of Health and Human Services (HHS), under the Title 21 of the Code of Federal Regulations (CFR Citation2016). Parts 70–82 and 101 contain rules on petitions and labelling and list the specifications and rules for use of approved colour additives, referring also to Part 58 on good laboratory practice for testing. The standards of identity for over 300 foods, listed in Parts 130–169 and in Part 319 of Title 9 under the jurisdiction of the Food Safety and Inspection Service (FSIS) of the Department of Agriculture (USDA), contain additional restrictions for use in certain products.
Colour additives are regulated apart from other food additives as a specific class of substances added to food and animal feed with the purpose or capable of imparting colour to food. They are controlled more strictly than other food additives. For example, for colour additives a valid proof of safety needs to be provided during the authorisation procedure and they cannot be registered as ‘generally recognised as safe’ (GRAS), exempt from USFDA approval.
The definition does not differentiate between colours produced by synthesis and pigments extracted or isolated with or without modifications from vegetable, animal, mineral or other sources. However, the approved colours are divided into two groups: certified colours which are all artificial and those exempt from certification that contain pigments obtained from natural sources, of which some can also be produced by synthesis. Colourants subject to certification require every batch be certified by the USFDA. Those listed exempt from certification also must comply with the identity and purity specifications and use limitations described in their listing regulations, but this is the responsibility of the user, not of the USFDA (Barrows et al. Citation2003).
Coloured food ingredients, which contribute their own colour when mixed with other foods, such as chocolate in chocolate milk or cherries in cherry yoghurt, are not considered as colour additives (21 CFR §70.3). Applying cherry juice to colour cherry yoghurt, however, is defined as colour addition (Matulka & Tardy Citation2014). Similarly, ingredients such as riboflavin and beta-carotene are only considered to be colour additives when they are intended to impart colour, not when they are added for nutritive value as additives with GRAS status; the colouring effect must then remain unimportant (21 CFR §70.3(g)).
Unlike in the EU, where feed additives and food contact materials are regulated separately, the ingredients in animal feed, which are added with the purpose to colour meat, milk or eggs, are also included in the regulations for colouring additives and colours used on packaging are considered to be colour additives if they may be expected to migrate to the contents of the package.
In the US, the states regulate all foods within the state, and foods that are not intended for interstate commerce and are consumed in the state where they are produced are not subject to federal law. The states may thus have additional requirements and even local administrations can impose their own rules. For example, Oregon authorises its administration to prescribe conditions of use or maximum limits, whether or not in accordance with federal rules (49 ORS §616.366). The state laws are, however, in principle pre-empted by federal laws on requirements for food standards, labelling, health claims and ingredient declaration (Kushner et al. Citation2014; Wilhelms Citation2014).
Authorised colours
This study investigates the rules on colour additives covered by the EU food additives regulation and US colour additive regulations excluding those that would be considered feed additives in the EU. For the EU the colouring foods that are contained in the US listing regulations are included.
The authorised food colours in the EU and the US are listed in . Presently, in the EU 39 colours are authorised as colour additives for use in foods together with canthaxanthin that is no longer authorised in food but is still included for its use in drugs. In the US, there are 36 approved food colour additives of which nine, mainly of artificial or synthetic origin, are subject to certification.
Table 2. Correspondence of approved colours in the EU and the US. Permitted use of the lake forms and the US attribution of subject to certification are also indicated.
The overlap between the US and EU food colour authorisations is limited even if almost 30 colours or groups of colours are approved for use in food in both the EU and US. Only six colours of synthetic origin are authorised by both jurisdictions. Six food colours are authorised for use in the EU as other food improvement agents or colouring foods. Four colour additives approved in the US are not permitted in the EU: the three synthetic colours, namely Orange B, Citrus Red No. 2 and FD&C Green No. 3 (Fast Green FCF) and toasted partially defatted cooked cottonseed flour. In turn, 16 colour additives authorised in the EU are not allowed in the US, including nine colours of synthetic origin and lutein, vegetable carbon, aluminium, silver and gold, chlorophylls and chlorophyllins and calcium carbonate. Pearlescent mica-based pigments, manufactured by coating mica platelets with titanium dioxide authorised in the US and used in, for example, fun foods, decorations and frostings are not listed in the EU as colour additives but can be labelled as mixtures of the approved carrier mica (E 555) and titanium dioxide (E 171) and/or iron oxide (E 172). In the US, iron oxide-coated mica is not permitted, though.
The use of lakes to increase stability of certain colours by precipitating them with aluminium or calcium compounds is authorised in the US for seven straight colours subject to certification (). In the EU, aluminium lakes are allowed for 21 additives, including most of the synthetic colours but only for foods for which maximum limits on aluminium coming from lakes are given.
For trade, the use of Orange B, Citrus Red No. 2 and FD&C Green No. 3 in the US is not an issue. Even though Orange B is authorised no new batches of Orange B have been certified since 1975 (Harp & Barrows Citation2015). Citrus Red No. 2 is authorised for marking skins of oranges only and FD&C Green No. 3 is the least commonly used of certified colours since green is typically produced by mixing blue and yellow colours (Harp & Barrows Citation2015). Further, silver and gold can be replaced by, for example, pearlescent pigments or mixtures of glazing agents, gum arabic and approved colourings for export to US.
The impact of banning several synthetic colours in the US, potentially requiring EU exporters to reformulate their products, is alleviated by progressive replacing of colours of synthetic origin by natural alternatives in the EU market. US exporters on the other hand would generally be expected to adjust to the shift to natural colours in the EU markets, and presently also increasingly in the US. Therefore, switching to natural alternatives seems to be the most promising option to harmonise use of colour additives even though not all of them are authorised on both markets either. For instance, the yellow safflower extract, used as a colouring food in the EU, is not approved for colouring in the US and the use of blue Spirulina extract is restricted to, for example, confections, frostings, ice cream and beverage mixes.
Approval processes
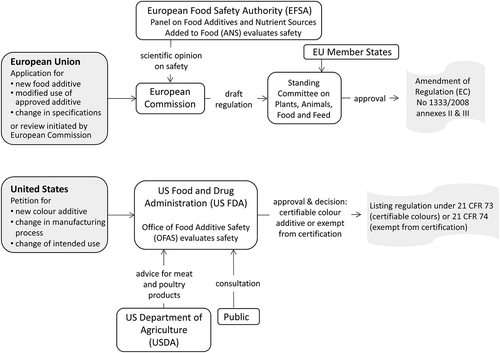
In the EU, an application to authorise a new food additive is sent to the European Commission (EC) which asks the EFSA to carry out a risk assessment. The EC can also act on its own initiative, e.g., requesting a review of approved additives. To modify the intended use and conditions of use of an approved additive or to remove it from the list of approved additives, the same procedure is followed. At EFSA, the Panel on Food Additives and Nutrient Sources Added to Food (ANS), made up of scientists from expert organisations in the EU member states, evaluates the safety of the food additive or new proposed uses of an additive.
The EFSA sends its opinion to the EC and the member states and publishes the opinion to the public. The opinion includes the identity and characterisation of the food additive, the assessment of the biological and toxicological data, a dietary exposure assessment for the European population taking into account other possible sources of dietary exposure, an overall risk assessment establishing a health-based guidance value, such as an acceptable daily intake (ADI) value, and the contribution of each food category or foodstuff for which the use is authorised or has been requested, to the total exposure.
A food additive may be approved if there are no safety concerns at proposed legal-use level, a technological need is considered justified, there are advantages and benefits for the consumer, and the consumer is not mislead. In that case the EC submits a draft regulation, taking account of the EFSA opinion, any relevant provisions of EU law and any other legitimate factors, to the Standing Committee on Plants, Animals Food and Feed (PAFF Committee).
Upon approval, the food additive is entered into the list in Annexes II and III of the food additive regulation and a unique identifier, the E number, is assigned for it. The list also specifies the name of the food additive, the foods to which the additive may be added, the conditions under which the food additive may be used and, if appropriate, whether there are any restrictions on the sale of the food additive directly to the consumer. If concerns about safety arise after long-term consumption, the colour may be re-evaluated by the EFSA; presently chlorophyllins, copper chlorophyllins and iron oxides and hydroxides are being reviewed.
In the US, a petition for the use of a colour additive is addressed to the USFDA. Upon receipt, the USFDA publishes the request in the federal register, including a brief description of the proposal in general terms. Additionally, all safety and functionality data from the petition, a protocol for a test/study, a list of all ingredients contained in a colour additive, an assay or analytical method, all USFDA tests/actions, and adverse reaction reports can be made available for the public to comment upon. The evidence accompanying the application, provided by the petitioner, is reviewed by the USFDA’s team of scientists from its Office of Food Additive Safety (OFAS), including toxicologists, chemists, environmental experts and statisticians.
The USFDA considers probable consumption or exposure from a colour additive, cumulative effects in the diet, evaluations by experts, and the availability of analytical methods for determining its purity and acceptable levels of impurities (FFDCA). A colour additive is deemed safe if ‘there is convincing evidence that establishes with reasonable certainty that no harm will result from the intended use of the color additive’ (21 CFR §70.3(i)). However, no additive is considered safe if found to cause cancer in humans or animals (Delaney Clause) (Sanchez Citation2015). If the colour additive is intended for use in meat or meat or poultry products, the USFDA asks the USDA to advise whether the substance would be permitted in products under its jurisdiction.
Taking into account all factors and comments the USFDA decides whether to approve the colour additive and determines whether batch certification is necessary. If approved, the USFDA will publish the approval as a listing regulation in 21 CFR Part 73 or 74, including specifications and any conditions and limitations of use. Certifiable colours receive a FD&C identification code upon approval (e.g. FD&C Yellow No. 5) and each certified batch has to be marked accordingly. A new application or petition for amendment would be needed when the manufacturing process changes significantly, a new way of production or a new source material is introduced, or the intended use changes.
, which shows the main stages and actors of the approval process in the EU and the US, points to an important difference in the application process. In the US, the USFDA, responsible for rulemaking, approval and control, is also responsible for the risk assessment; the safety assessment is carried out by the experts of its administration. In the EU, there is a separation between risk assessment and risk management: the EFSA is responsible for the risk assessment and the EC is responsible for risk management through the committee procedure involving the representatives of the member states, ensuring wider political oversight than in the US. On the other hand, in the US the public can access most relevant documentation and comment on the proposal before a decision is taken.
Another significant difference in the procedure is that in the EU the applicant needs to justify the need for the additive, demonstrate its effectiveness, present the benefits for the customer and explain how use of the additive is not misleading the consumer. In the US there are no such requirements; the approval is based on safety assessment only.
In the US, the applicant can also be requested to provide five test samples to USFDA that may carry out its own safety testing and to provide the USFDA with samples of labelling (USFDA Citation2009). In the EU, the EFSA may commission studies but does not require samples from the applicant and compliance with labelling rules is verified by post-market control.
In the EU, the applicant needs to provide information about the analytical methods for quantification of the additive, its residues and degradation and reaction products in food (EFSA Citation2012), while the US requires methods also for all intermediates, subsidiary colours and other components of the colour additive (USFDA Citation2009). Both, however, provide detailed guidance regarding the requirements appropriate methods must fulfil. For instance, the EFSA requires methods that are specific and fit for purpose and single-laboratory validated at minimum; the USFDA guidelines also mention the potential for collaborative studies and the ability to withstand scrutiny in a courtroom.
Safety evaluation of food colours
Both administrations base their approval of a colour additive on risk assessment in line with international good practise laid down by JECFA. Both require a large amount of information to enable evaluation of the safety of a new colour additive and to define the necessary risk-management measures. These information requirements are laid down in the regulations and in more detail in the EFSA and USFDA guidance documents for applicants (USFDA Citation2000, Citation2006b, Citation2009, Citation2014; EFSA Citation2012) and include detailed descriptions of the identity and properties of the substance and the manufacturing process, proposed foods or food categories, expected use levels and exposure assessment, supported by documented studies and raw data.
The safety evaluations review, for example, the composition of the proposed colour additive and potential presence of harmful impurities and processing residues like solvents or heavy metals and information on stability and degradation products to identify substances needing toxicological assessment. The available toxicological data are examined to define health-based guidance values and estimates of probable exposure levels are determined to enable the risk assessment.
The safety evaluation is largely based on toxicological data and both the EU and the US set minimum test requirements, complemented with more specific and elaborate testing depending on the properties of the substance. The EU relies on a tiered approach having as a minimum toxicokinetics studies and studies on subchronic, reproductive and developmental toxicity. There are also two 90-day genotoxicity in vivo tests performed on rats, which include a repeated-dose 28-day oral toxicity study. If these show absorption, toxicity or genotoxicity, then further tests from the second tier are required. If the conditions of the second tier are not satisfied, the tests of the third tier are required on a case-by-case basis.
The US has a system of classification according to the level of concern based on the chemical structure and exposure. If the substance is expected to be of low concern, then the minimum amount of testing includes genetic toxicity tests and short-term toxicity tests with rodents comprising also the screens for neurotoxicity and immunotoxicity. The US requires three different genotoxicity tests of 28 days and only two if the dose is less than 50 μg kg–1. For novel additives, however, studies normally recommended for the highest concern level may be required and toxicological testing may be extended to cover also metabolites or degradation products and impurities.
Both authorities address special properties of engineered nanomaterials. This is considered especially important in view of the present uncertainty about the health effects of nanoscale materials and as food colours such as titanium dioxide may contain nanoscale particles. In the EU, information on additional characteristics like surface properties, are to be supplied for both engineered and non-engineered nanomaterials used as food additives. The US only requires information of such physical properties if relevant for the colouring effect, and linked to manufacturing method/technology, with the intention of modifying particle characteristics.
It is evident that the safety of colour additives is thoroughly evaluated prior to approval in both the EU and in the US. Despite the similarity of requirements, however, the evaluation of available safety data results in different decisions concerning their authorisation and use. For instance, some of the synthetic colours were banned in the US on the basis of claims of carcinogenicity, applying the Delaney Clause, but are still permitted in the EU as later evaluations by the JECFA and the EFSA concluded their use is safe.
The health-based guidance values derived from toxicity data are different for many comparable colours in the EU and the US (see the examples in ) and many are also different from those allocated by the JECFA. Furthermore, combining the health-based guidance values with different probable consumption estimates results in variable maximum limits of colours in foods and different restrictions for use. Reaching a consensus on the guidance values and cooperation in reviewing available data could help improve harmonisation in this respect. Besides, investigating the safety evaluation processes in detail could give useful insights when addressing concerns over the comparability of the national or regional safety standards, commonly expressed in the context of trade negotiations.
Table 3. Available ADI values for certain colours approved both in the EU and the US, and those from JECFA. Where US column contains (GMP), USFDA has found no evidence of hazard to the public at estimated use and intake levels; for β-apo-8ʹ-carotenal use limits are set.
Identity and quality requirements of colours – the specifications
In the EU, all approved food additives must comply with the specifications laid down in Regulation (EC) No. 231/2012. For each additive, the specifications list names and synonyms, define the substance through chemical and physical characteristics, its origin/source and a description of the preparation process, and contain a description of the appearance of the substance, parameters for identification tests like a specific wavelength for spectrophotometric analysis, and purity criteria. A minimum colouring matter content is set out for all additives but caramel colours and anthocyanins, the two colour additive groups of natural origin and variable composition.
In the US, the listing regulation of each colour additive contains names and descriptions of the substances. They may also contain information about the origin and preparation, the minimum colouring matter content or range, and criteria for purity. For ferrous gluconate and lactate and riboflavin the listing regulations refer to the specifications in the US Food Chemicals Codex.
Comparison of the specifications shows:
The minimum contents of the colouring matter are the same for many colour additives but in almost 40% of those approved in both the EU and the US, either US gives no specification or the minimum content is set higher than in the EU. A comparison with JECFA values shows that 95% of the minimum content values in the EU are identical with JECFA specifications or cases where JECFA does not provide recommendations. A total of 70% are the same, while in the US only 30% confer with the JECFA value. Typically, the differences are not large. Nevertheless they are laid down in the regulations and relevant for claims of adulteration.
The EU sets maximum limits for four heavy metal contaminants in almost all colour additives: arsenic, lead, cadmium and mercury. The US does not regulate the content of cadmium. Furthermore, maximum limits are different from those of the EU and regularly differ from those of the JECFA where available. Importers of colour additives need to consider these differences to comply with specifications.
In both the EU and the US the regulations define the origin and the preparation method of the additive. For instance, only indicated solvents can be used for extraction or the source of the material is specifically mentioned like plants in general, specific algae or strains of fungi for carotenes. The range of acceptable solvents as well as residues to be tested and their maximum limits are different. Deviation from the specifications would render the additive and the foods where it is used adulterated.
Frequently, the EU regulation refers to the JECFA for standards and tests for purity and identity. The USFDA mostly incorporates references to AOAC International (AOAC) and Food Chemicals Codex (FCC) in its regulations.
Even though the specifications are similar for most of the colour additives approved in both the EU and US, the small differences may create increased compliance costs in the form of additional testing or imply modifying the product composition for the specific market. Harmonising the specifications with JECFA in both the EU and the US could be a way to increase the regulatory compatibility.
Restrictions for use of food colours
To keep the intake of colour additives at safe use level, regulations define in which foods their use is permitted and where appropriate also set maximum limits for their content (). In the EU, the use of each individual colour additive is limited to defined food categories and the addition of food colorants to unprocessed food, food labelled as organic, baby food and, for example, unflavoured milk, bread and oil is not permitted at all. Use of colour additives is banned in some EU traditional foods too. A total of 15 colours, listed as group II additives in the regulation, are authorised in specific food categories listed in at quantum satis, meaning no maximum level is specified. These food colours that are mostly of natural origin, are to be used in accordance with good manufacturing practice (GMP), at a level not higher than necessary to achieve the intended purpose.
Table 4. Restrictions to use of colour additives in the EU and the US.
Table 5. Food categories in which the EU’s group II and III colour additives are permitted.
Five approved azo-dyes tartrazine, azorubine, Allura Red, Brilliant Black PN and Brown HT together with curcumin, cochineal, Patent Blue V, indigotine, Brilliant Blue, Green S, beta-apo-8′-carotenal and lutein can be used within maximum limits for their combined content (group III additives) in specified food categories listed in . The reddish azo-dye Litholrubine BK is authorised for use only in edible cheese rind at quantum satis. The other colours are permitted within individually defined maximum limits in specified foods or within limits set for a combination of specified other colours; only erythrosine and annatto are regulated individually for each permitted food category.
Colouring foods, such as concentrates of fruit and vegetable juice or spirulina extract, are food ingredients and may therefore be added to all food categories without restrictions. Where use of aluminium lakes is permitted, combined maximum limits for aluminium are set.
In the US, most authorised colours are permitted in foods generally in accordance with GMP. Usually the phrase ‘may be safely used for colouring foods generally (including dietary supplements) in amounts consistent with good manufacturing practice …’ is used. However, a ‘self-limiting level of use’ where the food may become unpalatable, unappealing or otherwise unfit for consumption, defining the maximum usage level, should be provided for the safety evaluation (USFDA Citation2014). Specific use restrictions are set in the listing regulations for Orange B, Citrus Red No. 2, sodium copper chlorophyllin, grape colour and grape skin extract, iron oxide, mica-based pearlescent pigments, ferrous gluconate and lactate and spirulina extract. A maximum allowed content is defined for Orange B, Citrus Red No. 2, sodium copper chlorophyllin, beta-apo-8ʹ-carotenal, canthaxanthin, titanium oxide, mica-based pearlescent pigments and iron oxide.
Additional limitations are set by standards of identity issued to promote honesty and fair dealing. The standards of identity define the ingredients, quality and manufacturing processes for certain foods. Over 300 standards of identity are defined in the US food regulations (21 CFR 130–169; 9 CFR 319). For example, Cheddar cheese, which is a standardised food product, may contain a food colour. In turn, cottage cheese, a standardised food as well, must not contain a colour. Altogether, 60% of the standards of identity do not allow the addition of colour. In some cases like cream and milk, only fruit juice is approved for colouring purpose.
The approach the two administrations apply to restrictions stem from the founding legislative principles. In the EU the precautionary principle prescribes detailed rules to manage the risk, whereas in the US the emphasis is on limiting constitutional freedoms as little as possible. While in the EU maximum limits are set for 600 different colour additive/food category combinations, there are fewer legal maximum limits in the US. On the other hand, in the US the use of colour additives is prohibited in about 200 standardised foods while colour additives are excluded only in few EU food categories.
Overlaying the two sets of rules results in an intricate framework of restrictions an exporter needs to navigate. US exporters could avoid some of the effects by voluntarily applying maximum limits as set in the EU and the EU exporters could favour using group II (quantum satis) colours. Furthermore, additives that are less used or subject to specific concerns such as some azo-colours could be replaced by other authorised substances and their approvals could be gradually revoked to increase regulatory coherence. However, harmonisation in this field of regulation seems challenging.
Labelling of food colours
In the EU, the colour additives used in food products must be declared in the ingredients list, giving their full name and/or their E number. In 2007, a study carried out by McCann et al. (Citation2007), widely known as the Southampton study, generated concerns about harmful effects of some artificial food colours on children’s behaviour. As a consequence, the EU applied the precautionary principle and presently foods containing tartrazine, Quinoline Yellow, Sunset Yellow, Ponceau 4R, Allura Red and carmoisine need to be accompanied by warning of potential adverse effects on activity and attention in children. Additionally, special rules apply to labelling of products for professional use.
In the US, colours subject to certification are required to be declared by listed names (e.g. FD&C Yellow No. 5). Their lakes can be listed with a simple name, dropping the FD&C prefix (e.g., Yellow 5 lake). Alternative names such as E numbers may be added in parenthesis. Colour additives exempt from certification can be labelled as ‘artificial color’, ‘artificial color additive’, ‘color added’ or an equally informative term and can be combined with the listed name. Colouring added to butter, cheese or ice cream does not need to be declared in the ingredient list unless required by the listing regulation, yet voluntary declaration is recommended. However, as potential allergen or sensitiser cochineal extract, carmine and FD&C Yellow No. 5 need to be declared on all food labels. The USFDA did review the claims arising from the Southampton study, too, but took no action (USFDA Citation2011). Like in the EU, labelling of colour batches for professional use is regulated requiring information about the use limitations and warnings and additionally for certified colours the lot or a lot-bound code needs to be available (21 CFR §70.25).
Neither in the EU nor in the US is the advertising message ‘natural colour or natural colourant’ legally recognised. The regulations do not differentiate between artificial colours and colours obtained from natural sources. Indeed, naturally occurring colours may be derived directly from animal, vegetable or mineral sources, being chemically modified or synthetically reproduced following their natural examples. For instance, the majority of the naturally occurring compound beta-carotene used for colouring purpose is synthesised industrially for economic reasons; carmine is the aluminium lake of a cochineal extract; and copper chlorophyllin the more stable derivative of the natural pigment.
Eventually, declaring colour additives in the ingredients list is just a detail in the discussion on labelling requirements, dominated by issues relating to information on nutrition, health claims and allergens. Aligning of rules on declaration of using colour additives would be possible and will require adaptations by both administrations. In addition, the recommendation in the EU of declaring colouring foods that are added for colouring purposes in the ingredient list could be turned into a requirement.
Enforcement and control of food colours
Generally, food business operators are legally responsible for the safety of their products. EU member states are responsible for maintaining systems to monitor and verify the fulfilment of the relevant requirements covering all stages of production (Regulation (EC) No. 178/2002), as well monitoring the consumption and use of food additives and reporting their findings to the EC and EFSA (Regulation (EC) No. 1333/2008). According to Regulation (EC) No. 882/2004 (EC Citation2004), official controls must use a risk-based approach. When a food product that poses a risk to human and animal health is detected in a member state, an alert is distributed across the EU to allow other member state authorities to react. In 2002–14, for instance, the EU alert system contained 110 alerts based on official controls on the market and 152 notifications of rejections at the borders because of unauthorised colour additives or unauthorised use of colour additives in food products (RASFF Portal).
In the US, the USFDA is responsible for the control of food products except for meat and poultry. In addition to the five regional and 20 district USFDA offices across the country that handle inspections under its jurisdiction, the USFDA cooperates with 400 state agencies, which carry out most of the control (Johnson Citation2014; Sanchez Citation2015). Under the Bioterrorism Act and the FSMA the USFDA controls are applied to foreign facilities that import food to the US. Abroad, food inspection can be done by USFDA staff or recognised accreditation bodies and accredited third-party certification bodies (Johnson Citation2014; Sanchez Citation2015). For meat and poultry products that are under the jurisdiction of the USDA, FSIS inspectors control products for adulteration and misbranding, including use of colour additives where stipulated by the USFDA and USDA rules (Sanchez Citation2015). When a USFDA-regulated product is either defective or potentially harmful, the USFDA can suspend facility registration or issue restraining orders and injunctions, import refusals and alerts, recalls, seizures and administrative detentions (Sanchez Citation2015). For instance, in 2002–14 almost 16,000 import refusals were issued because the article appeared to contain an unsafe colour additive (Grundke & Moser Citation2016), this being the sixth most common reason for import refusals.
In addition, the USFDA is responsible for certifying all colour-additive batches that are subject to certification, including mainly the synthetic colour additives. This premarket certification system in the US is an additional safeguard measure to ensure the purity of the additives on the market. It is also an additional complication for EU exporters to the US, on top of the limited number of approved synthetic origin/artificial additives.
Besides the ability to carry out inspections, the enforcement and control depends on the ability to detect and quantify colour additives in a great variety of foods to assess regulatory compliance. In the EU especially, this concerns over 600 colour additive/food category combinations with set maximum limits, presenting a considerable challenge, in particular for quantification. For instance, lack of appropriately validated quantitative methods for Brown HT, which is a group III additive, can weaken enforcement of the EU’s combined maximum limits and introduce uncertainty in monitoring data.
In the US, the variety of combinations is less. For most colour additives, the compliance with GMP can be verified during inspections by observing the preparation and examining the used colour additives to detect delisted or uncertified colour additives. Only when severe misuse or violation is suspected would samples be taken or theoretical levels in the final product calculated.
Conclusions
Food colours are strictly regulated both in the EU and the US. Despite the different regulatory frameworks and underlying principles, the overall approach to ensuring food safety is similar, applying well-established risk-assessment procedures and risk-management measures. Nevertheless, some differences worthy of attention in the context of free movement of goods can be found in the details and implementation of regulations by the two administrations. Failure to comply gives rise to regulatory action for adulteration, misbranding or non-compliance, rejection at the border or removal or recall from the market.
For instance, the large number of maximum limits for additives and limitations for use in specified food categories only, set as a result of the EU precautionary principle, may deter US producers from exporting to the EU market. On the other hand, the majority of the standards of identity (e.g., cottage cheese) for over 300 foods in the US prohibit use of colour additives in certain foods. The US requirement for certifying colour batches prior to use, also applicable to foreign operators, is an additional complication and results in increased regulatory compliance costs. Further, using additives approved only in one of the jurisdictions implies that producers need to adjust their product composition specifically to the export market and the differences in specifications and in labelling requirements may result in allegations of adulteration or misbranding.
Some of these effects could be overcome by aligning regulation better with the internationally agreed JECFA specifications and safety assessments and their updates. While regional particularities, e.g., local dietary intake patterns, need to be considered, the safety margins may be able to accommodate a more harmonised approach by both administrations. Therefore, implementing the already existing policies on international harmonisation on both sides, collaboration in international fora and the uptake of international standards should be strongly encouraged. Designating primarily internationally harmonised analytical methods for official control would reduce compliance costs for exporters further.
Reassessing the (technical) need of additives that are less used, replaceable by other authorised substances or allowed for few foods only, and revoking their approval could also help aligning the regulations. Examples of such additives are some azo colours authorised in the EU only like Brown HT and Brilliant Black PN. The European trend of using colours from natural sources or colouring foods instead of artificial colours is supportive of such development (Scotter Citation2011; Stich Citation2016).
Issues in labelling of colour additives could be solved by requiring food colours to be named individually, instead of allowing additives of natural origin to be labelled as a group, reducing the burden for exporters and better meeting the information requirements of customers.
Finally, a careful comparison of the level of protection provided by the two sets of regulations based on monitoring and intake data, GMP inspections and certification requirements could be key to aligning the rules and to negotiating mutual recognition agreements.
Acknowledgements
S. Bratinova and T. Wenzl, of the European Commission’s Joint Research Centre, are thanked for providing information on analytical methods for food colours, as is E. Anklam for her contributions and valuable comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Barrows JN, Lipman AL, Bailey CJ. 2003. Color additives: FDA’s regulatory process and historical perspectives [Internet]. Food Saf Mag. [cited 2016 Sep 8]. Available from: http://www.foodsafetymagazine.com/magazine-archive1/octobernovember-2003/color-additives-fdas-regulatory-process-and-historical-perspectives/
- Baumgartner T, Uebe W. 2014. European union. In: Kirchsteiger-Meier E, Baumgartner T, editors. Global food legislation: an overview. Weinheim: Wiley; p. 11–135.
- Burrows AJD. 2009. Palette of our palates: A brief history of food coloring and its regulation. Compr Rev Food Sci and Food Saf. 8:394–408.
- Clydesdale FM. 1991. Color perception and food quality. J Food Qual. 14:61–74.
- [CFR] Code of Federal Regulations [Internet]. 2016. Washington: National Archives and Records Administration, Office of the Federal Register & Government Publications Office. Available from: http://www.ecfr.gov/cgi-bin/ECFR?page=browse
- Downham A, Collins P. 2000. Colouring our foods in the last and next millennium. Int J Food Sci and Technol. 35:5–22.
- Ederington J, Ruta M. 2016. Non-tariff measures and the world trading system. Washington (DC): World Bank Group, Policy Research Working Paper No. WPS 7661.
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). 2012. Scientific opinion. Guidance for submission for food additive evaluations. EFSA J. 10:2760.
- [EC] European Commission. 1997. Regulation (EC) No. 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients. Off J Eur Union. L43:1–6.
- [EC] European Commission. 2002. Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off J Eur Union. L31:1–24.
- [EC] European Commission. 2004. Regulation (EC) No. 882/2004 of the European Parliament and of the Council of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules. Off J Eur Union. L165:1–141.
- [EC] European Commission. 2006. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union. L364:5–24.
- [EC] European Commission. 2008a. Regulation (EC) No. 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings. Off J Eur Union. L354:1–6.
- [EC] European Commission. 2008b. Regulation (EC) No. 1332/2008 of the European Parliament and of the Council of 16 December 2008 on food enzymes and amending Council Directive 83/417/EEC, Council Regulation (EC) No. 1493/1999, Directive 2000/13/EC, Council Directive 2001/112/EC and Regulation (EC) No. 258/97. Off J Eur Union. L354:7–15.
- [EC] European Commission. 2008c. Regulation (EC) No. 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off J Eur Union. L354:16–33.
- [EC] European Commission. 2009. Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients (Recast). Off J Eur Union. L141:3–11.
- [EC] European Commission. 2011. Commission Regulation (EU) No. 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No. 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off J Eur Union. L295:1–177.
- [EC] European Commission. 2012. Commission Regulation (EU) No. 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No. 1333/2008 of the European Parliament and of the Council. Off J Eur Union. L83:1–295.
- [EC] European Commission. 2013. Guidance notes on the classification of food extracts with colouring properties. Version 1. Brussels: European Commission.
- [EC] European Commission. 2015. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No. 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No. 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No. 1852/2001. Off J Eur Union. L327:1–22.
- Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) [Internet]. 2010. Geneva: WHO. Available from: http://apps.who.int/food-additives-contaminants-jecfa-database/
- Food additive re-evaluations [Internet]. Parma: European Food Safety Authority; [ cited 2016 Sep 14]. Available from: http://www.efsa.europa.eu/en/topics/topic/additivesreevaluations
- Frick D. 2003. The coloration of food. Rev Prog Color. 33:15–32.
- Griffiths JC. 2005. Coloring foods & beverages. Food Technol Mag. 59:38–44.
- Grundke R, Moser C 2016. Hidden protectionism? Evidence from non-tariff barriers to trade in the United States. Salzburg: University of Salzburg, Working Papers in Economics and Finance No. 2016-02.
- Harp BP, Barrows JN. 2015. US regulation of color additives in foods. In: Scotter MJ, editor. Colour additives for foods and beverages. Cambridge (UK): Woodhead Publishing; p. 75–88.
- [IOM] Institute of Medicine. 2001. Dietary reference intakes for Vitamin A, Vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): The National Academies Press.
- Johnson J, Clydesdale FM. 1982. Perceived sweetness and redness in colored sucrose solutions. J Food Sci. 47:747–752.
- Johnson R. 2014. The federal food safety system: a primer. Washington (DC): Congressional Research Service, CRS Report 7-5700, RS22600.
- Kushner GJ, Gradison Hermida M, Eyink BD. 2014. United States of America. In: Kirchsteiger-Meier E, Baumgartner T, editors. Global food legislation: an overview. Weinheim: Wiley; p. 279–305.
- Magnuson B, Munro I, Abbot P, Baldwin N, Lopez-Garcia R, Ly K, McGirr L, Roberts A, Socolovsky S. 2013. Review of the regulation and safety assessment of food substances in various countries and jurisdictions. Food Addit Contamin Part A. 30:1147–1220.
- Matulka RA, Tardy A-L. 2014. Global focus: food colours vs colouring foods in the USA, EU, China, Russia and Brazil. Agro Food Ind Hi Tech. 25:7–9.
- McCann D, Barrett A, Cooper A, Crumpler D, Dalen L, Grimshaw K, Kitchin E, Lok K, Porteous L, Prince E, Sonuga-Barke E, Warner JOStevenson J. 2007. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. The Lancet. 370:1560-1567.
- Northcutt JK, Parisi MA. 2013. Major food laws and regulations. In: Curtis P, editor. Guide to US food laws and regulations. Chishester: Wiley Blackwell; p. 73–96.
- RASFF Portal [Internet]. Version 1.81. Brussels: European Commission. Available from: https://webgate.ec.europa.eu/rasff-window/portal/
- Reinhart A. 2014. Colouring foods versus food colours. Guidance notes on the classification of food extracts with colouring properties. Eur Food Feed Law Rev. 9:105–113.
- Sanchez MC. 2015. Food law and regulation for non-lawyers: a US perspective. Cham (CH): Springer International Publishing.
- Scotter MJ. 2011. Emerging and persistent issues with artificial food colours: natural colour additives as alternatives to synthetic colours in food and drink. Quality Assur Saf Crops Foods. 3:28–39.
- Scotter MJ, editor. 2015. Colour additives for foods and beverages. Cambridge (UK): Woodhead Publishing.
- Sheldon I. 2009. Food standards and international trade. In: Lusk J, Roosen J, Shogren J, editors. The Oxford handbook of the economics of food consumption and policy. Oxford: Oxford University Press; p. 393–415.
- Spence C. 2016. The psychological effects of food colors. In: Carle R, Schweiggert R, editors. Handbook on natural pigments in food and beverages: industrial applications for improving food color. Cambridge (UK): Woodhead Publishing; p. 29–58.
- Stich E. 2016. Food color and coloring food: quality, differentiation and regulatory requirements in the European Union and the United States. In: Carle R, Schweiggert R, editors. Handbook on natural pigments in food and beverages: industrial applications for improving food color. Cambridge (UK): Woodhead Publishing; p. 3–27.
- [USFDA] US Food and Drug Administration. 1998. Listing of color additives exempt from certification; canthaxanthin. Fed Regist. 63:14814–14817. Available from: https://www.gpo.gov/fdsys/pkg/FR-1998-03-27/pdf/98-8127.pdf
- [USFDA] US Food and Drug Administration. 2000. Guidance for industry and other stakeholders. Toxicological principles for the safety assessment of food ingredients. Redbook 2000 [Internet]. College Park (MD): US Food and Drug Administration. [ revised 2007 Jul]. Available from: http://www.fda.gov/downloads/Food/GuidanceRegulation/UCM222779.pdf
- [USFDA] US Food and Drug Administration. 2006a. Listing of color additives exempt from certification; food, drug, and cosmetic labeling: cochineal extract and carmine declaration. Fed Regist. 71:4839–4851. Available from: http://www.fda.gov/ohrms/dockets/98fr/e6-1104.htm
- [USFDA] US Food and Drug Administration. 2006b. Guidance forindustry: summary table of recommended toxicological testing for additives used in food [Internet]. College Park (MD): US Food and Drug Administration; [ updated 2016 Jul 1; cited 2016 Apr 25]. Available from: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm054658.htm
- [USFDA] US Food and Drug Administration. 2009. Guidance for industry: color additive petitions – FDA recommendations for submission of chemical and technological data on color additives for food, drugs, cosmetics, or medical devices [Internet]. 1997 Jan; revised 2009 Jul. College Park (MD): US Food and Drug Administration; [ updated 2016 Jul 1; cited 2016 Apr 25]. Available from: http://www.fda.gov/ForIndustry/ColorAdditives/GuidanceComplianceRegulatoryInformation/
- [USFDA] US Food and Drug Administration. 2011. Background document for the food advisory committee: certified color additives in food and possible association with attention deficit hyperactivity disorder in children. Silver Spring (MD): US Food and Drug Administration. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/FoodAdvisoryCommittee/ucm250901.htm
- [USFDA] US Food and Drug Administration. 2014. Guidance for industry: assessing the effects of significant manufacturing process changes, including emerging technologies, on the safety and regulatory status of food ingredients and food contact substances, including food ingredients that are color additives. June 2014 [Internet]. College Park (MD): US Food and Drug Administration. [ updated 2016 Jul 1; cited 2016 Apr 24]. Available from: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/IngredientsAdditivesGRASPackaging/
- Wilhelms J. 2014. Comparison of European and US food law regulations in the context of the Transatlantic Trade and Investment Partnership (TTIP) [Master’s thesis]. Münich: Grinn Publishing.