ABSTRACT
We performed a safety evaluation using the procedure devised by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) of the following four flavouring substances that belong to the class of ‘aliphatic primary alcohols, aldehydes, carboxylic acids, acetals, and esters containing additional oxygenated functional groups’ and are uniquely used in Japan: butyl butyrylacetate, ethyl 2-hydroxy-4-methylpentanoate, 3-hydroxyhexanoic acid and methyl hydroxyacetate. Although no genotoxicity study data were found in the published literature, none of the four substances had chemical structural alerts predicting genotoxicity. All four substances were categorised as class I by using Cramer’s classification. The estimated daily intake of each of the four substances was determined to be 0.007–2.9 μg/person/day by using the maximised survey-derived intake method and based on the annual production data in Japan in 2001, 2005 and 2010, and was determined to be 0.250–600.0 μg/person/day by using the single-portion exposure technique and based on average-use levels in standard portion sizes of flavoured foods. Both of these estimated daily intake ranges were below the threshold of toxicological concern for class I substances, which is 1800 μg/person/day. Although no information from in vitro and in vivo toxicity studies for the four substances was available, these substances were judged to raise no safety concerns at the current levels of intake.
Graphical Abstract
Introduction
Flavouring substances used in Japan
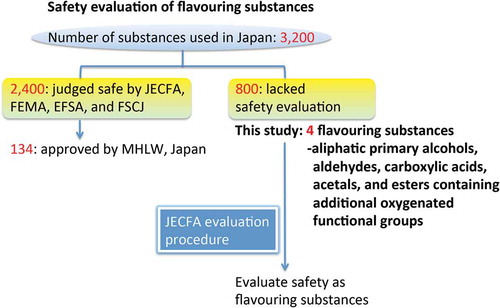
Various flavouring substances have been found or developed that improve the taste and aroma of food products but have no nutritional value. The wide variety of these substances enables the food industry to meet the needs of mimicking the ‘naturally occurring’ flavours of foods. According to an annual use survey by the Japan Flavour and Fragrance Materials Association (JFFMA), approximately 3200 flavouring substances have been used commercially in Japan (Someya Citation2012). Among them, 134 substances are currently approved as designated additives by the Ministry of Health, Labour and Welfare in Japan. The remaining substances have been classified into 18 designated chemical structural groups and approved for use as food additives under the compliance of the Food Sanitation Act of Japan; however, science-based safety evaluation of these substances is lacking.
Safety evaluation procedures
In 1996, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) adopted a novel safety evaluation procedure for examining flavouring substances (WHO Citation1996). Under this procedure, the safety of many flavouring substances can be efficiently evaluated utilising the safety evaluation data for substances that have similar chemical structures or metabolic pathways, even if the substances under investigation have no safety data. Evaluation protocols similar to the JECFA procedure are used in the United States by the Flavour and Extract Manufacturers Association of the United States (FEMA) Expert Panel and in the European Union by the European Food Safety Authority (EFSA). In addition, the results of the safety evaluations by JECFA, the FEMA Expert Panel and EFSA are now widely utilised in more than 70 countries (Konishi et al. Citation2014).
Of the approximately 3200 flavouring substances used in Japan, about 2400 have been judged safe under their intended conditions of use for flavouring by JECFA, the FEMA Expert Panel and/or EFSA. However, this leaves about 800 flavouring substances whose safety has not been evaluated by these organisations. Thus, in 2009, the JFFMA established a Safety Re-evaluation Committee composed of expert scientists in the fields of toxicology and food chemistry as well as representative members of the JFFMA to evaluate substances uniquely used in Japan based on the JECFA evaluation procedure (see Figure S1 in the supplemental data online), with the order of priority being determined by the annual volume of production of the substances.
In the present study, using the JECFA evaluation procedure, we evaluated the safety of the following four flavouring substances that are uniquely used in Japan and that based on the classification of flavouring substances by JECFA belong to the class ‘aliphatic primary alcohols, aldehydes, carboxylic acids, acetals, and esters containing additional oxygenated functional groups’: butyl butyrylacetate, ethyl 2-hydroxy-4-methylpentanoate, 3-hydroxyhexanoic acid and methyl hydroxyacetate. These four substances have unique and characteristic odours and are used with other flavouring substances to improve the flavour of processed foods, such as jams, jellies and soft drinks.
Collecting information for the evaluation procedure
Chemical information for four flavouring substances and their metabolites
The Chemical Abstracts Service (CAS) number, molecular weight and chemical structure of the four flavouring substances to be subjected to toxicity evaluation (butyl butyrylacetate, ethyl 2-hydroxy-4-methylpentanoate, 3-hydroxyhexanoic acid and methyl hydroxyacetate) are shown in . These substances have not been reported to occur naturally, and, thus, all four substances are expected to be ingested only in foods containing them as flavouring agents (JFFMA Citation2003, Citation2006, Citation2011). These four substances are metabolised to various hydrolysis or oxidation products. presents the chemical information of these produced substances to be subjected to toxicity evaluation.
Table 1. Chemical information on the four flavouring substances and their metabolites.
Predicting genotoxic potential using chemical structure and in silico models
The genotoxic potential of each substance was evaluated by analysing the biological potential of its chemical structure. While the JECFA decision-tree approach does not cover genotoxicity in its evaluation procedure (WHO Citation1996), the potential of genotoxicity is now evaluated before the steps of the decision tree are undertaken by incorporating structural alerts and other available information on genotoxicity of structurally related chemicals. In the present study, following the JECFA procedure, the structural alerts for chemical substructure groups were examined, as is generally done for chemicals lacking genotoxicity information (Ashby & Tennant Citation1988, Citation1991; Tennant et al. Citation1990). While genotoxicity study data were not available for any of the four substances, there were no structural alerts on their chemical structures. The genotoxic potential of the four substances was also examined using structure–activity relationship (SAR) analysis (Hansch Citation1969) with in silico prediction software that has been previously applied to the regulatory evaluation of flavouring substances (Cronin et al. Citation2003; Ono et al. Citation2012). Three SAR software programs with different algorithms were used for the analysis: DEREK (v. 10.0.2; Lhasa Ltd, Leeds, UK), MultiCASE (v. 1.90; Multicase Inc., Cleveland, OH, USA), and ADMEWORKS (v. 4.0; Fujitsu Kyusyu Systems Ltd, Fukuoka, Japan). All three programs indicated that all four substances had no genotoxic concern. The available genotoxicity information for structurally related substances was also referred to during our evaluation. Based on these results, we concluded that the JECFA evaluation procedure could be applied to these four flavouring substances.
Estimating daily intake of flavouring substances using SPET and MSDI methods
The development of the maximised survey-derived intake (MSDI) procedure was based on disappearance data from periodic surveys of ingredient manufacturers using the volume of ingredients produced during the survey year (Young et al. Citation2006). Because the production volume varied every survey year, the maximum production volume of each substance was selected from past surveyed data to avoid any underestimation of the intake. According to the annual production data in Japan from 2001, 2005 and 2010, the annual production volumes as flavourings of butyl butyrylacetate, ethyl 2-hydroxy-4-methylpentanoate, 3-hydroxyhexanoic acid and methyl hydroxyacetate were within the ranges of 0.2–12.0, 0.030–0.088, 4.8–11.0 and 0.021–0.030 kg year−1 respectively (JFFMA Citation2003, Citation2006, Citation2011). Based on these data, the daily intake of each of the four flavouring substances was calculated using the MSDI method. Daily intakes of butyl butyrylacetate, ethyl 2-hydroxy-4-methylpentanoate, 3-hydroxyhexanoic acid and methyl hydroxyacetate were estimated to be 2.9, 0.021, 2.4 and 0.007 μg/person/day respectively ().
Table 2. Estimated daily intake data of four flavouring substances uniquely used in Japan.
The daily intake of each substance was calculated using the single-portion exposure technique (SPET) (WHO Citation2008) and based on the estimated use (food category) and use-level survey conducted by JFFMA (unpublished information 2013–16) and single portion sizes of each food category from the predefined food categories in the Codex General Standard for Food Additives. As a result, the estimated daily intakes of butyl butyrylacetate, ethyl 2-hydroxy-4-methylpentanoate, 3-hydroxyhexanoic acid and methyl hydroxyacetate were calculated as 15.0, 0.900, 600.0 and 0.250 µg/person/day respectively ().
Because the daily intake volumes estimated by SPET were higher than those determined by the MSDI method, a safety evaluation was conducted using the values calculated by SPET.
Estimating absorption, metabolism and excretion
No information was available on the absorption, metabolism and excretion of the four substances. Therefore, their structural components or structurally related substances were examined for their metabolic fate. Three of four flavouring substances, butyl butyrylacetate, ethyl 2-hydroxy-4-methylpentanoate and methyl hydroxyacetate, are esters, whereas the remaining substance, 3-hydroxyhexanoic acid, is a fatty acid. Hydrolysis of esters mainly occurs in the intestinal tract, blood and liver and is catalysed by carboxylesterases or esterases in most tissues (Heymann Citation1980; Anders Citation1989). Acetyl esters are the preferred substrates of carboxylesterases (Heymann Citation1980), and the presence of a second oxygenated functional group has little effect on the hydrolysis of esters. Evidence for hydrolysis of esters has come from various types of experiments. For example, incubation of aqueous methyl 2-oxo-3-methylpentanoate with a 2% pancreatin solution (pH 7.5) results in virtually complete hydrolysis (> 98%) within 80 min (Leegwater & van Straten Citation1979). Dibutyl sebacate is also hydrolysed in vitro in a 10% crude pancreatic lipase solution (Smith Citation1953). The 14C-tributylacetyl citrate administered by gavage to male Sprague–Dawley rats at a single dose of 70 mg kg−1 body weight (bw) is rapidly absorbed (half-life, 1 h) and partially hydrolysed in the blood. More than 87% of the radiolabelled tributylacetyl citrate is eliminated within 24 h of dosing. At least nine urinary metabolites representing 59–70% of the dose have been detected. Five metabolites have been identified as the partially hydrolysed mono-, di- and trialkylesters of citric acid. Three metabolites representing 25–36% of the dose have been identified in the faeces. Approximately 2% is eliminated as 14CO2 (Hiser et al. Citation1992). With regard to the metabolic fate of fatty acids, they are metabolised after ingestion in organs such as the liver, heart and skeletal muscle to fatty acyl adenylate by fatty acid-coenzyme A ligase, and fatty acyl adenylate reacts with free coenzyme A to give fatty acyl-coenzyme A. The fatty acyl-coenzyme A enters the mitochondrial matrix via the carnitine shuttle to be metabolised to acetyl-coenzyme A by β-oxidation and is completely oxidised to CO2 and water (Houten & Wanders Citation2010).
Butyl butyrylacetate is expected to be hydrolysed to butyrylacetic acid and butanol. Butyrylacetic acid and 3-hydroxyhexanoic acid can be oxidised in vivo to acetoacetic acid, which is endogenous in humans and is formed from the condensation of two acetyl-coenzyme A units in the fatty acid pathway. Acetoacetic acid is released from the liver into the bloodstream and transported to peripheral tissues where it is converted to acetyl-coenzyme A and is completely metabolised (Voet & Voet Citation1990). When endogenous levels are high, acetoacetic acid yields acetone and carbon dioxide through non-enzymatic decarboxylation of endogenous β-keto acids (Voet & Voet Citation1990). Ethyl 2-hydroxy-4-methylpentanoate is an α-keto ester, is hydrolysed to 2-hydroxy-4-methylpentanoic acid and is expected to be metabolised in the same manner as endogenous α-keto esters formed from oxidative deamination of leucine in vivo (Voet & Voet Citation1990). Methyl hydroxyacetate is expected to be hydrolysed to glycolic acid (hydroxyacetic acid), with most of the glycolic acid excreted directly in the urine. A small proportion of the glycolic acid is metabolised to glyoxylic acid and oxalic acid (DuPont Citation1963).
Safety evaluation of the four substances using the JECFA evaluation procedure
Decision-tree approach
Details of the JECFA evaluation procedure are described in a JECFA evaluation report (WHO Citation1996). A summary of the decision-tree approach for this procedure is shown in Figures S1 and S2 in the supplemental data online.
Step 1
In the first step, each flavouring substance was classified into one of three chemical structure classes (I, II or III) using the Cramer decision tree, as shown in Figure S2 in the supplemental data online (Cramer et al. Citation1978). Substances in class I have simple chemical structures with known efficient metabolic pathways that suggest low oral toxicity. Substances in class II are less innocuous than class I, but do not contain structural features suggestive of those that produce toxicity, such as substances in class III. Substances in class III have a chemical structure that may cause significant toxicity.
Based on the results of the Cramer decision-tree analysis (See Figure S2 in the supplemental data online) (Cramer et al. Citation1978), all four substances were assigned to structural class I ().
Table 3. Safety evaluation of four flavouring substances using the JECFA evaluation procedure: Step 1, Cramer decision-tree approach.
Step 2
In the second step, the metabolic fate of each substance was predicted by its chemical structure and applied using a decision-tree approach consisting of two branches (see Figure S1, Step A3, in the supplemental data online). Based on estimates of the metabolism of the structurally related substances or their metabolites determined by the JECFA procedure, all four substances were predicted to go to ‘A’ side of the decision tree.
Step A3
In this step, the estimated daily intake of each substance was compared with the threshold level of human exposure based on Cramer’s three structural classes (Cramer et al. Citation1978), with estimated threshold levels of 1800, 540 and 90 µg/person/day for structural classes I, II, and III respectively (Munro et al. Citation1996, Citation1999).
As mentioned above, the estimated daily intakes of the four flavouring substances were calculated using SPET because these values were higher than those calculated using the MSDI method. The daily intake of the four class I substances were 0.007–600.0 μg/person/day (), which was below the threshold of toxicological concern applied to structural class I substances, which is 1800 μg/person/day.
Table 4. Safety evaluation of four flavouring substances using the JECFA evaluation procedure: Steps 2 and A3.
Considering the combined intake from their use as flavouring agents
Because the JECFA considers evaluation of the combined intake to be unnecessary when the highest MSDI value is less than 20 μg/person/day (WHO Citation2011), the evaluation examining the combined intake of all four substances was omitted here ().
Assessing toxicological information for the metabolites and for structurally related substances of the four flavouring substances
Because no in vitro or in vivo toxicity data were available for any of the four substances, data from their metabolites and structurally related substances reported by the JECFA (WHO Citation2000, Citation2011) were examined ( and see Table S1 in the supplemental data online). The four flavouring substances had many structurally related substances, and so data were selected based on those showing close structural relation.
Sub-acute toxicity/carcinogenicity
Regarding toxicity studies of the metabolites and structurally related substances of the four flavouring substances, the results on ethyl acetoacetate (Cook et al. Citation1992), levulinic acid (Tischer et al. Citation1942), glycolic acid (USEPA Citation2000) and propylene glycol (Morris et al. Citation1942; Weil et al. Citation1971; Gaunt et al. Citation1972) have been reported in rats and are shown in .
Table 5. Sub-acute toxicity and carcinogenicity data summary for the metabolites and structurally related substances of four flavouring substances.
In one study, Sprague–Dawley rats, 16 males and 16 females/group, were administered ethyl acetoacetate via their diet at 100, 300 or 1000 mg kg−1 bw day−1 for 28 or 29 days. Although proteinaceous aggregates in the urinary bladder of males and calcium deposits in the kidneys of females were found in the group ingesting 1000 mg kg−1 bw day−1, renal function was unimpaired in the treated male and female animals. Thus, the no observed adverse effect level (NOAEL) of ethyl acetoacetate was determined to be > 1000 mg kg−1 bw day−1 (Cook et al. Citation1992).
In another study, albino rats (three animals/group) were administered levulinic acid at 0, 1000 or 2000 mg kg−1 bw day−1 via their diet for 16 days. The treated animals showed no indication of toxicity at these doses, and the NOAEL of levulinic acid was determined to be > 2000 mg kg−1 bw day−1 (Tischer et al. Citation1942).
In a study of rats administered glycolic acid at 0, 150, 300 or 600 mg kg−1 bw day−1 for 90 days, decreases in bw and bw gain were observed at 300 mg kg−1 bw day−1. Thus, the NOAEL of glycolic acid was determined to be 150 mg kg−1 bw day−1 (USEPA Citation2000).
In a study in which rats received propylene glycol in their diet at a dose of 0, 310, 630, 1300 or 2500 mg kg−1 bw day−1 for 2 years, no treatment-related adverse effects on bw gain or organ weight or on haematological, urinary or clinical chemical endpoints were found. Thus, the NOAEL of propylene glycol in that study was determined to be > 2500 mg kg−1 bw day−1 (Gaunt et al. Citation1972). In a study in which rats were given propylene glycol as 0%, 2.45% or 4.90% of their dietary intake (equivalent to doses of 0, 900 and 1800 mg kg−1 bw day−1 respectively) for 2 years, no treatment-related adverse effects were found on body growth or histological changes in the examined organs and tissues. Thus, the NOAEL of propylene glycol in that study was determined to be > 1800 mg kg−1 bw day−1 (Morris et al. Citation1942). In a study in which male and female beagle dogs received propylene glycol in the diet at a dose of 0, 2000 or 5000 mg kg−1 bw day−1 for 2 years, increased erythrocyte destruction was found at a dose of 5000 mg kg−1. However, no significant treatment-related effects on haematological, clinical chemical or urinary endpoints, or on gross or histological appearance were found at a dose of 2000 mg kg−1. Thus, the NOAEL of propylene glycol in that study was determined to be 2000 mg kg−1 bw day−1 (Weil et al. Citation1971).
Genotoxicity
Several in vivo and in vitro genotoxicity studies have examined either the metabolites or the structurally related substances of four flavouring substances, namely lactic acid, ethyl acetoacetate, 1-hydroxypropan-2-one, L-malic acid, pyruvic acid, prop-1-ene-1,2,3-tricarboxylic acid, ethyl lactate, ethyl pyruvate, diethyl malonate, butyl-O-butyryllactate, ethyl 3-hydroxybutyrate and methyl acetoacetate. The results of these studies are summarised in .
Table 6. In vivo and in vitro genotoxicity data summary for the metabolites and structurally related substances of four flavouring substances.
Lactic acid displayed a negative result in the reverse mutation assay (Ames test) (Al-Ani & Al-Lami Citation1988) but a positive result in the chromosome aberration test. However, this positive result was found using non-physiological (low) pH levels (Morita et al. Citation1990). Lactic acid is an endogenous substance and is well estimated to be metabolised into endogenous products.
Propylene glycol showed positive results only in minor number of in vitro assays, i.e., two chromosomal aberration tests, one sister chromatid exchange assay and one host-mediated mutation test, and negative results in most in vitro assays and all in vivo assays (Weir Citation1974; McCann & Ames Citation1976; Clark et al. Citation1979; Stolzenberg & Hine Citation1979; Florin et al. Citation1980; Sasaki et al. Citation1980; Vargova et al. Citation1980; Kawachi et al. Citation1981; Haworth et al. Citation1983; Ishidate et al. Citation1984; Hayashi et al. Citation1988).
Ethyl acetoacetate, an ester of β-keto acid, displayed negative results in both the chromosomal aberration and reverse mutation assays (Ishidate et al. Citation1984; Fujita & Sasaki Citation1987). But ethyl acetoacetate also produced negative results in two studies testing DNA repair using rec assays (Oda et al. Citation1978; Kuroda et al. Citation1984). However, the results of another DNA repair test were positive, and in that same study a gene mutation assay also provided a positive result (Yoo Citation1986). Ethyl acetoacetate includes a centre of Michael reactivity, which is recognised by the JECFA as one of the structural alerts for genotoxicity. However, the JECFA concluded that this substance is readily metabolised in vivo (WHO Citation2000). Thus, esters of β-keto or β-hydroxy acids are hydrolysed to acetoacetic acid or its β-hydroxy or aldehyde precursor. The latter two can be oxidised in vivo to acetoacetic acid, which is an endogenous product in humans and is formed from the condensation of two acetyl-coenzyme A units in the fatty acid pathway. It is released from the liver into the bloodstream and transported to peripheral tissues, where it is converted to acetyl-coenzyme A and is completely metabolised. When endogenous levels are high, β-keto acids may undergo non-enzymatic decarboxylation, which for acetoacetic acid yields acetone and carbon dioxide (Voet & Voet Citation1990).
Positive results were found using several reverse mutation assays for 1-hydroxypropan-2-one (Yamaguchi Citation1982; Garst et al. Citation1983; Yamaguchi & Nakagawa Citation1983; Marnett et al. Citation1985). However, 1-hydroxypropan-2-one is an endogenous metabolite of acetone that is endogenously formed from the degradation of body fat and fatty acids, and it occurs at low concentrations in the blood of healthy humans without acetone exposure (Wang et al. Citation1994). In this major metabolic pathway, the majority of the acetone in blood would be oxidised to 1-hydroxypropan-2-one (WHO Citation1998). Taking these data together, we determined that the daily intake of 1-hydroxypropan-2-one as flavouring substances does not affect human health.
Pyruvic acid, L-malic acid, prop-1-ene-1,2,3-tricarboxylic acid, ethyl lactate, ethyl pyruvate, diethyl malonate, butyl-O-butyryllactate, ethyl 3-hydroxybutyrate and methyl acetoacetate showed negative results with respect to mutagenicity (Bjeldanes & Chew Citation1979; Florin et al. Citation1980; Yamaguchi Citation1982; Wild et al. Citation1983; Andersen & Jensen Citation1984; Shimizu et al. Citation1985; Al-Ani & Al-Lami Citation1988; Clary et al. Citation1998; Zeiger & Margolin Citation2000).
Based on the results of sub-acute toxicity/carcinogenicity and genotoxicity studies, we concluded that the metabolites of the four flavouring substances as well as substances structurally related to these flavouring substances do not pose a safety concern when used at low levels of intake or as flavouring substances.
Evaluation of substances based on the JECFA procedure
This review presented the key scientific data relevant to a safety evaluation using the JECFA procedure for the safety evaluation of flavouring agents of four flavouring substances that are uniquely used in Japan and belong to the JECFA classification of ‘aliphatic primary alcohols, aldehydes, carboxylic acids, acetals, and esters containing additional oxygenated functional groups’. Regarding predicting genotoxicity, none of the four substances had structural alerts, and none showed genotoxic potential based on in silico software analyses. Although no metabolism data were available for any of these four substances, it is well estimated that esters and fatty acids in this group can be metabolised to endogenous products. All four substances were categorised as class I using the Cramer decision tree (Cramer et al. Citation1978). The estimated daily intake for each substance was within 0.007 and 2.9 µg/person/day as determined by the MSDI method and within 0.250 and 600.0 by SPET, which was below the toxicological threshold of concern for this class (1800 μg/person/day). Thus, it was concluded that none of the four substances raised safety concerns when used for flavouring foods at the current estimated intake levels.
Conclusions
In conclusion, the four flavouring substances, butyl butyrylacetate, ethyl 2-hydroxy-4-methylpentanoate, 3-hydroxyhexanoic acid and methyl hydroxyacetate, pose no health risk to humans when used to flavour foods, and the intake of each substance at the present levels of use as a food-flavouring ingredient is safe.
Supplemental Data
Download PDF (1.4 MB)Acknowledgments
The authors thank Professor Takeshi Yamazaki, Department of Food and Health Sciences, Jissen Women’s University, for his expert advice in preparing the manuscript.
Disclosure statement
Kenji Saito, Fumiko Sekiya, Shim-mo Hayashi, Yoshiharu Mirokuji, Hiroyuki Okamura and Shinpei Maruyama are employed by flavour manufacturers whose product lines include flavouring substances. The views and opinions expressed in this article are those of the authors and not necessarily those of their respective employers. Yasuko Hasegawa-Baba, Atsushi Ono, Madoka Nakajima, Masakuni Degawa, Shogo Ozawa, Makoto Shibutani and Tamio Maitani declare that no conflicts of interest exist.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Al-Ani FY, Al-Lami SK. 1988. Absence of mutagenic activity of acidity regulators in the Ames Salmonella/microsome test. Mutat Res. 206:467–470.
- Anders MW. 1989. Biotransformation and bioactivation of xenobiotics by the kidney. In: Hutson DH, Caldwell J, Paulson GD, editors. Intermediary xenobiotic metabolism in animals. New York (NY): Taylor and Francis Inc; p. 81–97.
- Andersen PH, Jensen NJ. 1984. Mutagenic investigation of flavourings: dimethyl succinate, ethyl pyruvate and aconitic acid are negative in the Salmonella/mammalian-microsome test. Food Addit Contam. 1:283–288.
- Ashby J, Tennant RW. 1988. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the US NCI/NTP. Mutat Res. 204:17–115.
- Ashby J, Tennant RW. 1991. Definitive relationships among chemical structure, carcinogenicity and mutagenicity for 301 chemicals tested by the US NTP. Mutat Res. 257:229–306.
- Bjeldanes LF, Chew H. 1979. Mutagenicity of 1,2-dicarbonyl compounds: maltol, kojic acid, diacetyl and related substances. Mutat Res. 67:367–371.
- Clark CR, Marshall TC, Merickel BS, Sanchez A, Brownstein DG, Hobbs CH. 1979. Toxicological assessment of heat transfer fluids proposed for use in solar energy applications. Toxicol Appl Pharmacol. 51:529–535.
- Clary JJ, Feron VJ, Velthuijsen JA. 1998. Safety assessment of lactate esters. Regul Toxicol Pharmacol. 27:88–97.
- Cook WM, Purchase R, Ford GP, Creasy DM, Brantom PG, Gangolli SD. 1992. A 28-day feeding study with ethyl acetoacetate in rats. Food Chem Toxicol. 30:567–573.
- Cramer GM, Ford RA, Hall RL. 1978. Estimation of toxic hazard-decision tree approach. Food Cosmet Toxicol. 16:255–276.
- Cronin M, Walker JD, Jaworska JS, Comber M, Watts CD, Worth AP. 2003. Use of QSARs in international decision-making frameworks to predict health effects of chemical substances. Environ Health Perspect. 111:1391–1401.
- DuPont. 1963. Hydroxyacetic acid 70% technical: final report. Biological activity following single oral administration to rats. Newark (DE), Haskell Laboratory. (Report No. MR-680-4, HL-20-63).
- [FAO] Food and Agriculture Organization of the United Nations. 2011. Codex Stan 192-1995. General Standard For Food Additives. Adopted in 1995; [accessed 2017 May 1]. http://www.fao.org/gsfaonline/docs/CXS_192e.pdf.
- Florin I, Rutberg L, Curvall M, Enzell CR. 1980. Screening of tabacco smoke constituents for mutagenicity using the Ames’ test. Toxicology. 15:219–232.
- Fujita H, Sasaki M. 1987. Mutagenicity test of food additives with Salmonella typhimurium TA97 and TA102 (II). Ann Rep Tokyo Metr Res Lab P H. 38:423–430. Japanese.
- Garst J, Stapleton P, Johnston J. 1983. Mutagenicity of alpha-hydroxy ketones may involve superoxide anion radical. In: Greenwald RA, Cohen G, editors. Oxy radicals and their scavenger systems. Vol. II. Cellular and medical aspects. Amsterdam: Elsevier Science Publishing; p. 125–130.
- Gaunt IF, Carpanini FM, Grasso P, Lansdown AB. 1972. Long-term toxicity of propylene glycol in rats. Food Cosmet Toxicol. 10:151–162.
- Hansch C. 1969. Quantitative approach to biochemical structure-activity relationships. Acc Chem Res. 2:232–239.
- Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E. 1983. Salmonella mutagenicity test results for 250 chemicals. Environ Mutag. 5:3–49.
- Hayashi M, Kishi M, Sofuni T, Ishidate M Jr. 1988. Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food Chem Toxicol. 26:487–500.
- Heymann E. 1980. Carboxylesterases and amidases. In: Jacoby WB, editor. Enzymatic basis of detoxication. 2nd ed. New York (NY): Academic Press; p. 291–323.
- Hiser MF, Markley BJ, Reitz RH, Nieusma JL. 1992. Metabolism and disposition of acetyl tributyl citrate in male Sprague-Dawley rats. In: Abstracts of the 31st annual meeting. toxicologist. An official publication of the society of toxicology; 1992 Feb 23–27; Seattle. USA: Seattle Convention Center.
- Houten SM, Wanders RJ. 2010. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis. 33:469–477.
- Ishidate M Jr, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M, Matsu A. 1984. Primary mutagenicity screening of food additives currently used in Japan. Food Chem Toxicol. 22:623–636.
- [JFFMA] Japan Flavour and Fragrance Materials Association. 2003. Usage survey of food-flavouring ingredients in Japan. Health and Labour Sciences Research Grant, 2003. Japanese; [accessed 2017 May 1]. http://mhlw-grants.niph.go.jp
- [JFFMA] Japan Flavour and Fragrance Materials Association. 2006. Survey for production, usage and intake of food-flavouring ingredients in Japan. Health and Labour Sciences Research Grant, 2006. Japanese; [accessed 2017 May 1]. http://mhlw-grants.niph.go.jp
- [JFFMA] Japan Flavour and Fragrance Materials Association. 2011. Survey for usage and intake of food-flavouring ingredient in Japan. Health and Labour Sciences Research Grant, 2011. Japanese; [accessed 2017 May 1]. http://mhlw-grants.niph.go.jp
- Kawachi T, Komatsu T, Kada T, Ishidate M, Sasaki M, Sugiyama T, Tazima Y. 1981. Results of recent studies on the relevance of various short-term screening tests in Japan. In: Williams GM, Kroes R, Waaijers HW, van der Poll KW, editors. Workshop on the predictive value of short-term screening tests in carcinogenicity evaluation (applied methods in oncology, Vol. 3). Amsterdam: Elsevier/North Holland Biomedical Press; p. 253–267.
- Konishi Y, Hayashi S-M, Fukushima S. 2014. Regulatory forum opinion piece. Supporting the need for international harmonization of safety assessments for food-flavouring substances. Toxicol Pathol. 42:949–953.
- Kuroda K, Tanaka S, Yu YS, Ishibashi T. 1984. Rec-assay of food additives. Nippon Kosyu Eisei Zasshi. 31:277–281. Japanese.
- Leegwater DC, van Straten S. 1979. In vitro digestion test on methyl-2-keto-3-methyl valerate. Unpublished report from Central Institute for Nutrition and Food Research. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States.
- Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, Ames BN. 1985. Naturally occurring carbonyl compounds are mutagens Salmonella tester strain TA104. Mutat Res. 148:25–34.
- McCann J, Ames BN. 1976. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proc Natl Acad Sci USA. 73:950–954.
- Morita T, Takeda K, Okumura K. 1990. Evaluation of clastogenicity of formic acid, acetic acid and lactic acid on cultured mammalian cells. Mutat Res. 240:195–202.
- Morris HJ, Nelson AA, Calvery HO. 1942. Observations on the chronic toxicities of propylene glycol, ethylene glycol, diethylene glycol, ethylene glycol mono-ethyl-ether and diethylene glycol mono-ethyl-ether. J Pharmacol Exp Ther. 74:266–273.
- Munro IC, Ford RA, Kennepohl E, Sprenger JG. 1996. Correlation of structural class with no-observed-effect levels: a proposal for establishing a threshold of concern. Food Chem Toxicol. 34:829–867.
- Munro IC, Kennepohl E, Kroes R. 1999. A procedure for the safety evaluation of flavouring substances. Food Chem Toxicol. 37:207–232.
- Oda Y, Hamono Y, Inoue K, Yamamoto H, Niihara T, Kunita N. 1978. Mutagenicity of food flavours in bacteria. Shokuhin Eisei Hen. 9:177–181. Japanese.
- Ono A, Takahashi M, Hirose A, Kamata E, Kawamura T, Yamazaki T, Sato K, Yamada M, Fukumoto T, Okamura H, et al. 2012. Validation of the (Q)SAR combination approach for mutagenicity prediction of flavor chemicals. Food Chem Toxicol. 50:1538–1546.
- Sasaki M, Sugimura K, Yoshida MA, Abe S. 1980. Cytogenetic effects of 60 chemicals on cultured human and Chinese hamster cells. Kromosomo II. 20:574–584.
- Shimizu H, Suzuki Y, Takemura N, Goto S, Matsushita H. 1985. The results of microbial mutation test for forty-three industrial chemicals. Jpn J Ind Health. 27:400–419.
- Smith CC. 1953. Toxicity of butyl stearate, dibutyl sebacate, dibutyl phthalate, and methoxyethyl oleate. Arch Ind Hyg Occup Med. 7:310–318.
- Someya T. 2012. The safety evaluation procedure for flavoring substances in Japan: overview and a future prospect. Foods Food Ingredients J Jpn. 217:151–156. Japanese.
- Stolzenberg SJ, Hine CH. 1979. Mutagenicity of halogenated and oxygenated three-carbon compounds. J Toxicol Environ Health. 5:1149–1158.
- Tennant RW, Spalding J, Stasiewicz S, Ashby J. 1990. Prediction of the outcome of rodent carcinogenicity bioassays currently being conducted on 44 chemicals by the national toxicology program. Mutagenesis. 5:3–14.
- Tischer RG, Fellers CR, Doyle BJ. 1942. The non-toxicity of levulinic acid. J Am Pharm Assoc. 31:217–220.
- [USEPA] United States Environmental Protection Agency. 2000. Toxicity review of subchronic oral toxicity study [870.3100 (82-1)] with immunotoxicity [870.7800 (85-5)], neurotoxicity [870.6200 (83-1, 82-7, 81-8)] and one-generation reproduction in the rat with glycolic acid; [accessed 2017 May 1]. https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-000101_19-Oct-00_003.pdf.
- Vargova M, Polakova H, Podstavkova S, Siskova A, Dolan L, Vicek D, Miadokova E. 1980. The mutagenic effect of the new insecticide and acaricide pyridathion. Mutat Res. 78:353–360.
- Voet D, Voet JG. 1990. Biochemistry. New York (NY): Wiley. Chapter 19, Citric acid cycle. Chapter 23, Lipid metabolism. Chapter 24, Amino acid metabolism; p. 506–527, 632–634, 690–691.
- Wang G, Maranelli G, Perbellini L, Raineri E, Brugnone F. 1994. Blood acetone concentration in “normal people” and in exposed workers 16 h after the end of the workshift. Int Arch Occup Environ Health. 65:285–289.
- Weil CS, Woodside MD, Smyth HF Jr, Carpenter CP. 1971. Results of feeding propylene glycol in the diet to dogs for two years. Food Cosmet Toxicol. 9:479–490.
- Weir RJ. 1974. Mutagenic evaluation of compound FDA 71-56, propylene glycol. Unpublished report from Litton Bionetics Inc. Submitted to WHO by Flavor and Extract Manufacturers Association of the United States.
- [WHO] World Health Organisation. 1996. A procedure for the safety evaluation of flavouring substances. Forty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series No. 35, Annex 5; [accessed 2017 May 1]. http://www.inchem.org/documents/jecfa/jecmono/v35je21.htm.
- [WHO] World Health Organisation. 1998. Acetone. Environmental Health Criteria (EHC) 207. International Programme on Chemical Safety (IPCS); [accessed 2017 May 1]. http://www.inchem.org/documents/ehc/ehc/ehc207.htm.
- [WHO] World Health Organisation. 2000. Safety evaluation of certain food additives and contaminants. The fifty-third meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series 44; [accessed 2017 May 1]. http://www.inchem.org/documents/jecfa/jecmono/v44jec10.htm.
- [WHO] World Health Organisation. 2008. Safety evaluation of certain food additives and contaminants. The sixty-eighth meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series 59; [accessed 2017 May 1]. http://whqlibdoc.who.int/publications/2008/9789241660594_eng.pdf.
- [WHO] World Health Organisation. 2011. Evaluation of certain food additives. Seventy-third report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No. 960; p. 1–8; [accessed 2017 May 1]. http://whqlibdoc.who.int/trs/WHO_TRS_960_eng.pdf?ua=1.
- Wild D, King M-T, Gocke E, Eckhardt K. 1983. Study of artificial flavouring substances for mutagenicity in the salmonella/microsome, Basc and micronucleus tests. Food Chem Toxicol. 21:707–719.
- Yamaguchi T. 1982. Mutagenicity of trioses and methyl glyoxal on Salmonella typhimurium. Agric Biol Chem. 46:849–851.
- Yamaguchi T, Nakagawa K. 1983. Mutagenicity of and formation of oxygen radicals by trioses and glyoxal derivatives. Agric Biol Chem. 47:2461–2465.
- Yoo YS. 1986. Mutagenic and antimutagenic activities of flavoring agents used in foodstuffs. J Osaka City Med Center. 34:267–288.
- Young KW, Danielewska-Nikiel B, Munro IC. 2006. An evaluation of the maximized survey-derived daily intake (MSDI) as a practical method to estimate intake of flavouring substances. Food Chem Toxicol. 44:1849–1867.
- Zeiger E, Margolin BH. 2000. The proportions of mutagens among chemicals in commerce. Reg Toxicol Pharmacol. 32:219–225.
