ABSTRACT
Various studies have shown that bee-collected pollen sold as nutritional supplements may contain toxic pyrrolizidine alkaloids (PAs) and, thus, pose a potential health risk for consumers. The level of contamination may vary according to its geographical and botanical origin. Here, the PA content of pollen produced in Switzerland was studied and 32 commercially available bee-collected pollen supplements produced between 2010 and 2014 were analysed. In addition, at what time period bees collect PA-containing pollen was investigated. Hence, this study looked into the occurrence of PAs in pollen samples collected daily during two-to-three consecutive seasons. Furthermore, the PA spectrum in pollen was compared to the spectrum found in flower heads of PA-plants to unambiguously identify plants responsible for PA contamination of pollen. The PA concentration of commercial and daily collected pollen was determined by target analysis using an HPLC-MS/MS system, allowing the detection of 18 different PAs and PA N-oxides found in the genera Echium, Eupatorium and Senecio, while the comparison of the PA spectrum in pollen and flower heads was performed by LC-HR-MS, allowing the detection of all PA types in a sample, including saturated, non-carcinogenic PAs. Of the commercially available pollen, 31% contained PAs with a mean concentration of 319 ng/g, mainly Echium- and Eupatorium-type PAs, while the PA concentrations were below the limit of quantitation (LOQ) in 69% of the pollen samples. Bees collected pollen containing Echium-type PAs mainly in June and July, while they gathered pollen containing Eupatorium-type PAs from mid-July to August. Senecio-type PAs appeared from June to September. Comparison of the PA array in pollen and plants identified E. vulgare and E. cannabinum as the main plants responsible for PA contamination of Swiss bee-collected pollen, and to a lesser extent also identified plants belonging to the genus Senecio.
Introduction
Honey bees collect pollen from the anthers of the flowers, add nectar or honeydew from their honey sac to form pollen loads, and bring the loads back to their hives as the major protein source in their diet. Beekeepers collect pollen loads with traps that they install at the entrance of the beehives. Bee-collected pollen is sold as a dietary supplement and marketed as a frozen or dried product. It is rich in flavonoids and polyphenols and is well known for its antioxidative properties (Campos et al. Citation2010). Several studies have reported that bee-collected pollen, like many other food products originating from plants, can contain toxic pyrrolizidine alkaloids (PAs) (Beales et al. Citation2007; Boppré et al. Citation2008; Kempf et al., Citation2010a, Citation2010b; Dübecke et al. Citation2011; Mulder et al. Citation2015; EFSA, Citation2016) and can, therefore, pose a risk for human consumption. PAs are mainly produced by plant species belonging to the families Asteraceae (tribes Senecioneae and Eupatorieae), Fabaceae (genus Crotolaria) and Boraginaceae (all genera) (Hartmann and Witte Citation1995; EFSA, Citation2011). Several hundred 1,2-unsaturated PAs have been identified and these compounds are toxic to animals and humans, causing liver and lung damage or cancer (Culvenor et al. Citation1976; Fu et al. Citation2001; Chou et al. Citation2003; Molyneux et al. Citation2011; Wiedenfeld Citation2011). Dietary exposure of the European population to PAs in plant-derived food has recently been evaluated by the EFSA (European Food Safety Authority, Citation2016). So far, no legal limits for PAs in food have been established, either in the European Union or in Switzerland. However, the UK Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) (2008), the German Federal Institute for Risk Assessment (BfR) (2011, 2016) and the EFSA (2011) recommend ingesting no more than 0.007 µg of 1,2-unsaturated PAs per day and kg bodyweight (BW). As a nutritional supplement, 5–10 grams (1–2 tablespoons) of bee-collected pollen is usually consumed per day. Therefore, the maximum PA concentration of bee-collected pollen should not exceed 84 µg/kg when calculated for a person of 60 kg BW consuming 5 g of pollen daily. These limits would be even lower if intake from other sources were also taken into account. Commercially available bee-collected pollen contains PAs with a higher frequency and often at substantially higher concentrations than does honey (Kempf et al. Citation2010a, Citation2010b; Dübecke et al. Citation2011; Edgar et al. Citation2011). In a survey of 55 commercial pollen products, 31% of the pollen samples contained PAs at an average PA concentration of 5170 µg/kg (Kempf et al. Citation2010a), while in another study on 119 bee-collected pollen samples, 60% of the samples were PA-positive (median = 192 µg/kg; average = 1846 µg/kg) (Dübecke et al. Citation2011).
The highest concentrations of PAs, up to 37,855 µg/kg, were measured in bee-collected pollen samples of unknown origin (Dübecke et al. Citation2011) and PA concentrations up to 16,350 µg/kg have been reported for a pollen sample from Spain containing mainly Echium pollen (Kempf et al. Citation2010a). These values are several 100-times above the recommendations of the BfR, which means that such pollen products are critical for food safety and may not be suitable for human consumption.
The type and abundance of PA-producing plants that grow in a specific region depends on several factors, but mainly on the climatic conditions, geology and land use. Furthermore, environmental conditions affect PA concentration in plants (Selmar and Kleinwächter Citation2013). Hence, the PA concentrations in pollen products from different biogeographical origins can be very variable. Pollen products so far studied often originated from southern European countries or regions outside Europe and information about pollen products produced in continental and alpine biogeographical regions of Europe is sparse. Switzerland has a very diverse flora and many plant species are found that have their main distribution areas within the surrounding countries. First, we aimed to study the PA content of bee-collected pollen produced commercially in Switzerland. This allowed us to determine if commercial pollen samples exceeded the BfR recommendations with respect of PA contamination. Second, we investigated how PAs vary during the pollen production season. Our special focus was on the time period when bees collect such pollen in order to advise beekeepers on how to avoid PA contamination in bee products. Third, we unambiguously identified the plant species responsible for PA contamination of bee-collected pollen by comparing the PA spectrum in pollen samples to the spectrum found in flower heads.
Materials and methods
Chemical reagents
Standards used for LC-MS/MS were echimidine and lycopsamine from Cfm Oskar Tropitzsch (Marktredwitz, Germany), heliotrine from Latoxan (Valence, France), seneciphylline obtained from Carl Roth (Karlsruhe, Germany), senkirkine from Phytolab (Vestenbergsgreuth, Germany), and monocrotaline, retrorsine and senecionine from Sigma-Aldrich (Steinheim, Germany). The solvents used for LC-MS/MS were of analytical grade for residue analysis. Standards used for LC-HR-MS were echimide, a mixture of 7-acetylintermedine and 7-acetyllycopsamine from PlantaAnalytica (Danbury, US) and seneciphylline from Cfm Oskar Tropitzsch (Marktredwitz, Germany). Lycopsamine, monocrotaline, senecionine, heliotrine, senkirkine, erucifoline, erucifoline-N-oxide and lasiocarpine were from PhytoLab GmbH (Vestenbergsgreuth, Germany). Retrorsine and intermedine were obtained from Sigma-Aldrich GmbH (Buchs, Switzerland), while echivulgarine was isolated in-house from leaves and inflorescences of E. vulgare and PA N-oxides were converted to tertiary bases (Lucchetti et al. Citationforthcoming). Additionally, the N-oxides of these PAs, except of erucifoline, echivulgarine and senkirkine, were prepared in-house in analogy to Cymerman Craig and Purushothaman (Citation1970), but trichloromethane was replaced with dichloromethane. This resulted in a total of 26 reference alkaloids for identification purposes. D2-Retronecine bis-butyrate was custom-synthesised (Marti and Meyer Citation2010).
Swiss bee-collected pollen samples on the market
In total, 32 commercial samples were obtained. Of these, 31 samples were from members of the Swiss Association of Beekeepers producing bee-collected pollen (n = 7 in 2010, n = 14 in 2011 and n = 10 in 2014) and one additional sample was collected in 2011 from the Jura region. The association’s samples originated from various regions of Switzerland north of the Alps, such as locations in the cantons St. Gallen (SG; n = 2), Appenzell (AI; n = 1), Basel (BL; n = 3, same beekeeper in 2010, 2011 and 2014), Aargau (AG; n = 9), Lucerne (LU; n = 3), Obwalden (OW; n = 1) and Zurich (ZH; n = 2, same beekeeper in 2010 and 2011) and from the mountain areas in the northern part of the Alps, such as Grisons (GR; n = 3, same beekeeper in 2010, 2011 and 2014) and Bern (BE; n = 7). All sites belong to the continental or alpine biogeographical region, but in Switzerland small-structured landscapes are dominating.
Observation sites
The first site was near Basel located in the northern part of Switzerland, north of the Alps, close to the border between Switzerland and France (hereafter Basel) and belonging mainly to the continental biogeographical region. This site was chosen, based on the fact that commercial bee-collected pollen produced in 2010 at this location contained PAs at concentrations above the BfR recommendation. During the bee seasons 2010–2014, pollen for the retail market was produced from April to July, with the pollen batches collected over the entire season being mixed. The second site was in the Verzasca valley in the southern part of Switzerland (hereafter Verzasca), south of the Alps, belonging to the alpine biogeographical region. This site was chosen since honey produced in 2011 at this location contained PAs (Echium- and Eupatorium-type) above the BfR recommendation (Kast et al. Citation2014). At the Verzasca site, two beekeepers participated in the project (hereafter Verzasca 1 and Verzasca 2). Their apiaries were approximately 400 metres apart (beeline distance). The beekeepers produced honey, but did not produce any bee-collected pollen for the retail market.
Daily collected pollen samples from the observation sites
In Basel, the pollen traps were integrated in the beehive systems, while at the Verzasca observation sites the traps were attached in front of the entrances of the beehives. If weather conditions permitted, beekeepers collected pollen 1 day per week from two-to-four beehives during the months of April to September. Samples were collected for 24 hours; the traps were emptied at the end of the day and the samples immediately frozen at −20°C. Samples were dried at 30°C for 48 hours and subsequently lyophilised. In total, 108 daily collected pollen samples were from Basel (n = 49 in 2012 and n = 59 in 2013) and 132 samples from Verzasca (Verzasca 1: n = 12 in 2012, n = 24 in 2013, n = 36 in 2014 and Verzasca 2: n = 26 in 2013, n = 34 in 2014). Daily collected pollen samples from the observation sites were analysed individually by LC-MS/MS for their PA content (see below) and averages were calculated for each collection day.
Seven daily collected pollen samples (three from Basel collected in June 2012 (sample 2) and July 2013 (samples 1, 3), respectively, and three from Verzasca 1 collected in July (samples 4 and 5) and September 2013 (sample 6), one from Verzasca 2 collected in August 2013 (sample 7) were further analysed by LC-HR-MS (see below) for comparison of their PA pattern with the pattern found in PA-containing flowers.
Collection of flowers
General reference samples of PA-containing plants were collected at different locations in Switzerland, including our observation sites. For species of the Boraginaceae family, usually corollas including stamens were sampled; in the case of very small blossoms, whole blossoms were sampled. For species of the Asteraceae family, floral heads were sampled. The samples were dried in a hot air oven, first for 2 hours at 50°C followed by 48 hours at 40°C.
The collection consisted of six samples of Eupatorium cannabinum L., 16 samples of Echium vulgare L. and 15 samples of the genus Senecio. Senecio species comprised samples of S. alpinus (L.) Scop. (n = 2), S. doronicum (L.) L. (n = 2), S. erucifolius L. (n = 2), S. jacobaea L. (n = 3), S. ovatus (P. Gaertn. et al.) Willd. (n = 2), S. viscosus L. (n = 2) and S. vulgaris L. (n = 2). Sample in this context means one collection at a specific location. Each collection contains the parts of at least five individual plants. The species names used are those listed in Info flora (Citation2017). To achieve higher confidence that no other PA-containing plant could confound the identification of the above species, all or at least nearly all PA-containing plants occurring in Switzerland were also sampled and analysed. These were the following species: Adenostyles alliariae (Gouan) A. Kern., Adenostyles glabra (Mill.) DC., Anchusa officinals L., Borago officinalis L., Brunnera macrophylla (Adams) I. M. Johnst., Cynoglossum germanicum Jacq., Homogyne alpina (L.) Cass., Myosotis alpestris F. W. Schmidt, Myosotis arvensis Hill, Myosotis scorpioides L., Myosotis sylvatica sensu Welten & Sutter, Omphalodes verna Moench, Petasites albus (L.) Gaertn., Petasites hybridus (L.) P. Gaertn. et al., Pulmonaria australis (Murr) W. Sauer, Pulmonaria obscura Dumort., Pulmonaria officinalis L., Symphytum officinale L., Symphytum x uplandicum Nyman and Tussilago farfara L. For each species, at least two samples were collected and identified by O. and B. Zoller. This approach was chosen, because most of the main PA of these species are described in the literature, but are not commercially available. It was the only feasible method to get reference signals of these missing PA. Although unequivocal assignment of a structure to a certain signal was often not possible, the assignment of a respective PA signal found in pollen samples to a certain plant species would have been possible.
Preparation of pollen and quantification of PAs with LC-MS/MS analysis
Pollen samples were prepared as described by Dübecke et al. (Citation2011). Pollen (10 g) was homogenised with an electric mill (Retsch Grindomix GM 200) for 20 seconds at 10,000 rpm. Pollen (1 g), 100 ng of heliotrine and 20 ml of 0.05 M sulphuric acid were vigorously shaken for 60 minutes. Samples were centrifuged (10 minutes at 2200 g), the solid residue was extracted once more with 10 ml of 0.05 M sulphuric acid and the supernatants were combined and filtered (2 µm mesh) overnight, followed by solid-phase extraction (SPE; HF Bond Elut LRC (500 mg/3 ml) SCX, Varian, Palo Alto, CA), according to Kempf et al. (Citation2008). After washing with 3 ml methanol, the cartridges were conditioned with 9 ml 0.05 M sulphuric acid. The samples were entirely applied onto the cartridges and subsequently eluted into 8 ml glass vials using 7.5 ml of ammoniated methanol. The eluates were dried at 40°C in a stream of ambient air, reconstituted in deionised water and filtered using a 0.45 µm syringe filter.
LC-MS/MS analysis was performed using an HTC PAL autosampler from CTC Analytics AG, a Shimadzu LC system with a Thermo Hypersil Gold C18 column (50 × 2.1 mm, 1.9 µm particle size) and an Applied Biosystems API4000 QTRAP triple quadrupole mass spectrometer. Concentrations were corrected against the recovery of the internal standard. Echimidine, senkirkine, heliotrine, monocrotaline, lycopsamine, retrorsine, senecionine and seneciphylline were quantified using external calibration with reference substances. The limit of quantitation (LOQ) was 1 ng/g for echimidine and senkirkine, 2 ng/g for heliotrine and 3 ng/g for monocrotaline, lycopsamine, retrorsine, senecionine and seneciphylline. A number of other PAs and PA N-oxides, such as echimidine N-oxide, acetyl-echimidine (and N-oxide), echiumine (and N-oxide), acetyl-echiumine N-oxide, echiuplatine (and N-oxide) and echivulgarine (and N-oxide) and lycopsamine N-oxide and isomers were indirectly quantified by using the calibration of echimidine and lycopsamine, respectively, and assuming the same response factors (Dübecke et al. Citation2011). These results have to be regarded as approximate results. The total PA concentration was calculated as the sum of 18 different PAs. PAs were grouped as Echium-type PAs (echimidine (and N-oxide), acetyl-echimidine (and N-oxide), echiumine (and N-oxide), acetyl-echiumine N-oxide, echiuplatine (and N-oxide) and echivulgarine (and N-oxide)), Eupatorium-type PAs (lycopsamine, lycopsamine isomers (and N-oxides)) and Senecio-type PAs (senecionine, retrorsine, seneciphylline, senkirkine).
Preparation of pollen and flowers and identification of PAs by LC-HR-MS analysis
D2-Retronecine bis-butyrate (c = 100 ng/g) was added as an internal standard to each sample (1 g pollen or 0.1–0.2 g plant material), as well as 20 ml of 0.05 M aqueous sulphuric acid solution. The samples were sonicated for 15 minutes, followed by centrifugation for 10 minutes at 4500 rpm and subsequent vacuum filtration through a 10 µm sieve plate. The cleanup was achieved by automated extraction on an Aspec GX-274 (Gilson, Mettmenstetten, Switzerland). Sample solution (15 ml) was passed through a solid-phase extraction SCX cartridge (SampliQ, Agilent, MSP Kofel, Zollikofen, Switzerland), previously conditioned with 3 ml methanol and 3 ml 0.05 M aqueous sulphuric acid solution. Consecutively, the column was washed with 3 ml aqueous sulphuric acid solution, 3 ml water and 5 ml methanol. The analytes were eluted by 8.5 ml ammonia in methanol (1.5%, v/v) and evaporated under nitrogen at 40°C and dissolved to an end volume of 0.5 ml in methanol/water/formic acid (50:50:0.1%, v/v/v).
The chromatographic system consisted of a Shimadzu Prominence binary gradient system (Shimadzu, Reinach, Switzerland), degasser, autosampler and column heater. Chromatographic separations were performed on a Kromasil C18 (125 × 2 mm, 3.5 µm particle size) analytical column (Macherey-Nagel, Buchs, Switzerland). The flow rate was 0.2 ml/min and the column temperature was maintained at 30°C. A gradient programme was used consisting of water and 10% methanol, both with 0.1% formic acid (v/v), ramped linearly over the course of 6 minutes to 40% methanol, then within 6 minutes to 90% methanol, held for 5 minutes at this condition, then re-equilibrated for 4 minutes at 90% water. Injection volume was 5 µl, resulting in 500 pg of standards on column.
Mass spectrometric detection was achieved by a Bruker maXis 4G Qq-TOF mass spectrometer (Bruker, Bremen, Germany), equipped with an electrospray ionisation interface operated in positive ion mode. Source parameters were plate offset 500 V, capillary voltage 4.5 kV, dry temperature 200°C, nebulizer gas pressure 150 kPa and nitrogen dry gas flow rate 8 l/min. Internal calibration was achieved by incorporating sodium formate solution as a calibrant at the beginning of every run with a loop injection. For instrument control, data acquisition and processing, Compass 1.5 and TargetAnalysis 1.3 were used.
Identification of compounds was accomplished by high resolution mass determination (deviation ≤ 2 mDa allowed), MS/MS fragmentation with nitrogen as collision gas at 35 eV ion energy and comparing the retention time to the standard compound if available.
For estimation purposes, a concentration adapted one-point calibration was applied and values were related to the internal standard. The absolute recovery was alkaloid- and matrix dependent and ranged from 30–80%. The applied internal standard calibration ensures for an adequate correction. Under unfavourable matrix conditions, however, a worst-case under-estimation error of a factor of 2–5 is possible, as shown from comparisons of internal standard vs spiking experiments. The limit of detection was 1 ng/g, the limit of quantitation was 3 ng/g.
Results
About a third of the analysed commercially-available Swiss pollen samples contained PAs. The average PA concentration of the positive samples was 319 ng/g and the average concentration of PAs in all pollen samples was 100 ng/g (). The positive samples contained alkaloids that are present, for example, in E. vulgare (hereafter Echium-type PAs; six pollen samples), E. cannabinum (hereafter Eupatorium-type PAs; four pollen samples) or Senecio spp. (hereafter Senecio-type PAs; three pollen samples) (Supplementary Table S1). In seven out of 32 samples (22%), the PA concentrations were above the BfR recommendations. The highest PA concentration in a commercial sample was 1185 ng/g, all Eupatorium-type PAs, measured in a pollen sample from the Bernese alpine region. Three positive samples with PAs above the BfR recommendations contained Echium-type PAs at concentrations between 155 ng/g and 586 ng/g, while two additional samples surpassing the recommendations contained Eupatorium- and Echium-type and one sample Echium- and Senecio-type PAs (Supplementary Table S1). We also followed, over several consecutive years (2010–2014), commercial pollen produced at our observation site in Basel. The PA content varied between the production years, ranging from below the LOQ to 586 ng/g ().
Table 1. PA concentrations in Swiss pollen on the retail market.
Table 2. Pollen for the retail market produced at our observation site in Basel.
In order to investigate the time at which bees collect pollen containing PAs, once a week from May to September we gathered daily collected pollen from one-to-four bee colonies located at our observation sites ( and ). In Basel, E. vulgare bloomed during the month of June until mid-July and E. cannabinum flowered starting mid-July. In 2012, bees collected pollen containing Echium-type PAs during the flowering period of E. vulgare, up to a maximum average concentration of 1519 ng/g in pollen collected on 18 June 2012 (; 2012). Although E. vulgare bloomed to a similar extent again in 2013, pollen collected in 2013 contained much lower average concentrations of Echium-type PAs (; 2013), probably due to the fact that other, more attractive plants were flowering at the same time as E. vulgare. Later in the season, mainly from mid-July and in August, bees collected pollen that contained a different spectrum of PAs, alkaloids that are present for example in E. cannabinum. A maximum average Eupatorium-type PA concentration of 1311 ng/g was observed in pollen collected on 31 July 2012 (). Bees also collected pollen that contained alkaloids, which are present, for example, in various Senecio plant species (hereafter Senecio-type PAs) up to a maximum average concentration of 568 ng/g in pollen collected on 6 September 2012 ().
Figure 1. Appearance of PAs in daily collected pollen samples from Basel. In total, 49 pollen samples were collected in 2012 and 59 samples in 2013. Numbers express the number of pollen samples collected from different hives at a specific day from which the average PA concentration is calculated. Blue: Echium-type PAs; dark red: Eupatorium-type PAs; yellow: Senecio-type PAs.
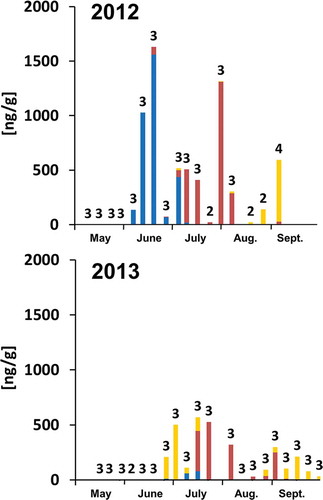
Figure 2. Appearance of PAs in daily collected pollen samples from Verzasca. In total, 12 pollen samples were collected in 2012, 24 samples in 2013 and 36 samples in 2014 in Verzasca 1. Twenty-six samples were collected in 2013 and 34 samples in 2014 in Verzasca 2. Numbers express the number of pollen samples collected from different hives at a specific day from which the average PA concentrations were calculated. Blue: Echium-type PAs; dark red: Eupatorium-type PAs; yellow: Senecio-type PAs.
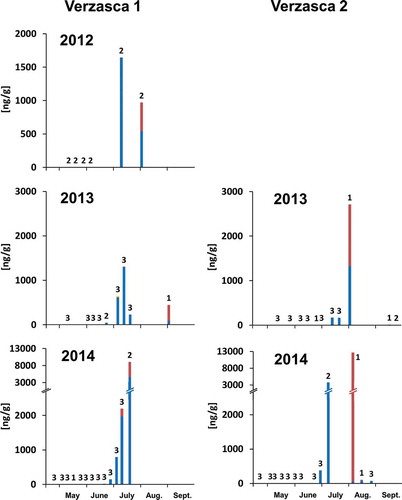
Pollen samples collected in 2012 in Verzasca contained Echium- and Eupatorium-type PAs at maximal average concentrations up to 1644 ng/g and 429 ng/g, respectively (, 2012), concentrations that were similar to PA concentrations in samples collected in the same year in Basel (, 2012). In the following years, pollen samples were collected at two apiaries (Verzasca 1 and 2). E. vulgare bloomed mainly in June and July and many fewer plants were still blooming later on in the summer. Bees collected pollen with Echium-type PAs mainly during July and early August (, 2013, 2014), probably when other attractive pollen sources, such as chestnut, were not available anymore. E. cannabinum flowered in July and August. During this time, bees collected pollen containing Eupatorium-type PAs (, 2013, 2014). Some pollen samples contained low amounts of Senecio-type PAs (maximum average concentration of 47 ng/g, barely visible in , Verzasca 1 2013). Maximal average concentrations of Echium- (4584 ng/g; Verzasca 1, 2014) and Eupatorium-type PAs (12,609 ng/g; Verzasca 2, 2014) in daily collected pollen samples were higher in 2014 than in previous years.
In order to unambiguously identify Echium vulgare, Eupatorium cannabinum and Senecio spp. as the plants responsible for PA contamination of daily bee-collected pollen from our observation sites, we performed an in-depth analysis of PA types, including non-carcinogenic PAs (samples one to three from Basel, samples four to seven from Verzasca) and compared their PA spectrum to the pattern found in flower heads (). Echium-type PAs that are present in flower heads of Echium vulgare were found in pollen samples one to seven. Echivulgarine N-oxide, echimidine N-oxide, vulgarine N-oxide, acetyl-echimidine N-oxide and/or acetyl-vulgarine N-oxide and the corresponding tertiary bases were the most prominent Echium-type PAs found in pollen and flower heads. The PA spectrum of pollen corresponded to the typical pattern found in E. vulgare (Betteridge et al. Citation2005; Boppré et al. Citation2005; Cairns et al. Citation2015; Lucchetti et al. Citation2016), therefore strongly suggesting that these alkaloids originate from E. vulgare. PAs in pollen samples 1, 6 and 7 containing intermedine N-oxide, lycopsamine N-oxide, their corresponding tertiary amine isomers and echinatine, corresponded to the typical Eupatorium pattern (Edgar et al. Citation1992; Roeder Citation1995; Boppré et al. Citation2008) found in flower heads of E. cannabinum, supporting the conclusion that E. cannabinum is the plant source of these alkaloids found in pollen. Senecio flower heads were collected from different Senecio species. The major alkaloids in S. ovatus are fully saturated (Boppré et al. Citation2008) and, hence, non-carcinogenic. The non-carcinogenic sarracine N-oxide present in pollen sample no 7 from the Verzasca site is typically found in S. ovatus. Senecionine N-oxide present in pollen sample no 3 from Basel, however, is a characteristic alkaloid for S. jacobaea (Hartmann and Toppel Citation1987; Boppré et al. Citation2008). The chemical fingerprinting identified E. vulgare, E. cannabinum and Senecio spp. as important plants responsible for PA contamination of pollen harvested in moderate climatic European regions such as Switzerland.
Table 3. PA pattern in daily collected pollen samples compared to the PA pattern in flower heads of E. vulgare, E. cannabinum and Senecio spp. Daily collected pollen samples were from Basel (sample 2: June 2012; samples 1 and 3: July 2013), from Verzasca 1 (samples 4 and 5: July 2013; sample 6: September 2013) and from Verzasca 2 (sample 7: August 2013). Median and concentration range are listed for flower heads of Echium vulgare L. (n = 16), Eupatorium cannabinum L. (n = 6) and Senecio species (n = 15; comprising S. alpinus (L.) Scop., S. doronicum (L.) L., S. erucifolius L., S. jacobaea L., S. ovatus (P. Gaertn. et al.) Willd., S. viscosus L. and S. vulgaris L.). blue: Echium-type PAs; dark red: Eupatorium-type PAs; yellow: Senecio-type PAs. (1) putative identification; (2) PAs present in E. vulgare and E. cannabinum; nd: not detected (< LOD); Ac, acetylated; O, N-oxide.
Discussion
About a third of the commercially-sold Swiss pollen samples tested in this study contained PAs, while two thirds of the samples tested negative for PAs. The PA concentrations were above the BfR recommendations in 22% of the pollen samples. Echium-type PAs were present in 19% of the commercial samples, while 13% of the samples contained Eupatorium-type PAs and 9% contained low concentrations of Senecio-type PAs. Eupatorium-type PAs can easily be avoided in pollen if beekeepers stop pollen collection before E. cannabinum flowers in July; however, it is more challenging to avoid PAs originating from E. vulgare, since this plant blooms during the main pollen collection season. The PA pattern in our daily collected pollen samples corresponded to the pattern found in flowers of E. vulgare, E. cannabinum or Senecio spp., thus identifying these plant species as mainly responsible for the contamination of Swiss bee-collected pollen.
The COT (Committee on toxicity of chemicals in food, consumer products and the environment Citation2008) concluded that PA doses up to 0.007 µg/kg BW per day would be unlikely to be of concern for carcinogenicity. The BfR and the EFSA (Citation2016) came to similar conclusions in their respective evaluations and, therefore, PA concentrations below 0.007 µg/kg BW per day are of low concern from a public health point of view. Only 1,2-unsaturated PAs are of relevance and undergo metabolic activation to reactive pyrrolic species responsible for hepatotoxicity, genotoxicity and carcinogenicity. The mentioned tolerable daily intake is based on the carcinogenicity of lasiocarpine in an animal study (NTP Citation1978). There are indications that certain PAs, especially the monoesters, might be less carcinogenic than lasiocarpine. However, the available data is insufficient to allow the derivation of toxicological equivalency factors. On the other hand, there is also a certain possibility that some of the PAs, especially some of the macrocyclic diesters, might be more carcinogenic than lasiocarpine. For non-cancer effects the COT (Citation2008) derived a tolerable daily intake of 0.1 µg/kg BW from an animal study with riddelliine. For a person of 60 kg BW, this corresponds to a daily intake of 6 µg.
Based on the BfR recommendation (0.007 µg/kg BW per day), the maximum PA concentration of pollen should not exceed 84 µg/kg, calculated for a person of 60 kg BW consuming 5 g of pollen (one tablespoon) per day. For non-cancer effects, PA concentrations in pollen up to 1200 µg/kg might be within the limit (for 60 kg BW and 5 g pollen/day). The commercial samples with PA concentrations higher than 84 µg/kg contained Echium-type PA up to 586 µg/kg (586 ng/g) or Eupatorium-type PA up to 1185 µg/kg (1185 ng/g). Assuming an intake of 5 g per day and a BW of 60 kg, even the samples with the highest concentrations pose no risk for non-cancer effects. In contrast, the regular intake of such supplements may increase the risk of cancer to some extent; in other words: one cannot be sure of being on the safe side. At the moment, it is impossible to quantify the extra risk with reasonable certainty and, if feasible, any extra risk should be avoided. In conclusion, Swiss bee-collected pollen usually does not pose a risk to consumers. However, efforts should be made to reduce PA contamination as much as possible.
In our study, the average PA concentration of 319 µg/kg (319 ng/g) in commercially-available PA-positive pollen samples was substantially lower than PA levels presented in previous studies reporting average PA concentrations of 5170 µg/kg or 1846 µg/kg in PA-positive pollen samples (Kempf et al. Citation2010a; Dübecke et al. Citation2011) and were in the same range as reported for pollen-based food supplements by Mulder et al. (Citation2015) (12 pollen products were analysed; in 11 of these pollen products PA were detectable; the arithmetic mean over all 12 pollen products was 576 µg/kg). Hence, PA-containing plants may be less abundant in Central European countries belonging to the continental and alpine biogeographical regions as compared to the Mediterranean or tropical biogeographical regions and, therefore, the risk of high PA levels may be distinctly lower for bee products produced in Central Europe. In Switzerland only E. vulgare is present, whereas in the Mediterranean region several Echium species are abundant and also E. vulgare is probably more abundant than in Central Europe. Nevertheless, beekeepers are advised to minimise PA contamination in pollen, especially since other types of food, such as tea or honey, may additionally contribute to the daily intake of PAs.
We performed chemical mapping using LC-HR-MS analysis to correlate PA types in pollen with the corresponding flower species. Liquid chromatography coupled with high resolution mass spectrometry (LC-HR-MS) profiles both non-saturated and saturated PAs in a non-targeted manner (Avula et al. Citation2015) and, hence, is an ideal method for chemical fingerprinting. Since there are more PAs than commercial standards available, each chromatographic peak of sample extract has to be analysed for possible contaminants. For this purpose, the applied LC-HR-MS instrument, with its capability to run full scan mass spectra with a high sampling rate at high resolution, is a good choice. We performed unselective MS/MS experiments to confirm specific and common PA fragments. By this means, additional PAs were unambiguously resolved, although their trivial names remain to be determined. In our samples, this additional contribution to the whole PA content is of importance (see , putatively identified PAs) and may not be neglected for toxicological and/or taxonomical interpretation.
Previous studies reported that Echium-type PAs are the most frequently encountered PAs in European honeys (Kempf et al. Citation2010b; Dübecke et al. Citation2011; Kast et al. Citation2014; Martinello et al. Citation2014; Huybrechts and Callebaut Citation2015; Lucatello et al. Citation2016). This is in line with our current study on Swiss pollen. In terms of frequency and average concentrations, Echium-type PAs were the most important PA types in commercial pollen samples. Our study on daily collected pollen samples confirmed that bees collect pollen from Echium plants during its blooming period. However, it remains difficult to predict to what extent. If more attractive pollen sources are available, bees may not collect Echium pollen, even if Echium plants are abundant around the hives. E. vulgare is sometimes intentionally planted by beekeepers as a foraging plant and is also included in some seed mixtures for flower strips or field margins to increase abundance and species richness of insects. Since E. vulgare blooms in June during the main pollen collection, we advise beekeepers to refrain from planting this plant around their apiaries. In the alpine region and climatic areas north of the Alps, E. cannabinum blooms from mid-July onwards, a time period when pollen sources are less abundant and, hence, most beekeepers usually discontinue pollen collection. This is an ideal way to avoid contamination of pollen with Eupatorium-type alkaloids. In the southern part of Switzerland this may also help to reduce Echium-type alkaloids in pollen, since in Verzasca bees collected Echium-type PAs mainly in July, although E. vulgare started to bloom earlier. Senecio spp. are not only problematic for PAs in bee products such as honey (e.g. Neumann and Huckauf Citation2016) or pollen, but are also toxic for horses and cattle. Currently, we observe only minor contamination of Swiss bee products with Senecio-type PAs (for honey: Kast et al. Citation2014; for pollen: this study). However, for a few years Senecio inaequidens, an invasive plant species, has been spreading in Europe (Vacchiano et al. Citation2013; Blanchet et al. Citation2015). Hence, eradication programmes should step up efforts to reduce the spread of all types of Senecio plants.
Our study shows that E. vulgare, E. cannabinum and different species of Senecio are the most important plants responsible for the contamination of pollen products in Switzerland and possibly also in Central Europe. To ensure the production of pollen products with low PA-contamination, the harvest time-window is of major importance and the bee keepers have to be advised correspondingly.
Kast_Table_S2_updated2.xlsx
Download MS Excel (20.4 KB)Table_S1.docx
Download MS Word (14.5 KB)Acknowledgments
We would like to thank the beekeepers from Basel and the Verzasca valley for providing us with bee-collected pollen samples. We thank Barbara Zoller for the assistance in the collection and identification of plant specimens.
Matteo Lucchetti has been financially supported in his doctorate studies by a grant from Agroscope, the Swiss Federal Research Institute for Agriculture and Food Sciences.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
References
- Avula B, Sagi S, Wang YH, Zweigenbaum J, Wang M, Khan IA. 2015. Characterization and screening of pyrrolizidine alkaloids and N-oxides from botanicals and dietary supplements using UHPLC-high resolution mass spectrometry. Food Chem. 178:136–148.
- Beales K, Betteridge K, Boppré M, Cao Y, Colegate SM, Edgar JA. 2007. Hepatotoxic pyrrolizidine alkaloids and their N-oxides in honey and pollen. In: Panter KE, Wierenga TL, Pfister JA, editors. Poisonous plants: global research and solutions. Wallingford: CABI; p. 94–100.
- Betteridge K, Cao Y, Colegate SM. 2005. Improved method for extraction and LC-MS analysis of pyrrolizidine alkaloids and their N-oxides in honey: application to Echium vulgare honeys. J Agric Food Chem. 53(6):1894–1902.
- BfR (German Federal Institute for Risk Assessment). 2011. Stellungnahme Nr. 038/2011 des BfR vom 11. August 2011. http://www.bfr.bund.de/cm/343/analytik-undtoxizitaet-von-pyrrolizidinalkaloiden.pdf
- BfR (German Federal Institute for Risk Assessment). 2016. Stellungnahme Nr. 030/2016 des BfR vom 28. September 2016. http://www.bfr.bund.de/cm/343/pyrrolizidinalkaloide-gehalte-in-lebensmitteln-sollen-nach-wie-vor-so-weit-wie-moeglich-gesenkt-werden.pdf
- Blanchet É, Penone C, Maurel N, Billot C, Rivallan R, Risterucci AM, Maurice S, Justy F, Machon N, Noël F. 2015. Multivariate analysis of polyploid data reveals the role of railways in the spread of the invasive South African Ragwort (Senecio inaequidens). Conserv Genet. 16:523–533.
- Boppré M, Colegate SM, Edgar JA. 2005. Pyrrolizidine alkaloids of Echium vulgare honey found in pure pollen. J Agric Food Chem. 53:594–600.
- Boppré M, Colegate SM, Edgar JA, Fischer OW. 2008. Hepatotoxic pyrrolizidine alkaloids in pollen and drying-related implications for commercial processing of bee pollen. J Agric Food Chem. 56:5662–5672.
- Cairns E, Hashmi MA, Singh AJ, Eakins G, Lein M, Keyzers R. 2015. Structure of echivulgarine, a pyrrolizidine alkaloid isolated from the pollen of Echium vulgare. J Agric Food Chem. 63(33):7421–7427.
- Campos MGR, Frigerio C, Lopes J, Bogdanov S. 2010. What is the future of Bee-Pollen? J ApiProd ApiMed Sci. 2(4):131–144.
- Chou MW, Wang YP, Yan J, Yang YC, Beger RD, Williams LD, Doerge DR, Fu PP. 2003. Riddelliine N-oxide is a phytochemical and mammalian metabolite with genotoxic activity that is comparable to the parent pyrrolizidine alkaloid riddelliine. Toxicol Lett. 145(3):239–247.
- COT (Committee on toxicity of chemicals in food, consumer products and the environment). 2008. COT statement on pyrrolizidine alkaloids in food. https://cot.food.gov.uk/sites/default/files/cot/cotstatementpa200806.pdf
- Culvenor CCJ, Edgar JA, Jago MV, Outteridge A, Peterson JE, Smith LW. 1976. Hepato- and pneumotoxicity of pyrrolizidine alkaloids and derivatives in relation to molecular structure. Chem-Biol Interact. 12(3–4):299–324.
- Cymerman Craig J, Purushothaman KK. 1970. Improved preparation of tertiary amine N-oxides. J Org Chem. 35(5):1721–1722.
- Dübecke A, Beckh G, Lüllmann C. 2011. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit Contam Part A. 28(3):348–358.
- Edgar JA, Colegate SM, Boppré M, Molyneux RJ. 2011. Pyrrolizidine alkaloids in food: a spectrum of potential health consequences. Food Addit Contam Part A. 28(3):308–324.
- Edgar JA, Lin HJ, Kumana CR, Ng MMT. 1992. Pyrrolizidine alkaloid composition of three Chinese medicinal herbs, Eupatorium cannabinum, E. japonicum and Crotalaria assamica. Am J Chin Med. 20(3–4):281–288.
- EFSA (European Food Safety Authority). 2011. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA panel on contaminants in the food chain (CONTAM). Efsa J. 9(11):2406. doi:10.2903/j.efsa.2011.2406
- EFSA (European Food Safety Authority). 2016. Dietary exposure assessment to pyrrolizidine alkaloids in the European population. Efsa J. 14(8):4572. doi:10.2903/j.efsa.2016.4572
- Fu PP, Chou MW, Xia Q, Yang YC, Yan J, Doerge DR, Chan PC. 2001. Genotoxic pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides – mechanisms leading to DNA adduct formation and tumorigenicity. J Environ Sci Health Part C. 19(2):353–385.
- Hartmann T, Toppel G. 1987. Senecionine N-oxide, the primary product of pyrrolizidine alkaloid biosynthesis in root cultures of Senecio vulgaris. Phytochemistry. 26(6):1639–1643.
- Hartmann T, Witte L. 1995. Chemistry, biology and chemoecology of the pyrrolizidine alkaloids. In: Pelletier SW, editor. Alkaloids: chemical and biological perspectives. Vol. 9. Oxford (UK): Pergamon Press; p. 155–233.
- Huybrechts B, Callebaut A. 2015. Pyrrolizidine alkaloids in food and feed on the Belgian market. Food Addit Contam Part A. 32(11):1939–1951.
- Info flora. 2017. The National Data and Information Center on the Swiss Flora. February 2017. https://www.infoflora.ch/en. Consulted.
- Kast C, Dübecke A, Kilchenmann V, Bieri K, Böhlen M, Zoller O, Beckh G, Lüllmann C. 2014. Analysis of Swiss honeys for pyrrolizidine alkaloids. J Apicult Res. 53(1):75–83.
- Kempf M, Beuerle T, Bühringer M, Denner M, Trost D, von der Ohe K, Bhavanam VBR, Schreier P. 2008. Pyrrolizidine alkaloids in honey: risk analysis by gaschromatography-mass spectrometry. Mol Nutr Food Res. 52:1193–1200.
- Kempf M, Heil S, Hasslauer I, Schmidt L, Von der Ohe K, Theuring C, Reinhard A, Schreier P, Beuerle T. 2010a. Pyrrolizidine alkaloids in pollen and pollen products. Mol Nutr Food Res. 54:292–300.
- Kempf M, Reinhard A, Beuerle T. 2010b. Pyrrolizidine alkaloids (PAs) in honey and pollen-legal regulation of PA levels in food and animal feed required. Mol Nutr Food Res. 54:158–168.
- Lucatello L, Merlanti R, Rossi A, Montesissa C, Capolongo F. 2016. Evaluation of some pyrrolizidine alkaloids in honey samples from the Veneto region (Italy) by LC-MS/MS. Food Anal Methods. 9(6):1–12.
- Lucchetti AM, Glauser G, Kilchenmann V, Dübecke A, Beckh G, Praz C, Kast C. 2016. Pyrrolizidine Alkaloids from Echium vulgare in honey originate primarily from floral nectar. J Agric Food Chem. 64(25):5267–5273.
- Lucchetti AM, Kilchenmann V, Glauser G, Praz C, Kast C. Forthcoming. Larval nursing protects honeybee larvae from pollen secondary metabolites. submitted.
- Marti R, Meyer D. 2010. Deuterated derivatives of retronecine: Project report 26455. EIA Fribourg, Switzerland: Institut Chimie et Sciences de la Vie.
- Martinello M, Cristofoli C, Gallina A, Mutinelli F. 2014. Easy and rapid method for the quantitative determination of pyrrolizidine alkaloids in honey by ultra performance liquid chromatography-mass spectrometry: an evaluation in commercial honey. Food Control. 37:146–152.
- Molyneux RJ, Gardner DL, Colegate SM, Edgar JA. 2011. Pyrrolizidine alkaloid toxicity in livestock: a paradigm for human poisoning? Food Addit Contam Part A. 28(3):293–307.
- Mulder PPJ, López Sánchez P, These A, Preiss-Weigert A, Castellari M. 2015. Occurrence of Pyrrolizidine Alkaloids in food. EFSA Supporting Publ. EN-859:1–114.
- Neumann H, Huckauf A. 2016. Tansy ragwort (Senecio jacobaea): a source of pyrrolizidine alkaloids in summer honey? J Verbr Lebensm. 11(2):105–115.
- NTP (National Toxicology Program). 1978. Bioassay of Lasiocarpine for possible carcinogenicity. NTP Tech Rep. 39:1–66.
- Roeder E. 1995. Medicinal plants in Europe containing pyrrolizidine alkaloids. Pharmazie. 50:83–98.
- Selmar D, Kleinwächter M. 2013. Stress enhances the synthesis of secondary plant products: the impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol. 54(6):817–826.
- Vacchiano G, Barni E, Lonati M, Masante D, Curtaz A, Tutino S, Siniscalco C. 2013. Plant invasion in Southern Europe: monitoring and modeling the invasion of the fast spreading alien Senecio inaequidens DC in an alpine region. Plant Biosyst. 147(4):1139–1147.
- Wiedenfeld H. 2011. Plants containing pyrrolizidine alkaloids: toxicity and problems. Food Addit Contam Part A. 28(3):282–292.
