ABSTRACT
Inorganic arsenic (iAs) is a contaminant present in food, especially in rice and rice-based products. Toxicity of arsenic compounds (As) depends on species and oxidative state. iAs species, such as arsenite (As(III)) and arsenate (As(V)), are more bioactive and toxic than organic arsenic species, like methylarsonic acid (MMA(V)) and dimethylarsinic acid (DMA(V)) or arsenosugars and arsenobetaine. An ion chromatography-inductively coupled-plasma-mass spectroscopy method was developed to separate the four following arsenic anions: As(III), As(V), MMA(V) and DMA(V). Sample preparation was done in mild acidic conditions to ensure species preservation. The predominant arsenic species found in rice and rice-based products, except for rice drinks, was As(III), with 60–80% of the total As content, followed by DMA(V) and As(V). MMA(V) was measured only at low levels (<3%). Analyses of rice products (N = 105) intended for toddlers, including special products destined for infants and toddlers, such as dry form baby foods (N = 12) or ready-to-use form (N = 9), were done. It was found in this study that there is little or no margin of exposure. Risk assessment, using the occurrence data and indicated intake scenarios compared to reference BMDLs as established by EFSA, demonstrated toddlers with a high consumption of rice based cereals and rice drinks are at risk of high iAs exposure, for which a potential health risk cannot be excluded.
Introduction
Worldwide rice consumption has increased in the last few years (OECD-FAO Citation2015). Rice is considered to be staple food in many countries, especially in Southeast Asia (EC Citation2015). Rice has also gained in popularity in the Western diet. Due to its bland taste, nutritional value (source of carbohydrate) and the absence of gluten, rice is widely consumed. It is also used in baby foods because of its low allergenic potential. Rice-based products may be recommended for adults and children suffering coeliac disease or cows’ milk allergy (Munera-Picazo et al. Citation2014a, Citation2014b; Pedron et al. Citation2016).
Unfortunately, rice is known to be a source of the toxic metalloid arsenic (As) (Williams et al. Citation2007). Arsenic not only occurs naturally in rock, soil and groundwater but is also released by anthropogenic activities such as agriculture, mining or industry. Rice has a high ability to accumulate arsenic in both shoot and grain (Williams et al. Citation2007; Meharg et al. Citation2009; Zhao et al. Citation2013), resulting in a 10-fold higher concentration compared to other cereals (Davis et al. Citation2017). The arsenic profile in rice is dependent on the geological nature of the soil, the groundwater used to irrigate paddy fields, the plant genotype and the growing conditions (Lamont Citation2003; Wu et al. Citation2011; Signes-Pastor et al. Citation2016a).
Diet represents the major route of arsenic exposure for humans. Apart from rice, arsenic is mostly found in seafood, including shellfish and seaweeds (0.5–50 mg kg−1), in some mushroom species (mg kg−1 range, depending on the soil composition) and in drinking water (concentration dependent on rock composition) (FAO/WHO Citation1998). Seafood contains mostly organic compounds such as arsenobetaine (AB), arsenosugars or arsenolipids (Taylor et al. Citation2017), but also a low concentration of inorganic arsenic (iAs) and methylated As compounds. It is estimated that at least 140 million people are exposed through drinking water to iAs levels above the limit (10 µg L−1) set by WHO guideline for drinking water (WHO Citation2011b).
Arsenic has been listed as a human carcinogen since 1980 by the International Agency for Research on Cancer. Carcinogenicity is related to the exposure to iAs: arsenite (As(III)) and arsenate (As(V)), which are classified in Group 1, while methylated organic species, such as methylarsonic acid (MMA(V)) and dimethylarsinic acid (DMA(V)), are possibly carcinogenic to humans (Group 2B) (IARC Citation2012). Chronic exposure to high level of arsenic may lead to harmful effects on human health, like cancers, such as urinary tract, skin, liver or lung cancer, as well as respiratory impairment (bronchiectasis), cardiovascular dysfunctions and severe skin lesions (keratosis). In addition, chronic low-dose exposure poses also a risk of developing chronic diseases such as respiratory symptoms, skin lesions such as hyperpigmentation, gastrointestinal troubles or diabetes (Huang et al. Citation2004; Kapaj et al. Citation2006; Ruangwises et al. Citation2014; Chavez-Capilla et al. Citation2016). Early-life exposure to arsenic has been shown to have a negative impact on brain weight and brain development (Tolins et al. Citation2014), as well as having long-term health implications (Farzan et al. Citation2013; Naujokas et al. Citation2013).
According to current knowledge, the majority of As(V) is converted to As(III), in the body. Arsenic metabolism takes place mainly in the liver. As(V) is rapidly reduced into the trivalent state, under anaerobic conditions. As(III) has a high affinity with sulfhydryl groups, such as glutathione (GSH – antioxidant agent involved in many biological process) and cysteine – rich proteins, an ability not shared with As(V). Then As(III), bound to GSH, is metabolised by As-methyltransferase (AS3MT) and converted into organic methylated compounds, MMA(V), and then after a second methylation into DMA(V). The specific steps of As-methylation are not clearly elucidated yet (EFSA Citation2009; Watanabe and Hirano Citation2013).
Currently, the iAs forms are not usually differentiated in risk assessment. Based on key epidemiological studies with the critical endpoints lung cancer, dermal cancer, bladder cancer and skin lesions, EFSA used 1% excess risk for dose–response modelling and identified a range of benchmark dose lower confidence limit (BMDL01) values between 0.3 and 8 µg iAs kg−1 b.w. d−1 (EFSA Citation2009). The lowest BMDL01 values are for lung cancer from a relatively small study in Chile (Ferreccio et al. Citation2000). This study has the advantage that the population is likely to have a nutritional and genetic background that is more similar to that of EU populations than those of the rural Asian populations. EFSA noted that iAs is not DNA reactive and there are a number of proposed mechanisms of carcinogenicity, for each of which a mechanism with a threshold could be postulated. However, taking into account, the uncertainty with respect to the shape of the dose–response relationships, EFSA considered it not appropriate to identify, from the human data, a dose of iAs with no appreciable health risk, i.e. a tolerable daily or weekly intake. Therefore, the margins of exposure (MOEs) should be assessed between the identified reference points from the human data and the estimated dietary exposure to iAs in the EU population (EFSA Citation2009).
For DMA(V), the most harmful effect is carcinogenicity in the urinary bladder, observed in rats. The mode of action is considered to involve cytotoxicity and sustained increased cell proliferation rather than direct DNA damage (USEPA Citation2005; Cohen et al. Citation2006). The US EPA modelled the dose–response relationships for cell proliferation (labelling with bromodeoxyuridine), hyperplasia and tumour incidence, resulting in BMDL10 values of 0.29, 1.61 and 5.96 mg kg−1 b.w. d−1, respectively (EPA Citation2005). It is common practice in the evaluation of non-genotoxic carcinogens to demand a MOE of 100 between the human exposure and lowest BMDL10 for carcinogenic effects from in vivo studies (CoC Citation2012, Citation2014; EFSA Citation2017). This corresponds to levels of 2.9, 16.1 and 59.6 µg kg−1 b.w. d−1, respectively. It should be noticed that the rat is considered to be particularly sensitive to DMA(V) due to its much slower elimination and greater potential for metabolism to DMA(III) compared to other species including human (Cohen et al. Citation2006; ATSDR, Citation2007). In comparison, the US Agency for Toxic Substances and Disease Registry derived a minimal risk level of 20 µg DMA(V) kg−1 b.w. d−1 for non-carcinogenic effects after chronic oral exposure (ATSDR Citation2007). The critical effect was vacuole formation in the urothelium of the bladder of female mice. For MMA(V), a reference point for the chronic toxic effects has not yet been established.
Children, especially toddlers, aged 1–3 years, are considered at risk with regards to arsenic dietary exposure. During the weaning period, young children are usually fed with a variety of food including rice. Rice, due to its low allergenic particularities can be therefore consumed in relatively high quantity, for example by children suffering of coeliac disease, resulting in increased arsenic intake (Meharg et al. Citation2008; Munera-Picazo et al. Citation2014a). According to EFSA, children are two- to threefold more exposed to arsenic than adults, since they have a greater intake of food and fluids relative to their body weight (EFSA Citation2014).
Toxic properties of arsenic are related to molecular species and oxidation states (Huang et al. Citation2004). Inorganic species are known to be more toxic and bioactive than organic species, such as MMA(V), DMA(V), AB, arsenolipids or arsenosugars. As(III)-based species are considered to have the most harmful potential for humans (Hughes Citation2002). Until now, little information is available about As(III) and As(V) proportion in rice and rice-based products. To characterise the risk, it seems obvious to distinguish iAs species from the total arsenic content in food, in this case, in rice and rice-based products. X-ray absorption spectroscopy analysis can provide important information about speciation (Lombi et al. Citation2009), but this method is not suitable for large numbers of samples.
At the beginning of 2016, maximum values for iAs in rice and rice-based products were established in the European Union (EU Citation2015), followed by Switzerland, in May 2017, in the ‘Ordinance on Maximum Values for Contaminants in Food’ (SR 817.022.15) (DFI Citation2016). The maximum value of iAs in rice for the production of food for infants and toddlers is set at 0.1 mg kg−1. For rice biscuits, including wafers and crackers, the limit is set at 0.3 mg kg−1, whereas for non-parboiled (polished and white) rice, the limit is set at 0.2 mg kg−1. It is set at 0.25 mg kg−1 for parboiled and husked rice.
The aim of this study was to provide validated analytical methods with low limits of quantification that is capable to assess all arsenic speciation. Numerous methods for total arsenic quantification have been developed, but there is a lack of robust methods to characterise and quantify the different iAs species. Arsenic speciation (As(III), As(V), MMA(V), DMA(V)) was provided by coupling ion chromatography to inductively coupled plasma mass spectroscopy (IC-ICP-MS). In addition, total arsenic was assessed using ICP-MS.
Using the methodology developed, a survey of arsenic speciation in rice and rice-based products, especially baby foods, was performed, to assess the risk for toddlers. The occurrence data generated, together with food intake data from the German VELS study, were used to estimate the exposure of toddlers (aged 1–3) and to assess the level of concern to potential health risk. The VELS study (‘Verzehrsstudie zur Ermittlung der Lebensmittelaufnahme von Säuglingen und Kleinkindern’) was conducted between 2001 and 2002 in 816 infants and young children aged between 6 months and under 5 years. The parents kept two 3-d nutritional protocols for each child covering all consumed food (Banasiak et al. Citation2005).
Materials and methods
Chemicals and reagents
All solutions were prepared using deionised water with a specific resistance >18 MΩ cm from a Purelab Ultra (LabWater, ELGA, Marlow, UK). Nitric acid (HNO3) 65% (Suprapur, Merck KGaA, Darmstadt, Germany) was used for sample preparation as well as for chromatographic eluent preparation. A mixed arsenite and arsenate standard was purchased as 1000 mgL−1 from Spex Certiprep (Metuchen, NJ, USA). Rice flour standard, NIST 1568b, was purchased from LGC (Wesel, Germany). arsenic trioxide (As(III), ≥99% purity), arsenic acid sodium (As(V), ≥99% purity), disodium methyl arsonate hexahydrate (MMA(V), ≥99% purity) and dimethylarsinic acid sodium salt trihydrate (DMA(V), ≥98% purity) were obtained from Sigma Aldrich (Buchs, Switzerland).
Samples and standard reference materials
Samples of baby foods with rice (dry form, N = 12 and ready-to-eat form, N = 9), rice cereals (flakes and crispies) (N = 7), rice crackers (N = 25), milk rice (N = 6), rice drinks (N = 15) and rice grains (white rice, N = 27 and brown rice, N = 4) were purchased from grocery stores, drugstores, pharmacies and health food stores, in the Berne area, Switzerland. Rice-based products were collected and sampled directly after purchase between August 2016 and March 2017.
Solid samples, such as rice crackers, rice cereals and rice grains, were powdered using a Retsch Grindomix GM 200 with plastic vessel and platinum cutter and stored at RT. Baby foods (dry form) were sampled and stored at RT. Milk rice and baby foods (ready-to-eat form) samples were mixed using the same system as above, samples were stored at −20°C. Sampled rice drinks were also stored at −20°C.
NIST rice flour SRMs 1568b was used as quality control material for As speciation (total As, iAs, MMA(V) and DMA(V)). Spex Certiprep Dual-As (As(III) and As(V)) was used as quality control to evaluate iAs species preservation (As(III), As(V)) as well as being used as calibrator.
Total arsenic analysis
Sample preparation
Prior to analysis, frozen samples were placed in the refrigerator at 4°C overnight and equilibrated for at least 2 h to RT. Each sample was prepared and analysed in duplicate (N = 2).
An aliquot of 0.2 g rice-based product (rice cereals, milk rice, baby foods, rice crackers or rice grains, rice drinks) and 1 g of 65% HNO3 Suprapur were directly weighed in a 15-mL quartz tube. Samples were placed in an autoclave (ultraCLAVE III, MLS GmbH, Microwave-Labor-System, Germany). The digestion method was performed as follows: 30 min up to 220°C and held at 220°C for 45 min, with a pressure of 150 bar. After cooling, the solution was diluted with 10 g (except for rice drink: 4.9 g) of deionised water. Final sample, ready to analyse, was made of 1.0 g of the above solution spiked with 200 µL (except for rice drink: 100 µL) of internal standard solution of rhodium (20 ppb), diluted with 8.8 g (no dilution step needed for rice drink) of 1% HNO3 Suprapur. NIST 1568b was used as a control sample to assess method validation.
Analysis
Total arsenic determination was carried out using a sector field ICP-MS (ICP-MS Element-XR, Thermo Scientific, USA). Arsenic was monitored at a m/z of 75. The operating conditions were as follows: RF power 1270 W, cooling gas flow rate at 16 L min−1, auxiliary gas flow rate 0.75 L min−1, sample gas flow rate 1.2 L min−1, sample intake speed 13.5 rpm, spray chamber temperature held at ambient temperature, dwell time 0.01 s and 1% HNO3 Suprapur as rinse solution between samples. Data were acquired in low resolution setting (R/ΔR = 300, 10% valley) through scan survey between m/z = 74.734 and 75.108 (arsenic determination) and 102.648 and 103.162 (internal standard, rhodium). The instrument was tuned daily.
Total arsenic concentrations were quantified using external calibration: 0.2, 0.4, 0.6, 0.8 µg kg−1 in 1% HNO3 from Merck Certipur (Merck KGaA, Darmstadt, Germany in 5% HNO3). Calibrators were prepared freshly from Merck standard.
Validation for the total As
The molecular ion 40Ar35Cl, which interferes with 75As, was not present under measurement conditions. It could be excluded by comparison to an additional high-resolution measurement (R2 = 0.983), in which the 40Ar35Cl was separated. Hence, no bias was found in these sets of data because the 95% confidence interval for the regression coefficients (0.89–1.07) includes the value one.
Repeatability of the method was verified using certified rice flour NIST 1568b, in between runs. %RSD of 1.93 was obtained. Accuracy was determined in terms of multiple analysis of NIST 1568b, mean As concentration ± standard deviation (SD) of six independent analyses amounted to 283.7 ± 5.5 μg kg−1 compared with the certified value of 285 ± 14 μg kg−1. LOD (3 SD) was measuring with a blank solution as 3.44 μg kg−1 and LOQ (10 SD) of 10.3 μg kg−1.
Arsenic species analysis
Validation for the total arsenic
Prior to analysis, frozen samples were placed in the refrigerator at 4°C overnight and equilibrated for at least 2 h to RT.
Sample preparation procedure was adapted from Huang et al. (Citation2012). Each sample was prepared and analysed in duplicate. An amount of 0.5 g of rice-based product (rice cereals, milk rice, baby foods, rice crackers or rice grains) and 1.5 g of 0.28 M HNO3 were weighed in a 50-mL polypropylene tube. Closed sample was mixed on a vortex for 10–30 s and afterwards in an oven at 90°C for 1 h. Then, 10 g of deionised water was added to the cooled sample and centrifuged for 10 min at 4000 rpm. An amount of 1.0 g aliquot of the supernatant was diluted with 7.0 g of deionised water. The solution was filled into a sealed autosampler vial. Rice drink samples were diluted fivefold and directly analysed. Analysis was monitored using NIST 1568b, as control sample.
Analysis
Speciation analysis was performed using ion chromatography coupled with ICP-MS. Compared to HPLC-ICP-MS, widely used for speciation analysis, IC-ICP-MS is a metal-free system and thus no contamination with metallic compounds arises from the chromatographic system. This characteristic has an influence on the analysis background. IC-ICP-MS shows a signal-to-noise ratio superior to that of a HPLC-based system, resulting in a low LOD. Furthermore, metal deposition in the analytical column is prevented and thus its lifetime extended.
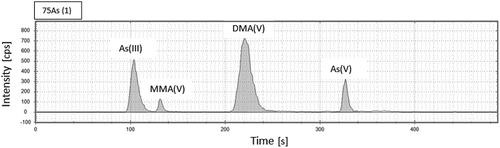
Arsenic species were analysed using a Thermo Fisher ion chromatography (ICS 5000, Dionex Thermo Scientific, USA) coupled with a Thermo Fisher ICP-MS (iCAP Q, Thermo Scientific, USA). Chromatographic separation was carried out in an IonPac AS7 anion exchange column (2 × 250 mm) (Thermo Scientific, USA) and the matching guard-column IonPac AG7 (2 × 50 mm) (Thermo Scientific, USA) and gradient mobile phase (A: 0.1 mM HNO3, B: 50 mM HNO3). The injection volume was 25 µL and the flow rate was 0.3 mL min−1. Column compartment was kept at 30°C. Gradient programme started with 100% A (for 1.0 min) and ramped linearly over the course of 2 min to 100% B, held for 3 min at this condition and then ramped back within 0.1 min and left in the starting condition (100% A) until 8.0 min. Following this condition, the obtained retention times for the As species were 130 s (As(III)), 140 s (MMA(V)), 226 s (DMA(V)) and 326 s (As(V)) (). The chromatographic method was adapted from Jackson and Bertsch (Citation2001). Arsenic detection was performed using an ICP-MS, ICAP Qc ICP-MS (Thermo Fisher Scientific, USA), that monitored at m/z = 75, using He gas in collision cell mode (KED mode), dwell time 0.01 s. The instrument was tuned daily for maximum sensitivity.
Figure 1. IC-ICP-MS chromatogram of arsenic species of certified rice flour NIST 1568b. As(III): Arsenite, MMA(V) monomethylarsonic acid, DMA(V): dimethylarsinic acid, As(V): arsenate.
Calibrators for arsenic speciation were prepared as follows. Individual stock solutions (100 mg kg−1 in 2.5 mM HNO3) of the four different As species (As(III) and As(V), MMA(V), DMA(V)) were prepared, kept at 4°C without light. Multispecies calibration solutions 0.1, 0.5, 1.0 µg kg−1 for As(III), As(V), DMA(V) and 0.05, 0.1, 0.25 µg kg−1 for MMA(V) were made fresh weekly from respective stock solutions. The present method was also developed to separate AB (retention time: 411 s). But no specific calibration was done for this compound, as it was not likely that AB would be present in rice samples.
Validation for the arsenic speciation
Linearity of the current arsenic speciation method was assessed by analysis of six equidistant calibration points, in a range of 0.05–2.0 µg kg−1 for As(III), As(V) and DMA(V) and from 0.02 to 1.0 µg kg−1 for MMA(V). Accuracy of the method was assessed by analysing certified rice flour NIST 1568b. Mean arsenic concentration (µ kg−1) ± SD of compounds of interest (iAs (As(III) + As(V)): 104 ± 2, MMA(V): 11.1 ± 0.9, DMA(V): 173 ± 3) were calculated using 10 independent analyses, performed over 5 d (N = 2) and compared with certified values (iAs: 92 ± 10, MMA: 11.6 ± 3.5, DMA: 180 ± 12) (Conklin and Chen Citation2012; Llorente-Mirandes et al. Citation2014).
According to a previous study (Huang et al. Citation2012), species may be reduced or oxidised during extraction. Unfortunately, no certified rice flour, regarding As(III) and As(V), is actually available. Therefore, to verify the species preservation of As(III) and As(V) during the whole process, a real rice sample (baby food dry form) was spiked with a certified As(III) and As(V) solution (As(III): 20 µg kg−1, As(V): 20 µg kg−1) (Spex Certiprep Dual As). The following results were obtained: As(III): 108%, As(V): 117%, MMA(V): 115%, DMA(V): 100%. Analyses were repeated over days, in between runs. It can be concluded that the present sample preparation and analysis methods were able to ensure that the As(III) and As(V) concentrations were not affected. To determine the repeatability of measures, NIST 1568b was analysed over days, during running batches. Then, the following precision values (%RSD) were found: iAs: 6.6%, MMA(V): 15% and DMA(V): 6.3%. The following limits of detection LOD (3 SD) were calculated using a blank solution: As(III): 0.29 µg kg−1; As(V): 0.37 µg kg−1; MMA(V): 0.50 µg kg−1; DMA(V): 0.81 µg kg−1 and LOQ (10 SD): As(III): 0.87 µg kg−1; As(V): 1.13 µg kg−1; MMA(V): 1.50 µg kg−1; DMA(V): 2.45 µg kg−1.
Consumption of rice and rice-based products
Since no consumption data on rice and rice products for toddlers have yet been collected in Switzerland, this evaluation refers to food consumption data of 1–2 year-old children collected from the German VELS study (Banasiak et al. Citation2005) which was also used by the German Institute for Risk Assessment (BfR Citation2015). Rice and rice-based products were grouped into eight different food categories. The food category ‘rice grains’ comprises white rice, brown rice, parboiled rice and wild rice. Category ‘snacks with rice, sweet/savoury’ include foods containing rice, e.g. breakfast cereals, muesli bars, biscuits, rice crispies or puffed rice. Rice crackers form a separate group and were evaluated separately. Other groups are rice dishes offered as finished products, rice drinks and milk rice. Baby foods still used by toddlers are separated into dry form and ready-to-eat form. ‘Consumers’ in the present study refers only to the consumption of rice and rice-based products among toddlers. The denomination ‘all consumers’ means all toddlers (1–3 years old) in Switzerland who might consume or might not consume rice and/or rice-based products. ‘Only consumers’ means toddlers (1–3 years old) in Switzerland excluding those who do not consume rice and rice-based products. Chronic consumption (mean and 95th percentile) of the different food categories is listed in and for ‘all consumers’ and and , showing ‘only consumers’ of the selected products.
Table 1. Concentration of total arsenic and arsenic species, expressed as µg As kg−1 on total mass product, for each group of rice-based products.
Table 2. Median and range of iAs concentration (µg kg−1) in rice-based products (baby foods, dry form and ready-to-use form, rice cereals and rice crackers) destined for young children, from various study (US FDA, Citation2013; EFSA (European Food Safety Authority) Citation2014; Signes-Pastor et al. Citation2016b) and from the present study.
Table 3. Estimated dietary exposure to iAs for toddlers (all consumers)a.
Table 4. Estimated dietary exposure to iAs for toddlers (only consumers)a.
Table 5. Estimated exposure to iAs for toddlers via drinking water consumption.
Table 6. Estimated dietary exposure to DMA(V) for toddlers (all consumers).
Table 7. Estimated dietary exposure to DMA(V) for toddlers (only consumers).
Results and discussion
Chemical analysis
A total of 105 samples of rice and rice-based products from the Swiss market were analysed. In all of the samples, the three predominant arsenic species characterised were As(III), As(V) and DMA(V).
Rice and rice-based products analysis
Data of the different groups of rice and rice-based products are shown in . Concentrations in the products as bought were expressed without preparation for consumption. It was observed that arsenic concentration is dependant on the rice percentage in the final product. Mean values of the concentration of the summation of the arsenic species (∑As species) in rice grains, brown (N = 4) and white (N = 27), are 182 and 136 µg kg−1, respectively. The majority of the baby foods dry form (N = 12) contains between 25% and 100% rice, representing a ∑As species mean value of 84.3 µg kg−1. Rice crackers (N = 25) and rice cereals (N = 7), which are almost exclusively rice based (rice content around 100%), have mean values of 154 and 267 µg kg−1, respectively. Rice-based products like milk rice (N = 6), baby foods ready-to-eat form (N = 9) or rice drinks (N = 15), which contained low amounts of rice (ca. 10%, ca. 15%, ca. 15%), have relatively low concentration of ∑As species (11.2, 10.9, 18.6 µg kg−1). The concentration of arsenic is correlated with the concentration of rice. The more rice the product contained, the more arsenic was found.
Species profile in rice and rice-based products
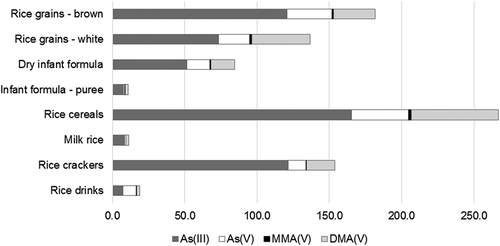
The proportion of the four arsenic species (As(III), As(V), MMA(V), DMA(V)) in rice and rice-based products is presented in . The predominant arsenic species found in rice and rice-based products was As(III), followed by DMA(V) and As(V). MMA(V) was measured only in low levels (<3%). As(III) proportion has a mean value of 63% of the total arsenic in rice and rice-based products (brown rice grains 66%, white rice grains 54%, baby foods dry form 61%, baby foods ready-to-eat form 70%, rice cereals 62%, milk rice 75%, rice crackers 80% and rice drinks 38%). The measured samples have approximately 20% DMA(V) and 15% As(V), except rice drinks which contain 11% and 50%, respectively. A similar arsenic profile was observed in all rice and rice-based products. The only exception was rice drinks. On average, the content of As(V) was higher than that of As(III). Rice drinks, a liquid product, contain between 2% and 14% of rice, some aroma and at least 80% water. The iAs species composition may not be stable under aqueous conditions. Therefore, an iAs interconversion of species can occur. Processing steps may also play a role in species interconversion.
Figure 2. The arsenic species profile (As(III), As(V), MMA(V), DMA(V)) among rice and rice-based products: rice grains – brown: 120.6, 31.1, 1.1, 28.7; rice grains – white: 73.5, 21.4, 1.4, 40.4; dry infant formula: 51.3, 16.2, 0.7, 16.1; infant formula – purée: 7.6, 1.0, 0.3, 2.0; rice cereals: 165.4, 39.3, 2.0, 60.3; milk rice:8.4, 0.6, 0.3, 1.9; rice crackers: 121.5, 12.4, 0.6, 19.2 and rice drinks: 7.1, 9.2, 0.3, 2.0. Concentrations are given in µg kg−1.
The present IC-ICP-MS method for the arsenic speciation provided robust and reliable results. For comparison, total arsenic was also measured by ICP-MS. The total arsenic measurements agreed reasonably well with the sum of the species. The summarised species expressed in fractions of total arsenic: brown rice grains 89%, white rice grains 94%, baby foods dry form 101%, baby foods ready-to-eat form 77%, rice cereals 96%, milk rice 75%, rice crackers 91% and rice drinks 96%. Deviations from 100% are either analytical inaccuracies or other species that were analytically not accessible besides As(III), As(V), MMA(V) and DMA(V). It was shown in a previous study that tetramethylarsonium may constitute as much as 5.8% of the total arsenic in rice grains. It has also been suggested that other arsenic species such as trivalent methyl arsenic and As–S species seem to be unstable under oxidative acidic condition at high temperature (Huang et al. Citation2012).
Correlation between both analytical methods, total arsenic and ∑As species, is illustrated by the coefficient of determination (R2), which is 0.9865. The ∑As species method outlines the most prevalent anionic species, whereas total arsenic method shows all the arsenic content of a product.
Discussion
Arsenic in rice and rice-based products was analysed in several recent studies (Meharg et al. Citation2008; Carbonell-Barrachina et al. Citation2012; Juskelis et al. Citation2013; Llorente-Mirandes et al. Citation2014; Rintala et al. Citation2014; BfR Citation2015; Signes-Pastor et al. Citation2016b; Ciminelli et al. Citation2017). But until now, there is little information on the As(III)–As(V) proportion. This study has shown that As(III) represents approximately 63% of the species pattern in all types of rice and rice-based products. Predominance of As(III) in rice was shown by other studies as well (Sanz et al. Citation2005; Guzman Mar et al. Citation2009; Huang et al. Citation2012). Signes-Pastor et al. (Citation2016b) conducted a study in which they analysed iAs, MMA(V) and DMA(V) concentrations in several food items, such as baby foods dry form with rice (N = 29), rice cereals (N = 53) and rice crackers (N = 97), destined to infant and toddlers. They found, for the three groups cited above, the following iAs concentration: 121, 75, 111 µg kg−1. US FDA (Citation2013) for the same rice-based products found the following results for these three categories: 114 µg kg−1 (N = 85), 91 µg kg−1 (N = 105), 79 µg kg−1 (N = 199). Similar results were collected by EFSA. In EFSA’s scientific report about ‘Dietary Exposure to iAs in the European Population’, results from over 28 surveys from 17 European countries were compiled (EFSA Citation2014). iAs concentrations in various types of food, including rice and rice-based products, were assessed. The mean values for brown rice and white rice were 151.9 µg kg−1 (N = 94) and 88.7 µg kg−1 (N = 189). Regarding baby foods, mean values were found of 107.5 µg kg−1 (N = 6) in ready-to-use form and 133.1 µg kg−1 (N = 20) in dry forms. In , the results of the three studies cited above and the present study are compared. The studies, done by Signes-Pastor et al. (Citation2016a), US FDA (Citation2013) and EFSA (Citation2014), were done before the application of the maximum level for iAs. It can be observed that iAs levels decreased since the new regulation is in force.
In 2016, the EU (EC No 1881/2006) followed by Switzerland (Ordinance on Maximum Values for Contaminants in Food), in 2017, set the same maximal values for iAs concentration in rice and rice-based products, which are 0.25 mg kg−1 for parboiled rice, 0.2 mg kg−1 for white or polished rice, 0.3 mg kg−1 for rice-based products (e.g. crackers). About baby foods, the regulation specifies that the limit (0.1 mg kg−1) is set for the rice used for the production of food for infants and young children and not for the final product. The analyses were done on the final product, without any access to the raw material used for the preparation of the formula. Based on the assumption that all iAs come from rice and that the process has no influence in the arsenic content, most of the baby foods dry form were not in compliance with the regulatory limit of 0.1 mg kg−1. Based on the same assumption, all baby foods ready to eat were in compliance with the law. All the other rice grains samples and rice-based products were in compliance with the regulatory limits.
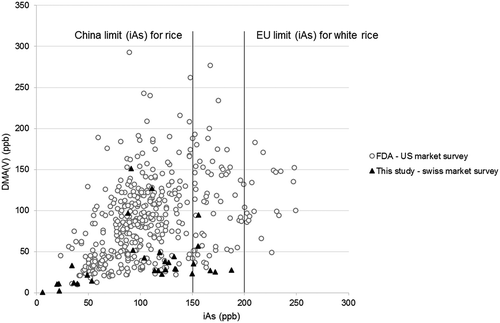
Another interesting aspect pointed out by the US FDA market survey is about DMA(V). Rice from the USA, especially rice grown in the South central region, is known to contain high levels of DMA(V) (Zavala et al. Citation2008; Meharg et al. Citation2009) (US FDA Citation2013). To illustrate this geographical specificity, compares US FDA market survey (Citation2013) and rice grains results of the present study. In 2013, US FDA conducted a national market survey about rice-based products, including 486 samples of rice grains. In the present study, only one of the pool sample (parboiled rice) was from USA, which contains a high level of DMA(V): 94.3 µg kg−1 (37.2% of the As species distribution). Three samples made with rice from Brazil have also shown high DMA(V) concentration (96.6, 127, 151.3 µg kg−1).
Figure 3. Concentrations of DMA(V) in relationship with iAs concentrations in rice grains from the FDA US market survey and from the current study (Swiss market survey). Concentrations are given in µg kg−1 or ppb. An amount of 150 µg kg−1 is the limit for iAs in rice set by China, the EU limit is 200 µg kg−1.
Different batches of the same sample type may exhibit fluctuations in arsenic species concentrations. This is illustrated in rice pops samples (rice cereals), which were obtained at different times and hence differed in lot numbers. One of the rice cereal samples, organic rice pops (origin of rice: Italy), also showed significant variation in DMA(V) concentrations. The DMA(V) level was particularly high in one sample: 247.8 µg kg−1, compared to two other samples: 21.5 and 28.5 µg kg−1. Regarding actual knowledge about As accumulation in rice, the reason of these differences of DMA(V) concentration remains unclear. Cultivated in flooded paddy soil, complex anaerobic conditions influence arsenic assimilation in the rice plant (Lu et al. Citation2008). Conditions of irrigation, seasonal factors and genotype of the plant may also play a role in the arsenic concentration and in species distribution (Wu et al. Citation2011; Zhao et al. Citation2013).
Exposure assessment
Based on the measured iAs concentrations and the German food intake data scenarios, the iAs exposure for toddlers (1–3 years) was estimated. Mean values of food consumption were multiplied by mean iAs concentrations (scenario A). Ninety-fifth percentiles of food consumption were multiplied by mean iAs concentrations (scenario B), and 95th percentiles of food consumption were multiplied by maximum values of iAs concentrations (scenario C). For all consumers, the total iAs intake through consumption of rice and rice-based food was estimated to be 0.044, 0.184 and 0.312 µg kg−1 b.w. d−1 in scenario A, B and C, respectively. The highest intake was estimated to be via rice grains consumption that was in the worst case (scenario C) at 0.118 µg kg−1 b.w. d−1 (). For only consumers, rice drinks were the predominant source for iAs intake in all three scenarios. In the scenario with mean consumption and mean concentrations (scenario A), the exposure was found to be 0.265 µg kg−1 b.w. d−1 (). In scenario C, rice drinks contributed most to iAs intake followed by rice grains and rice dishes (0.567, 0.182 and 0.076 µg kg−1 b.w. d−1, respectively). In contrast to the evaluation by BfR (Citation2015), we had no access to the empirical data sets of the VELS study with the consumption patterns of the individuals. Therefore, the consumption of all food categories for the corresponding scenarios was added. It was estimated to be at 0.546, 0.958 and 1.649 µg kg−1 b.w. d−1 in scenario A, B and C, respectively. However, this is an absolute worst-case assumption in the case of the high exposure (95th percentiles), which is extremely unlikely because of energy-based restrictions and saturation of the toddlers by rice and rice-based food. It would be much more precise if the actual individual consumption levels were linked to the measured iAs concentrations in the consumed products and the individual consumption patterns could be taken into account when summing up all iAs intakes from the different food categories. In comparison to this evaluation, the total iAs intake for 1–2 year-old children (all consumers) via rice and rice products was estimated by BfR to be 0.026 µg kg−1 b.w. d−1 and in the 95th percentile 0.093 µg kg−1 b.w. d−1 (BfR Citation2015). For only consumers of the same age group, iAs intake was calculated to be 0.056 µg kg−1 b.w. d−1 and in the 95th percentile 0.140 µg kg−1 b.w. d−1.
According to EFSA (Citation2014), the main contributors to iAs exposure for all toddlers were ‘milk and dairy products’ (13–24%, median 17%) and ‘grain-based processed products (non-rice-based)’ with an average contribution at the middle bound (MB) in the range of 9–17% (median 11%). ‘Drinking water’ (3–16%, median 10%) was also an important source of dietary iAs in this population group. In this age class, the contribution to total iAs exposure of the food group ‘food for infants and young children’ (1–23%, median 6%) was lower compared to infants in most dietary surveys; in three dietary surveys, the contribution at the MB ranged from 27% to 34% of the overall exposure. As observed in infants, ‘milk and fermented foods’ were the main contributors to total iAs from the food group ‘milk and dairy products’, while ‘wheat bread and rolls’ were the main contributors to total iAs exposure from ‘grain-based processed products (non-rice-based)’. Rice was also an important contributor to the total exposure to iAs in the toddler population. A median contribution at the MB among the different surveys of 7% was observed, although in some cases, the contribution was up to 14% of the total exposure to iAs. This contribution was even higher at the lower bound assumption where rice contributed up to 25% to the exposure (median 12%). Fish and seafood had, in general, a very low contribution to the total exposure to iAs in toddlers except in the two surveys in Spain and Italy where they contributed 5% and 7% to the overall exposure to iAs, respectively. However, it is unclear to which extent, the population at higher risk groups (e.g. children with coeliac disease or cows’ milk allergy) for iAs exposure via rice and rice-based products were covered in the EFSA evaluation.
In addition to the iAs exposure via rice and rice-based products, exposure via drinking water containing arsenic has to be taken into account. Arsenic in drinking water is almost exclusively iAs. Different scenarios with 0.2, 1, 5 and 10 μg iAs L−1 in drinking water were used in the calculation (mean, 95th percentile). Data on consumption amounts and body weights from the German DONALD study (Hilbig and Kersting Citation2006) were used instead of the WHO default scenario of 1 L drinking water consumption per day and 10 kg b.w. (WHO Citation2011a). Assuming the drinking water concentration at the maximum limit of 10 µg iAs L−1, the iAs intake by toddlers via drinking water is considerable. For average drinking water consumers, it is 0.37, 0.18 and 0.14 µg kg−1 b.w. d−1 for toddlers of 12, 24 and 36 months of age, respectively (). For high consumers (95th percentile), it is 0.83, 0.49 and 0.36 µg kg−1 b.w. d−1 for 12, 24 and 36 month old children, respectively. In Switzerland, the median iAs value in water is 0.2 µg L−1. The iAs intake due to water consumption is therefore considered to be low. However, iAs concentration in ground-water is inhomogenously spread across the country. In some alpine regions, high iAs levels were measured, above the limit of 10 µg L−1 and the corresponding iAs intake can be significant.
Total DMA(V) intake for all consumers was calculated to be 0.013, 0.060 and 0.227 µg kg−1 b.w. d−1 in scenario A, B and C, respectively (). Rice grains were the most important source in scenario C with 0.105 µg DMA(V) kg−1 b.w. d−1 intake. For only consumers, total DMA(V) intake was estimated to be 0.112, 0.221 and 0.887 µg kg−1 b.w. d−1 in scenario A, B and C, respectively (). Again, the summation of all these worst-case scenario is highly unrealistic. In scenario C, DMA(V) intake was 0.147 µg kg−1 b.w. d−1 via rice grains consumption, 0.190 µg kg−1 b.w. d−1 via rice drinks consumption and 0.061µg kg−1 b.w. d−1 via rice dishes consumption.
Risk assessment
In several scenarios, iAs intake was estimated to be higher than EFSA’s lower BMDL01 of 0.3 µg kg−1 b.w. d−1, but in no scenario higher than EFSA’s upper BMDL01 of 8 µg kg−1 b.w. d−1. In scenario A for only consumers with mean consumption and mean iAs concentrations, the total iAs intake was 1.8 times higher than EFSA’s lower BMDL01. In scenario C for only consumers, iAs intake via rice drinks was 1.9-fold and via rice cereals 1.6-fold higher. The iAs intake exceeded the lower BMDL01 via drinking water consumption at a concentration of 10 µg L−1 at the age of 1 year 1.2 times for average consumers and 2.8 times by high consumers. In these cases, there is little or no MOE and the possibility of a health risk for some toddlers cannot be excluded.
For DMA(V), in no scenario was the MOE to the BMDL10 from in vivo experiments (range of 0.29–5.96 mg kg−1 b.w. d−1) lower than the default uncertainty factor of 100. For that reason, no health risks by DMA(V) could be identified via the consumption of rice and rice products.
Conclusion
The main cause of concern with respect to dietary exposure is due to inorganic species. Future studies for food toxicology assessment should take into account that information about As(III) and As(V) speciation in rice would be an asset. This work has shown that about 60–80% of the total arsenic content in rice and rice-based products is As(III). For this reason, preservation of the oxidative state of inorganic species was the main concern in the development of the analytical method. The ratio between arsenate and arsenite has been preserved because of the mild conditions of both sample preparation procedure and analysis process. Most of the current analytical methods are not able to differentiate inorganic species (Meharg et al. Citation2008; Carbonell-Barrachina et al. Citation2012; Llorente-Mirandes et al. Citation2014; Rintala et al. Citation2014; Signes-Pastor et al. Citation2016b). The four following species have been quantified: As(III), As(V), MMA(V) and DMA(V). In addition, IC-ICP-MS method was also able to encompass AB, but none of the rice and rice-based samples contained this organic As compound, which is normally found in fish and sea food or mushrooms. Based on our analysis, the dietary exposure of toddlers has been established. Based on previous assumptions, most baby foods dry forms were not in compliance with the regulatory limits. In the future, food producers should ensure a correct interpretation and application of the maximum limit of iAs. Referring to our data, there is little or no MOE and the possibility of a health risk caused by iAs for certain toddlers by the consumption of rice and rice products, particularly rice drinks, rice grains and rice cereals, cannot be excluded. In particular specific groups of toddlers are at risk of arsenic exposure via rice and rice-based products. These include children (1) with coeliac disease, when rice and rice-based products are consumed instead of gluten-containing cereals, (2) with cows’ milk allergy, for whom rice drinks replace cows’ milk, (3) vegans, who don’t drink cows’ milk and consume rice drink instead, (4) from specific ethnic groups, for example Asians, who cover their carbohydrate needs mainly through rice consumption. For these risk groups, targeted dietary recommendations regarding rice and rice-based products consumption might be the most effective measure to reduce risks for toddlers.
Acknowledgements
The authors are grateful to Vincent Dudler (Division of Risk Assessment, Federal Food Safety and Veterinary Office) for his scientific expertise and valuable support during the whole study, Liz Stalder (Division of Risk Assessment, Federal Food Safety and Veterinary Office) for her interest in the manuscript and valuable remarks and Lucia Klauser (Division of Food and Nutrition, Federal Food Safety and Veterinary Office) for her valuable expertise on risk management.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [ATSDR] Agency for Toxic Substances and Disease Registry. 2007. Public health statement for arsenic. Public health service. https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf.
- Banasiak U, Heseker H, Sieke C, Sommerfeld C, Vohmann C. 2005. Abschätzung der Aufnahme von Pfanzenschutzmittel-Rückständen in der Nahrung mit neuen Verzehrsmengen für Kinder. Bundesgesundheitsbl - Gesundheitsforsch -Gesundheitsschutz. 48:84–98.
- [BfR] Bundesinstitut für Risikobewertung, Deutschland. 2015. Arsen in Reis und Reisprodukten. Nr 018/2015. http://mobil.bfr.bund.de/cm/343/arsen-in-reis-und-reisprodukten.pdf.
- Bognar A. 2002. Tables on weight yield of food and retention factors of food constituents for the calculation of nutrient composition of cooked foods (dishes). Berichte Des Bundesforschungsanstalt Für Ernährung. http://www.fao.org/uploads/media/bognar_bfe-r-02-03.pdf.
- Carbonell-Barrachina AA, Wu X, Ramirez-Gandolfo A, Norton GJ, Burlo F, Deacon C, Meharg AA. 2012. Inorganic arsenic contents in rice-based infant foods from Spain, UK, China and USA. Environ Pollut. 163:77–83.
- Chavez-Capilla T, Beshai M, Maher W, Kelly T, Foster S. 2016. Bioaccessibility and degradation of naturally occurring arsenic species from food in the human gastrointestinal tract. Food Chem. 212:189–197.
- Ciminelli VS, Gasparon M, Ng JC, Silva GC, Caldeira CL. 2017. Dietary arsenic exposure in Brazil: the contribution of rice and beans. Chemosphere. 168:996–1003.
- Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD. 2006. Methylated arsenicals: the implication of metabolism and carcinogenitcity studies in rodents to human risk assessment. Crit Rev Toxicol. 36:99–133.
- [CoC] Committee on Carcinogenicity. 2012. Risk characterisation methods. COC/G 06 – Version 1.0, 2012. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/315883/Risk_characterisation_methods.pdf.
- Committee on Carcinogenicity (CoC). 2014. Defining a point of departure and potency estimates in carcinogenic dose response, COC/G 05 – version 1.0, 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/359324/Defining_a_point_of_departure_and_potency_estimates_in_carcinogenic_dose_response.pdf.
- Conklin SD, Chen PE. 2012. Quantification of four arsenic species in fruit juices by ion-chromatography-inductively coupled plasma-mass spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 29:1272–1279.
- Davis MA, Signes-Pastor AJ, Argos M, Slaughter F, Pendergrast C, Punshon T, Gossai A, Ahsan H, Karagas MR. 2017. Assessment of human dietary exposure to arsenic through rice. Sci Total Environ. 586:1237–1244.
- [DFI] Département Fédéral de l’Intérieur, Suisse. 2016. Ordonnance du DFI sur les teneurs maximales en contaminants. https://www.admin.ch/opc/fr/classified-compilation/20143406/201705010000/817.022.15.pdf. French.
- [EFSA] European Food Safety Authority. 2017. Update: use of the benchmark dose approach in risk assessment. Efsa J. 15(1):4658. https://www.efsa.europa.eu/en/efsajournal/pub/4658.
- [EFSA] European Food Safety Authority. 2009. EFSA panel on contaminants in the food chain (CONTAM): scientific opinion on arsenic in food. Efsa J. 7(10):1351.
- [EFSA] European Food Safety Authority. 2014. Dietary exposure to inorganic arsenic in the European population. Efsa J. 12(3):3597.
- [EC] European Commission. 2015. EU rice economic fact sheet. https://ec.europa.eu/agriculture/sites/agriculture/files/cereals/trade/rice/economic-fact-sheet_en.pdf.
- [FAO/WHO] Food and Agriculture Organization/World Health Organization. Joint FAO/WHO Food Standards Programme. 1998. Position paper on arsenic. ftp://ftp.fao.org/codex/meetings/CCFAC/CCFAC31/fa99_22e.pdf.
- Farzan SF, Karagas MR, Chen Y. 2013. In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol. 272:384–390.
- Ferreccio C, Gonzales C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. 2000. Lung cancer and arsenic concentration in drinking water in Chile. Epidemiology. 11:673–679.
- Guzman Mar JL, Ryes LH, Mizanur Rhaman GM, Skip Kingston HM. 2009. Simultaneous extraction of arsenic and selenium species from rice products by microwave-assisted enzymatic extraction and analysis by ion chromatography-inductively coupled plasma-mass spectroscopy. J Agric Food Chem. 57:3005–3013.
- Hilbig A, Kersting M. 2006. Effects of age and time on energy and macronutrient intake in German infants and young children: results of the DONALD study. J Pediatr Gastroenterol Nutr. 43:518–524.
- Huang C, Ke Q, Costa M, Shi X. 2004. Molecular mechanisms of arsenic carcinogenesis. Mol Cell Biochem. 255:57–66.
- Huang JH, Fecher P, Ilgen G, Hu KN, Yang J. 2012. Speciation of arsenite and arsenate in rice grain—Verification of nitric acid based extraction method and mass sample survey. Food Chem. 130:453–459.
- Hughes MF. 2002. Arsenic toxicity and potential mechanism of action. Toxicol Lett. 133:1–16.
- [IARC] International Agency for Research on Cancer. 2012. Arsenic and arsenic compounds. http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-6.pdf.
- Jackson BP, Bertsch PM. 2001. Determination of arsenic speciation in poultry wastes by IC-ICP-MS. Environ Sci Technol. 35:4868–4873.
- Juskelis R, Li W, Nelson J, Cappozzo JC. 2013. Arsenic speciation in rice cereals for infants. J Agric Food Chem. 61:10670–10676.
- Kapaj S, Peterson H, Liber K, Bhattacharya P. 2006. Human health effects from chronic arsenic poisoning—a review. J Environ Sci Health A Tox Hazard Subst Environ Eng. 41:2399–2428.
- Lamont WH. 2003. Concentration of inorganic arsenic in samples of white rice from the United States. J Food Compos Anal. 16:687–695.
- Llorente-Mirandes T, Calderon J, Centrich F, Rubio R, Lopez-Sanchez JF. 2014. A need for determination of arsenic species at low levels in cereal-based food and infant cereals. Validation of a method by IC-ICPMS. Food Chem. 147:377–385.
- Lombi E, Scheckel KG, Palloon J, Carey AM, Zhu Y, Meharg AA. 2009. Speciation and distribution of arsenic and localization of nutrients in rice grains. New Phytologist. 184:186–201.
- Lu Y, Adomako EE, Solaiman ARM, Rafiqul Islam M, Deacon C, Williams PN, Rahman GKMM, Meharg AA. 2008. Baselin soil variation is a major factor in arsenic accumulation in Bengal Delta paddy rice. Environ Sci Technol. 23:1724–1729.
- Meharg AA, Sun G, Williams PN, Adomako E, Deacon C, Zhu YG, Feldmann J, Raab A. 2008. Inorganic arsenic levels in baby rice are of concern. Environ Pollut. 152:746–749.
- Meharg AA, Williams PN, Adomako E, Lawgali Y, Deacon C, Villada A, Cambell RCJ, Sun G, Zhu Y-G, Feldmann J, et al. 2009. Geographical variation on total and inorganic arsenic content of polished (white) rice. Environ Sci Technol. 43:1612–1617.
- Munera-Picazo S, Burlo F, Carbonell-Barrachina AA. 2014a. Arsenic speciation in rice-based food for adults with celiac disease. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 31:1358–1366.
- Munera-Picazo S, Ramirez-Gandolfo A, Burlo F, Carbonell-Barrachina AA. 2014b. Inorganic and total arsenic contents in rice-based foods for children with celiac disease. J Food Sci. 79:T122–128.
- Naujokas MF, Anderson B, Ahsan H, Vasken Aposhian HV, Graziano JH, Thompson C, Suk WA. 2013. The broad scope of health effects from chronic arsenic exposure update on a worldwide public health problem. Environ Health Perspect. 121.
- [OECD-FAO] Organisation for Economic Co-operation and Development-Food and Agriculture Organization of the United Nations.. 2015. Rice projections: consumption, per capita. http://www.oecd-ilibrary.org/docserver/download/512015021e1t022.pdf?expires=1505984967&id=id&accname=guest&checksum=203B5B15E29D477ED392119A5B008173.
- Pedron T, Segura FR, Ferreira da Silva F, de Souza AL, Maltez HF, Lemos Batista B. 2016. Essential and non-essential elements in Brazilian infant food and other rice-based products frequently consumed by children and celiac population. J Food Compos Anal. 49:78–86.
- Rintala EM, Ekholm P, Koivisto P, Peltonen K, Venalainen ER. 2014. The intake of inorganic arsenic from long grain rice and rice-based baby food in Finland—low safety margin warrants follow up. Food Chem. 150:199–205.
- Ruangwises S, Saipan P, Ruangwises N. 2014. Wheat and rice in disease prevention and health. Book: benefits, risks and mechanisms of whole grains in health promotion. New York (NY): Elsevier.
- Sanz E, Munoz R, Camara C. 2005. A rapid and novel alternative to conventional sample treatment for arsenic speciation in rice using enzymatic ultrasonic probe. Analytica Chimica Acta. 535:227–235.
- Signes-Pastor AJ, Carey M, Carbonell-Barrachina AA, Moreno-Jimenez E, Green AJ, Meharg AA. 2016a. Geographical variation in inorganic arsenic in paddy field samples and commercial rice from the Iberian Peninsula. Food Chem. 202:356–363.
- Signes-Pastor AJ, Carey M, Meharg AA. 2016b. Inorganic arsenic in rice-based products for infants and young children. Food Chem. 191:128–134.
- Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, Karagas MR, Francesconi KA. 2017. Human exposure to organic arsenic species from seafood. Sci Total Environ. 580:266–282.
- Tolins M, Ruchirawat M, Landrigan P. 2014. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health. 80:303–314.
- [EPA] Environmental Protection Agency. 2005. Guidelines for carcinogen risk assessment. https://www3.epa.gov/airtoxics/cancer_guidelines_final_3-25-05.pdf.
- [FDA] Food and Drug Administration. 2013. Analytical results from inorganic arsenic in rice and rice products sampling September 2013. https://www.fda.gov/downloads/food/foodborneillnesscontaminants/metals/ucm352467.pdf.
- Watanabe T, Hirano S. 2013. Metabolism of arsenic and its toxicological relevance. Arch Toxicol. 87:969–979.
- [WHO] World Health Organisation. 2011a. Arsenic in drinking-water. http://www.who.int/water_sanitation_health/dwq/chemicals/arsenic.pdf.
- [WHO] World Health Organisation. 2011b. Guidelines for drinking-water quality. http://www.who.int/water_sanitation_health/dwq/arsenicun5.pdf.
- Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA. 2007. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol. 41:6854–6859.
- Wu C, Ye Z, Shu W, Zhu Y, Wong M. 2011. Arsenic accumulation and speciation in rice are affected by root aeration and variation of genotypes. J Exp Bot. 62:2889–2898.
- Zavala YJ, Gerards R, Gürleyük H, Duxbury JM. 2008. Arsenic in rice: II. Arsenic speciation in USA grain and implication for human health. Environ Sci Technol. 42:3861–3866.
- Zhao FJ, Zhu YG, Meharg AA. 2013. Methylated arsenic species in rice: geographical variation, origin, and uptake mechanisms. Environ Sci Technol. 47:3957–3966.
