ABSTRACT
Leuco crystal violet (LCV) and leuco malachite green (LMG) are the main metabolites of two dyes that are forbidden for use in food production, but can be present at low background concentration in novel Atlantic salmon feed ingredients such as processed animal proteins (animal by-product [ABP]). In this study, the potential transfer of dietary LCV or LMG to the fillet of farmed Atlantic salmon was investigated. The uptake and elimination rate kinetics were determined in seawater-adapted Atlantic salmon (initial weight 587 ± 148 g) fed two levels of either LCV- or LMG-enriched diets (~500 and 4000 µg kg−1, respectively) for 40 days, followed by a 90-day depuration period with feeding on control diets (<0.15 μg kg−1 LCV and LMG). A three-compartmental model was developed, based on a fillet fat, fillet muscle and a central body compartment comprising all other tissues. Model calibrations showed a good fit with measured values during overall uptake and elimination period; however, the model poorly predicted the short-term (days) peak measured values at the end of the exposure period. The model was used to simulate the long-term (>16 months) LCV and LMG feed-to-fillet transfer in Atlantic salmon under realistic farming conditions such as the seasonal fluctuations in feed intake, growth and fillet fat deposition. The model predictions gave highest expected LCV and LMG fillet concentrations of approximately 0.12 and 0.45 μg kg−1, depending on the dietary levels of ABP and background level of LCV and LMG contamination. These levels are under the reference point for action of 2 µg kg−1 for the sum of MG and LMG that EFSA assessed as adequate to protect public health. However, for LCV, the predicted highest levels exceed the analytical decision limit (CCα) of 0.15 µg kg−1 for the method used in this paper.
Introduction
Due to a rapid growth in aquaculture and limited access to marine resources, an increasing part of the fish oil and fish meal in feeds for carnivorous marine species such as Atlantic salmon (Salmo salar) has been partially replaced with plant ingredients over the past decade (Ytrestoyl et al. Citation2015). Animal by-products (ABP) from the rendering industry constitute one of the largest sources of high-quality animal protein and lipid available for animal feed production (Toldra et al. Citation2012). Several nutritional studies have showed ABP to be valuable feed ingredients for fish species including Atlantic salmon (Rosenlund et al. Citation2001; Wilson et al. Citation2007; Liland et al. Citation2015). The EU has recently set out a working plan for the re-authorisation of the use of processed animal protein (PAP) in animal feeds in 2013, initially for aquafeeds (EC Citation2010, Citation2013).
The use of PAP in marine fish farming can introduce residue levels of therapeutic agents such as antibacterials, which are used in poultry and swine farming. Wide-scope qualitative screening for permitted pharmaceutical residues in commercially available EU-produced PAPs showed the presence of pharmaceutic agents like monensin, flumequine, enrofloxacin, trimethoprim and tylosin A (Nacher-Mestre et al. Citation2015). The PAPs also included forbidden substances such as leuco crystal violet (LCV), a metabolite of crystal violet (CV) also named gentian violet (Nacher-Mestre et al. Citation2015). The structurally similar triphenylmethane dye malachite green (MG) has been used in fish farming as an agent against microbiota, fungi and parasite, mostly in the form of water baths (Sudova et al. Citation2007). However, MG has been banned for use in the EU (EC Citation1996) due to its carcinogenic properties (EFSA CONTAM Panel Citation2005; Mani and Bharagava Citation2016), and the determination requirement has been set at a minimum required performance limit (MRPL) of 2 ppb for the sum of MG and leuco malachite green (LMG) in meat of aquaculture products (EC Citation2004). CV is an unauthorised dye in food production (EC Citation1996), although CV has been reported in EU-produced farmed fish (EFSA Citation2016a).
Whether residues in feed ingredients also become a food safety issue depends on the transfer of these substances from feed to fillet. In general, both MG and CV are readily metabolised into the leuco forms (LMG and LCV), and in PAPs, the leuco forms are the dominant forms present and not the parent compounds (Nacher-Mestre et al. Citation2015). The leuco forms of MG and CV have been shown to be eliminated less quickly and are hence more persistent than their mother compounds in several farmed fish species (Chan et al. Citation2012; Roybal et al. Citation1995; Plakas et al. Citation1996; Thompson et al. Citation1999; Bergwerff et al. Citation2004; Jiang et al. Citation2009; Bajc et al. Citation2011). As opposed to CV and MG itself, the leuco forms seem to persist in fish muscle long after MG and CV dosing has stopped (Bergwerff et al. Citation2004; Bajc et al. Citation2011; Chan et al. Citation2012). The relative high Kow (approximately 5.7 for LCV and LMG) makes them dissolve in the lipid tissue of an organism, and LMG levels in fish significantly correlate with lipid content in fish tissue (Jiang et al. Citation2009). However, LCV and LMG will also partly accumulate in the non-fat muscle fraction, albeit at lower concentrations (Schermerhorn and Munns Citation1994; Jiang et al. Citation2009). Most studies on (leuco) MG and CV have been performed on a short-term (1–24 h) basis and high waterborne therapeutic doses (1.5–0.1 mg l−1) (Plakas et al. Citation1996, Citation1998; Bergwerff et al. Citation2004; Bajc et al. Citation2011; Chan et al. Citation2012). Few transfer kinetic information exist on long-term (>1 month) dietary exposures at non-therapeutically contamination levels of the leuco forms (Seel-Audom et al. Citation2013). Although several studies have assessed the tissue distribution (Jiang et al. Citation2009) and muscle LCV and LMG half-lives after waterborne exposure (Alderman and Cliftonhadley Citation1993; Plakas et al. Citation1996; Thompson et al. Citation1999; Bajc et al. Citation2011; Tan et al. Citation2011; Chan et al. Citation2012), the combined fillet uptake and elimination kinetics of LMG and LCV after prolonged dietary exposure have not been yet assessed.
Dietary transfer models are developed to predict levels of fat-soluble contaminants in the edible part of farmed Atlantic salmon when exposed to low background dietary contamination over prolonged periods (Berntssen et al. Citation2011, Citation2013, Citation2014, Citation2016). Fish transfer models are based on uptake and elimination kinetics and vary from simple one-compartmental transfer models (Berntssen et al. Citation2007, Citation2011, Citation2016; Brambilla et al. Citation2007) to multi-compartmental physiological-based kinetic (PBK)- based models (Nichols et al. Citation2004; Berntssen et al. Citation2011, Citation2013). An earlier two-compartmental Atlantic salmon model described the distribution of fat-soluble persistent components (the brominated flame retardant and hexabromocyclododecane [HBCD]) over a central compartment (blood and internal organs such as liver and kidney) and a fat compartment after oral exposure (Berntssen et al. Citation2011). As LCV and LMG are likely to partly partition in both the fat and muscle parts of the salmon fillet, a compartmental distribution can be envisaged in which LMG and LCV distribute from a central compartment to a fat compartment with a further distribution to the muscle compartment of the fillet. Earlier pharmacokinetic models on tissue MG distribution in waterborne-exposed rainbow trout suggest a three-compartmental distribution for the parent compound (Alderman and Cliftonhadley Citation1993).
In the present study, fillet uptake and elimination rate kinetics of LCV and LMG in adult consumption-sized Atlantic salmon, with standard growth and feed intake, are assessed after prolonged LCV and LMG dietary exposure followed by a period of depuration. A three-compartmental (central, fat and muscle compartments) PBK model was developed. The toxicokinetic model was used to predict fillet LMG and LCV levels when Atlantic salmon were fed a diet with low background levels of LCV and LMG. The LCV and LMG levels selected were based on levels found in PAP in recent years and fish were held under standard seawater farming conditions.
Materials and methods
Feeding trial
Seawater-adapted Atlantic salmon (S. salar) were fed LCV- or LMG-spiked diets at two levels for 40 days followed by a 90-day depuration period where the fish were fed control feeds, which were free of LCV and LMG. The experiment was performed at Cargill Innovation Center in Dirdal, N-4335, Norway, in the period of April 2015–September 2015. Post-smolt (Salmobreed, both genders) were randomly distributed among eight fibreglass tanks (500 l; ~120 fish per tank). At the beginning of the experiment, the weight and length (fork-tail) of fish were 587 ± 148 g and 36.9 ± 2.38 cm, respectively (mean ± standard deviation [SD]; n = 60). The fish were acclimated to the holding facilities for 1 month while being fed a commercial diet that had no detectable levels of LCV or LMG (LOD <0.15 μg kg−1). After the acclimatisation period, fish in randomly selected duplicate tanks were fed LMG- or LCV-enriched diets with nominal levels of 500 or 4000 µg kg−1 for 40 days. The feed concentrations represented an oral dose of approximately 4 and 31 µg kg−1 body weight per day. Following the dietary LCV and LCV exposure period, the fish were given LCV- and LMG-free diets during and depuration period of 90 days. As no detectable LCV and LMG levels were present in the acclimatised fish or the basal diet, no control group was needed to compensate for potential background LCV and LMG levels. Fish were reared under a 12-h light:12-h dark regime during the exposure period and fed by automatic feeders in three meals a day to a level approximating 0.77% of body weight per day. The feeding rate was adjusted for growth biomass increase, which was assessed by measured average weight gain of the sampled fish per sampling time point, and biomass reduction due to sampling. Uneaten pellets were collected in a flow-over system and registered daily, thus providing daily feed intake values. Water temperature, salinity and oxygen saturation were monitored continuously and varied over the course of the trial from 8°C to 10°C, 33 to 35 g l−1 and 75% to 84%, respectively. To avoid potential water contamination of LCV and LMG from the diet and faeces, a high water flow (15–20 l min−1) through the tanks was maintained. During the accumulation period, five fish from each tank were sampled at day 0, 1, 2, 3, 4, 5, 7, 11, 22 and 40. Similarly, during the depuration period, five fish per tank were sampled at day 0, 2, 3, 4, 5, 6, 7, 10, 13, 16, 21, 31, 55 and 90. At fish sampling, the fish were anaesthetised with 3-amino benzoic acid ethyl ester (MS-222; ~1 g l−1) before body weight and fork-tail length were measured. Fish were stored at −28°C, and at the end of the experiment, all fish were filleted, homogenised (whole fillet muscle plus skin on the left side of the salmon) and analysed for LCV and LMG. For all time points, except at the end of the accumulation period (day 40), fish from each tank were pooled. At day 40, individual fish were analysed (n = 10 per diet) to assess the individual variability.
LCV- and LMG-enriched diets
The LCV- and LMG-fortified diets were produced by top coating LCV- and LMG-free commercial salmon feeds with LMG- or LCV-enriched (Sigma-Aldrich ≥98% HPLC graded, Eichenzell, Germany) fish oil. The lowest level of LCV and LMG supplementation (500 µg kg−1) was chosen to give detectable levels of LCV and LMG in fish muscle plus skin, while low enough to represent the upper range of what could be expected in PAP-containing feeds. The higher LMG and LCV level (4000 µg kg−1) was chosen to provide quantifiable data of LMG and LCV in the edible part of the fish during the final phase of the depuration period. Immediately after production, six subsamples of feed were analysed for LMG and LCV to test homogeneity of the feed. Measured levels (mean ± SD, n = 6) were 860 ± 9 and 4800 ± 25 µg kg−1 for LCV and 770 ± 9 and 4730 ± 21 µg kg−1 for LMG for the nominal 500 and 4000 µg kg−1 feed, respectively. The fortified feeds were stored for 2 weeks at 4°C before and during the dietary LCV and LMG exposure period. Feed samples were taken each week and analysed for LCV and LMG, and the analysis showed no LCV and LMG loss during the cold storage.
Analyses of LCV and LMG
Homogenised feed and fish samples were spiked with internal standards (LMG-D6 and LCV-D6; Witega Laboratorien Berlin-Adlershof GmbH, Germany) and extracted with 0.01 mM perchloric acid in acetonitrile and dichloromethane (4:1, v/v). The mixtures were vortex-mixed, sonicated and centrifuged before the extracts were transferred to a new vial. A second extraction was performed with 0.01 mM perchloric acid in acetonitrile. After extraction, the samples were evaporated by pressurised vaporisation at 38°C (Turbovap IITM; Zymark, Hopkinton, MA, USA). The samples were dissolved in acetonitrile containing 5% acetic acid before the extracts were cleaned-up by solid-phase extraction according to Tarbin et al. (Citation1998). The eluents were concentrated at 38°C under nitrogen flow. The residues were dissolved in 0.25 M ammonium acetate–acetonitrile (1:1) and filtered through a 0.45-µm filter. Analysis was performed by an Agilent 1200 LC-system (Agilent Technologies, Waldbronn, Germany) coupled to an Agilent 6410 triple quadrupole mass spectrometer (Agilent Technologies, Waldbronn, Germany). The instrument was equipped with an ESI source operated in a positive mode. The analytes were separated by a reverse-phase Zorbax C18-column (150 mm × 2.1 mm i.d., 5-μm particle size (Agilent Technologies, Waldbronn, Germany) using a 0.5-ml/min flow). The mobile phases used in the assay were acetonitrile and 62.5 mM ammonium acetate in water. Chromatography was performed according to a stepwise gradient: 0–2 min, 30% acetonitrile; 4–7 min, 90% acetonitrile; 8 min, 70% acetonitrile; and 10 min, 30% acetonitrile. All gradient steps were linear. The following experimental parameters were used: Drying gas temperature, 300°C; drying gas flow, 11 l/min; nebuliser pressure, 35 psi; and capillary voltage, 4000 V. The analytes were monitored using the following transitions: LMG, 331.3 m/z→239.3 m/z (quantifier) and 331.3 m/z→315.2 m/z (qualifier); LMG-d6, 37.3 m/z→240.3 m/z; LCV, 374.3 m/z→358.4 m/z (quantifier) and 374.3 m/z→364.3 m/z (qualifier); and LCV-d6, 380.3 m/z→364.3 m/z. Procedural blank, matrix blank, matrix-matched calibration curve and controls were prepared for each series. The LOD was determined as 0.15 ng/g for both LMG and LCV, and the method was linear over the range studied (0.15–9000 ng/g). Recovery ranged from 90% to 105%, and inter-run precision varied from 5% to 20% for both compounds.
Modelling approach
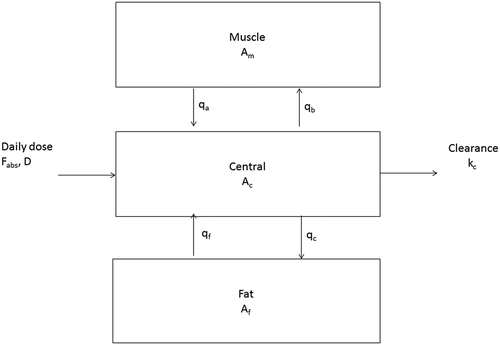
A three-compartmental model was used which was based on an earlier established two-compartmental HBCD PBK model in which the body was considered to consist of a fat compartment for storage of highly lipophilic compounds and a central compartment comprising all other tissues, among them muscle (Berntssen et al. Citation2011). As LMG and LCV in fish and other farmed animals such as chicken are known to distribute not only to fatbut also to a considerable degree to muscle (Schermerhorn and Munns Citation1994), the two-compartmental model was expanded to a three-compartmental model, in which the body was divided into a central compartment, a fat compartment and a separate muscle compartment (). As shown, LCV (or LMG) enters the system in the central compartment, flows from the central to the muscle or fat compartment and backwards. LCV (or LMG) leaves the system from the central compartment, i.e. by faeces and urine. As in the HBCD PBK model (Berntssen et al. Citation2011), the fillet compartment was assumed to be a fixed mixture of fat and muscle. Hence, the concentration of LCV (or LMG) in the fillet compartment was calculated as the weighted mean of the calculated concentrations in the fat and muscle compartments (see below). Specific for the salmon model is that the salmon has a continuous growth until harvest and that fillet contaminant levels are explained by both the fat and muscle compartment levels.
Figure 1. Schematic representation of a physiological-based kinetic (PBK) model for the disposition of leuco crystal violet and leuco malachite green in the muscle of adult consumption-sized Atlantic salmon (Salmo salar), using a three-compartmental approach. ‘A’ denotes the amount of contaminant in a compartment (muscle: Am, central: Ac and fat: Af). Fraction F of the dose is absorbed (Fabs, D) over the gut wall into the central compartment. Kc represents the elimination through clearance. Qb and qc represent the compartmental transfer parameters from central to muscle (qb → qcm) and fat compartments (qc → qcf), respectively, while qa and qf, respectively, represent the transfer from the muscle (qa → qmc) and fat compartments (qf → qfc) to the central compartment.
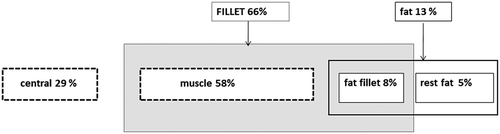
Compartmental weight data from a large-scale farming trial with Atlantic salmon (Berntssen et al. Citation2010a; Lock et al. Citation2011) were described as a function of time (supplementary data Figure 1). In addition, both the muscle and fat deposition data during a large-scale farming production cycle were modelled likewise (supplementary data Figure 1 and describing relationships). Relative liver weight growth was modelled from data of the experiment and was modelled as a relationship between weight and body weight from start to the end of the experiment (supplementary data Figure 1). shows the fractions of the different compartments in the three-compartmental model approach, expressed as percentage of total body weight of a whole fish at a weight class of ~1–2 kg. The whole fish can be divided into a central (29%), muscle (58%) and fat compartment (13%). The central compartment consists of organs such as liver, kidney, gills, etc. The fat compartment in which fat-soluble contaminants will accumulate can be divided into a fillet part (‘fat fillet’ 8%) and fat in other organs such abdominal fat and bone marrow (‘rest fat’ 5%). The non-fat muscle (58%) is the third compartment, and the fillet is described as a combination of the muscle and fat compartments, and LCV and LMG accumulation are described as a separate combination of both.
Figure 2. The body composition in percentage of total weight of a whole fish. As an example, relative volumes are given of an approximately 1 kg Atlantic salmon. For a three-compartmental model approach, the whole fish can be divided into a central (29%), muscle (58%) and fat compartment (13%). The central compartment can be divided into organs such as liver, kidney, gills, etc. The muscle compartment in total is part of the fillet. The fat compartment in which LCV and LMG will accumulate can be divided into a fillet part (fillet fat 8%) and fat located outside the fillet such as abdominal fat and bone marrow fat (‘remaining fat’ 5%). The accumulation of LCV and LMG in fillet is described as the separate accumulation in the muscle and fat compartments of the fillet.
Model equations
In concordance with the two-compartmental PBK model used before (Berntssen et al. Citation2011), can be formulated mathematically as a set of three differential equations that describe the change of the amounts of LCV (or LMG) in each of the three distinguished compartments, i.e. central (C), muscles (M) and fat (F).
with:
Af(t) amount in the fat compartment (μg, calculated), Af(0) = 0
Ac(t) amount in the central compartment (μg, calculated), Ac(0) = 0
Am(t) amount in the muscle compartment (μg, calculated), Am(0) = 0
Bw(t) body weight (g, known)
D(t) administered dose rate (μg/day, known)
Wc(t) weight of the central compartment (g, known)
Wm(t) weight of the muscle compartment (g, known)
Wf(t) weight of the fat compartment (g, known)
Wl(t) weight of liver compartment (g, known)
QcBw cardiac output (ml, known)
qc relative flow rate from central to fat compartment (/ml; calibrated on data)
qf relative flow rate from fat to central compartment (/ml, calibrated on data)
qa relative flow rate from muscle to central compartment (/ml, calibrated on data)
qb relative flow rate from central to muscle compartment (/ml, calibrated on data)
Fabs proportion of LCV taken up in the system (dimensionless, calibrated on data)
kc elimination rate from central compartment (-/day, calibrated on data)
Given all model parameters, the known concentration of LCV (or LMG) in fillet was calculated as:
with φ the fraction of total body fat in fillet (based on fillet fat fraction versus total fat fraction of the whole body) (Berntssen et al. Citation2011).
Parameter estimation
The data were assumed to be distributed skewed to the right. Hence, they were transformed by taking the square root and used to fit the PBK model on the transformed data concentration values using the non-linear model fitting procedure nls of CRAN R (R Core Team, 2017, version 3.4.0). All parameters were transformed to prevent calculating unrealistic values. The logit-transformed values of the parameter Fabs and other parameters were calculated. Because muscle and fat are infinitely woven in fillet, it was assumed that the perfusion rates from the central compartment to the muscle and that to the fat are equal. For all but one time point, mean concentration data were available over all fishes within one tank. For the final time point when stopping the dose, individual data were available, i.e. for each fish separately. Since the former concentration values are calculated from the number of fishes (five) in each tank, the data set was expanded by replacing each mean concentration value by five equal values, with each data point in the expanded data set having equal value. The expanded data set then was used to calibrate the model’s unknown parameters on the fillet data.
Statistics
Statistically significant differences in LCV and LMG muscle levels over time were assessed with by one-way Analysis of variance (ANOVA). To account for the variance among experimental tanks within a dietary treatment, as well as variance among fish within an experimental tank, nested ANOVA followed by Tukey’s HSD post hoc test was used (p < 0.01). A Kolmogorov–Smirnov test was used to assess normality of distribution of each treatment. All data were found to be normally distributed. All statistics were performed using the program Statistica (Statsoft Inc., Tulsa, USA).
Model application
The model fitting procedure for fish fed low, medium and high (0.28, 0.55, 1.1 µg kg−1 ww (wet weight)) LCV and LMF feed simulation resulted in parameter estimations, i.e. the mean values (μ) and the co-variance matrix (Σ). The parameter estimations were assumed to be normally distributed. Hence, μ and Σ were used to generate random parameter values, i.e. b = μ + Σ½ . e
with: b new random parameter value
μ mean of parameter estimations
Σ½ square-root of co-variance matrix of parameter estimations
e standard normally distributed stochastic variable
These random parameter values were used to solve the differential equations and thus to calculate random concentrations of LCV (or LMG) in the fillet compartment over time. These random concentration values were used to calculate the 95% confidence interval of the calculated concentrations of LCV (or LMG) in the fillet compartment.
Results and discussion
shows the uptake and elimination rate kinetics for Atlantic salmon fed low and high levels of LCV and LMG. The dietary LMG and LCV accumulated significantly between all sampling points, and no steady state in LCV and LMG accumulation was observed in fillet. Although dietary LMG and LCV levels were similar (4.7 and 4.8 mg kg−1, for high LMG and LCV, respectively), the fillet levels in the LMG groups were significantly lower than the fillet LCV-exposed fish at the end of the exposure period (501 ± 54 and 637 ± 59 µg kg−1, respectively). The biomagnification factor, expressed as the level in fillet divided by level in feed at steady state, was lower for LMG than LCV (0.1 ± 0.01 versus 0.13 ± 0.02, respectively), indicating higher accumulation potential for LCV compared to LMG.
Figure 3. (a–d) Fillet concentrations (µg kg−1 WW) in Atlantic salmon fed two levels of LCV (0.86 and 4.8 mg kg−1, a and b, respectively) and LMG (0.77 and 4.7 mg kg−1, c and d, respectively) for 40 days, followed by a 90-day depuration (mean ± SD, n = 2 of 5 pooled fish samples per time point, at day 40, n = 10 from 10 individual fish samples).
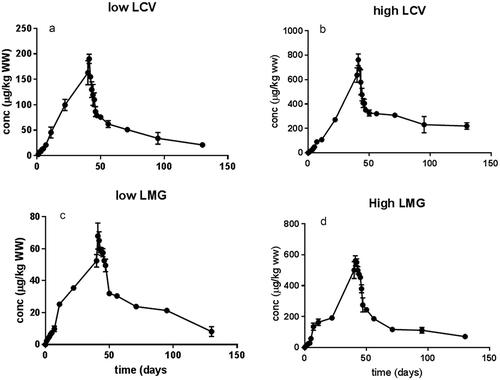
Whereas the uptake behaves mono-phasic, nearly linearly, the elimination is surprisingly described as a bi-phasic kinetic elimination with a quick initial elimination followed by a slower second elimination. Earlier trials with fat-soluble contaminants such as dioxins in chicken (Van Eijkeren et al. Citation2006) or HBCD in salmon (Berntssen et al. Citation2011) showed a mono-exponential elimination which is typically for elimination from fat storage which behaves as a large capacitor with slowly reacting kinetics. A bi-phasic elimination is more typically for low-fat tissues which have a small capacity for storage and often fast initial kinetics that reflect blood levels or release from a non-fat compartment of a tissue (Van Eijkeren et al. Citation2006). The bi-phasic fillet elimination kinetic in the present trial indicates that the muscle compartment plays an important role in fillet elimination. Earlier trials establishing the muscle elimination kinetics in waterborne-LCV-exposed Atlantic salmon (Chan et al. Citation2012) and waterborne-LMG-exposed rainbow trout (Bajc et al. Citation2011) showed a bi-phasic muscle elimination for both components with a rapid elimination in the first 10 days followed by a slow terminal elimination, similar to the present study. For farmed animals such as chicken and several cultured fish species, LMG and LCV are known to distribute not only to fat but also partly to muscle (Schermerhorn and Munns Citation1994; Jiang et al. Citation2009). In general, the highest levels of MG and its metabolite (LMG) were found in the fat tissue of waterborne-exposed channel catfish, but MG and its metabolite were also distributed to other tissues, including muscle (Plakas et al. Citation1996; Jiang et al. Citation2009). Accumulation of LMG after dietary exposure is initially rapid in low-fat muscle (0.94%) from the herbivorous fish Parabramis pekinensis, followed by a later but more dominant deposition in adipose fat, and the depuration from fat is slower than from the low-fat muscle (Jiang et al. Citation2009). The carnivorous Atlantic salmon has a relative high fillet fat (~10%), and in the present study, the LMG and LCV kinetic in fillet is a combination of both muscle and fat kinetics. Despite the high fat solubility of both LCV and LMG, these compounds seem to be distributed to both the muscle and the fat part of the Atlantic salmon fillet. In the present study, an attempt was made to assess the concentrations in the fat part and the muscle part of the fillet after fat extraction with ethyl acetate. Despite higher concentrations in the extracted fat, low LCV and LMG concentration was still observed in the fat-free muscle part of the fillet. The current model predictions show a high distribution of the total amount of LMG and LCV in the muscle compartment compared to the fat compartment of the fillet (see ). As the total volume of the muscle part of the fillet is larger by far than the fat volume (95% versus 5% in the present trial), the muscle can carry more LCV and LMG than the fat compartment despite lower concentrations in the muscle than the fat tissue. Figure 3 of the supplementary data shows both concentration and total amount of LMG and LCV in the different compartments of the three-compartmental model used in the present study.
Figure 4. (a–d) Measured concentration amount (µg kg−1, data points consist of two pooled samples from five fish per time point, at day = 40 data points consists of 10 individual fish samples) of leuco crystal violet (LCV) and leuco malachite green (LMG) in the fillet of Atlantic salmon fed two levels of LCV (0.86 and 4.8 mg kg−1, a and b, respectively) and LMG (0.77 and 4.7 mg kg−1, c and d, respectively) for 40 days, followed by a 90-day depuration, and period model-simulated concentration–time curve based on fitted parameters. Model simulation is even line (results) and measured values (data) are data points with broken line.
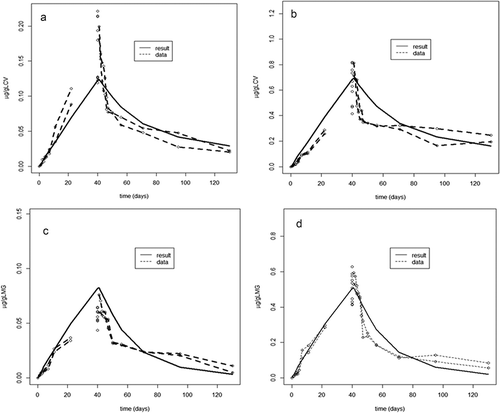
Figure 5. (a–d) Model predicted total amount (µg) of leuco crystal violet (LCV) and leuco malachite green (LMG) in the fillet of Atlantic salmon fed two levels of LCV (0.86 and 4.8 mg kg−1, a and b, respectively) and LMG (0.77 and 4.7 mg kg−1, c and d, respectively) for 40 days, followed by a 90-day depuration, and period model-simulated concentration–time curve based on fitted parameters. Model-simulated amounts in fillet are given as uneven line (–.–.), model predictions for muscle part of the fillet as broken line (.......), model predictions for the fat part of the fillet as dashed line (–––) and central compartment as straight line (——). For the definition of fillet fat and filet muscle, see .
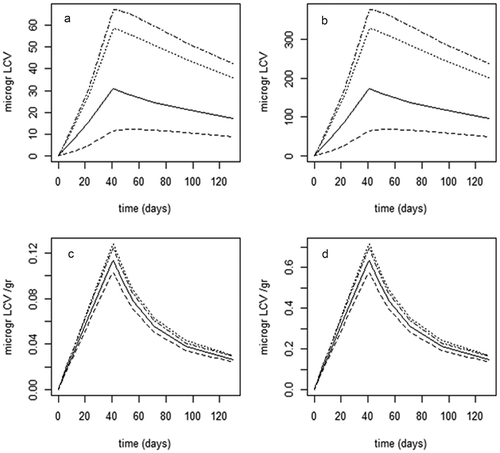
The half-lives of LMG and LCV depend on temperature (Bajc et al. Citation2011) and lipid content (Jiang et al. Citation2009). Earlier studies reported a muscle LMG half-life of 16 days for channel catfish (Plakas et al. Citation1996), and 10–40 days for rainbow trout (Bauer et al. 1988), while in juvenile Atlantic salmon, LCV had a half-life of 15–16 days (Chan et al. Citation2012). In the present study, the half-life of LMG and LCV at low dietary exposure level was around 73–76 days, respectively, and at high exposures, 64 and 105 days, respectively. The slower elimination in the present study compared to the other studies is likely the combined results of the use of adult fish in the present study, with relative high fillet fat content and lower water temperatures.
Model performance
shows the fit of the calibrated model to the experimental data. As shown, for both LMG and LCV, the model initially follows the measured data approximately linear until the end of the 40-day exposure period (). After the exposure period, the model clearly underestimated the initial rapid elimination phase. However, later in the elimination period (>10 days), the model-predicted values followed the measured slow terminal elimination phase. The latter is essential for simulating the long-term accumulation of LMG and LCV in the salmon. The under- and overestimation of LMG and LCV around the time of ending the exposure are also seen in the distribution of the model residuals, i.e. the differences between the calculated and measured data, to the normal distribution (data not shown). With regard to the model parameter estimation, for LMG, a fraction absorbed of 0.56 (SD: 0.02) was found to be compared with 0.54 (SD: 0.005) for LCV. Intrinsically, LMG was eliminated from the body four times as efficient as LCV (compared clearances in supplementary material, Table 1). The complete lists of model parameter value estimations, both the means and SDs, are presented in supplementary data Table 1. The model calibration indicated that the perfusion rates from the muscle and the fat to the central compartment (respectively ga and gf ) were found remarkably equal, indicating that the results shown in can readily be approximated by a two-compartmental approach. The used three-compartmental model structure can be reduced to a two-compartmental model in either of two ways: (combined fat and central) + muscle fat (two-compartmental model A) or fat + (combined central and muscle) (two-compartmental model B). Additional model simulations (data not shown) indicate that the two-compartmental model in which the muscle compartmental model is combined with the fat compartment (two-compartmental model A) gives a model fit deviating from measured values; however, the two-compartmental model in which muscle is part of the central compartment (two-compartmental model B) gives a model fit that approaches the three-compartmental model used in the present study. The latter indicates that the muscle and central compartment behave similar, and/or the muscle compartment is dominating the central compartment, which is likely due to the large muscle volume compared to the central compartment. Although mathematically correct, the two-compartmental model variants are difficult to interpret in terms of the biological properties of LCV and LMG, i.e. both fat and muscle partitioning. As shown in , LCV/LMG deposition in the fillet’s muscle dominated the deposition in fillet fat, with approximately 80% of the total amount in the fillet estimated to reside in its muscle sub-compartment.
Production cycle model simulation and food safety assessment
CV is an unauthorised dye in food production (EC Citation1996), although it has been reported in EU-produced farmed fish (EFSA Citation2016a). In addition to CV, MG has also been banned for use in the EU (EC Citation1996) due to its carcinogenic properties. Determination requirement for the chemical analyses of MG and its main metabolite LMG has been set at an MRPL of 2 ppb for the sum of MG and LMG in meat of aquaculture products (EC Citation2004). The EFSA has recently evaluated whether a reference point for action (RPA) of 2 µg/kg for the sum of MG and LMG is adequate to protect public health. It was concluded that it is unlikely that exposure to food contaminated with MG/LMG at or below the RPA of 2 µg/kg represents a health concern (EFSA Citation2016b). In the present study, the RPA of 2 µg/kg for MG and LMG will be used to estimate an adequate protection for public health for LMG in Atlantic salmon fed background levels of these substances in PAP products when used in aquafeeds during an entire seawater production cycle. For CV and its main metabolites, no RPA has been established yet. However, in a recent EFSA risk assessments on dyes in aquaculture (EFSA Citation2017), EFSA concluded that CV and LCV should be regarded as genotoxic. Both substances consequently belong to Group I (non-allowed pharmacologically active substances for which there is direct evidence of genotoxicity), for which a toxicological screening value (TSV) of 0.0025 µg kg−1 body weight (bw) per day should be used, for both MG and LMG. The TSV is then used to calculate a Toxicologically Based Limit of Quantification (TBLOQ), which is compared with the Reasonably Achievable Lowest Limit of Quantification (RALLOQ) using a decision tree to establish an appropriate RPA (EFSA CONTAM Panel Citation2013). In addition to the human health-related food assessments (e.g. RPA), an analytical decision limit (CCα) has been defined for forbidden substances by the EU (EC Citation2002). The CCα is dependent on the method used and describes the analytical concentrations detected in a food product that requires reporting of the presence of forbidden substances. For the currently analytical method used, the CCα was 0.15 µg kg−1 for both LCV and LMG.
The highest expected inclusion of PAP as replacement of the protein fraction of fish feed is around 20% although levels up to 30% for combined poultry and blood meal PAP products have been tested in experimental trials (Hatlen et al. Citation2015). The highest levels of LMG and LCV in commercially available PAP samples present on the European market were 5.5 µg kg−1 (Nacher-Mestre et al. Citation2015) (unpublished data), giving potential salmon feed concentrations of LCV and LMG of 1.1 µg kg−1 when using a 20% PAP inclusion level in salmon feeds. The LCV/LMG transfer model was employed to estimate fillet LCV and LMG levels in Atlantic salmon under realistic farming conditions with the use of commercially available PAPs (). Farming conditions from a commercial-scale feeding trial were used as input data in the LCV/LMG transfer model (Berntssen et al. Citation2010a, Citation2010b, Citation2016). The input data to the model included seasonal variations in daily LCV and LMG intake, growth rate and fat deposition in Atlantic salmon farmed from about 80 g to about 5 kg during an 18-month period (Lock et al. Citation2011). The daily dietary LCV and LMG intake per salmon, weight gain and relative fillet fat deposition during a seawater production cycle vary based on season and water temperature as well as fish size. The model predictions show that, after exposure to LCV or LMG of 1.1 µg kg−1, maximum LCV and LMG levels are approximately 0.12 (0.10–1.3, 95% confidence intervals) µg kg−1 for LMG and 0.45 (0.39–0.51, 95% confidence intervals) µg kg−1 for LCV, indicating that the background levels of LMG found in PAP are unlikely to give salmon fillet levels that exceed the RPA of 2 µg kg−1. For LMG, and especially LCV, the use of PAPs might cause the official detection of this forbidden substance in food products when using a CCα of 0.15 µg kg−1. At detection, the presence of these forbidden substances in food products has to be reported to food authorities (EFSA Citation2016a).
Figure 6. (a, b)Model-simulated concentration of (µg kg−1 ww) leuco crystal violet (LCV) (a) and leuco malachite green (LMG) (b) over time in the fillet of Atlantic salmon farmed under realistic conditions during a full seawater production cycle with either a high feed level (1.1 µg kg−1; even line), medium feed level (0.55 µg kg−1; dashed line) or a low feed level (0.28 µg kg−1; broken line). Predictions are given as medium estimated levels (middle curve per feed level group) as well as 95% confidence intervals (upper and lower curve per feed group).
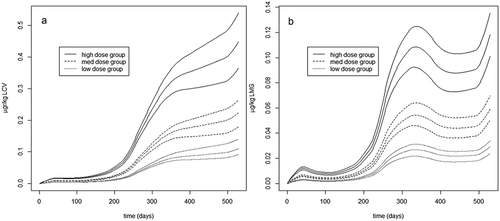
In conclusion, long-term model predictions show that when salmon are fed background levels of LMG, the fillet LMG levels are unlikely to exceed the RPA of 2 μg kg−1. For LMG, and especially LCV, the use of PAPs might cause the official detection of this forbidden substance in food products when using a CCα of 0.15 µg kg−1. At detection, the presence of these forbidden substances in food products has to be reported to food authorities.
Supplemental Material
Download MS Word (69.8 KB)Acknowledgements
Project financed by the Norwegian Research Council project ‘Safe-PAP’ (NFR 227387/E40). Tore Tjensvoll and Anette Bjordal contributed to the analyses of LCV and LMG.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Alderman DJ, Cliftonhadley RS. 1993. Malachite green – a pharmacokinetic study in rainbow-trout, Oncorhynchus-mykiss (walbaum). J Fish Dis. 16:297–311.
- Bajc Z, Jencic V, Gacnik KS. 2011. Elimination of malachite green residues from meat of rainbow trout and carp after water-born exposure. Aquaculture. 321:13–16.
- Bergwerff AA, Kuiper RV, Scherpenisse P. 2004. Persistence of residues of malachite green in juvenile eels (Anguilla anguilla). Aquaculture. 233:55–63.
- Berntssen MHG, Giskegjerde TA, Rosenlund G, Torstensen BE, Lundebye AK. 2007. Predicting world health organization toxic equivalency factor dioxin and dioxin-like polychlorinated biphenyl levels in farmed Atlantic salmon (Salmo salar) based on known levels in feed. Environ Toxicol Chem. 26:13–23.
- Berntssen MHG, Julshamn K, Lundebye AK. 2010a. Chemical contaminants in aquafeeds and Atlantic salmon (Salmo salar) following the use of traditional versus alternative feed ingredients. Chemosphere. 78:637–646.
- Berntssen MHG, Lock EJ, Zeilmaker MJ, Van Eijkeren JCH. 2013. Toxicokinetic model assessment on the dechlorination of dietary toxaphene CHB-62 into CHB-44 in Atlantic salmon (Salmo salar L.). Food Addit Contam Part A. 30:1581–1589.
- Berntssen MHG, Olsvik PA, Torstensen BE, Julshamn K, Midtun T, Goksoyr A, Johansen J, Sigholt T, Joerum N, Jakobsen JV, et al. 2010b. Reducing persistent organic pollutants while maintaining long chain omega-3 fatty acid in farmed Atlantic salmon using decontaminated fish oils for an entire production cycle. Chemosphere. 81:242–252.
- Berntssen MHG, Sanden M, Helge H, Ø L. 2016. Modelling scenarios on feed-to-fillet transfer of dioxins and dioxinlike PCBs in future feeds to farmed Atlantic salmon (Salmo salar). Chemosphere. 163:413–421.
- Berntssen MHG, Valdersnes S, Lunestad BT, Hatlen B, Alm M, Waagbo R, Buttle L. 2014. Residue levels of enrofloxacin and ciprofloxacin in processed animal by-products used in Atlantic salmon feeds and their long-term carry-over to the edible part of the fish. Aquac Nutr. 20:712–721.
- Berntssen MHG, Valdersnes S, Rosenlund G, Torstensen BE, Zeilmaker MJ, van Eijkeren JCH. 2011. Toxicokinetics and carry-over model of alpha-hexabromocyclododecane (HBCD) from feed to consumption-sized Atlantic salmon (Salmo salar). Food Addit Contam Part A. 28:1274–1286.
- Brambilla G, Dellatte E, Fochi I, Iacovella N, Miniero R, di Domenico A. 2007. Depletion of selected polychlorinated biphenyl, dibenzodioxin, and dibenzofuran congeners in farmed rainbow trout (Oncorhynchus mykiss): a hint for safer fish farming. Chemosphere. 66:1019–1030.
- Chan D, Tarbin JA, Stubbings G, Kay J, Sharman M. 2012. Analysis of incurred crystal violet in Atlantic salmon (Salmo salar L.): comparison between the analysis of crystal violet as an individual parent and leucocrystal violet and as total crystal violet after oxidation with 2,3-dichloro-5,6-dicyanobenzoquinone. Food Addit Contam Part A-Chem. 29:66–72.
- [EC] European Commission. 1996. Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. Off J. L125:10–32.
- [EC] European Commission. 2002. Commission Decision 2002/657/EC of 12 August 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Union L. 221:8–29
- [EC] European Commission. 2004. Commission Decision of 22 December 2003 amending Decision 2002/657/EC as regards the setting of minimum required performance limits (MRPLs) for certain residues in food of animal origin. Off J Eur Union L. 6:38–39.
- [EC] European Commission. 2010. European commission, evaluation of the EU legislative framework in the field of medicated feed. Food Chain Evaluation.s Consortium (Editors). Final Report. European Commission DG SANCO, Brussels; p. 188.
- [EC] European Commission. 2013. Commission Regulation (EU) No 56/2013 of 16 January 2013 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Off J Eur Union. 21:3–16.
- [EFSA CONTAM Panel] European Food Safety Authority Panel on Contaminants in the Food Chain. 2005. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the commission to review the toxicology of a number of dyes illegally present in food in the EU. EFSA J. 263:1–71.
- [EFSA CONTAM Panel] European Food Safety Authority Panel on Contaminants in the Food Chain. 2013. Guidance on methodological principles and scientific methods to be taken into account when establishing Reference Points for Action (RPAs) for non-allowed pharmacologically active substances present in food of animal origin. EFSA J. 11:3195.
- [EFSA] European Food Safety Authority. 2016a. Chemicals in food 2016 Overview of selected data collection. https://www.efsa.europa.eu/sites/default/files/corporate_publications/files/161215chemicalsinfoodreport.pdf.
- [EFSA] European Food Safety Authority. 2016b. Malachite green in food. EFSA panel on contaminants in the food chain (CONTAM). EFSA J. 14:4530.
- [EFSA] European Food Safety Authority. 2017. Dyes used in aquaculture. EFSA J. 15:4920.
- Hatlen B, Jakobsen JV, Crampton V, Alm M, Langmyhr E, Espe M, Hevroy EM, Torstensen BE, Liland N, Waagbo R. 2015. Growth, feed utilization and endocrine responses in Atlantic salmon (Salmo salar) fed diets added poultry by-product meal and blood meal in combination with poultry oil. Aquac Nutr. 21:714–725.
- Jiang Y, Xie P, Liang GD. 2009. Distribution and depuration of the potentially carcinogenic malachite green in tissues of three freshwater farmed Chinese fish with different food habits. Aquaculture. 288:1–6.
- Liland NS, Hatlen B, Takle H, Venegas C, Espe M, Torstensen BE, Waagbo R. 2015. Including processed poultry and porcine by-products in diets high in plant ingredients reduced liver TAG in Atlantic salmon, Salmo salar L. Aquac Nutr. 21:655–669.
- Lock EJ, Fjelldal PG, Torstensen BE, Bjornevik M, Breck O, Johansen J, Reynolds P, Sigholt T, Joerum N, Jakobsen JV, et al. 2011. Dietary decontaminated fish oil has no negative impact on fish performance, flesh quality or production-related diseases in Atlantic salmon (Salmo salar). Aquac Nutr. 17:E760–E772.
- Mani S, Bharagava RN. 2016. Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. In: Reviews of environmental contamination and toxicology. Vol. 237. P. DeVoogt. Cham, Springer Int Publishing Ag. 237: 71-104.
- Nacher-Mestre J, Serrano R, Beltran E, Perez-Sanchez J, Silva J, Karalazos V, Hernandez F, Berntssen MHG. 2015. Occurrence and potential transfer of mycotoxins in gilthead sea bream and Atlantic salmon by use of novel alternative feed ingredients. Chemosphere. 128:314–320.
- Nichols JW, Fitzsimmons PN, Whiteman FW. 2004. A physiologically based toxicokinetic model for dietary uptake of hydrophobic organic compounds by fish – II. Simulation of chronic exposure scenarios. Toxicol Sci. 77:219–229.
- Plakas SM, Doerge DR, Turnipseed SB. 1998. Disposition and metabolism of malachite green and other therapeutic dyes in fish. Abstr Pap Am Chem Soc. 215:U55–U55.
- Plakas SM, ElSaid KR, Stehly GR, Gingerich WH, Allen JL. 1996. Uptake, tissue distribution, and metabolism of malachite green in the channel catfish (Ictalurus punctatus). Can J Fish Aquat Sci. 53:1427–1433.
- Rosenlund G, Obach A, Sandberg MG, Standal H, Tveit K. 2001. Effect of alternative lipid sources on long-term growth performance and quality of Atlantic salmon (Salmo salar L.). Aquac Res. 32:323–328.
- Roybal JE, Pfenning AP, Munns RK, Holland DC, Hurlbut JA, Long AR. 1995. Determination of malachite green and its metabolite, leucomalachite green, in catfish (Ictalurus punctatus) tissue by liquid-chromatography with visible detection. J AOAC Int. 78:453–457.
- Schermerhorn PG, Munns RK. 1994. Determination of leucogentian violet in chicken fat by liquid-chromatography with electrochemical and ultraviolet detection – interlaboratory study. J AOAC Int. 77:1454–1460.
- Seel-Audom M, Krongpong L, Futami K, Goncalves AT, Katagiri T, Areechon N, Endo M, Maita M. 2013. Toxicity and absorption of dietary leucomalachite green in Nile tilapia Oreochromis niloticus. Fish Sci. 79:119–127.
- Sudova E, Machova J, Svobodova Z, Vesely T. 2007. Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Vet Med. 52:527–539.
- Tan ZJ, Xing LH, Guo MM, Wang HY, Jiang YH, Li ZX, Zhai YX. 2011. Persistence of malachite green and leucomalachite green in perch (Lateolabrax japonicus). Chin J Oceanol Limnol. 29:647–655.
- Tarbin JA, Barnes KA, Bygrave J, Farrington WHH. 1998. Screening and confirmation of triphenylmethane dyes and their leuco metabolites in trout muscle using HPLC-vis and ESP-LC-MS. Analyst. 123:2567–2571.
- Thompson HC, Rushing LG, Gehring T, Lochmann R. 1999. Persistence of gentian violet and leucogentian violet in channel catfish (Ictalurus punctatus) muscle after water-borne exposure. J Chromatogr B. 723:287–291.
- Toldra F, Aristoy MC, Mora L, Reig M. 2012. Innovations in value-addition of edible meat by-products. Meat Sci. 92:290–296.
- Van Eijkeren JCH, Zeilmaker MJ, Kan CA, Traag WA, Hoogenboom LAP. 2006. A toxicokinetic model for the carry-over of dioxins and PCBs from feed and soil to eggs. Food Addit Contam. 23:509–517.
- Wilson CM, Friesen EN, Higgs DA, Farrell AP. 2007. The effect of dietary lipid and protein source on the swimming performance, recovery ability and oxygen consumption of Atlantic salmon (Salmo salar). Aquaculture. 273:687–699.
- Ytrestoyl T, Aas TS, Asgard T. 2015. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture. 448:365–374.
