ABSTRACT
Identifying and monitoring dietary toxicants is an important component of contemporary food safety systems. To characterise their potential dietary risks, analysis was undertaken of 10 elements: antimony, barium, beryllium, boron, bromine, lithium, nickel, strontium, thallium and uranium in 132 different food types. All 10 elements were reported as quantified in aportion of the analysed foods, with prevalence ranging from less than 1% for antimony to 98% for barium. Dietary exposure assessment was undertaken for 10 New Zealand population cohorts using apublished simulated diet, and proportionality of food groups to total exposure identified. Characterisation against health-based guidance values identified no dietary risk from exposures to beryllium, boron, bromine, lithium, strontium and uranium to any of the population cohorts. For antimony and thallium, the exposure range for infants was calculated to exceed the health-based guidance value, and for barium and nickel, all mean exposures were above the health-based guidance value for younger population cohorts. Although some conservatism in applying upper-bound mean exposures reduces the likelihood of asignificant dietary risk, further laboratory method development and analysis of these elements in the New Zealand diet would be beneficial to ensure protection of New Zealand public health.
Introduction
Chemical elements are aubiquitous, and in many cases essential, part of the diet. However, alarge number of the elements in the periodic table provide no proven nutritional benefit and if ingested in excess present arisk to health (Emsley Citation2001). Elements such as arsenic, cadmium, lead and mercury are the most widely known and studied as food contaminants, although aluminium and tin have also received appreciable attention. There are, however, anumber of other elements which can be toxic that have not received the same degree of interest. The reasons for this may include alower rate of occurrence in the diet, reduced toxic potency, or apoorer understanding of the implications of dietary exposure. While the priority for risk assessment and risk management of these elements in food may be lower than elements such as arsenic and lead, there are still public health benefits in quantifying and characterising dietary exposure.
The Total Diet Study is avaluable tool in dietary risk assessment for its broad examination of the diet, covering many groupings of foods, of both domestic and imported origin (WHO, FAO and EFSA Citation2011). From the obtained occurrence values, typical exposures for various population cohorts can be calculated and any exposure characterised as comprising arisk identified for risk management measures. In New Zealand, the Total Diet Study programme (Pearson etal. Citation2018b) has been undertaken every 4–5 years since 1975 up to the latest publication in 2016. The analysis of long-term trends in occurrence and exposure to prominent toxic elements and agricultural chemicals informs much of New Zealand risk management activities in chemical food safety.
A difficulty of the Total Diet Study however results from the need to concentrate resources to interpret trends of the most prominent toxic elements in the diet, for example, arsenic, cadmium and mercury. One outcome is that other elements of potential concern have not had the same degree of scrutiny, and as aresult, less is understood regarding their occurrence across the diet and potential exposures to the population. Certainly, the hazard of elements such as antimony and thallium means that wide occurrence in the diet would present asignificant risk (ATDSR Citation1992, Citation2017).
An additional study to the core programme of the 2016 New Zealand Total Diet Study was to analyse and interpret the dietary concentrations of anumber of other elements of potential health concern. The aim of this research was to identify the need to prioritise risk management activities on dietary elements currently outside the core programme of the New Zealand Total Diet Study. The results would also provide dietary baselines for investigations of contamination with any of the elements of interest, as well as benchmark New Zealand baselines to international ranges.
A further consideration was that the New Zealand food supply encompasses awide variety of imported foods, including those exclusively imported such as bananas, seasonally imported such as strawberries and tomatoes, or imported to supplement domestic production such as nuts, wheat and wine. As aresult, abroader aim was to contribute to international understanding of the occurrence of these lesser scrutinised contaminants in global food supplies and support their prioritisation by other national food regulators.
Elemental selection for this study was informed by lack of proven nutritional role, ability to analyse through inductively coupled plasma-mass spectrometry (ICP-MS) methodology and availability of previous hazard characterisation reports for oral toxicity to enable risk characterisation. Elements where chemical speciation plays asignificant role in interpreting dietary levels, such as chromium and vanadium, were excluded from this study. The 10 selected elements were antimony, barium, beryllium, boron, bromine, lithium, nickel, strontium, thallium and uranium. Of these elements to our knowledge, only uranium has previously had New Zealand dietary levels and exposures published (Pearson etal. Citation2018a).
Materials and methods
Food sampling
All of the foods analysed were collected as part of the 2016 New Zealand Total Diet Study (Pearson etal. Citation2018b). Eight composite samples each of 132 food types were collected from New Zealand food retailers over the 2016 calendaryear. Each sample was acomposite of between 2 and 12 individual samples. Foods were prepared in accordance with normal cooking practices at the Institute of Environmental Science and Research Ltd (ESR), Christchurch, New Zealand, prior to chemical analysis.
Sample analysis
Samples were analysed for 10 toxic elements by ICP-MS at Hill Laboratories, Hamilton, New Zealand. Typical limits of analytical reporting for each of the elements are reported in . All mean results and ranges are presented in the supplementary material.
Table 1. Analytical limits of reporting for different food matrices of 10 elements analysed in the New Zealand food supply.
Data handling
Mean concentrations were calculated for each of the elements in each of the 132 food groups. Left-censoring of results was managed through derivation of lower-bound (LB) means, where values below the limit of reporting were substituted by zero, and upper-bound (UB) means, where values below the limit of reporting were substituted by the limit of reporting concentration.
Exposure assessment
Mean dietary exposure values were calculated for all the tested elements against 10 different New Zealand population cohorts, which are presented in . Dietary exposures were derived through multiplication of the mean concentration of an element against the estimated daily intake value for that food by the specific cohort. Daily intakes were derived from simulated fortnightly diets which are published as part of the 2016 New Zealand Total Diet Study (Pearson etal. Citation2018b). All exposure estimates are expressed in µg/kg bw/day. Where there was arange in the mean dietary exposure between LB and UB, the LB value was used for identifying key contributors, as this places the emphasis on the concentrations found in the sampled foods rather than on the proxy values used for high intake foods.
Table 2. New Zealand population cohorts considered in dietary exposure assessment for 10 elements (Pearson etal. Citation2018b).
Risk characterisation
Risk assessment of the analysed elements was completed through characterising exposures as apercentage of apublished health-based guidance value. The selected health-based guidance values are recorded in . In anumber of cases, arange of potential values were available for consideration, the rationale for selecting the hazard characterisation behind certain health-based guidance values is detailed in the discussion for each element.
Table 3. Health-based guidance values used for risk characterisation of exposures to 10 elements in the New Zealand diet.
Results
Antimony
Antimony was quantified above the limit of reporting in <1% (10 out of 1056) of the composite samples analysed. Composite samples with reported results included four out of eight of the prawn and shrimp samples, and two out of eight of the bottled water samples. The reported concentrations ranged from 0.0002mg/kg in bottled water to 0.04mg/kg in prawns and shrimps.
Barium
Barium was quantified above the limit of reporting in 98% (1033 out of 1056) of the composite samples analysed. Food types with no reported barium concentrations included oil (all 8 samples), sugar (7 out of 8 samples) and table spreads (3 out of 8 samples). The reported concentrations ranged from 0.0001mg/kg in bottled water to 8.9mg/kg in bran flake cereal.
Beryllium
Beryllium was quantified above the limit of reporting in 2% (25 out of 1056) of the composite samples analysed. Four of the white wine samples had detected beryllium; however, no other food type had more than two samples with detections. The reported concentrations ranged from 0.001mg/kg in soya milk to 0.022mg/kg in salad dressing.
Boron
Boron was quantified above the limit of reporting in 84% (885 out of 1056) of the composite samples analysed. Meat and dairy-based foods most frequently had no detectable concentrations of boron, with levels below the limit of reporting in all analysed composite samples of butter, cheese, beef rump steak and infant formula. All analysed composite samples of oil, sugar, pasta and white rice were also negative for quantified boron. The reported concentrations ranged from 0.01mg/kg in bottled water to 46.2mg/kg in avocado.
Bromine
Bromine was quantified above the limit of reporting in 82% (870 out of 1056) of the analysed composite samples. Quantified bromine concentrations were absent in all analysed composite samples of almonds, avocados, caffeinated and carbonated beverages, and in 7 out of 8 composite samples of tea. The reported concentrations ranged from 0.009mg/kg in tap water to 61.3mg/kg in white rice.
Lithium
Lithium was quantified above the limit of reporting in 48% (502 out of 1056) of the analysed composite samples. Lithium levels were reported broadly across all of the tested food groups, although seafood had consistent findings with all samples of canned fish, fresh fish, mussels, oysters, and prawns and shrimps having quantified concentrations. In contrast, grain-based foods were largely lacking quantified concentrations of lithium with none of the composite samples of the three types of bread (mixed grain, wheatmeal, and white) and only asingle sample from any of the three types of biscuit (chocolate, cracker and plain sweet) with areported concentration of lithium. The reported concentrations ranged from 0.0007mg/kg in tap water to 0.54mg/kg in mussels.
Nickel
Nickel was quantified above the limit of reporting in 57% (597 out of 1056) of the analysed composite samples. Nickel was largely absent from the meat and dairy-based foods, with no quantified concentrations reported in beef rump steak, butter, cheese, lamb and mutton and lambs liver. Plant-based foods were largely all positive for quantified nickel concentrations, with nut-based foods in particular having consistently higher concentrations (peanuts and peanut butter: 0.94–10.2mg/kg). The reported concentrations ranged from 0.0006mg/kg in tap water to 10.2mg/kg in whole peanuts.
Strontium
Strontium was quantified above the limit of reporting in 98% (1031 out of 1056) of the analysed composite samples. All composite samples of oil, 7 out of 8 composite samples of table spread, 4 out of 8 composite samples of white rice and 3 out of 8 composite samples of coconut cream were notable for the absence of quantified levels of strontium. Amongst the food groups in which strontium was quantified, almonds were of note for being consistently higher in concentration (11–21.3mg/kg). The reported concentrations ranged from 0.009mg/kg in tea to 21.3mg/kg in whole almonds.
Thallium
Thallium was quantified above the limit of reporting in 16% (164 out of 1056) of the analysed composite samples. The predominant food type for quantified concentrations of thallium were foods from vegetables where aroot or tuber is consumed, with thallium present in all eight composite samples of canned beetroot, carrots, potato crisps, potato hot chips, potatoes with skin and taro, and 7 out of 8 of composite samples for both peeled potato and onions. The reported concentrations ranged from 0.0005mg/kg in vegetable soup to 0.17mg/kg in potato crisps.
Uranium
Uranium was quantified above the limit of reporting in 21% (217 out of 1056) of the analysed composite samples. Seafood was the most common food type to have quantified concentrations of uranium reported, with all eight composite samples of mussels, oysters and sushi having reported concentrations. In addition, all samples of hamburger, muffins and scones, and 6 out of 8 composite samples of cakes and slices had quantified concentrations of uranium. The reported concentrations ranged from 0.0001mg/kg in tap water to 0.03mg/kg in oysters.
Discussion
Antimony
Antimony was the most infrequently reported toxic element of the 10 elements tested across the analysed foods, with only 10 samples reporting quantified levels (1%). This frequency of detection for antimony is notably below that reported in thesecond French Total Diet Study (Millour etal. Citation2011) and 20th Australian Total Diet Study (FSANZ Citation2003), where samples had quantified levels of 33% and 15%, respectively. The quantified levels of antimony in bottled water are consistent with findings reported overseas of the potential for migration of the residual antimony catalyst in polyethylene terephthalate (PET) plastic bottles (CFS Citation2007).
The estimated exposures for antimony ranged from the lower-bound means of <0.014 µg/kg bw/day for all of the population cohorts to the highest upper-bound estimate of 6.43 µg/kg bw/day for infants (). The influence of ahigh proportion of left-censored results (99% of samples) likely causes the UB mean exposures for antimony being an overestimate. At LB, mean exposures dairy products and dairy substitutes were the largest contributor to intake (50-85%; Supplementary data Figure1). In comparison to the New Zealand estimates, Australia had noted LB-UB ranges between <0.01–0.07 µg/kg bw/day for teenage girls to 0.01–0.25 µg/kg bw/day for infants (FSANZ Citation2003); similarly, the mean adult exposure (0.03 µg/kg bw/day) in the UK total diet study (Rose etal. Citation2010) was aligned with the LB mean estimates for the New Zealand exposures.
Table 4. Estimated lower-bound to upper-bound mean dietary exposures for 10 toxic elements in New Zealand population cohorts.
For the hazard characterisation of antimony, aTolerable Daily Intake (TDI) of 6 µg/kg bw/day established in the World Health Organisation (WHO) guidelines for drinking water quality (WHO Citation2003a) was selected as the health-based guidance value. Only for the UB mean exposure of infants was the health-based guidance value exceeded (107%; ). For all other population cohorts, the UB means were less than 50% of the health-based guidance value, and LB exposure less than 0.2%. The mean exposures are consistent with those reported in HongKong (CFS Citation2007), equating to 0.5–0.7% of the TDI for average teenage consumers.
Figure 1. Characterised lower-bound– upper-bound mean exposure ranges for four elements where New Zealand population cohorts may exceed the health-based guidance value (marked by adotted line).
AF: Adult Female (25 years and over); AF(P): Adult Female– Pacific Island ethnicity (15 years and over); AM: Adult Male (25 years and over); AM(P): Adult Male– Pacific Island ethnicity (15 years and over); YM: Young Adult Male (19–24 years); TB: Teenage Boy (11–14 years); TG: Teenage Girl (11–14 years); C: Child (5–6 years); T: Toddler (1–3 years); I: Infant (6–12 months).
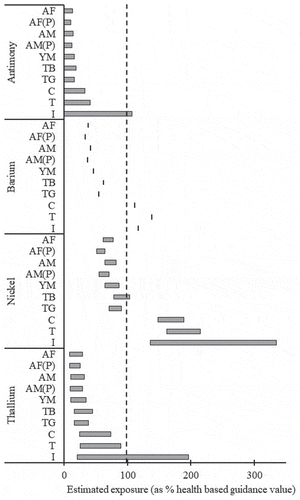
While it is unlikely the small exceedance of the health-based guidance value for infants when using the UB exposure reflects an actual health concern, given the large conservatism in assuming concentrations are present in the majority of foods up to the limit of reporting, it does indicate that afurther investigation of dietary antimony, using more sensitive methods, could be of value to better refine the actual population exposures.
Barium
Barium was the most prevalent of the analysed elements across the diet, with only 2% of food samples having concentrations below the limit of reporting. This prevalence of barium is much greater than the 66% of the samples that were able to be quantified for barium in thesecond French Total Diet Study (Millour etal. Citation2012). However, this is likely to be explained by the use of amore sensitive method for the New Zealand analysis, with limits of reporting in the French survey reported at 0.09mg/kg. Generally, the New Zealand food concentrations for barium were consistent with those reported in France. For example, the mean concentration in milk chocolate (1.1mg/kg) aligned with the French mean for milk chocolate (~1.25mg/kg) and similarly for kiwifruit (NZ range: 1.0–1.7mg/kg, French result: 1.4mg/kg). Similarly, concentrations were equivalent to those of composite food group samples in the 2006 UK Total Diet Study (Rose etal. Citation2010), for example in potatoes (UK: 0.17mg/kg; NZ 0.13–0.24mg/kg) and milk (UK: 0.07mg/kg; NZ 0.10–0.12mg/kg). Anotable difference was for nuts where 131mg/kg barium was reported in the composite sample in the UK Total Diet Study, which was far above the means of 4mg/kg in New Zealand almonds or peanuts, this could reflect the presence of different nut varieties in the UK composite, with Brazilian nuts noted to be high accumulators of barium (Parekh etal. Citation2008).
As there was alow number of left-censored results, the mean exposures for barium did not vary between LB and UB (). The exposure estimates across the cohorts range between 6.5 µg/kg bw/day for adult females of Pacific Island ethnicity to 27.5 µg/kg bw/day for toddlers. Cereal grain-based foods were the largest contributor (33-47%; Supplementary data Figure2) to exposures across all of the population cohorts, with vegetables also anotable contributor (11-26%). Nuts only contributed to 3-5% of the exposures for children and adults, this contrasts with the contribution of this food group to 46% of the total exposure in the UK total diet study (Rose etal. Citation2010). The mean adult exposure in the UK was estimated at 9.4 µg/kg bw/day which aligns well with the estimated adult exposures in New Zealand.
For the hazard characterisation of barium, aTDI of 20 µg/kg bw/day established in the WHO guidelines for drinking water quality (WHO Citation2004) was selected as the health-based guidance value. This value was derived from the findings of an epidemiological study where water containing 7.3mg/L barium was found to have no adverse effects in the consuming population. The World Health Organisation assessment did not characterise the dose at which adverse effects could be expected, as aresult, exceedance of the established TDI is not necessarily indicative that exposures will be ahealth concern. Exposures to dietary barium for all adult and teenage population cohorts fell below the health-based guidance value (37–62%; ); however, this value was slightly exceeded by the estimated exposures for children, toddlers and infants (111–138%). Asimilar conclusion for pre-school age groups was reached in the UK diet (111% of the health-based guidance value) (COT Citation2008).
Due to the conservatism applied in assigning the health-based guidance value for barium, it is unlikely to present adietary concern given the only marginal exceedance calculated for the younger age group diets. However, further surveillance could be warranted on barium in the New Zealand diet to better understand the sources of entry in the food chain (natural or anthropogenic) and identify any trends of exposure.
Beryllium
The majority of the analysed samples were negative for reported concentrations of beryllium. Areview of beryllium concentrations in the diet was published by the U.S Agency for Toxic Substances and Disease Registry (ATSDR) (ATDSR Citation2002)., which identified most foods had beryllium concentrations below 100 µg/kg, this aligns with the New Zealand findings where levels did not exceed 22 µg/kg
The estimated dietary exposures to beryllium ranged between LB means of 0.001 µg/kg bw/day to UB means of 0.12 µg/kg bw/day for adults and teenage population cohorts (). For the child, toddler and infant age groups, the range the UB means ranged up to 0.665 µg/kg bw/day. As the majority of foods had left-censored results the ranges between LB and UB means were considerable. At the LB, mean exposures for adults and the dietary contributors for beryllium were evenly distributed across most of the food groupings. However, some food groups stood out as predominant contributors for teenage boys and children (meat and eggs: 32-34%; Supplementary data Figure3), toddlers (non-alcoholic beverages: 36%) and infants (infant foods: 40%). Comparable exposures from overseas for beryllium are limited; however, the estimated New Zealand exposures fit the dietary ranges of 5–100 µg/day reported on by the ATSDR (Citation2002).
For the hazard characterisation of beryllium, aTDI of 2 µg/kg bw/day established in the WHO guidelines for drinking water quality (WHO Citation2009a) was selected as the health-based guidance value. The TDI has been derived from the benchmark dose for the lower confidence limit for a10% incidence for gastrointestinal lesions in dogs, with the application of a300 fold uncertainty factor. None of the estimated UB mean exposures for the New Zealand population cohorts exceeded the health-based guidance value for beryllium (Supplementary data Figure4), with the highest exposure of 0.65 µg/kg bw/day calculated in infants, corresponding to only 32% of the health-based guidance value. As even through the use of the more conservative UB mean approach, the exposures are below the health-based guidance value, it can be concluded that dietary beryllium is not acurrent food safety risk for the New Zealand population.
Boron
Boron was widely quantified across the sampled foods, with only 16% of the samples being below the limits of reporting. Ahigh rate of occurrence for boron has also been reported in the HongKong diet (CFS Citation2014), with only 2% of the samples not having reportable levels. The concentrations in certain foods in the HongKong study also aligned with the New Zealand results, with high boron levels reported in peanuts (NZ mean: 20.1mg/kg, HongKong mean: 22.0mg/kg) and red wine (NZ mean: 7.1mg/kg, HongKong mean: 8.8mg/kg). The highest levels of boron in the New Zealand foods were present in avocados; boron deficiency is anoted concern with cultivating avocado as aresult this could reflect greater boron fertiliser application rates (Smith etal. Citation1997).
The lower proportion of left-censored results caused minimal variation between LB and UB mean exposures for boron (). The estimated adult dietary exposures to boron across the cohorts ranged between 19.1 and 20.4 (LB-UB) µg/kg bw/day for adult females of Pacific Island ethnicity, to 26.4–28.1 µg/kg bw/day for young adult males. Estimated exposures in the younger age groups were higher with toddlers reporting arange of 82–86 µg/kg bw/day. The greatest contributors to boron intake were plant-based foods, with fruit accounting for 23–45% of intake and vegetables 17–26% (Supplementary data Figure5). For HongKong’s adults (CFS Citation2014), fruit was estimated to contribute 31% of the 1.5mg/day (equivalent to 17 µg/kg bw/day for an NZ adult male) boron intake and vegetables for 24%, which aligns well with the New Zealand findings.
For the hazard characterisation of boron, aTDI of 170 µg/kg bw/day established in the WHO guidelines for drinking water quality (WHO Citation2009b) was selected as the health-based guidance value. The TDI has been derived from the benchmark dose for the lower confidence limit for a5% incidence of developmental toxicity in rats, with the application of a60 fold uncertainty factor. None of the estimated UB mean exposures for the New Zealand population cohorts exceeded the health-based guidance value for boron (Supplementary data Figure4), with the highest exposure of 104 µg/kg bw/day calculated in infants, corresponding to only 61% of the health-based guidance value. As aresult, it can be concluded that dietary boron is not acurrent food safety risk for the New Zealand population.
Bromine
Quantified concentrations of bromine were present in alarge proportion of the tested foods encompassing most of the food groups analysed. Apattern of bromine occurrence appears amongst some of the foods likely to be imported into New Zealand, for example, in strawberries sampled during New Zealand’s winter (4 samples: 19–26mg/kg) and in imported grains such awhite rice (9–61mg/kg). This likely results from the quarantine treatment of these foods with methyl bromide which leaves aresidual concentration of inorganic bromide.
Similar to boron there is alimited influence of the left-censored results on the estimated mean exposures, with LB-UB ranges being small (). Exposure ranges for adult population cohorts were between 63 and 65 (LB-UB) µg/kg bw/day for adult females of Pacific Island ethnicity, to 94–97 µg/kg bw/day for young adult males. The highest estimated exposure was for toddlers with aLB-UB mean range of 289–291 µg/kg bw/day. For all age groups, except toddlers and infants, cereal grain-based foods contributed the most to bromine intake (Supplementary data Figure6), with dairy products also an important contributor and amajor contributor for toddlers and infants. An estimate of adult exposures to bromine was made in the 1997 UK Total Diet Study (Rose etal. Citation2001). The mean bromide intake of 3.6mg/day (equivalent to 41 µg/kg bw/day for aNew Zealand adult male) reported fell below that estimated for the New Zealand adult population cohorts.
For the hazard characterisation of bromine, an Acceptable Daily Intake of 1000 µg/kg bw/day established in the Joint WHO and Food and Agricultural Organisation of the United Nation Meeting on Pesticide Residues assessment of methyl bromide (JMPR Citation1989) was selected as the health-based guidance value. None of the estimated UB mean exposures for the New Zealand population cohorts exceeded the health-based guidance value for bromine (Supplementary data Figure4), with the highest exposure of 294 µg/kg bw/day calculated in infants, corresponding to only 29% of the health-based guidance value. As aresult, it can be concluded that dietary bromine is not acurrent food safety risk for the New Zealand population.
Lithium
Approximately half (48%) of the analysed samples had reported concentrations of lithium, with about half again of these samples reporting concentrations between 0.01 and 0.1mg/kg. Prevalence of lithium was lower than reported in the French diet (92% of the samples with quantified levels; ANSES Citation2011). While the majority of the French results for foods appear comparable to those reported in New Zealand some food groups showed notable disparity, for example, crustaceans and molluscs (NZ mean: 0.15mg/kg, French mean 0.07mg/kg) and water (NZ mean: 0.007mg/kg, French mean 0.069mg/kg). Amongst the analysed New Zealand foods kumara appeared to be higher in lithium (mean: 0.1mg/kg), much more so than the other root vegetables where lithium was infrequently reported.
The influence of the left-censored results was that LB means were generally around 50% of the UB means, with only infants having agreater range (0.42–1.59 µg/kg bw/day) resulting from the absence of lithium in infant formula (). Dietary lithium was contributed to by most of the food groups; however, for adults shellfish (13-16%; Supplementary data Figure7), alcoholic (5-20%) and non-alcoholic beverages (15-25%) were the most important sources. In younger age groups non-alcoholic beverages were the majority source (27-38%) of lithium intake. The important role of non-alcoholic beverages was also reported on in the French diet (ANSES Citation2011), with water being aslightly larger contributor than reported in the New Zealand diet (French child 34%, NZ child 23%). Overall intakes of lithium in the New Zealand diet were lower than those in France (New Zealand adults UB mean: 20–29 µg/day; French mean: 38–55 µg/day).
The hazard characterisation of lithium is less clear-cut than many of the other elements as there is ongoing consideration over whether lithium plays an essential role in the diet (Schrauzer Citation2002). To characterise the hazard of dietary lithium the provisional subchronic and chronic reference dose (p-RFD) established by the US EPA was selected (US EPA Citation2008). Ap-RFD value of 2 µg/kg bw/day was derived from the intake necessary to achieve the lower-bound serum lithium concentration associated with adverse effects reported during therapeutic dosing. A1000 fold uncertainty factor was applied to derive the final health-based guidance value. All of the estimated New Zealand population intakes fell below the health-based guidance value for lithium (Supplementary data Figure4), with only the UB mean exposure for infants exceeding 50% of this value. As even through the use of the more conservative UB mean approach, the exposures are below the health-based guidance value, it can be concluded that dietary lithium is not acurrent food safety risk for the New Zealand population.
Nickel
Nickel was widespread amongst the foods tested, being most prevalent in plant-based foods. The reporting rate for nickel in the sampled New Zealand foods (57%) was less than that in the HongKong diet (90% samples > LOD) (CFS Citation2013); however, there was agood agreement with the concentrations found in the food groups. For example, whole peanuts stood out with higher nickel concentrations (NZ mean: 4.1mg/kg, HongKong mean: 5.3mg/kg), afinding also reported in European monitoring (LB-UB mean 3.5–3.6mg/kg; EFSA Citation2015).
The ranges between LB and UB mean exposures were generally small for most of the population cohorts, with the total range being from 1.4 µg/kg bw/day for adult females of Pacific island ethnicity to 6.0 µg/kg bw/day for toddlers (). Only the infant population cohort had alarge range of estimated exposures (3.8–9.4 µg/kg bw/day), which was driven by the absence of reported nickel concentrations in infant formula. Cereal grain-based foods were an important contributor to nickel exposure for all of the population cohorts (14-33%; Supplementary data Figure8), while for adult age groups non-alcoholic beverages also contributed appreciably to total intake (17-32%). Very similar findings regarding dietary contributors were reported for adult exposures to nickel in HongKong (CFS Citation2013), with cereals and non-alcoholic beverages contributing each to 25% of the total intake. Additionally, the European Food Safety Authority (EFSA) (EFSA Citation2015) had also highlighted non-alcoholic beverages, in particular, cocoa and coffee as an important dietary source of nickel. Reported nickel exposures overseas are generally equivalent to those in New Zealand. For example, in the UK diet (COT Citation2008) adults were estimated to receive 1.5–1.6 µg/kg bw/day (NZ: 1.4–2.4 µg/kg bw/day) and toddlers 4.2–4.9 µg/kg bw/day. (NZ: 4.5–6.0 µg/kg bw/day), and in HongKong diet (CFS Citation2013) the adult mean exposure was 3.1 µg/kg bw/day. The breakdown of mean dietary exposures in European diet survey reported adults had amedian LB-UB range of 2.7–3.4 µg/kg bw/day and toddlers 7.4–10.3 µg/kg bw/day (EFSA Citation2015).
For risk assessment of nickel in the diet, the TDI of 2.8 µg/kg bw/day established by EFSA (EFSA Citation2015) was adopted as the hazard characterisation value. This value was derived through benchmark dose modelling for the lower confidence level of a10% incidence in post-implantation loss, with the application of a100 fold uncertainty factor. In addition to its general toxicity, nickel induces acute hypersensitivity reactions in individuals sensitised to it, with indications that symptoms can occur at levels below toxicity in the general population. As aresult, exposures were also compared to areference point for induction of systemic contact dermatitis, this being avalue of 1.1 µg/kg bw. This value was reported on by EFSA (EFSA Citation2015) from benchmark dose modelling for the lower confidence level of a10% incidence of systemic contact dermatitis in sensitised individuals. EFSA values were used over other international organisations due to the incorporation of the more contemporary benchmark modelling in their calculation.
Characterisation of the dietary exposures to nickel has established that for adults and teenage girls all of the LB and UB mean intakes are currently within health-based guidance values, ranging up to 91% of the tolerable daily intake. The estimated exposure range for teenage boys slightly exceeds the TDI (104%; ) at the UB mean intake, while for the younger age groups of children, toddlers and infants the TDI was estimated to be exceeded by all LB values (136-162% of the TDI; ). The TDI for nickel is derived from an increased incidence of post-implantation loss (EFSA Citation2015) and thus the exceedance in younger age groups may not present as significant aconcern as it would in adults.
For all populations, the LB mean exposures exceeded the reference dose for systemic contact dermatitis. As the simulated diet used in estimating exposures is based on the daily average for acomplete fortnightly diet, some caution has to be taken in relating this to intakes that could occur from astandard meal from which the acute hypersensitivity reaction could results. (Pearson etal. Citation2018b). However, it is clear that certain food types in the diet contain sufficient nickel to potentially cause this reaction if consumed by sensitised individuals. For example, in the adult female population cohort, the cohort with most prevalence of sensitivity (Thyssen and Menné Citation2010), consuming just 18 gof whole peanuts with the mean nickel concentration (4.1mg/kg), or 8 gwith the maximum concentration (10.3mg/kg), would lead to an exceedance of the hypersensitivity reference point. presents the portions sizes for certain nickel-rich foods that could cause exceedance of the reference dose for nickel hypersensitivity.
Table 5. Portion sizes, up to 100 g, for nickel-rich foods necessary to lead to an exceedance of the hypersensitivity reference point for adult females (25 years and above).
There would be value in further monitoring of dietary nickel concentrations, including consideration of trends over time and input from cooking practices, to establish if current levels are reflective of background or may be contributed to from sources that can be mitigated to reduce the level of exposure.
Strontium
Strontium was quantified in almost all of the food samples tested, with only 25 samples having no reported levels. This wide prevalence in the diet has also been seen in the Australian and French diets (FSANZ Citation2011; Millour etal. Citation2012). Anotable split in strontium ranges was seen in the canned fish samples, with half the samples ranging between 0.4 and 0.8mg/kg and the other half 6–9mg/kg. On review of the sample details, this difference resulted from comparing canned tuna against canned salmon, with the latter showing the higher strontium values.
No difference was evident between LB and UB mean exposures; reflecting the limited influence, the small number of samples with no reported strontium had on overall exposures (). Exposures in the adult population cohorts ranged from 16 to 23 µg/kg bw/day, and those in the younger age groups from 24 to 69 µg/kg bw/day. Strontium exposures were contributed to through most of the main food groups;however, cereal grain-based foods (21-36%; Supplementary data Figure9) were amajor contributor to strontium intakes across the population cohorts, afinding also reported in the Australian diet (FSANZ Citation2011). The estimated strontium exposures reported overseas align closely with those for New Zealand. For example, in Australia UB mean exposures of 26 µg/kg bw/day were reported for adults (NZ average: 19 µg/kg bw/day), 29 µg/kg bw/day for teenagers (NZ average: 26 µg/kg bw/day) and 58 µg/kg bw/day for 9-month-old infants (NZ: 63 µg/kg bw/day). Similarly in the UK diet, strontium exposures for adults were reported at 16 µg/kg bw/day (COT Citation2008). This consistency in exposures between countries suggests dietary strontium levels may be largely reflective of natural background occurrence in foods, with limited influence of agricultural practices or anthropogenic contamination in each country.
For the hazard characterisation of strontium, aTDI of 130 µg/kg bw/day established in the WHO Concise International Chemical Assessment Document (WHO Citation2010) was selected as the health-based guidance value. This TDI has been derived from ano observed adverse effect level of 40mg/kg bw/day in weanling rats, with thyroid and liver chemistry effects seen at the next dose group, with the application of a30 fold uncertainty factor. All of the estimated strontium exposures for the New Zealand population cohorts fell below the health-based guidance values (Supplementary data Figure4), with the highest 69 µg/kg bw/day in toddlers equating to only 53% of the health-based guidance value. As aresult, it can be concluded that dietary strontium is not acurrent food safety risk for the New Zealand population.
Thallium
Approximately asixth of the sampled foods had reported the occurrence of thallium. The reported levels of thallium were generally very low, with 87% of the quantified results being less than 0.01mg/kg. Patterns of occurrence in the New Zealand diet are comparable with those reported in the UK (Rose etal. Citation2010), with poultry and offal reporting similar concentrations in both studies. Anotable difference is in the concentrations reported in potatoes, with the results for acomposite sample of potatoes in the UK Total Diet Study (0.001mg/kg), falling below that reported in New Zealand for potatoes with skin(mean: 0.009mg/kg) and without (mean: 0.005mg/kg). Given the higher results for potatoes with skin, which may be due to adherence of soil, the basis of the higher levels in New Zealand potatoes may reflect different thallium concentrations in the soils of New Zealand growing regions.
The range between LB and UB mean exposures was moderate for most of the population cohorts; however, due to no quantified thallium being reported in infant foods, the range covered one order of magnitude for infants (0.04–0.35 µg/kg bw/day; ). The estimated exposures were highest for toddlers (0.05–0.16 µg/kg bw/day), whereas adult exposure ranges were lower ranging between the LB of 0.02 µg/kg bw/day for adult females of Pacific Island ethnicity to the UB mean exposure of 0.06 µg/kg bw/day for young adult males. Vegetables contributed to majority of exposure for all of the population cohorts (48-67%; Supplementary data Figure 10), with snack foods, which encompasses the findings in potato crisps, also an important contributor for younger age groups (14-33%). Exposures for New Zealand population cohorts were greater than estimates published for the UK diet (Adults: 0.01 µg/kg bw/day; Pre-school children 0.02–0.03 µg/kg bw/day; Rose etal. Citation2010); however, they are equal to or slightly below those reported in Canada (Adults: 0.08 µg/kg bw/day; Toddlers: 0.21 µg/kg bw/day; Infants: 0.15 µg/kg bw/day; CCME Citation1999).
Thallium has the highest toxicity of the elements assessed in the present study, it also has adegree of uncertainty regarding ahealth-based guidance value. Candidate or provisional TDIs ranging from 0.0003 to 0.2 µg/kg bw/day have been derived in Canada, the United States and the Netherlands (RIVM Citation1998; CCME Citation1999; US EPA Citation2009). All of these values are based on either the medium or high dose from a90-day study of rats, with various approaches to applying uncertainty factors leading to the magnitude of the difference in the candidate TDIs. ANew Zealand review of establishing soil guideline values for thallium adopted adifferent approach to estimating ahealth-based guidance value (Golder Associates Citation2012). Ahealth-based guidance value was calculated instead from the point of departure in the World Health Organisation Environmental Heath Criteria that aurinary thallium concentration of up to 5 µg/L was unlikely to lead to any adverse outcomes (WHO Citation1996). The value derived was within the margin of error of the Dutch provisional TDI of 0.2 µg/kg bw/day (RIVMCitation1998) and thus this value was proposed. Maintaining consistency with this outcome, ahealth-based guidance value of 0.2 µg/kg bw/day is used for hazard characterisation in the present study. Only one of the exposure estimates exceeded the health-based guidance value, this being the UB exposure for infants (176%; ). However, as noted above, the absence of quantified concentrations of thallium in infant foods has led to alarge range of potential exposures thus the UB is potentially conservative for the actual exposures. Exposures for all of the other population cohorts fell below the health-based guidance value.
Given the uncertainty in establishing asafe dietary dose for thallium and the large ranges evident in potential exposures for younger age groups, further consideration of thallium in the New Zealand Total Diet Study would be beneficial. Method development to achieve greater sensitivity would be of value to refine the potential exposure ranges and also provide better information on any trends in increase over time.
Uranium
Uranium was quantified in approximately afifth of the analysed food samples. This is less than the 81% of composite samples in which the uranium isotope uranium-238 was quantified in aprevious New Zealand food study in 2012 (Pearson etal. Citation2015). However, the discrepancy is aresult of the reduced sensitivity offered by the mass spectrometric methods in the present study compared to the radiometric methods used previously. The quantified uranium concentrations in both studies, however, are consistent, for example, amean concentration of 0.014mg/kg (0.171 Bq/kg 238U) was reported in shellfish, which closely matches the mean of 0.018mg/kg for oysters and mussel samples in the present study.
The left censoring evident amongst the reported uranium concentrations resulted in amoderate range between the LB and UB mean exposures (). For adults the LB-UB exposure ranges fall within 0.006–0.027 µg/kg bw/day, and for younger age groups between 0.009 and 0.138 µg/kg bw/day. In adult population key dietary contributors to uranium, intake were composite foods (22-30%; Supplementary data Figure 11), offal and shellfish (18-24%) and cereal grain-based foods (20-21%). Cereal-based foods (22-32%) remained an important contributor for the younger age groups; however, dairy substitutes (18-27%) were important across all non-infant age groups, while dairy products (25-38%) were also akey contributor to intake for toddlers and infants. In contrast, an estimate of mean European exposures (EFSA Citation2009) was heavily contributed to by water and beverages, with these making up between 51% (UB) to 83% (LB) of the total diet exposure.
For the hazard characterisation of uranium, aTDI of 0.6 µg/kg bw/day established in the WHO guidelines for drinking water quality (WHO Citation2003b) was selected as the health-based guidance value. This health-based guidance value is based on the lowest dose causing kidney toxicity in a3-month study of rats, with a100 fold uncertainty factor applied. All of the estimated mean exposures for uranium across the population cohorts fell below the health-based guidance values (Supplementary data Figure4). Previous exposure assessment of dietary uranium ranged between 3% and 7% of the health-based guidance value for New Zealand cohorts (Pearson etal. Citation2018a), these values correspond well with the estimates reported in the present study. As aresult, it can be concluded that dietary uranium is not currently afood safety risk for the New Zealand population and that exposures have remained consistent between 2012 and 2016.
Conclusions and future research
Analysis and risk assessment has been undertaken for 10 elements of potential human health concern in the New Zealand diet. For beryllium, boron, bromine, lithium, strontium and uranium the results of exposure assessment are characterised as being within health-based guidance values and therefore unlikely to reflect adietary concern. For antimony and thallium parts of the estimated range of exposure for infants exceeds the health-based guidance value, although aresult of the estimation of concentration in infant foods due to the left-censoring of results. Similarly, barium mean exposures slightly exceeded the health-based guidance value for children, toddler and infants. However, the conservatism of the hazard characterisation for barium that means it is unlikely to pose arealistic dietary risk. Finally, dietary exposures of nickel suggest apotential dietary risk for younger age groups. In addition, nickel concentrations in anumber of foods could lead to intakes above the reference point for dermatitis in sensitised individuals. Further method development, to refine exposure ranges, and future monitoring could be useful for antimony, barium, nickel and thallium to ensure that dietary sources are well characterised, and if necessary risk management measures implemented to limit trends of elevation in certain dietary sources. The findings of this paper support surveying and risk assessing awider range of elements of potential toxic concern as beneficial in identifying and prioritising future work on dietary toxicants.
Supplemental Material
Download MS Word (17 KB)Supplemental Material
Download MS Word (16.6 KB)Supplemental Material
Download MS Word (16.6 KB)Supplemental Material
Download MS Word (17.1 KB)Supplemental Material
Download MS Word (17.1 KB)Supplemental Material
Download MS Word (17.3 KB)Supplemental Material
Download MS Word (17 KB)Supplemental Material
Download MS Word (17.1 KB)Supplemental Material
Download MS Word (78.7 KB)Supplemental Material
Download MS Word (16.9 KB)Supplemental Material
Download MS Word (17.1 KB)Supplemental Material
Download MS Word (16.3 KB)Supplemental Material
Download MS Excel (79.4 KB)Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material
Supplemental data of this article can be accessed on the publisher’s website.
References
- [ANSES] French Agency for Food, Environmental and Occupational Health & Safety. 2011. Second French Total Diet Study (TDS2) Report 1. Inorganic contaminants, minerals, persistent organic pollutants, mycotoxins and phytoestrogens. Paris (France): ANSES.
- [ATSDR] Agency for Toxic Substances and Disease Registry. 1992. Toxicological profile for thallium. Atlanta (GA): Agency for Toxic Substances and Disease Registry.
- [ATSDR] Agency for Toxic Substances and Disease Registry. 2002. Toxicological profile for beryllium. Atlanta (GA): Agency for Toxic Substances and Disease Registry.
- [ATSDR] Agency for Toxic Substances and Disease Registry. 2017. Toxicological profile for antimony. Atlanta (GA): Agency for Toxic Substances and Disease Registry.
- [CCME] Canadian Council of Ministers of the Environment. 1999. Canadian soil quality guidelines for the protection of environmental and human health: Thallium (1999). In: Canadian environmental quality guidelines. Winnipeg (Canada): CCME.
- [CFS] Centre for Food Safety. 2007. Risk Assessment Studies Report No. 26 Chemical Hazard Evaluation dietary exposure to antimony of secondary school students. HongKong (China): Centre for Food Safety.
- [CFS] Centre for Food Safety. 2013. The First HongKong Total Diet Study: Metallic Contaminants. HongKong (China): Centre for Food Safety.
- [CFS] Centre for Food Safety. 2014. The First HongKong Total Diet Study: Minerals. HongKong (China): Centre for Food Safety.
- [COT] Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. 2008. COT Statement on the 2006 UK Total Diet Study of Metals and Other Elements. London (UK): COT.
- [EFSA] European Food Safety Authority. 2009. Scientific Opinion of the Panel on Contaminants in the Food Chain on arequest from German Federal Institute for Risk Assessment (BfR) on uranium in foodstuff, in particular mineral water. The EFSA J. 2009(1018):1–59.
- [EFSA] European Food Safety Authority. 2015. Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J2015. 13(2):4002, 202.
- EmsleyJ. 2001. Nature’s building blocks: An A–Z guide to the elements. Oxford (UK): Oxford University Press.
- [FSANZ] Food Standards Australia New Zealand. 2003. The 20th Australian Total Diet Study. Canberra (Australia): FSANZ.
- [FSANZ] Food Standards Australia New Zealand. 2011. The 23rd Australian Total Diet Study. Canberra (Australia): FSANZ.
- Golder Associates. 2012. Soil Guideline Value for Thallium. Moanataiari Subdivision, Thames. Golder Associates, New Zealand. [accessed 2018 Sep 21]. https://www.tcdc.govt.nz/PageFiles/8254/Thallium%20soil%20guideline%20value%20for%20Moanataiari%20Golder%20Report%20-%203%20Aug%202012.pdf.
- [JMPR] Joint Food and Agricultural Organization of the United Nations (FAO) and World Health Organization Meeting on Pesticide Residues. 1989. Bromide ion. In: Pesticide residues in food– 1988. Evaluations. Part II. Toxicology. FAO Plant Production and Protection Paper 93. Rome (Italy): FAO.
- MillourS, NoëlL, KadarA, ChekriR, VastelC, SirotV, LeblancJC, GuérinT. 2011. Pb, Hg, Cd, As, Sb and Al levels in foodstuffs from the 2nd French total diet study. Food Chem. 126:1787–1799.
- MillourS, NoëlL, KadarA, ChekriR, VastelC, SirotV, LeblancJC, GuérinT. 2012. Strontium, silver, tin, iron, tellurium, gallium, germanium, barium and vanadium level in foodstuffs from theSecond French Total Diet Study. JFood Compos Anal. 25:108–129.
- ParekhPP, KhanAR, TorresMA, KittoME. 2008. Concentrations of selenium, barium, and radium in Brazil nuts. JFood Compos Anal. 21(4):332–335.
- PearsonAJ, GawS, HermanspahnN, GloverCN. 2015. Natural and anthropogenic radionuclide activity concentrations in the New Zealand diet. JEnviron Radioact. 151(3):601–608.
- PearsonAJ, GawS, HermanspahnN, GloverCN. 2018a. Deterministic and semi-probabilistic modelling of the committed dose from radionuclides and the chemical burden from uranium in the New Zealand diet. JFood Prot. 81(9):1400–1410.
- PearsonA, GibbsM, LauK, EdmondsJ, AlexanderD, NicolasJ. 2018b. The 2016 New Zealand Total Diet Study. Wellington (New Zealand): Ministry for Primary Industries.
- [RIVM] Netherlands National Institute for Public Health and the Environment. 1998. Maximum permissible risk levels for human intake of soil contaminants, fourth series of compounds. Bilthoven (The Netherlands): RIVM.
- RoseM, BaxterM, BreretonN, BaskaranC. 2010. Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit Contam. 27(10):1380.
- RoseM, MillerP, BaxterM, AppletonG, CrewsH, CroasdaleM. 2001. Bromine and iodine in 1997 UK total diet study samples. JEnviron Monit. 3(4):361–365.
- SchrauzerGN. 2002. Lithium: occurrence, dietary intakes, nutritional essentiality. JAm Coll Nutr. 21(1):14–21.
- SmithTE, AsherCJ, StephensonRA, HetheringtonSE. 1997. Boron deficiency of avocado. 2. Effects on fruit size and ripening. In: BellRW, RerkasemB, editors. Boron in Soils and Plants. Developments in Plant and Soil Sciences. Vol. 76. Dordrecht: Springer; p. 135–137.
- ThyssenJP, MennéT. 2010. Metal allergy–a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 23(2):309–318.
- [US EPA] United States Environmental Protection Agency. 2008. Provisional Peer Reviewed Toxicity Values for Lithium. Washington (DC): US EPA.
- [US EPA] United States Environmental Protection Agency. 2009. Toxicological Review of Thallium and Compounds, In support of summary information on the Integrated Risk Information System (IRIS). Washington (DC): US EPA.
- [WHO] World Health Organization. 1996. Environmental Health Criteria 182: Thallium. International Programme on Chemical Safety. Geneva (Switzerland): WHO.
- [WHO] World Health Organization. 2003a. Antinomy in drinking-water. Background document for preparation of WHO Guidelines fordrinking-water quality. Geneva (Switzerland): WHO.
- [WHO] World Health Organization. 2003b. Uranium in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva (Switzerland): WHO.
- [WHO] World Health Organization. 2004. Barium in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva (Switzerland): WHO.
- [WHO] World Health Organization. 2009a. Beryllium in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva (Switzerland): WHO.
- [WHO] World Health Organization. 2009b. Boron in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva (Switzerland): WHO.
- [WHO] World Health Organization. 2010. Concise international chemical assessment document 77– Strontium and strontium compounds. Geneva (Switzerland): WHO.
- [WHO, FAO and EFSA] Food and Agriculture Organization of the United Nations and European Food Safety Authority. 2011. Towards aharmonised total diet study approach: aguidance document: joint guidance of EFSA, FAO and WHO. Geneva (Switzerland): WHO.
