ABSTRACT
To investigate the transfer of pyrrolizidine alkaloids (PAs) from feed to milk, rumen-cannulated dairy cows were intra-ruminally fed with 200 g/day of dried plant material of either ragwort (mixture of Jacobaea vulgaris and Senecio inaequidens), common groundsel (Senecio vulgaris) or viper’s bugloss (Echium vulgare) for a period of 4 days. PA levels in the plant materials were 3767, 2792 and 1674 µg g−1 respectively. Feed intake, milk yield and several blood parameters indicative for liver function were not influenced by the treatment. When fed ragwort, increased levels of PAs were detected in the milk, in particular jacoline and an unidentified cyclic diester, possibly a hydroxylated metabolite from retrorsine. The latter was the most important PA in milk from cows fed common groundsel. For viper’s bugloss, echimidine was the most abundant identified PA but in addition several hydroxylated PA metabolites were detected. For ragwort, the overall PA transfer was estimated at 0.05% and 1.4% for jacoline (N-oxide). Transfer rates were similar for viper’s bugloss (0.05%) but lower for common groundsel (0.01%). Only a small portion of the administered PAs was quantified in milk, urine and faeces, with an overall balance of 4.5%, 2.9% and 5.8%, for ragwort, common groundsel and viper’s bugloss, respectively. Samples taken from the rumen indicated that the N-oxides were converted into the free bases, which was confirmed by in vitro studies with the same plant species incubated with ruminal fluid. These results confirm that the transfer of PAs to milk is relatively low but may be of concern for human health regarding the genotoxic and carcinogenic properties of these compounds. The transfer rate depends on the type of PAs present in the weeds. The incomplete balance of input vs output stresses the need to further investigate the metabolism and the potential transfer of metabolites into edible products.
Introduction
Pyrrolizidine alkaloids (PAs) (), toxic secondary metabolites in many plants, present a potential risk for the health of animals and humans. A number of PAs show carcinogenic and genotoxic properties, implying that a large margin of exposure (104) is required to protect public health (EFSA Citation2011; JECFA Citation2015). EFSA recently evaluated these plant toxins and concluded that the current level of exposure of humans does not ensure this safety margin (EFSA Citation2017). For humans, the most important sources of PAs are contaminated honey, herbal tea and certain herbs used as food supplements and as kitchen herbs (Dübecke et al. Citation2011; Bodi et al. Citation2014; Mathon et al. Citation2014; Mulder et al. Citation2018; Picron et al. Citation2018). Occasionally, cereals or leafy vegetables (like rocket salad) may get contaminated with PA-containing weeds (Kakar et al. Citation2010; Wiedenfeld Citation2011). However, animal-derived food products such as milk, meat and eggs could also be a potential source of PAs due to the presence of weeds in feed and the subsequent transfer of PAs from the feed to edible products of animal origin (Hoogenboom et al. Citation2011; Mulder et al. Citation2016).
Figure 1. Representative pyrrolizidine alkaloids, present in ragwort, common groundsel and viper’s bugloss, showing the structural diversity
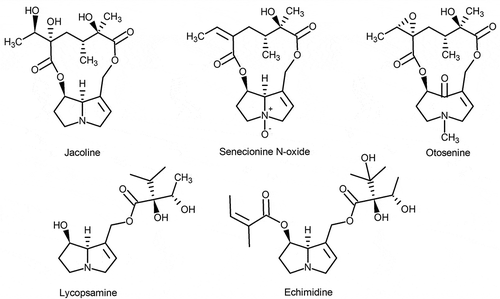
Previously, we studied the potential transfer of PAs from a mixture of common ragwort (Jacobaea vulgaris) and narrow-leaved ragwort (Senecio inaequidens) to milk, showing a relatively low overall transfer of around 0.1% (Hoogenboom et al. Citation2011). These results confirmed the results from an older study by Dickinson et al. (Citation1976). Hoogenboom et al. (Citation2011) observed a selective excretion of specific PAs to the milk. In particular, the transfer of jacoline and certain otonecine type PAs was high compared to other PAs. Since the PA composition differs between plant species, this implies that the overall transfer rate might also be rather different. It was decided to study this transfer in more detail by including, besides the previously used mixture of common ragwort and narrow-leaved ragwort, also common groundsel and viper’s bugloss, which all are common weeds in the Netherlands, as well as in other parts of the world.
Hoogenboom et al. (Citation2011) also observed that a major part of the PAs appeared to have been metabolised, based on the rather poor overall recovery of these compounds in urine, faeces and milk. Conversion or degradation of PAs is of interest to human health since potential metabolites may also contribute to the transfer to food products of animal origin. Furthermore, metabolism of PAs is a requirement for bioactivation and toxicity and it cannot be excluded that certain metabolites might still possess genotoxic properties. However, this conclusion could not be firmly drawn from the first study since urine and faeces were not collected quantitatively during the exposure experiment but only at one time point (4 h after administration). This could have resulted in a potential underestimation of excretion of intact PAs and therefore excreta were collected for a longer time period in the present study. Furthermore, the fate of PAs in the rumen was studied both by taking samples from the cow’s rumen after applying the weeds, and by incubating the various weeds in vitro with ruminal fluid.
Materials and methods
Preparation of plant materials
The ragwort used in this follow-up study was from the same batch as used in the previous transfer study (Hoogenboom et al. Citation2011), being a mixture of 84% (w/w) common ragwort (Jacobaea vulgaris, syn. Senecio jacobaea) and 16% narrow-leaved ragwort (Senecio inaequidens). The material was harvested in June and July 2008 in the province Gelderland in the Netherlands. Common groundsel (Senecio vulgaris) and viper’s bugloss (Echium vulgare) were collected in 2010 at various locations mostly in the vicinity of Wageningen in the Netherlands. All plants were air dried, subsequently milled to 1 mm using a Peppink 200 AN Grinding machine (Veerman, Olst, The Netherlands) and homogenised. The materials were stored in airtight plastic containers at room temperature until use.
Animal study and collection of samples
Animals
All experimental protocols and interventions were approved by the Ethical Committee on Animal Experiments of the Animal Sciences Group of Wageningen University and Research Centre (Lelystad, the Netherlands). Three multiparous Holstein-Friesian dairy cows (all third parity, live weight around 610, 720 and 790 kg, producing on average 35, 24 and 41 kg milk per day, respectively), equipped with rumen cannulas were selected (Supplementary Material, Table S1). Cows were housed in a tie-stall barn and had free access to water.
Feeding
Cows were fed twice daily at 8 AM and 4 PM with equal meals of a total mixed ration (TMR) of grass silage, maize silage, condensed distillers solubles, rape seed meal and concentrates in different proportions to match energy and protein requirements with ad libitum intake (Supplementary Material 1, Table S1). Refusals were weighed in the morning just before feeding fresh material, to calculate individual daily feed intake. Feed samples were collected once (concentrates) or twice (silage) a week and stored frozen at −18°C until analysis. In TMR samples taken during the feeding trial no PAs were detected.
Treatment
Each test period consisted of a four-day treatment period (day 1 to 4) and a three day washout period (day 5 to 7) before the next period started. Cows were treated directly before the morning feeding (once a day around 8 AM), by administering 200 g of the dried plant material directly in the rumen through the cannula. Animals received the ragwort mixture in Period 1, common groundsel in Period 2 and viper’s bugloss in Period 3.
Milk
Animals were milked twice daily; individual milk yield was registered and the total milk volume was homogenised and immediately cooled to 4°C. At each milking, two samples of 50 mL of the homogenised milk were taken per cow and frozen at −18°C for further PA analysis.
Rumen
Samples (2 x 500 g) were taken at day 4 of each period, just before administering the weeds at 8 AM, and subsequently at 10.30 AM, 1 PM, 4 PM and 9.30 PM. Samples were stored at −18°C.
Faeces
Faeces were collected from day 2 to 4 of each period. The amount of faeces collected was weighed two times per day (at 8 AM and 9.30 PM). After weighing, faeces were mixed and 2 samples of 500 g were taken per cow and stored at −18°C. The dry matter content of faeces was determined by drying approx. 100 g overnight in an electrical drying stove.
Urine
Cows were equipped with urine catheters during day 2 to 4 of each treatment period. Urine was collected by the urine catheters starting at 10.30 AM on day 3 until 8 AM on day 5 of each period. Collected urine was weighed at approx. 10.30 AM, 1 PM, 4 PM, 9.30 PM and 8 AM. After weighing, urine was mixed and two samples of 50 mL were taken per cow and stored at −18°C.
Blood
Blood was sampled from the tail vein in lithium heparin-coated tubes (Vacuette, type 455084; Greiner Bio-One, Frickenhausen, Germany) before the start of the experiment (day 0) and on day 4 of each period. Blood samples were centrifuged at 1500 × g for 10 min and blood plasma was stored in duplicate at −18°C until further analysis. Plasma was analysed for liver enzymes (aspartate aminotransferase (ASAT), gamma glutamyl transpeptidase (GGT), glutamate dehydrogenase (GLDH)) and bilirubin concentration as indicators for potential liver damage. Analyses of ASAT, GGT, direct and total bilirubin (using test kits of Synchron CX, Beckman Coulter, Ireland) and glutamate dehydrogenase (GLDH) (using a RANDOX kit, Randox, UK) were carried out at the Veterinary Diagnostic Laboratory (UVDL) of Utrecht University, Utrecht, The Netherlands.
Rumen fluid model experiments
Apart from the study with cows, in vitro studies were performed with rumen fluid, which was collected from 2 rumen cannulated lactating cows, 2 hours after the morning feeding. The cows were fed with a grass and maize silage and supplemented with concentrate according to requirements. The rumen fluid was transported to the laboratory in insulated flasks, prefilled with CO2. Rumen fluid was filtered through two layers of cheese cloth and mixed (1:2 v/v) with an anaerobic buffer/mineral solution, containing per litre 8.75 g NaHCO3, 1.00 g NH4HCO3, 1.43 g Na2HPO4, 1.55 g KH2PO4, 0.15 g MgSO4.7H2O, 0.52 g Na2S, 0.017 g CaCl2.2H2O, 0.015 g MnCl2.4H2O, 0.002 g CoCl3.6H2O, 0.012 g FeCl3.6H2O and 1.25 mg resazurin. The residue on the cheese cloth was discarded. All manipulations were done under continuous flushing with CO2 (Cone et al. Citation1996).
Incubations were conducted with four different weeds: common ragwort, narrow-leafed ragwort, common groundsel and viper’s bugloss. Triplicate fermentations were conducted in 250 mL serum bottles (Schott, Mainz, Germany) in which 500 mg ground plant material was incubated in 60 mL buffered rumen fluid. The headspace of the bottles was filled with CO2. The bottles were placed in a shaking water bath with 50 movements per minute at 39°C.
At t = 10 min, 20 min, 40 min, 1 h, 3 h, 6 h and 24 h, 1 mL from the content of the bottles was sampled and mixed with 2 mL ice-cold methanol, vortexed, put on ice for 10 min and stored at −20°C. As a control, at t = 0, 12 mL buffered rumen fluid was collected and mixed with 24 mL ice-cold methanol, vortexed and put 10 min on ice. Then, 100 mg of the ground plant material was added, and this mixture was vortexed and stored at −20°C. One control sample of buffered rumen fluid was prepared without the addition of plant material. No PAs were detected in the control sample upon LC-MS/MS analysis.
Pyrrolizidine alkaloid standards
Commercially available analytical PA standards were obtained from Phytolab (Vestenbergsgreuth, Germany) or from Phytoplan (Heidelberg, Germany). Florosenine was custom isolated from Senecio inaequidens plant material by PRISNA (Leiden, the Netherlands). All standards were at least 95% pure. A total of 18 PA standards (erucifoline, integerrimine, jacobine, retrorsine, riddelliine, senecionine, seneciphylline and the corresponding N-oxides; florosenine, jacoline, otosenine and senkirkine) were used for the study with ragwort and common groundsel, and 4 PA standards (lycopsamine, echimidine and the corresponding N-oxides) for the study with viper’s bugloss. Heliotrine (Latoxan, Valence, France) was used in all experiments as internal standard and had a purity of 90% according to LC-MS/MS analysis.
Analysis of PAs in plant material
The analysis of the homogenised plant materials was conducted according to the method described previously (Hoogenboom et al. Citation2011). To assess the PA composition and homogeneity of the materials, 10 samples were randomly taken and transferred to 50-mL test tubes. Heliotrine (100 µL of a 100 µg mL−1 solution in methanol) was added as internal standard. Twenty-five millilitres of a 2% formic acid solution in water were added and the samples were extracted by rotary tumbling for 1 h. Of the extracts, 2 mL was filtered over a 0.45 µm Teflon spin filter and a 25 µL aliquot was taken from the resulting clear extract, transferred to an HPLC vial and mixed with 975 µL of a 10 mM ammonia solution in water. The sample extracts were injected on the LC-MS/MS in a randomised order. Quantification was performed with internal standard correction against a 6-point calibration curve of PA standards (0–500 ng mL−1) in a diluted extract of Tanacetum vulgare (tansy or golden buttons). The extract of T. vulgare was prepared the same way as the ragwort extracts and was used to mimic a blank plant extract. Although the plant extracts were diluted 40 times to match the concentration of the major components with the linear range of the MS detector, many minor components could be adequately detected and (semi) quantified. The limit of quantification (LOQ) for the individual PAs and their N-oxides in dried plant material was between 0.2 and 0.5 µg g−1. No PAs were detected in the extract of T. vulgare.
Analysis of PAs in milk
The PA content in milk and milk products was assessed by means of LC-MS/MS. A modified procedure was followed compared to the previous study. Test portions of 3.0 mL of thawed milk were transferred to 50-mL test tubes. Seventy-five µL of heliotrine IS solution (100 ng mL−1) were added, followed by 15 mL 0.2% formic acid solution and 5 mL hexane. The mixture was agitated for 30 min by means of a rotary tumbler and centrifuged for 15 min at 3500 × g. The hexane layer, and most of the proteins that were present at the border between the aqueous and the hexane layer, were removed using a disposable pipette. The pH of the aqueous layer was adjusted to 10 by adding concentrated ammonia (25%), checked by using a pH test strip. The sample was purified and concentrated by solid phase extraction (SPE) over Strata-X 60 mg, 3 cc cartridges (Phenomenex, Torrance, CA, USA). Cartridges were conditioned with 3 mL methanol and equilibrated with 3 mL water. After application of the sample extracts, the cartridges were washed with 3 mL 1% aqueous formic acid solution, followed by 3 mL 1% aqueous ammonia solution. The cartridges were dried under reduced pressure and eluted with 3 mL methanol. The eluates were evaporated to dryness under a gentle stream of nitrogen at 50°C and the dry residues were reconstituted in 500 µL water/methanol 9:1 (v/v) and transferred to mini UniPrep filter vials (Whatman, Maidstone, UK). Subsequently, 10 µL was injected on the LC-MS/MS system. The sample extracts were quantified against an 8-point calibration curve of PA standards (0–25 ng mL−1) in blank milk extract of samples collected for the individual cows before the start of the study. The LOQ in milk for the individual PAs and their N-oxides was between 0.05 and 0.2 ng mL−1.
Analysis of PAs in urine and faeces
The analysis of PAs in urine and faeces was based on the procedure described in our previous study (Hoogenboom et al. Citation2011). Urine samples were thawed overnight at room temperature. Two aliquots (2 mL) of each sample were transferred to 10 mL test tubes and 4 mL 0.1% aqueous ammonia solution was added. The pH of the extract was checked with a pH test strip and adjusted to pH 10 if necessary. Following addition of heliotrine (50 µL of a 1 µg mL−1 solution in methanol), the extracts were shaken manually and purified by SPE over Strata-X 60 mg, 3 cc cartridges following the same protocol as described above for milk. The final extracts were prepared by reconstitution of the dry residues in 1 mL water/methanol 9:1 (v/v). Subsequently, 10 µL was injected on the LC-MS/MS system. Quantification was performed with internal standard correction against an 8-point matrix matched calibration curve of PA standards (0–250 ng mL−1) in blank urine. For each cow individual calibration curves were constructed using a blank urine sample collected during the pre-administration period. LOQs ranged between 0.2 and 0.5 µg L−1 for most PAs. In general, the free bases were somewhat more sensitive than the N-oxides.
Deconjugation of urine samples was carried out as follows: two aliquots (2 mL) of the urine sample were transferred to 10 mL test tubes. The pH of the urine was adjusted to 4.8 with concentrated acetic acid. Helix pomatia extract (20 µL, β-glucuronidase activity: 4.5 standard units per mL at 25°C, pH 4.5) (Merck, Darmstadt, Germany) was added and the sample extracts were incubated for 16 h at 37°C in a water bath. The extracts were processed as described above. For each cow an individual matrix matched calibration curve was included in the same concentration range as for the non-hydrolysed samples. The matrix matched standards were also subjected to the deconjugation procedure.
Faeces samples were thawed overnight at room temperature. Samples were homogenised by hand with a spoon. Two aliquots (2 g) were transferred to 50-mL test tubes. To the test samples, 40 mL 2% aqueous acetic acid was added together with heliotrine (50 µL of a 1 µg mL−1 solution in methanol). The mixtures were extracted by rotary tumbling for 1 h and subsequently centrifuged at 3500 × g for 10 min. Twenty mL of the supernatant was transferred to a 50 mL test tube and the pH was raised to 10 with concentrated ammonia. The extracts were purified by SPE as described above for milk. The final extracts were prepared in 500 µL water/methanol 9:1 (v/v). Quantification was performed with internal standard correction against an 8-point matrix matched calibration curve of PA standards (0–50 ng mL−1) in blank faeces. For each cow, individual calibration curves were constructed using a faeces sample from week 1 (pre-administration period). LOQs were comparable to those in urine.
Analysis of PAs in rumen fluid
Aliquots of the methanol-diluted rumen fluid (100 µL for common ragwort, common groundsel and viper’s bugloss and 25 µL for narrow-leaved ragwort) were diluted with water to 500 µL and transferred to mini UniPrep filter vials (Whatman, Maidstone, UK). Quantification was performed against a 6-point matrix matched calibration curve of PA standards (0–400 ng mL−1) in 50 µL blank rumen fluid diluted with 450 µL water.
Instrumentation
Analyses of plant extracts, rumen fluid, urine and faeces samples for PA content were performed on a Waters Acquity UPLC coupled to a Waters Quattro Premier XE tandem mass spectrometer (Waters, Milford, MA, USA), operated in positive electrospray mode. Analysis of milk samples was performed on a Waters Acquity UPLC coupled to a Waters Xevo TQ-S tandem mass spectrometer (Waters, Milford, MA, USA), operated in positive electrospray mode. With both systems, compounds were separated on a Waters Acquity BEH C18 150 × 2.1 mm, 1.7 µm analytical column, kept at 50°C and run at 0.4 mL min−1 with an acetonitrile/water gradient containing 6.5 mM ammonia. The gradient started at 100% water and was changed to 50% acetonitrile in 12 min. Total runtime of the method was 15 min. For both systems the MS/MS collision energy was optimised for the individual compounds using reference standards or plant extracts when standards were not available. Two precursor product ion transitions were selected and incorporated in a multiple reaction monitoring (MRM) method. Two MS/MS methods were constructed, one covering all potentially relevant PAs from ragwort and common groundsel and one method covering the PAs present in viper’s bugloss. An overview of the mass spectrometric settings used for the detection of the relevant PAs is given in Supplementary Material, Tables S2 and S3. Although the chemical structure of some metabolites could not be elucidated, all compounds could be unequivocally characterised on the basis of retention time and fragmentation transitions. With the final method, over 90 compounds (including over 10 metabolites) were monitored. For those compounds for which no reference standard was available, a semi-quantitative (indicative) concentration could be obtained by comparison with a closely related analogue (often an isomer), which exhibited a similar MS/MS fragmentation spectrum. In these cases the same transitions were used. For compounds such as metabolites with tentative or unknown structures, no closely related standard with similar MS/MS spectrum could be identified. In such cases, the concentration was estimated by taking the sum of the two most intense fragments and comparing this with the sum area of the transitions selected for a standard having the same or similar MW, form (free base or N-oxide) and retention time. It should be noted that for matrices with a substantial contribution of unknown PAs, e.g. urine samples, quantification is less reliable.
Results and discussion
Animal performance during the study
All three cows were treated sequentially for 4 days with 200 g dried material per day of either a ragwort mixture, common groundsel or viper’s bugloss, supplied via a rumen cannula. Assuming a daily dry matter intake of 20 kg, this corresponds to a contamination level of feed with PA-containing plants of 1%. This level and the direct application to the rumen is thought to represent a worst case situation.
The production parameters of the three animals at the start of the study and the relative composition of their feed rations during the study are presented in Supplementary Material, Table S1. The feed intake is shown in the Supplementary Material, Figure S1. The average daily feed intake was 22.5 ± 1.8 kg dm. Cow 1 showed a decreased feed intake and milk production when supplied with ragwort. However, it is unclear if this could have been caused by the treatment since it was not also observed with the other cows and during the consecutive treatments. Daily milk yield of the animals is shown in the Supplementary material, Figure S2. The average daily milk production was 31.0 ± 1.6 kg. Compared to the daily feed intake and milk yield per cow during the periods of administration of the plant materials was not different from that during the wash-out periods.
Urine and faeces were collected on day 3 and day 4 of the test periods. Neither urine nor faeces production changed over each of the two-day periods. The average urine production was 28.1 ± 1.9 L per day and the average amount of faeces collected was 38.6 ± 3.6 kg per day. The dry matter content of faeces during the study period averaged at 15.8 ± 1.4, 12.8 ± 0.6 and 17.0 ± 0.9%, for cow 1, 2, and 3, respectively. Faecal dry matter content did not change during the course of the study.
The results of the serum samples analysed for ASAT, GGT, GHDL and bilirubin showed no consistent increases suggestive for liver damage, that could be related to the administration of the plant toxins (Supplementary Material, Table S4). One animal showed a temporarily increased level of ASAT during administration of common groundsel.
Levels of pyrrolizidine alkaloids in plant materials and feed
Levels of PAs in the three weeds are shown in (ragwort mixture), (common groundsel) and (viper’s bugloss), together with those in samples of milk, urine and faeces. The data for the plants show that the composition of PAs was different between the three weeds. The ragwort mixture had the highest overall PA content, 3800 mg kg−1, with large contributions of the N-oxides of erucifoline, retrorsine, senecionine, seneciphylline and jacobine, and in the case of the free bases, jaconine (). In addition, this mixture contained relatively small amounts of a number of otonecine type PAs, derived from the narrow-leaved ragwort. Overall, the contribution of the N-oxides was higher than that of the free bases. This was even more the case for common groundsel, showing the highest contribution by the N-oxides of seneciphylline, senecionine, retrorsine, and to a lesser extent those of integerrimine, spartioidine and senecivernine. The total level of detected PAs in the common groundsel material was 2800 mg kg−1. The viper’s bugloss material contained an overall amount of 1700 mg PAs kg−1 with primarily the N-oxide of echimidine and the N-oxide of leptanthine or its isomer echimiplatine, which are 9-echimidinic esters of retronecine (Boppré et al. Citation2005; El-Shazly and Wink Citation2014).
Table 1. Pyrrolizidine alkaloid concentrations in ragwort, milk, urine and faeces. Mean ± SD for three cows for milk collected during 4 days and for urine and faeces collected during 2 days
Table 2. Pyrrolizidine alkaloid concentrations in common groundsel, milk, urine and faeces samples. Mean ± SD for three cows for milk collected during 4 days and for urine and faeces collected during 2 days
Table 3. Pyrrolizidine alkaloid concentrations in viper’s bugloss, milk, urine and faeces samples. Mean ± SD for three cows for milk collected during 4 days and for urine and faeces collected during 2 days
The TMR daily fed to the cows did not contain any PAs above the limit of detection (data not shown). Based on the identified and quantified PAs, the animals were exposed to, respectively, 753, 558 and 335 mg PAs/day during each of the 4-days treatment period with the ragwort mixture, common groundsel and viper’s bugloss.
PAs in milk
The total PA concentration in morning and evening milk during the three 4-day periods is shown in . The highest levels around 22 µg L−1 were observed for the ragwort mixture, followed by viper’s bugloss (8 µg L−1) and common groundsel (3 µg L−1). Levels were always higher in the evening than in the morning, the latter being collected before administering the new plant material. In the case of common groundsel, levels in the morning were practically non-detectable. –3 include the concentrations of individual PAs detected in milk during administration of the three plant materials. As shown before (Hoogenboom et al. Citation2011; de Nijs et al. Citation2017), jacoline was the main PA identified in milk during the treatment with ragwort, whereas in the plant material it was only a minor component (65% versus 1%). The second highest level was observed for a macrocyclic ester with protonated mass 368 (FB368), tentatively identified as a hydroxy metabolite originating from retrorsine. This PA was also the most important compound in the milk during the treatment with common groundsel. This putative metabolite was not detected in the plant materials nor in milk collected before the treatment. In milk produced during the treatment with ragwort small amounts of jacobine, jacobine N-oxide and jaconine were also detected, as well as the otonecine-type PAs senkirkine, otosenine and florosenine. Upon treatment with common groundsel, small amounts of known PAs were also detected in the milk, the most abundant being retrorsine and eruciflorine (21-hydroxyintegerrimine). Trace amounts of some N-oxides were also found, in particular eruciflorine-N-oxide.
Figure 2. Total PA concentrations in milk of dairy cows. Animals were treated after milking in the morning on days 1, 2, 3 and 4 (time points of administration are indicated with arrows) with 200 g of ragwort mixture, common groundsel or viper’s bugloss. Average ± SD of three cows
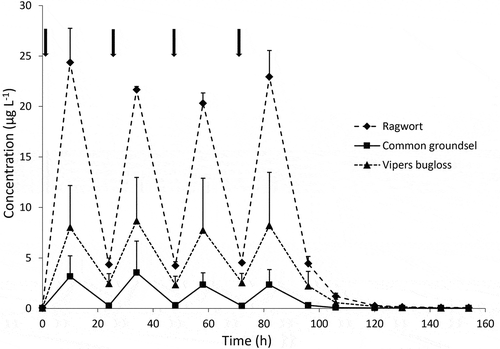
In the case of the treatment with viper’s bugloss, leptanthine (or echimiplatine) was the most abundant. In addition, echimidine was found, as well as an isomer (FB398-4; possibly the tigloyl analogue of echimidine (El-Shazly and Wink Citation2014)) and several putative hydroxy metabolites with protonated mass 414 (FB414-1, 2, 3). The latter were not observed in the plant material. In all experiments, N-oxides were detected in milk but they contributed 5% or less to the overall PA concentrations, due to degradation or reduction in the rumen or during first-pass metabolism (see below).
PAs in urine
The PA concentrations in urine collected during day 3 and 4 of the administration of the plant materials are shown in . Levels for the ragwort mixture and common groundsel were higher than those for viper’s bugloss. This seems only partly due to the lower levels in the plant material (see mass balance, below). For each of the three weeds, levels peaked within the first 8 h after administration of the weeds and were almost non-detectable 24 h later, both on day 4 (t = 24 h) and 5 (t = 48 h); for viper’s bugloss, the peak was lower and appeared slightly later than for the other weeds. –3 include the concentrations of individual PAs in urine, showing for the ragwort mixture about equal contributions of the free bases and the N-oxides. This indicates that the N-oxides are largely converted to free bases. Jacoline and jacobine-N-oxide contributed most to the levels, followed by jacobine, jaconine, retrorsine-N-oxide and its free base, and eruciflorine N-oxide and its free base. In comparison to other PAs, otonecines contributed to a relatively high extent to the total PA content in urine. In addition, the hydroxy metabolite FB368, also detected in milk but not in the plant material, was present. Overall, the contribution of PAs for which no standard was available increased in comparison to the plant material.
Figure 3. Total PA concentrations in urine of dairy cows at days 3 and 4. Animals were treated in the morning on days 1, 2, 3 and 4 (relevant time points of administration are indicated with arrows) with 200 g of ragwort mixture, common groundsel or viper’s bugloss. Average ± SD of three cows
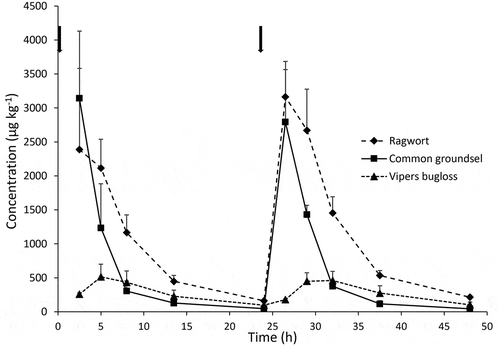
In the case of common groundsel, N-oxides, such as those of seneciphylline, retrorsine, and especially eruciflorine, contributed for 76% to the total PA content in urine. The latter compound is surprising since its contribution in the plant material is very minor (38% in urine versus 0.09% in plant material; based on the sum of free base and N-oxide). Eruciflorine could be formed by hydroxylation of integerrimine or senecionine (Wang et al. Citation2011). Only 40% of the PAs quantified in urine were available as standards, as compared to 99% in the plants, to a large extent due to eruciflorine and its N-oxide. In addition, some retronecine cyclic diesters and their N-oxides were observed, which could not be detected in the plant. This included the hydroxy metabolite FB368, which was the most important PA in milk when cows were administered common groundsel.
For viper’s bugloss, an approximately equal contribution of free bases and N-oxides to the PA levels was observed. However, only 5% could be attributed to PAs for which a standard was available, like echimidine and its N-oxide. Several hydroxylated analogues (FB414-1 to 414–5) were found. Only a small part of the PAs excreted in urine could be related to PAs already present in the plant material. Leptanthine and its N-oxide were important compounds excreted in urine. Interestingly, some retronecine monoesters, with MW of 238 (free base form) and MW 254 (N-oxide form) were also observed. These compounds were tentatively assigned as the angeloyl/tigloyl esters of retronecine, which could have been formed from echimidine and its tigloyl isomer by hydrolysis of the echimidinic ester at the C9 position.
The urine samples were subjected to deconjugation of possible glucuronides using Helix pomatia. Except for seneciphylline no significant differences were observed for the urine samples collected from the three treatments. In the deconjugated urine samples of ragwort and common groundsel the concentration of seneciphylline had increased approximately 8-fold and 10-fold, respectively. No effect was observed for seneciphylline N-oxide, which is an indication that glucuronidation takes place on the tertiary nitrogen of the necine base, as proposed by He et al. (Citation2010).
PAs in faeces
The PA concentrations in faeces collected in the morning and in the evening are shown in . Contrary to the urine, the variation between evening and morning samples was rather small. Highest PA concentrations were observed for the ragwort mixture, followed by viper’s bugloss and common groundsel. In –3 the concentrations of individual PAs detected in faeces are summarised. For all three plant materials, no N-oxides could be detected in faeces. In the case of the ragwort mixture, jacoline was by far the most important PA, followed by jacobine, otosenine, seneciphylline, floridanine, erucifoline, retrorsine and eruciflorine. Otonecine PAs, such as otosenine, floridanine and onetine were detected at relatively high levels when compared to plants. This again suggests a relatively poor metabolism of these PAs. Over 90% of the PAs detected in the faeces could be attributed to known standards.
Figure 4. Total PA concentrations in faeces of dairy cows at days 3 and 4. Animals were treated in the morning on days 1, 2, 3 and 4 (relevant time points of administration are indicated with arrows) with 200 g of ragwort mixture, common groundsel or viper’s bugloss. Average ± SD for three cows
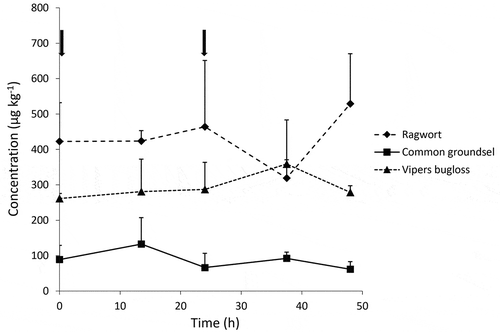
For common groundsel, seneciphylline was the most important PA in the faeces with smaller contributions of eruciflorine, retrorsine and senecionine. Overall, the contribution of known PAs in faeces was higher than in urine (73% vs 39%). For viper’s bugloss, echimidine was the major compound excreted via faeces, together with substantial amounts of a retronecine diester (FB414-5), and leptanthine/echimiplatine, lycopsamine/intermedine, and again some retronecine monoesters, with MW 238, which are possibly angeloyl or tigloyl esters of retronecine. The fraction of known PAs in faeces was relatively small (26%) compared to the other plant species, but larger than the proportion found in urine (5%).
Mass balance
The amounts of PAs administered and subsequently excreted via milk, urine and faeces were calculated for days 3 and 4 for each cow, averaged over the two days and three cows, and summarised in . The overall recovery of the PAs applied including PAs for which no standards were available was 4.5%, 2.9% and 5.8% for, respectively, the ragwort mixture, common groundsel and viper’s bugloss. For the latter, the mass balance is rather indicative, due to the large proportion of unknown PAs, particularly in urine and faeces. Despite the fact that several hydroxylated PAs were included in the analysis these mass balances are rather poor. This confirms our previous results (Hoogenboom et al. Citation2011) showing that most of the applied PAs appear to be metabolised to unknown compounds. In addition to low molecular weight molecules formed by hydrolysis, reactive metabolites may be bound to DNA and especially proteins, or excreted as glutathione adducts or their metabolites (Fu et al. Citation2004; Ruan et al. Citation2014; Geburek et al. Citation2020). The analysis of protein-bound metabolites in liver could be an important way to determine the actual exposure of animals to PA-containing weeds. Another option would be to use a sum parameter method that quantifies all PAs with the same necine backbone (Cramer et al. Citation2013; Mädge et al. Citation2015). Such an approach also has the advantage that metabolites that are not included in the targeted LC-MS/MS analysis are quantified. The main limitation of the published methods is that only metabolites with the retronecine (or heliotridine) backbone can currently be quantified. PA metabolites with a different necine backbone (e.g. otonecine, pyrrole, 1,2-saturated or hydroxylated retronecines) are still missed.
Table 4. Mass balance of PAs administered and excreted as identified PA metabolites in 24 h. Average of 2 days for urine and faeces, average of 4 days for milk (mean ± SD for three cows)
The amounts excreted via the milk were, respectively, 0.37, 0.05 and 0.16 mg per day for the ragwort mixture, common groundsel and viper’s bugloss, corresponding to transfer rates of 0.05%, 0.01% and 0.05%. For the ragwort mixture, this transfer rate is lower than the 0.1% observed in the previous study (Hoogenboom et al. Citation2011), but this can be due to differences in the study set up (one high daily dose instead of two lower daily doses) and to intra-species differences. The data for common groundsel show that even lower transfer rates may be obtained with a different mixture of PAs, but this also implies that the reverse could be the case for, e.g. weeds containing high levels of jacoline, or otonecines, which show a much higher transfer to milk. The differences in kinetics between PAs also resulted in different ratios of the amounts excreted via either faeces or urine.
Ruminal metabolism
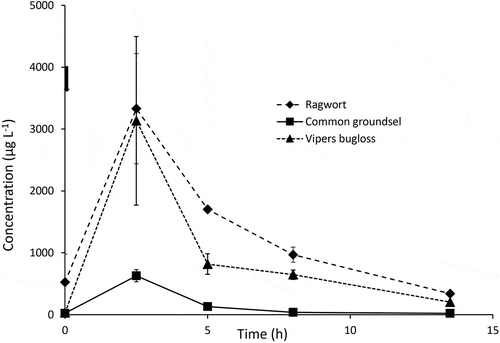
Ruminal metabolism was considered as a possible factor contributing to the poor overall mass balance. To study the effect of the rumen, at several time points after the administration of the plant material, samples were taken from the rumen. shows the concentrations in the rumen from 2.5 to 13.5 h after administration. Clearance from the rumen is fastest for the PAs from common groundsel: after 8 h the concentration of PAs in the rumen had decreased to 6% of the concentration after 2.5 h, while for ragwort this was 30% and for viper’s bugloss 20%. Of the PAs still present in the rumen after 8 h of administration of the ragwort mixture, jacoline accounted for 42%, followed by jaconine (22%), jacobine (8%) and the combined otonecines (17%). For common groundsel, after 8 h of administration eruciflorine was the most abundant compound (36%), followed by the hydroxy metabolite FB352-1 (19%). With respect to viper’s bugloss, echimidine and its isomer (FB398-4) were accounting for almost 90% of PAs still present in the rumen after 8 h (data not shown). At all time-points, only traces of N-oxides were detected in the rumen samples. These results either suggest a rapid conversion of the N-oxides into the free bases, or a rapid transport of the N-oxides through the gastrointestinal tract or a selective absorption of these PAs in the gastrointestinal tract. Furthermore, it cannot be excluded that some reduction occurred after the samples were taken from the animals, or when the samples were processed for chemical analysis (e.g. during thawing).
Figure 5. Total PA concentrations in rumen fluid taken from the cows via the rumen cannula at day 4. Animals were treated in the morning on days 1, 2, 3 and 4, with 200 g of ragwort mixture, common groundsel or viper’s bugloss. The t = 0 rumen sample was taken just before the plant material was supplied (indicated with an arrow). Average ± SD for three cows
Therefore, in a separate experiment, plant material of common ragwort, narrow-leaved ragwort, common groundsel and viper’s bugloss was incubated in vitro with ruminal fluid under controlled conditions to determine ruminal degradation in the absence of absorption or passage. Aliquots taken were immediately mixed with methanol to stop metabolic activity. The results for the time points from 0 to 3 h are shown in , while the results including the 6 h and 24 h time points are shown in Supplementary Material, Figure S3. The results indicate that regarding the overall PA concentration in the rumen, there is a slow but general decrease of the total content of known PAs. After 3 h of incubation, the overall PA concentration was 66% to 97% of the starting content. A more significant decrease in PA concentration was observed after 24 h of incubation (between 5% and 65% recovered, Supplementary Material, Figure S3). After 24 h, jacoline (73%) was the most important PA present in the ragwort incubation, in the common groundsel incubation this was retrorsine (43%). This PA was also the major PA (56%) found after 24 h in the narrow-leaved ragwort incubation. The viper’s bugloss incubation contained echimidine (57%) as major PA.
Figure 6. Rumen fluid in vitro incubations with selected plant materials. Total PAs (triangles), free bases (rectangles) and N-oxides (squares) concentration in the rumen fluid after addition of 500 mg ground common ragwort (A), narrow-leaved ragwort (B), common groundsel (C) or viper’s bugloss (D). Average ± SD of three incubations
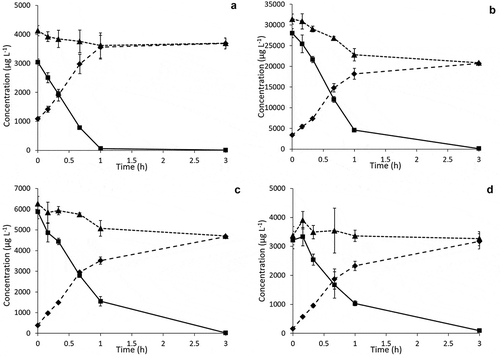
Formation of new PA metabolites was observed in none of the incubation experiments. However, a fast conversion from N-oxides into the corresponding free bases was observed for all plant materials. Reduction proceeded somewhat faster for the ragwort incubations (98% free base content after 1 h) compared to the other incubations (70–80% free base content after 1 h), but after 3 h of incubation essentially all PAs were present in their free base form, which is in good agreement with the experimental rumen samples taken from the cows after 2.5 h. The reduction rate appeared to be linear during the first hour of incubation, suggesting zero order kinetics, and the individual compounds showed very similar reduction behaviour within the individual incubations (Supplementary Material, Table S5). From the concentrations of the individual PAs in the incubations an efficiency of reduction in the order of 50% to 90% could be estimated.
The experiments with the rumen fluid model also indicate that besides the relatively fast N-oxide reduction, a second process takes place, in which the PAs are slowly degraded, probably by hydrolysis of ester linkages (Lanigan Citation1970; Lanigan and Smith Citation1970). Depending on the diet it can take up to 24 h before plant material is sufficiently digested in the rumen to be passed to the reticulum (Tamminga et al. Citation1994). During digestion, PAs are extracted from the plant material and dissolved in the rumen fluid. From 13 C-studies, it can be estimated that approximately 10% of the rumen fluid passes to the reticulum per hour (Daniel et al. Citation2014). This means that in 6–7 h approximately 50% of the rumen fluid is renewed and after 24 h ca 87%. From the in vitro rumen incubation it follows that there is a substantial variation in the PA conversion rate for the different plant materials (35% to 95% metabolised after 24 h, see above). It will strongly depend on the average retention time of PAs in the rumen, how much this process of ruminal hydrolysis contributes to the overall metabolism in the animal and whether it can explain the poor overall mass balance.
Conclusions
The current study confirms that the transfer of PAs to milk seems rather low. However, the overall balance obtained with different PA containing plants is poor and it remains to be determined whether certain unknown PAs or metabolites could be transferred to the milk. If so, it is important whether these still possess genotoxic and carcinogenic properties, and thus present a potential risk for the consumer. In fact, several unidentified PAs were detected in the plant material but also in the milk, based on fragmentation patterns obtained during LC-MS/MS analysis. It was also shown that the rumen plays an important role in the conversion of PA N-oxides into their free bases. However, part of the N-oxides appeared in the urine and to a very small extent in the milk. Some of the PAs seem rather resilient to ruminal metabolism. More work is needed to study the degradation, absorption and further metabolism of PAs in cows, the identification of unknown PAs in the PA-containing plants and on the genotoxic properties of PAs that are transferred into milk.
Supplemental Material
Download MS Word (153.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Bodi D, Ronczka S, Gottschalk C, Behr N, Skibba A, Wagner M, Lahrssen-Wiederholt M, Preiss-Weigert A, These A. 2014. Determination of pyrrolizidine alkaloids in tea, herbal drugs and honey. Food Addit Contam Part A. 31(11):1886–1895. doi:10.1080/19440049.2014.964337.
- Boppré M, Colegate SM, Edgar J. 2005. Pyrrolizidine Alkaloids of Echium vulgare Honey found in pure pollen. J Agric Food Chem. 53(3):594–600. doi:10.1021/jf0484531.
- Cone JW, Van Gelder AH, Visscher GJW, Oudshoorn L. 1996. Influence of rumen fluid and substrate concentration on fermentation kinetics measured with a fully automated time related gas production apparatus. Anim Feed Sci Technol. 61(1–4):113–128. doi:10.1016/0377-8401(96)00950-9.
- Cramer L, Schiebel HM, Ernst L, Beuerle T. 2013. Pyrrolizidine alkaloids in the food chain: development, validation, and application of a new HPLC-ESI-MS/MS sum parameter method. J Agric Food Chem. 61(47):11382–11391. doi:10.1021/jf403647u.
- Daniel JB, Van Laar H, Warner D, Dijkstra J, Navarro-Villa A, Pellikaan WF. 2014. Passage kinetics of dry matter and neutral detergent fibre through the gastro-intestinal tract of growing beef heifers fed a high-concentrate diet measured with internal d13C and external markers. Anim Prod Sci. 54(9):1471–1475. doi:10.1071/AN14354.
- de Nijs M, Mulder PPJ, Klijnstra MD, Driehuis F, Hoogenboom LAP. 2017. Fate of pyrrolizidine alkaloids during processing of milk of cows treated with ragwort. Food Addit Contam Part A. 34(12):2212–2219. doi:10.1080/19440049.2017.1364432.
- Dickinson JO, Cooke MP, King RR, Mohamed PA. 1976. Milk transfer of pyrrolizidine alkaloids in cattle. J Am Vet Med Assoc. 169(11):1192–1196.
- Dübecke A, Beckh G, Lullmann C. 2011. Pyrrolizidine alkaloids in honey and bee pollen. Food Addit Contam Part A. 28(3):348–358. doi:10.1080/19440049.2010.541594.
- [EFSA] European Food Safety Authority. 2011. EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Pyrrolizidine alkaloids in food and feed. Efsa J. 9(11):2406. [134 pp.]. 10.2903/j.efsa.2011.2406
- [EFSA] European Food Safety Authority. 2017. CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Statement on the risk for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. Efsa J. 15:4908. [34 pp.]
- El-Shazly A, Wink M. 2014. Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution and biological properties. Diversity. 6(2):188–282. doi:10.3390/d6020188.
- Fu PP, Xia Q, Lin G, Chou MW. 2004. Pyrrolizidine alkaloids - genotoxicity, metabolism, enzymes, metabolic activation, and mechanisms. Drug Metab Rev. 36(1):1–55. doi:10.1081/DMR-120028426.
- Geburek I, Preiss-Weigert A, Lahrssen-Wiederholt M, Schrenk D, These A. 2020. In vitro metabolism of pyrrolizidine alkaloids – metabolic degradation and GSH conjugate formation of different structure types. Food Chem Toxicol. 135:110868. doi:10.1016/j.fct.2019.110868.
- He YQ, Yang L, Liu HX, Zhang JW, Liu Y, Fong A, Xiong AZ, Lu YL, Yang L, Wang CH, et al. 2010. Glucuronidation, a new metabolic pathway for pyrrolizidine alkaloids. Chem Res Toxicol. 23(3):591–599. doi:10.1021/tx900328f.
- Hoogenboom LAP, Mulder PPJ, Zeilmaker MJ, van den Top HJ, Remmelink GJ, Brandon EFA, Klijnstra MD, Meijer GAL, Schothorst R, van Egmond HP. 2011. Carry-over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Addit Contam. 28(3):359–372. doi:10.1080/19440049.2010.547521.
- [JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2015. Joint FAO/WHO Expert Committee on Food Additives, eightieth meeting, Rome, 16–25 June. Report TRS 995-JECFA 80/65. http://www.fao.org/fileadmin/user_upload/agns/pdf/jecfa/Summary_report_of_the_80th_JECFA_meeting.pdf. Accessed 2020 Jun 2
- Kakar F, Akbarian Z, Leslie T, Mustafa ML, Watson J, van Egmond HP, Omar MF, Mofleh J. 2010. An outbreak of hepatic veno-occlusive disease in Western afghanistan associated with exposure to wheat flour contaminated with pyrrolizidine alkaloids. J Toxicol. 313280:1–7. doi:10.1155/2010/313280.
- Lanigan GW. 1970. Metabolism of pyrrolizidine alkaloids in ovine rumen.2. some factors affecting rate of alkaloid breakdown by rumen fluid in-vitro. Aust J Agric Res. 21(4):633–639. doi:10.1071/AR9700633.
- Lanigan GW, Smith LW. 1970. Metabolism of pyrrolizidine alkaloids in the ovine rumen. I. Formation of 7-α-hydroxy-1-α-methyl-8-α pyrrolizidine from heliotrine and lasiocarpine. Aust J Agric Res. 21(3):493–500. doi:10.1071/AR9700493.
- Mädge I, Cramer L, Rahaus I, Jerz G, Winterhalter P, Beuerle T. 2015. Pyrrolizidine alkaloids in herbal teas for infants, pregnant or lactating women. Food Chem. 187:491–498. doi:10.1016/j.foodchem.2015.04.067.
- Mathon C, Edder P, Bieri S, Christen P. 2014. Survey of pyrrolizidine alkaloids in teas and herbal teas on the Swiss market using HPLC-MS/MS. Anal Bioanal Chem. 406(28):7345–7354. doi:10.1007/s00216-014-8142-8.
- Mulder PPJ, de Witte SL, Stoopen G, van der Meulen J, Wikselaar P, Gruys E, Groot M, Hoogenboom LAP. 2016. Transfer of pyrrolizidine alkaloids from various herbs to eggs and meat in laying hens. Food Addit Contam Part A. 33(12):1826–1839. doi:10.1080/19440049.2016.1241430.
- Mulder PPJ, López P, Castellari M, Bodi D, Ronczka S, Preiss-Weigert A, These A. 2018. Occurrence of pyrrolizidine alkaloids in animal- and plant-derived food: results of a survey across Europe. Food Addit Contam Part A. 35(1):118–133. doi:10.1080/19440049.2017.1382726.
- Picron JF, Herman M, Van Hoeck E, Goscinny S. 2018. Analytical strategies for the determination of pyrrolizidine alkaloids in plant based food and examination of the transfer rate during the infusion process. Food Chem. 266:514–523. doi:10.1016/j.foodchem.2018.06.055.
- Ruan J, Yang M, Fu P, Ye Y, Lin G. 2014. Metabolic activation of pyrrolizidine alkaloids: insights into the structural and enzymatic basis. Chem Res Toxicol. 27(6):1030–1039. doi:10.1021/tx500071q.
- Tamminga S, Van Straalen WM, Subnel APJ, Meijer RGM, Steg A, Wever CJG, Blok MC. 1994. The Dutch protein evaluation system: the DVE/OEB-system. Livest Prod Sci. 40(2):139–155. doi:10.1016/0301-6226(94)90043-4.
- Wang C, Li Y, Gao J, He Y, Xiong A, Yang L, Cheng X, Ma Y, Wang Z. 2011. The comparative pharmacokinetics of two pyrrolizidine alkaloids, senecionine and adonifoline, and their main metabolites in rats after intravenous and oral administration by UPLC/ESIMS. Anal Bioanal Chem. 401(1):275–287. doi:10.1007/s00216-011-5075-3.
- Wiedenfeld H. 2011. Plants containing pyrrolizidine alkaloids: toxicity and problems. Food Addit Contam. 28(3):282–292. doi:10.1080/19440049.2010.541288.
