 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The substitution of fish oil and fishmeal with plant-based ingredients in commercial aquafeeds for Atlantic salmon, may introduce novel contaminants that have not previously been associated with farmed fish. The organophosphate pesticide pirimiphos-methyl (PM) is one of the novel contaminants that is most prevalent in commercial salmon feed. In this study, the feed-to-fillet transfer of dietary PM and its main metabolites was investigated in Atlantic salmon fillet. Based on the experimental determined PM and metabolite uptake, metabolisation, and elimination kinetics, a physiologically based toxicokinetic (PBTK) compartmental model was developed. Fish fed PM had a relatively low (~4%) PM retention and two main metabolites (2-DAMP and Desethyl-PM) were identified in liver, muscle, kidney and bile. The absence of more metabolised forms of 2-DAMP and Desethyl-PM in Atlantic salmon indicates different metabolism in cold-water fish compared to previous studies on ruminants. The model was used to simulate the long term (>1.5 years) feed-to-fillet transfer of PM + metabolite in Atlantic salmon under realistic farming conditions including seasonal fluctuations in feed intake, growth, and fat deposition in muscle tissue. The model predictions show that with the constant presence of the highest observed PM concentration in commercial salmon feed, fillet PM+ metabolite levels were approximately 5 nmol kg−1, with highest levels for the metabolite 2-DAMP. No EU maximum residue levels (MRL) for PM and its main metabolites exist in seafood to date, but the predicted levels were lower than the MRL for PM in swine of 32.7 nmol kg−1.
Introduction
Traditionally, aquafeeds for carnivorous farmed fish species have been based on marine protein and oil ingredients which are obtained from small pelagic fish species such as blue whiting, capelin, and sardines (FAO. Citation2018). The negative attention on the use of fish to produce seafood has led to the development of aquafeeds that require less feral fish to produce farmed fish (Naylor et al. Citation2000), the so-called “less fish in than fish out” principle (fish in< fish out;(Tacon and Metian Citation2008; IFFO Citation2009)). Plant-based ingredients from processed crops have been the main replacements for fish meal and oil in the development of more sustainable fish farming (Medale et al. Citation2013). Norway is one of the main producers of farmed Atlantic salmon and around 72% of the commercial salmon feeds currently used by the industry consist of plant-based products (Aas et al. Citation2019). The use of plant ingredients introduces novel contaminants, such as pesticides used to protect crops, which have not previously been associated with seafood. In order to protect fish health and food safety there is an extensive pesticide legislation which has established maximum residue levels (MRLs) for pesticides in plant-based food as well as terrestrial farm animal products which are reared on plant commodities (EC Citation2005). Seafood was added as a commodity category in EU legislation in Citation2013, with no MRLs established to date (EC, 2013), thus emphasising the need for risk assessments of pesticides in feed for Atlantic salmon.
Wide-scale chemical screening has revealed that the organophosphate (OP) pirimiphos-methyl (PM) is one of the most prevalent pesticides present in commercial Atlantic salmon feed (Nacher-Mestre et al. Citation2014, Citation2018; Portoles et al. Citation2017; Regueiro et al. Citation2017). The European Food Safety Authority (EFSA) has conducted a risk assessment on the acceptable background levels of PM in plants, processed commodities, rotational crops, and terrestrial livestock (EFSA Citation2015). Assessment of the toxicokinetics, and feed to food transfer are a prerequisite for establishing acceptable limits for PM with regards to food safety. Rats fed radiolabelled (C14) PM residues bound to wheat grain showed that 70–85% of PM and its metabolites were excreted in urine and faeces (Qureshi et al. Citation1992; Yeboah et al. Citation1992). Similarly, dietary PM studies in livestock such as goats and hens, showed that dietary PM has a high bioavailability and was extensively metabolised and excreted (~87–98% of the ingested PM) through urine and bile/faeces (EFSA Citation2015). In livestock, the parent PM compound is mainly found in the fat tissue, while five different metabolites, of which two dominate, are mainly identified in kidney, liver, and muscle (EFSA Citation2015). For farmed fish, limited information exists on the metabolism of PM from plant-based aquafeeds, and the kinetics of the parent PM compound and its main metabolites. Liver is one of the main organs of dietary xenobiotic metabolism, and bile is used for metabolite identification in dietary fish studies (Zhang et al. Citation2014; Bakke et al. Citation2016; Feng et al. Citation2016; Pampanin et al. Citation2016; Domoradzki et al. Citation2017).
Feed-to-food transfer assessments are used to establish acceptable background levels of pesticides in plant commodities which are used as feed ingredients for food-producing animals. To our knowledge, the assessment on the transfer of PM and its main metabolites during a food production cycle of farmed Atlantic salmon has not previously been performed. Toxicokinetic-based models used in feed-to-food transfer assessments, including simple tools such as one-compartment models, multi-compartment models, and physiologically based toxicokinetic (PBTK) models, are available for several fish species and a number of chemical classes (Grech et al. Citation2017). Recently, for food-producing farmed terrestrial animals (i.e. cattle, sheep, swine) generic multi-compartment PBTK models have been developed which predict tissue (i.e. liver, kidney, muscle) levels after oral exposure (Lautz et al. Citation2019, Citation2020). Similarly, generic PBTK models have been developed for fish which can predict multi-organ contaminant levels, independent from empirical kinetic data, based on generic physiological input data such temperature-dependent growth, cardiac output, and oxygen consumption as well as species-specific physiological data such as relative blood flows, organ volumes and tissue fat content (Grech et al. Citation2019).
In aquaculture studies, the relative feed intake, growth rate, and time of slaughter are highly relevant for the final concentrations of contaminants present in fish (Berntssen et al. Citation2016). Studies on persistent contaminants such as dioxins in farmed Atlantic salmon showed a great diversion in final levels and relative feed-to-fillet transfer (EFSA Citation2019), which may be explained by differences in feed intake, growth rate, exposure levels and duration of exposure among the different studies (EFSA Citation2019). These differences may be accounted for by PBTK models which include aquaculture parameters (feed intake, growth rate, and time to slaughter) to predict final levels under aquaculture conditions for an entire production cycle (EFSA Citation2019). For Atlantic salmon, simple empirical kinetic data-based compartmental models have been developed which describe the feed-to-fillet transfer of contaminants. These are based on physiological/aquaculture production parameters such as growth, fat deposition in muscle, and feed intake over time (Berntssen et al. Citation2011, Citation2013, Citation2018, Citation2019). These models are simplified PBTK models since they focus on the organ of importance for food safety, namely fillet. Atlantic salmon fillet consists mainly of anaerobic white muscle and has a rapid and high-fat deposition (Priede Citation2002). White muscle and fat generally compose the two compartments, which describe the deposition of lipo-or hydrophilic (or protein conjugated) xenobiotics with regards to food safety in salmon feed-to-fillet models. The changes in tissue volumes of white muscle and fat as well as feed intake are the physiological parameters included in the predictive whole cycle salmon feed-to-fillet modelling. PBTK feed-to-salmon fillet models which include metabolism, have been established for easily metabolised xenobiotics such as ethoxyquin (Berntssen et al. Citation2019) as well as semi-persistent toxicants such as toxaphene (Berntssen et al. Citation2013). PM is fat soluble, and the present study expands on previous two compartmental PBK feed-fillet salmon models based on toxicokinetic data for xenobiotics in aquafeed. The present trial provides empirical data on the uptake, metabolism and elimination of PM and its main metabolites in Atlantic salmon. These kinetic data are used in a PBK fillet model which predicts the chronic transfer of PM and its metabolites during an entire production cycle for salmon.
Material and methods
The feeding trial started on the 25th of October 2017 and ended on the 23rd of January 2018 and was conducted at Skretting ARC Research Station Lerang, Stavanger, Norway. Seawater adapted Atlantic salmon post-smolt (Salmo salar L.) of both genders (SalmoBreed strain) were distributed among six tanks (500 L; 1 m Ø x 0.9 m) with 32 fish per tank. Initial weight and length (fork-tail) were, respectively, 132 ± 25 g and 18 ± 2 cm (mean ± standard deviation; n = 192). To study the uptake, metabolism, and elimination of dietary PM, fish were fed PM-spiked feed for an exposure period, followed by a depuration period where fish were fed PM-free feed. Time-course samples were taken, and to avoid the possibility that sampling itself would affect feed intake, two series of triplicate tanks were sampled at altering sampling time points. Hence, a total of six tanks were used (twice triplicate). During a three-week acclimatisation period to the holding facilities, all fish were fed the control diet (without PM, no detectable levels with a detection limit of <0.01 mg kg−1). Following acclimatisation, fish were fed PM-spiked diets (5.0 mg kg−1) for a period of 81 days. After the exposure period, fish were fed the control diet for a depuration period of 39 days. Fish were fed to apparent satiety by automatic feeders in three meals per day (07:30–09:30, 12:00–14:00, 20:00–22:00). Feed intake per tank was measured by collecting and weighing feed waste 30 min after each meal, except for the evening meal which was collected the next morning, to assess daily feed intake (~0.9 % body weight (BW) day−1). The fish were reared in sea water (30 g L−1, 12°C) using a 12 h light, 12 h dark photoperiod regime. The oxygen saturation of the outlet water was always above 80%. Water samples were taken to assess possible leakage of the spiked PM from feed or faeces to the water (no detectable levels (<0.01 mg L−1) of PM were found in any of the experimental tanks). Mortality was recorded on a daily basis. The experiment complied with the guidelines of the Norwegian Regulation on Animal Experimentation and EC Directive 86/609/EEC. The Norwegian Food Safety Authority approved the protocol (identification number: ID 12091).
Preparation of pirimiphos-methyl -spiked diets and sampling
The PM-spiked diets were prepared by dissolving PM (Sigma-Aldrich AS, Norway) directly into the feed oil and further vacuum-coated on the basal pellet (3 mm diameter, lacking 4% oil) at a level of 4% oil inclusion. Control diets were vacuum-coated with 4% of PM free (< limit of quantification (LOQ) 0.01 of mg kg−1) feed oil. Livestock studies have shown that most of the ingested PM and metabolites are excreted (EFSA2015). The low retention of ingested PM makes the detection of PM and metabolite in tissues in a kinetic trial, especially muscle, challenging. The spiked PM feed levels (5 mg/kg) are higher than could be expected in commercial salmon feeds (see discussion), however were chosen to provide quantifiable data of PM and its main metabolites in the edible part of the fish during the final phase of the long-term depuration period. Immediately after production, samples were taken from each feed batch and analysed for supplementation levels which were 5.1 mg kg−1 feed wet weight (ww). Pellets were stored at −20°C until fed to the fish. Feed samples were taken at the end of the trial, and after 7 months of storage, and analysis showed no significant PM degradation. During the exposure period fish (three per tank) were sampled at 0, 3, 6, 18, 36, 69, and 81 days of exposure. During the depuration, period fish were sampled at 0.25, 0.5, 1, 2, 8, 28, and 39 days. Sampled fish were anaesthetized in a bath of tricaine methanesulfonate (MS-222) (Tricaine Pharmaq; ~40 mg L−1)(Popovic et al. Citation2012). The fish were sacrificed by a blow to the head, body weight and length of each fish were recorded and immediately frozen followed by storage at −28◦C. At the end of the experiment all fish were thawed, filleted and organs were sampled (whole fillet without skin, liver, bile, adipose tissue, and kidney). For organ distribution assessment at the end of the exposure (day 81) period, three individual fish per tank were sampled (n = 9) and analysed. For fillet kinetic assessment over time, three fish per tank were pooled (n = 3 per sampling point) at all time points, except at day 81 where individual samples were taken.
Pirimiphos-methyl and metabolite analyses
A more detailed method description of the extraction and quantification of PM and its two main metabolites, O-[2-(ethylamino)-6-methylpyrimidin-4-yl] O,O-dimethyl phosphorothioate (Desethyl-PM) and 2-(diethylamino)-4-hydroxy-6-methylpyrimidin (2-DAMP), in muscle liver, and kidney is published elsewhere (Garlito et al. Citation2019). Briefly, 0.5 g tissue was extracted in ACN:acetone (80:20) with 1% HCOOH (1 mL) and 0.5 g of MgSO4 per gram of sample. After centrifugation at 6000 rcf·g for 5 min, 200 µL of the supernatant was N2 evaporated and the residue was reconstituted with 200 µL of water and filtered through 0.45 µm nylon filters. Finally, 20 µL of the extract was analysed by UHPLC-MS/MS (UHPLC 50 × 2.1 mm, 1.7 μm particle size BEH C18 column by Acquity, Waters, Milford, MA, USA, interfaced with a triple quad MS/MS Xevo TQ-S, Waters Corporation, Manchester, UK) with water/acetonitrile mobile phases (both with 0.0025% HCOOH), at a flow rate of 0.3 mL·min−1, starting 50% acetonitrile which was linearly increased to 90% for 1.5 min. The following MS/MS parameters were used as described in detail by Garlito et al. (Citation2019). Different acquisition functions with different collision energies were applied after each other in each sample injection, In the first acquisition, the low energy function (LE), selecting a collision energy of 4 eV was used, and the second acquisition, the high energy function (HE), with a collision energy ramp ranging from 15 to 40 eV was used. The TOF resolution was 20.000 at FWHM at m/z 556.2771. The quantitative determination of PM and its metabolites was validation by performing studies on linearity, trueness and precision, limit of quantification (LOQ) and limit of detection (LOD), and specificity. A more detailed description of the results of this validation is given by Garlito et al. (Citation2019). In brief, for linearity a matrix-matched calibration was applied in the range 0.025–25 ng mL−1, trueness was evaluated by means of recovery experiments for all matrices in sixfold at three concentrations and precision was expressed as the method repeatability by establishing the relative standard deviation (RSD) from the recovery experiments at each fortification level (recoveries were between 70 and 120%, and RSDs below 20%), LOD was defined as the lowest fortification level, from which the LOQ was calculated (Garlito et al. Citation2019). The concentration of PM, 2-DAMP, and Desethyl-PM are expressed as nMol kg−1 to account for the difference in relative weight between the parent compound and its metabolites.
Model description
The transfer of dietary PM to the salmon fillet was initially described by a two-compartmental model which was based on a previously established two-compartmental PBTK-model for the fat-soluble persistent organic flame retardant, hexabromocyclododecane (HBCD). In this model the body was considered to consist of a fat compartment for storage of highly lipophilic compounds and a central compartment comprising all other tissues, among them muscle (Berntssen et al. Citation2011). As for HBCD, pirimiphos-methyl is fat-soluble although to a lesser degree (Log Kow 5.5 versus 3.9, respectively). PM is presumed to enter the system in the central compartment, flow from the central to the muscle or fat compartment, and backwards. PM leaves the system from the central compartment, i.e. by faeces and urine. The fillet compartment was assumed to be a fixed mixture of fat and muscle, and the concentration of PM in the fillet compartment was calculated as the weighted mean of the calculated concentrations in the fat and muscle compartment (Berntssen et al. Citation2011). In contrast to HBCD, PM is not persistent but is readily transformed into two main metabolites, namely Desethyl-PM and 2-DAMP (Bijlsma et al. Citation2019). Hence, the two-compartmental model was further amended into a serial two-compartment model which included the metabolism of a precursor into several successors (metabolites), like the serial compartment used for the feed xenobiotic toxaphene and the feed additive ethoxyquin and their main metabolites (Berntssen et al. Citation2013, Citation2019). The two main metabolites of PM, 2-DAMP, Desethyl-PM, are more water-soluble (around log Kow 2.9) than the parent compound. The precursor PM and the two formed successors distribute between the fat compartment and the larger none-fat central compartment (muscle fibres) of which the fillet consists.
In the model description (), PM, 2-DAMP, and Desethyl-PM are the amounts of PM and its main metabolites in the central and fat compartment, respectively. The entities D1, D2, D3 are the daily intakes of PM, 2-DAMP, Desethyl-PM from feed and fabs their fractions absorbed. The entities m1 and m2 are the metabolism rate constants of the precursor PM into the two successors 2-DAMP and Desethyl-PM, respectively. The transport rate constants of each compound from the central compartment to the fat compartment and vice versa are given by t12, t21, t34, t43, t56, t65. The excretion rate constants of PM, 2-DAMP, and Desethyl-PM are, respectively, as e1, e2, e3.
Figure 1. Two-compartment PBPK model for the disposition of dietary pirimiphos-methyl (PM) and metabolites (2-DAMP and Desethyl-PM) in the central (including white muscle fibres) and fat compartments of Atlantic salmon after exposure to a mixture of these compounds. See main text for definition of symbols
Model equations
The following rate equations were used in the model.
with:
PMc amount of pirimiphos-methyl in the central compartment (nmol)
PMf amount of pirimiphos-methyl in the fat compartment (nmol)
PM2c amount of 2-DAMP in the central compartment (nmol)
PM2f amount of 2-DAMP in the fat compartment (nmol)
PMDc amount of Desethyl-PM in the central compartment (nmol)
PMDf amount of Desethyl-PM in the fat compartment (nmol)
D1, D2, D3 daily dose of PM, 2-DAMP, and Desethyl-PM (nmol/day)
fabsfraction absorbed
m1, m2 metabolism rate constant (day−1)
rLW(t)relative liver weight (time dependent)
e1, e2, e3 excretion rate constants (day−1)
t12, t21, t34, t43, t56, t65 inter-compartment transport rate constants (day−1)
The model was implemented in R software (v.3.5.0) (R Core Development Team). The function expm, version 0.999–2, and deSolve, version 1.21 package were used. The R-code is available upon request.
Model parameterisation
The current feed-to-fillet transfer model is based on the simultaneous assessment of kinetic parameters from the exposure trial including the uptake of PM (fabs), metabolism of PM into the two metabolites (m1 and m2), as well as excretion of all three compounds (e1,e2,e3) (). Simultaneous assessment of uptake (fabs) and excretion (e1,e2,e3) kinetics of PM, and especially its metabolites, is challenging since metabolism is likely to continue during the depuration phase of the experiment (see section on Results below).
The model results are the amount of values for the set of given time-points. The estimated parameter values were those that resulted in the best fit of the calculated amount values to the data values. Because fitting models works best in case of parameters that can be assumed independent and normally distributed and because rate constant values must be positive, some re-parametrisation steps were applied. All parameters were log-transformed to guarantee positive values on the original scale, and introduced ratios of parameters to adjust for positive correlations on the original scale. The calculated amount values were fitted to the data amount values using the routine ‘nls’ of R. The estimated parameter values are normally distributed random values with the distributional characteristics being a result of the fitting procedure. Using these distributional characteristics back-transformation of the parameter values resulted in calculated values of the rate constant values. For the model extrapolation part, a Monte Carlo procedure with n = 200 was applied. That means parameter values were randomly drawn from the distribution defined above (200 times) and the model was run for the given feed characteristics, thereby generating a set of 200 randomly drawn model output values. From this set of output values the mean values and 95% confidence bounds were presented.
Model extrapolation to estimate fillet pirimiphos-methyl and its metabolites levels during a production cycle
The model was used to predict the fillet concentrations of PM, 2-DAMP, and Desethyl-PM in Atlantic salmon during a whole seawater production cycle fed on realistic background PM levels in feed. The input data used in the extrapolation model were based on the highest (0.019 mg kg−1) PM level surveyed in Norwegian commercial salmon feed in 2018 (Sele et al. Citation2019). Further input data for the model included average seasonal variation in daily feed intake, growth rate and fat deposition in Atlantic salmon farmed from about 80 g to 4 kg during an 18-month period, representative of a commercial production cycle (Berntssen et al. Citation2010b, Citation2016; Lock et al. Citation2011). The daily dietary PM intake per salmon, weight gain, and relative fillet fat deposition during a seawater production cycle vary based on season and water temperature as well as fish size. When making predictions for all time points during an entire production cycle, we assumed all estimated parameter values are unchanged over time, except hepatic clearance. We applied this assumption because the data did not indicate that the model parameters (compartment transition rates, metabolic rate, and excretion rates) were time-dependent.
Statistics
Significant differences in tissue PM, 2-DAMP, and Desethyl-PM concentrations were assessed by nested ANOVA, followed by Tukey’s HSD post hoc test. Significant differences among groups were set at p < .05. All statistics were performed using the programme Statistica (Statsoft Inc., Tulsa,USA).
Results
Pirimiphos-methyl and metabolite concentrations
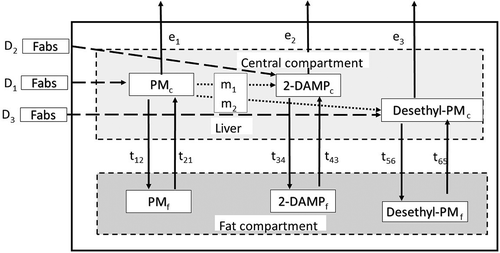
After dietary PM exposure, the two metabolites 2-DAMP and Desethyl-PM were identified in fillet, liver, kidney, and bile (Bijlsma et al. Citation2019; Garlito et al. Citation2019). The metabolite 2-DAMP was the dominant metabolite observed, while Desethyl-PM was present in lower concentrations in these tissues (). In both fillet and kidney ( the levels of PM and 2-DAMP were significantly higher than those of Desethyl-PM, with no significant differences between PM and 2-DAMP. In contrast, liver and bile had significantly higher levels of 2-DAMP than both PM and Desethyl-PM, and only the concentration of Desethyl-PM in bile was significantly different from PM (. Whole-body retention of the parent compound, PM, was 3.7 ± 0.44%, when including the main metabolites, the retention of PM+2-DAMP+Desethyl-PM was 4.4 ± 0.52%.
Figure 2. a-d) Concentration (nmol kg−1 ww) of the pesticide pirimiphos-methyl (PM) and its two main metabolites Desethyl-PM and 2-DAMP in fillet (a), kidney (b), liver (c) and bile (d) of Atlantic salmon (Salmo salar) fed PM spiked feeds (5.1 mg kg−1) for 81 days. Columns are the mean values and standard deviation (n = 9, mean ± SD). Columns with different superscripts are significantly different (p < .05, ANOVA, Tukey’s HSD t-test) from each other
Fillet kinetics
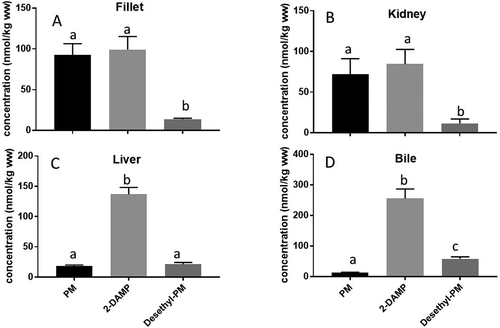
shows the uptake and elimination of PM and its two metabolites in the fillet of Atlantic salmon fed PM for 81 days followed by a 39-day elimination period, expressed as total amount of the substances in nmol. The uptake and elimination kinetics are expressed as total nmol (concentration multiplied with the total fillet mass over time) in order to account for the physiological changes in growth (increased fillet mass) and fat deposition (increased fat content in fillet mass) over time as described in the PBTK model. After 81 days, no steady state in PM levels was observed and during the depuration period, PM decreased in a two-phase elimination. Of the two metabolites, 2-DAMP was the dominant one in the muscle with the total amount equal to the parent compound, approximately 20 nmol in muscle. As opposed to PM, the metabolite 2-DAMP continued to increase during the entire elimination period ending with a total level of around 40 nmol in muscle. No steady-state in 2-DAMP formation was observed during the elimination period, when PM was absent in the diet. The second metabolite (Desethyl-PM) also increased during the PM exposure period, but had a fivefold lower total concentration (4 nmol) compared to 2-DAMP. As opposed to 2-DAMP, Desethyl-PM decreased in a one-phase pattern during the elimination period. shows the total level of PM, 2-DAMP and Desethyl-PM in the fat fraction of the fillet of Atlantic salmon. The most fat-soluble substance (PM) accumulated in the fat part which was eightfold lower than the total fillet which consisted of fat and muscle fibres (2.5 vs 20 nmol, respectively). As opposed to the PM in the fillet, PM in the fat fraction of the fillet continued to accumulate during the depuration period when no PM was present in the feed. The two metabolites were only found at low concentrations in the fat part of the fillet with an approximately 10-fold lower level in fat compared to fillet for Desethyl-PM and 2-DAMP, (0.4 and 0.16 nmol fat, respectively). As opposed to PM, the metabolite levels decreased in the fat fraction during the depuration period.
Figure 3. (a-c) Total amounts (in nmol) of PM (a) and the two metabolites (Desethyl-PM and 2-DAMP, b and c, respectively)) in the fillet of Atlantic salmon (Salmo salar) fed PM enriched feeds (5.1 mg kg−1) for 81 days followed by a 39-day elimination period (values are n = 3, mean ± SD). Vertical line indicates change from exposure into elimination period
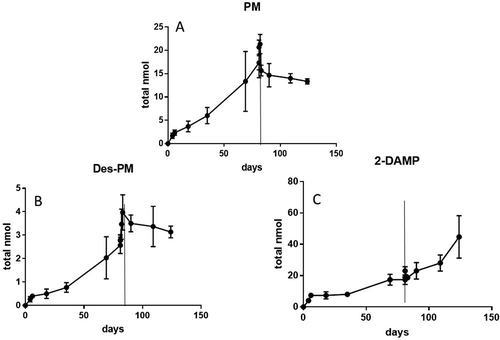
Figure 4. (a-c) Total amounts (in nmol) of PM (a) and the two metabolites (Desethyl-PM and 2-DAMP, b and c, respectively) in adipose tissue (fat) in Atlantic salmon (Salmo salar) fed PM enriched feeds (5.1 mg kg−1) for 81 days followed by a 39-day elimination period (values are n = 3, mean ± SD). Vertical line indicates change from exposure into elimination period
Model fit on experimental data
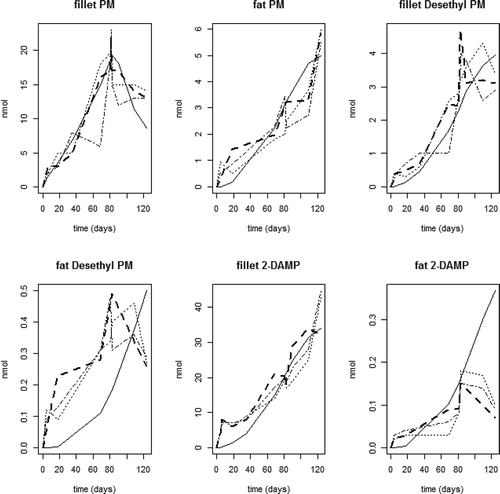
shows the fit of the model to the experimental data per individual tank (three tanks in total). The predicted values in the fillet are in accordance with the experimental data with a model-predicted increase in the main metabolite 2-DAMP during the depuration period (see also )). However, with regards to the fat compartment the present model was not able to predict the elimination from the fat compartment for the two metabolites, while for the more fat-soluble parent compound (PM) the model prediction was similar to the experimental data.
Figure 5. Model predicted fillet total level (nmol) (even line) and experimental data (three broken lines for the three different experimental tanks) for PM and the two metabolites (2-DAMP and Desethyl-PM) in the fillet and adipose tissue (fat) Atlantic salmon fed PM enriched feeds (5.1 mg kg−1) in triplicate tanks for 81 days followed by a 39-day depuration period
Discussion
Metabolites and organ concentrations
In the present study, 2-DAMP was the main metabolite formed after prolonged dietary PM exposure (Bijlsma et al. Citation2019; Garlito et al. Citation2019). Fillet and kidney 2-DAMP levels were similar to the parent compound (PM), and in liver and bile the 2-DAMP concentrations were significantly higher than both PM and the other metabolite (). The dominant formation of 2-DAMP was further seen from the time-course increase in the level of this metabolite in the muscle during the depuration period, while both the parent compound and the other metabolite decreased during this period (). In the present study only bile (and not urine) was assessed as an excretion route of the metabolites and the two main metabolites identified were 2-DAMP and Desethyl-PM (Bijlsma et al. Citation2019; Garlito et al. Citation2019). Earlier studies with rats fed (C14) PM residues bound to wheat grain, showed that the major part (42%) of total radioactivity was excreted by faeces, which was thought partly to come from biliary excretion (Akay et al. Citation1992). Also in the liver of PM-fed Atlantic salmon, 2-DAMP and Desethyl-PM, were the main metabolites, constituting ~ 90% of the total PM+metabolite concentration. The bile concentrations of 2-DAMP and Desethyl-PM, respectively, were ~20 or ~fourfold higher than the parent compound (). This indicates that Atlantic salmon readily metabolise dietary PM like terrestrial animals, and faeces is the main excretion route for these metabolites. Similar levels of the parent compound (PM) to the main metabolite (2-DAMP) were present in kidney, indicating urine as the other route for PM and metabolite elimination. In rats fed (C14) PM, around ~30% of the total ingested radioactivity was excreted by urine (Akay et al. Citation1992). Although fish, like mammals, readily metabolise PM, the main metabolites found in different organs appear to be different in mammals versus fish. In ruminants (lactating goat) the dominant metabolites identified in muscle, liver and kidney were de-ethylated metabolites of 2-DAMP and de-phosphorylated metabolites of Desethyl-PM, respectively (EFSA Citation2015). The dominant metabolite in Atlantic salmon, 2-DAMP, was identified only as a minor conjugated metabolite in goat liver and kidney (EFSA Citation2015). Furthermore, the second metabolite in Atlantic salmon tissues, Desethyl-PM, was also identified only as a minor metabolite in goat fat tissue. The higher (more de-ethylated and de-phosphorylated) metabolised forms of PM identified in goat could not be found in Atlantic salmon muscle, kidney, liver or bile (Bijlsma et al. Citation2019; Garlito et al. Citation2019). The absence of higher metabolised forms of 2-DAMP and Desethyl-PM in Atlantic salmon, and nearly absent lower metabolised Desethyl-PM and 2-DAMP in goat, indicates different metabolism of PM in cold-water fish compared to ruminant mammals.
Model parameterisation and model fit
The present model focused on the dietary transfer of pesticides into the organ of importance for food safety, namely the fillet, and its distribution among the muscle fibre (central compartment) and the fat content (fat compartment) of the fillet. In the present trial, both PM and Desethyl-PM were present in fat; however, the concentrations of PM and Desethyl-PM in fat were not higher than those in whole fillet, which is composed of both fat (7%) and muscle fibre (93%). The total levels of PM and Desethyl-PM in the fat fraction of the fillet was 8–10 fold lower than the total amount in the fillet, indicating that a larger proportion of the relatively fat-soluble PM was present in the non-fat muscle fibre fraction of the fillet () versus 4A). The fat compartment of the fillet accounted for 16% of PM and 14% and 0.5% for Desethyl-PM and 2-DAMP, respectively, of the total levels observed in fillet. The present PM feed-fillet transfer was hence best explained for the central compartment of the fillet model while the fat compartment contributed less. In contrast, for the persistent organic pollutant HBCD, on which the present model is based, the prediction of HBCD in the fillet was mainly explained by the fat part of the fillet while the central compartment of the fillet was of lesser importance. Feed-to-fillet transfer of HBCD was hence predicted by the accumulation in fat, with the minor fraction in the central compartment of the fillet (muscle fibre). For PM and its main metabolites the situation is reversed. Although PM has a Log Kow higher than 3 and is partly fat soluble (EFSA Citation2015), it is less fat soluble than HBCD (Log Kow 5.5 versus log Kow 3.9, respectively). For PM the fillet accumulation is dominantly explained by the non-fat central department, while the fat compartment of the fillet has a minor contribution.
The present model is based on a serial compartment model that includes the PM fractional uptake (fabs) and rates for the metabolism (m), and excretion (e) of PM and its main metabolites. Based on the experimental data from this trial, no simultaneous uptake and excretion rate assessment could be identified for PM and its metabolites. This is because for the main formed metabolite (2-DAMP), the concentrations increased rather than decreased during the depuration period ()), which is likely due to an ongoing metabolism of the circulating PM during the depuration period. Also, PM itself accumulates in the adipose tissue, although to a lesser extent than the non-fat part of the fillet. In addition, Desethyl-PM disappears from the central compartment and the adipose tissue. Despite the lack of decreasing 2-DAMP levels in the depuration phase, model calibration gave good model fits to the experimental data, especially regarding the ongoing increase of the main metabolite 2-DAMP during the depuration period (). In the absence of identification of effective elimination the parameter fabs in fact reflects the net transfer to muscle and fat. The model estimated a net transfer of around 2 ± 2% (Supplementary Data, Table 1), which was in the same order as the total retention (~4%) of the ingested PM and its main metabolites in the fillet of Atlantic salmon. Also in terrestrial livestock that were fed PM, ~2-13% of the ingested PM and its metabolites was retained in the body (EFSA Citation2015). In studies with rats fed C14-PM residues bound to wheat, excretion accounted for a large percentage (30–80%) of the ingested radioactivity, indicating a relative high uptake combined with a high excretion for PM and its metabolites which resulted in a low net absorption or retention of the ingested PM (Kacew et al. Citation1996). In order to evaluate whether a model allowing for identification of both uptake and excretion rates is feasible, fabs was set at a fixed value (20%) in concordance with observations in rat studies (Akay et al. Citation1992). However, this model setting gave a poor model fit to the experimental data with regards to the fillet levels in the depuration period (see Supplementary Data, and Table 2). Hence, the model was maintained with respect to the absence of elimination of PM and its metabolites from the fish body. However, with regard to the fat compartment, the model prediction is not able to predict the elimination from the fat compartment for the two metabolites, while for the more fat-soluble parent compound (PM) the model prediction is similar to the experimental data. Hence, the current model is likely to give an over-estimation of the two metabolites in the fat fraction as no elimination can be predicted. However as discussed above, the fat compartment contributed only to a minor extend to the total 2-DAMP and Desethyl-PM amount found in the fillet, thus the current model is expected to cause only a minor over-estimation of the fillet metabolite accumulation when making long-term exposure extrapolations during a whole seafood production cycle.
Long-term extrapolations
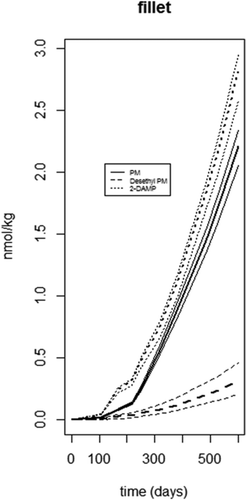
The model was used to predict the concentration of PM and its two metabolites in the fillet of farmed Atlantic salmon when fed background levels of PM for a full seawater production cycle. In addition to feed concentration, the long-term model input parameters included feed intake, growth, and development of the fat compartment which reflects the seasonal variation related to seawater temperature and maturation of a market-sized Atlantic salmon (Berntssen et al. Citation2010a, Citation2010b, Citation2016). Surveillance of PM in commercial Atlantic salmon feed in 2018 showed an average level of 0.014 mg/kg, and min.-max. values of 0.010–0.019 mg kg−1, respectively (Sele et al. Citation2019). The highest PM concentration was used as the model input, in addition to feed intake and growth rates representative of actual farming conditions as described in the material and method section. gives the long-term predictions of PM and its two main metabolites in the fillet of Atlantic salmon fed highest observed feed PM levels during an entire production cycle. The final predicted PM fillet levels were lower than the predicted 2-DAMP levels, as expected due to the high biotransformation of PM into 2-DAMP. The Desethyl-PM levels in fillet were much lower compared to PM and 2-DAMP, which is in accordance with the expected relatively low metabolism of PM into Desethyl-PM. Due to the low retention of the parent compound itself as well as the ready formation of 2-DAMP and reduced growth rate, and hence reduced growth dilution, of Atlantic salmon of slaughter-size (over 2 kg), there is a low continuous accumulation over time, with highest levels for 2-DAMP. Final estimated levels for 2-DAMP, PM, and Desethyl-PM were around 2.7, 2.2, and 0.3 nmol kg fillet−1, respectively. No MRL (maximum residue limit) has been established for PM in seafood to date; however, for swine, the MRL is 0.01 mg kg−1 or 32.7 nmol kg−1. The predicted levels in Atlantic salmon are at most around 5 nmol kg fillet−1 for the total amount of PM and its two metabolites. It appears that the background levels of PM found in fish feed are unlikely to cause an exceedance of the default MRL of 0.01 mg kg−1 for PM in meat from terrestrial livestock, thus indicating a low potential risk of PM and its metabolites with regards to food safety. However, MRLs for pesticides are only set for plant-based food products and terrestrial farm animals reared on plant commodities (EC Citation2005). MRLs for farmed seafood, reared on plant commodities (like salmon), are not established yet (EC 2013). Furthermore, as far as we know, no human risk assessment has been performed with regards to the specific PM metabolite profile identified in Atlantic salmon (see above), and a wide-scale monitoring of the potential presence and levels of PM and PM metabolites in farmed seafood is lacking. With regards to potential adverse effects on fish health, information on PM in aquafeeds is also lacking. Previous feeding studies on Atlantic salmon with another organophosphate pesticide, chlorpyrifos-methyl, have shown adverse effects on lipid metabolism in fish at feed concentrations comparable to background levels in commercial plant-based feeds (Sanden et al. Citation2018).
Figure 6. Whole seawater production cycle model predictions of fillet PM and its two metabolites 2-DAMP and N-Desethyl-PM concentrations (nmol kg−1 ww) in Atlantic salmon fed highest (0.019 mg kg−1) PM background levels surveyed in Norwegian commercial salmon feeds in 2018. Solid line is PM, dashed line is Desethyl-PM, dotted line is 2-DAMP. Thicker line in the middle is the mean and thinner lines are 95% lower and upper bounds
Conclusion
Dietary PM is readily metabolised and excreted in Atlantic salmon, as has been shown by other studies on terrestrial livestock. The main metabolites identified in several tissues, included fillet, are Desethyl-PM and especially 2-DAMP. The absence of more metabolised forms of 2-DAMP and Desethyl-PM in Atlantic salmon indicates different metabolism for cold-water marine fish compared to terrestrial livestock. The ready metabolism of PM into 2-DAMP caused an ongoing accumulation of fillet 2-DAMP in Atlantic salmon no longer fed PM, making an assessment of 2-DAMP excretion rates difficult. Separate dietary exposure-depuration studies with the pure 2-DAMP are needed to identify metabolite excretion rates. Although PM and to a lesser degree 2-DAMP are partly fat-soluble, the fillet levels of these compounds are mostly explained by the muscle part rather than the fat part of the fillet. The fillet uptake and elimination concentrations can be used in a serial compartment model that includes rates for the uptake (fabs), metabolism (m), and excretion (e) of PM and its main metabolites, when assuming a combined parameter for uptake and excretion rates. Model extrapolations to a full seawater production cycle of Atlantic salmon with realistic physiological parameters and dietary PM input values, predict PM and metabolite levels in Atlantic salmon fillet around 5 nmol kg−1, which is lower than the MRL set for meat from terrestrial livestock. However, a human risk assessment for PM and its metabolites in seafood, and a specific MRL for PM in fish feed or seafood, is lacking. Furthermore, an assessment of the potentially adverse effects of PM on fish health is needed.
Supplemental Material
Download MS Word (53.9 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Aas TS, Ytrestoyl T, Asgard T. 2019. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: an update for 2016. Aquacult Rep. 15:100216. doi:10.1016/j.aqrep.2019.100216.
- Akay MT, Yilmazoglu G, Yasacan S, Turk H, Kolanyaka D. 1992. Bioavailability and toxicological potential of wheat-bound pirimiphos-methyl residues in rats. Xenobiotica. 22(3):293–302. doi:10.3109/00498259209046641.
- Bakke MJ, Nahrgang J, Ingebrigtsen K. 2016. Comparative absorption and tissue distribution of C-14-benzo(a)pyrene and C-14-phenanthrene in the polar cod (Boreogadus saida) following oral administration. Polar Biol. 39:1165–1173. doi:10.1007/s00300-015-1816-7.
- Berntssen MHG, Hannisdal R, Buttle L, Hoogenveen R, Mengelers M, Bokkers BGH, Zeilmaker MJ. 2018. Modelling the long-term feed-to-fillet transfer of leuco crystal violet and leuco malachite green in Atlantic salmon. Food Addit Contam Part A. 35:1484–1496. doi:10.1080/19440049.2018.1487587.
- Berntssen MHG, Hoogenveen R, Bernhard A, Lundebye AK, Ornsrud R, Zeilmaker MJ. 2019. Modelling of the feed-to-fillet transfer of ethoxyquin and one of its main metabolites, ethoxyquin dimer, to the fillet of farmed Atlantic salmon (Salmon salar L. Food Addit Contam Part A. 36:1042–1054. doi:10.1080/19440049.2019.1605208.
- Berntssen MHG, Julshamn K, Lundebye AK. 2010a. Chemical contaminants in aquafeeds and Atlantic salmon (Salmo salar) following the use of traditional- versus alternative feed ingredients. Chemosphere. 78:637–646. doi:10.1016/j.chemosphere.2009.12.021.
- Berntssen MHG, Lock EJ, Zeilmaker MJ, Van Eijkeren JCH. 2013. Toxicokinetic model assessment on the dechlorination of dietary toxaphene CHB-62 into CHB-44 in Atlantic salmon (Salmo salar L. Food Addit Contam Part A. 30:1581–1589. doi:10.1080/19440049.2013.811544.
- Berntssen MHG, Olsvik PA, Torstensen BE, Julshamn K, Midtun T, Goksoyr A, Johansen J, Sigholt T, Joerum N, Jakobsen JV, et al. 2010b. Reducing persistent organic pollutants while maintaining long chain omega-3 fatty acid in farmed Atlantic salmon using decontaminated fish oils for an entire production cycle. Chemosphere. 81:242–252. doi:10.1016/j.chemosphere.2010.06.031.
- Berntssen MHG, Sanden M, Helge H, Lie Ø. 2016. Modelling scenarios on feed-to-fillet transfer of dioxins and dioxinlike PCBs in future feeds to farmed Atlantic salmon (Salmo salar). Chemosphere. 163:413. doi:10.1016/j.chemosphere.2016.08.067.
- Berntssen MHG, Valdersnes S, Rosenlund G, Torstensen BE, Zeilmaker MJ, van Eijkeren JCH. 2011. Toxicokinetics and carry-over model of alpha-hexabromocyclododecane (HBCD) from feed to consumption-sized Atlantic salmon (Salmo salar). Food Addit Contam Part A. 28:1274–1286. doi:10.1080/19440049.2011.587029.
- Bijlsma L, Berntssen MHG, Merel S. 2019. A refined nontarget workflow for the investigation of metabolites through the prioritization by in silico prediction tools. Anal Chem. 91:6321–6328. doi:10.1021/acs.analchem.9b01218.
- Domoradzki JY, Sushynski JM, Thackery LM, Springer TA, Ross TL, Woodburn KB, Durham JA, McNett DA. 2017. Metabolism of C-14-octamethylcyclotetrasiloxane (C-14 D-4) or C-14-decamethylcyclopentasiloxane (C-14 D-5) orally gavaged in rainbow trout (Oncorhynchus mykiss). Toxicol Lett. 279:115–124. doi:10.1016/j.toxlet.2017.03.025.
- [EC] European Commision. 2005. Pesticides EU-MRLs Regulation (EC) No 396/2005. Off J EU. 70:1–15.
- [EC] European Commission. 2013. Commission regulation (EU) No 212/2014 of 11 March 2014 replacing Annex I to regulation (EC) No 396/2005 of the European Parliament and of the Council as regards additions and modifications with respect to the products covered by that Annex. Off J EU. 68:30–52.
- [EFSA] European Food Safety Authority. 2015. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for pirimiphos-methyl according to Article 12 of Regulation (EC) No 396/20051. EFSA J. 13:3974. doi:10.2903/j.efsa.2015.3974.
- [EFSA] European Food Safety Authority. 2019. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J. 16:5333.
- [FAO] Food and Agriculture Organization. 2018. The state of world fisheries and aquaculture- meeting sustainable development goals. Rome. http://www.fao.org/3/i9540en/i9540en.pdf
- Feng JB, Huang DR, Zhong M, Liu P, Dong JD. 2016. Pharmacokinetics of florfenicol and behaviour of its metabolite florfenicol amine in orange-spotted grouper (Epinephelus coioides) after oral administration. J Fish Dis. 39:833–843. doi:10.1111/jfd.12416.
- Garlito B, Ibanez M, Portoles T, Serrano R, Amlund H, Lundebye A-K, Sanden M, Berntssen MHG, Hernandez F. 2019. LC-MS/MS method for the determination of organophosphorus 6 pesticides and their metabolites in salmon and zebrafish fed with plant-based feed ingredients. Anal Bioanal Chem. 411:7281–7291. doi:10.1007/s00216-019-02104-6.
- Grech A, Brochot C, Dorne JL, Quignot N, Bois FY, Beaudouin R. 2017. Toxicokinetic models and related tools in environmental risk assessment of chemicals. Sci Total Environ. 578:1–15. doi:10.1016/j.scitotenv.2016.10.146.
- Grech A, Tebby C, Brochot C, Bois FY, Bado-Nilles A, Dorne JL, Quignot N, Beaudouin R. 2019. Generic physiologically-based toxicokinetic modelling for fish: integration of environmental factors and species variability. Sci Total Environ. 651:516–531. doi:10.1016/j.scitotenv.2018.09.163.
- [IFFO] International Fish meal and Fish oil Organisation. 2009. Fish in - fish out ratios explained. https://wwwiffonet/system/files/EAS%20FIFO%20September2009%202_0pdf
- Kacew S, Akhtar MH, Khan SU. 1996. Bioavailability of bound pesticide residues and potential toxicologic consequences - An update. Proc Soc Exp Biol Med. 211:62–68. doi:10.3181/00379727-211-43952.
- Lautz LS, Hoeks S, Oldenkamp R, Hendriks AJ, Dorne J, Ragas AMJ. 2020. Generic physiologically based kinetic modelling for farm animals: part II. Predicting tissue concentrations of chemicals in swine, cattle, and sheep. Toxicol Lett. 318:50–56. doi:10.1016/j.toxlet.2019.10.008.
- Lautz LS, Oldenkamp R, Dorne JL, Ragas AMJ. 2019. Physiologically based kinetic models for farm animals: critical review of published models and future perspectives for their use in chemical risk assessment. Toxicol Vitro. 60:61–70. doi:10.1016/j.tiv.2019.05.002.
- Lock EJ, Fjelldal PG, Torstensen BE, Bjornevik M, Breck O, Johansen J, Reynolds P, Sigholt T, Joerum N, Jakobsen JV, et al. 2011. Dietary decontaminated fish oil has no negative impact on fish performance, flesh quality or production-related diseases in Atlantic salmon (Salmo salar). Aquac Nutr. 17:E760–E772.
- Medale F, Le Boucher R, Dupont-Nivet M, Quillet E, Aubin J, Panserat S. 2013. Plant based diets for farmed fish. Inra Prod Anim. 26:303–315.
- Nacher-Mestre J, Ballester-Lozano GF, Garlito B, Portoles T, Calduch-Giner J, Serrano R, Hernandez F, Berntssen MHG, Perez-Sanchez J. 2018. Comprehensive overview of feed-to-fillet transfer of new and traditional contaminants in Atlantic salmon and gilthead sea bream fed plant-based diets. Aquac Nutr. 24:1782–1795. doi:10.1111/anu.12817.
- Nacher-Mestre J, Serrano R, Portoles T, Berntssen MHG, Perez-Sanchez J, Hernandez F. 2014. Screening of pesticides and polycyclic aromatic hydrocarbons in feeds and fish tissues by gas chromatography coupled to high-resolution mass spectrometry using atmospheric pressure chemical ionization. J Agric Food Chem. 62:2165–2174. doi:10.1021/jf405366n.
- Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenco J, Mooney H, Troell M. 2000. Effect of aquaculture on world fish supplies. Nature. 405:1017–1024. doi:10.1038/35016500.
- Pampanin DM, Le Goff J, Skogland K, Marcucci CR, Oysaed KB, Lorentzen M, Jorgensen KB, Sydnes MO. 2016. Biological effects of polycyclic aromatic hydrocarbons (PAH) and their first metabolic products in in vivo exposed Atlantic cod (Gadus morhua). J Toxicol Environ Health Part A. 79:633–646. doi:10.1080/15287394.2016.1171993.
- Popovic NT, Strunjak-Perovic I, Coz-Rakovac R, Barisic J, Jadan M, Berakovic AP, Klobucar RS. 2012. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J Appl Ichthyol. 28:553–564. doi:10.1111/j.1439-0426.2012.01950.x.
- Portoles T, Ibanez M, Garlito B, Nacher-Mestre J, Karalazos V, Silva J, Alm M, Serrano R, Perez-Sanchez J, Hernandez F, et al. 2017. Comprehensive strategy for pesticide residue analysis through the production cycle of gilthead sea bream and Atlantic salmon. Chemosphere. 179:242–253. doi:10.1016/j.chemosphere.2017.03.099.
- Priede M editor. 2002. Biology of salmon chichester. UK:Springer Praxis Publishing.
- Qureshi MJ, Jamil FF, Haq A, Naqvi SHM. 1992. Bioavailability and toxicity to rats of bound residues of c-14 pirimiphos-methyl in stored wheat. J Environ Sci Health Part B-Pestic Contam Agric Wastes. 27:369–375. doi:10.1080/03601239209372787.
- Regueiro J, Negreira N, Hannisdal R, Berntssen MHG. 2017. Targeted approach for qualitative screening of pesticides in salmon feed by liquid chromatography coupled to traveling-wave ion mobility/quadrupole time-of-flight mass spectrometry. Food Control. 78:116–125. doi:10.1016/j.foodcont.2017.02.053.
- Sanden M, Olsvik PA, Softeland L, Rasinger JD, Rosenlund G, Garlito B, Ibanez M, Berntssen MHG. 2018. Dietary pesticide chlorpyrifos-methyl affects arachidonic acid metabolism including phospholipid remodeling in Atlantic salmon (Salmo salar L. Aquaculture. 484:1–12. doi:10.1016/j.aquaculture.2017.10.033.
- Sele V, Sanden M, Berntssen MHG, Storesund J, Lie KK, Espe M, Lundebye A-K, Hemre GI, Waagbø R, Ørnsrud R 2019. Program for overvåking av fiskefôr (in Norwegian). IMR reports.30. https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen–2019–2030
- Tacon AGJ, Metian M. 2008. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: trends and future prospects. Aquaculture. 285:146–158. doi:10.1016/j.aquaculture.2008.08.015.
- Yeboah PO, Semanhyia CB, Melfah PT. 1992. Bioavailability and biological-activity of bound residues of radiolabeled pirimiphos-methyl in maize grains. J Environ Sci Health Part B-Pestic Contam Agric Wastes. 27:377–386. doi:10.1080/03601239209372788.
- Zhang FH, Lu GH, Liu JC, Van ZH, Zhang ZH. 2014. Bioaccumulation, distribution and metabolism of BDE-153 in the freshwater fish Carassius auratus after dietary exposure. Ecotox Environ Safe. 108:16–22.
