ABSTRACT
Food contact materials (FCMs) can contain hazardous chemicals that may have the potential to migrate into food and pose a health hazard for humans. Previous studies have mainly focused on plastic materials, while data on packaging materials made from paper and cardboard are limited. We used a panel of cell-based bioassays to investigate the presence and impact of bioactive chemicals on human relevant endpoints like oxidative stress, genotoxicity, inflammation, xenobiotic metabolism and endocrine system effects in extracts made from paper and cardboard. In total, 23 methanol extracts of commonly used paper and cardboard available on the Swedish market were extracted as a whole product using methanol to retrieve polar substances, and tested at concentrations 0.3–10 mg/mL and 0.2–6 mg/mL. At the highest concentration bioactivities were observed in a high proportion of the samples: oxidative stress (52%), genotoxicity (100%), xenobiotic metabolism (74%), antiandrogenic- (52%) and antioestrogenic receptor (39%). Packages of potential concern included cake/pastry boxes/mats, boxes for infant formula/skimmed milk, pizza boxes, pizza slice trays and bag of cookies. It should be noted that the extraction for packages like cake/pastry boxes can be considered exaggerated, as the exposure usually is shorter. It can be hypothesised that the observed responses may be explained by inks, coatings, contaminants and/or naturally occurring compounds within the material. To summarise, an effect-based approach enables hazard identification of chemicals within FCMs, which is a valuable tool for ensuring safe use of FCMs.
Graphical Abstract
Introduction
Food contact materials (FCMs) are defined as materials intended to come into contact with food. These materials should be stable against varying temperatures, ensure prolonged shelf-life of foods, as well as protect against biological and chemical contaminations. They can be made from a range of materials such as plastic, glass, paper, cork and paperboard (Simoneau Citation2016).
Chemicals present in FCMs can either be intentionally added for a specific function or non-intentionally added. Non-intentionally added substances (NIAS) can originate from breakdown products, chemical interactions with the food item and the package material, or from contaminants (Peters et al. Citation2019). Examples of chemicals in FCMs are primary aromatic amines, mineral oil hydrocarbons, plasticisers (e.g. phthalates, adipates, terephthalates etc.), and bisphenol A (BPA), all of which have the potential to cause adverse health effects in humans (Lopez-Espinosa et al. Citation2007; Lorenzini et al. Citation2010; Campanella Citation2015). These chemicals have mainly been studied in plastic packaging materials and it is important to investigate other types of packaging materials, such as paper and cardboard (Campanella Citation2015; Severin et al. Citation2017; Park et al. Citation2018; Schweighuber et al. Citation2019).
There is currently no harmonised legislation within the European Union (EU) for chemical safety of paper and cardboard FCMs. The EU framework regulation states that FCMs should be in compliance with good manufacturing practice and not transfer their substances in amounts that could negatively affect human health or alter the food itself (EU Citation2004; EU Citation2011; EU Citation2016). Still, the framework does not regulate specific substances, and this is a limitation since migration of chemicals from the finished product containing inks, additives and adhesives may affect human health (Muncke Citation2010; Koster et al. Citation2015).
There is limited knowledge on toxicity of chemicals present in the environment. Toxicity testing of water samples has demonstrated that unknown compounds account for up to 99.1% of the biological effects for certain endpoints when tested in mammalian in vitro systems or in the bacterium Vibrio fischeri (Escher et al. Citation2013; König et al. Citation2016; Neale et al. Citation2020). Effect-based in vitro methods can be used to assess these effects. Our study did not focus on identifying chemicals, as known and unknown NIAS may exert the identified effects and these may not be identified by targeted chemical analyses.
Prior studies have reported effects on the aryl hydrocarbon- (AhR), oestrogen- (ER) and androgen (AR) receptors, as well as oxidative stress responses and genotoxicity by certain paper and cardboard FCMs (Bengtström et al. Citation2014; Rosenmai et al. Citation2017; Severin et al. Citation2017). This indicates that these endpoints are relevant human health outcomes to examine further when assessing the safety of FCMs.
There are a limited number of studies that use effect-based methods to identify bioactive chemicals in FCMs. Therefore, we used an effect-based approach in this study to evaluate the presence of hazardous chemicals in a large number of commonly used FCMs made of paper and cardboard. This panel of bioassays represented toxicity pathways of high relevance to human health. The endpoints investigated were oxidative stress (Nrf2 activity), genotoxicity (micronucleus test), nuclear factor kappa-light-chain-enhancer of activated B cells (NFκβ) signalling, oestrogen (ER), androgen (AR) and aryl hydrocarbon receptor (AhR). Sixty-seven commonly used paper and cardboard FCMs available on the Swedish market were chosen, and included materials like pizza boxes, microwave popcorn bags and fastfood packaging.
Material and methods
Sample preparation and extraction
A total of 67 food packages made from paper and cardboard were purchased by the Swedish Chemicals Agency from a variety of sources (e.g. supermarkets, bakeries and restaurants) in 2019 (). The selection of FCM samples were based on sales statistics of purchased materials on the Swedish consumer market (Kemikalieinspektionen Citation2020).
Table 1. Food packaging materials (FCMs) tested
The sample extraction procedures are described in the Supplementary information (Section SI-1). Briefly, samples were cut into small pieces, with inner and outer surfaces containing inks, glue, coatings, lacquers, coatings, etc., consisting of approximately 1 g of material, except for the extract paper for baking and baking moulds which received a weight of 0.6 g. The 67 materials were categorised into groups and similar materials (like pizza boxes) were pooled together and this resulted in a total of 23 extracts (). Each pool of materials contained in total 1 g, except for baking and baking moulds. The samples were placed in Teflon-coated test tubes and microwaved for 20 min at 80°C in 15 mL of methanol, before being transferred into glass tubes. An additional extraction by ultrasonication was performed for 15 min in the Teflon-coated tubes using an additional 10 mL of methanol (Alin and Hakkarainen Citation2012; Melski et al. Citation2003). Thereafter, the pooled extracts were evaporated under a nitrogen stream to 0.5 mL, and thereafter diluted with 0.5 mL ultrapure water (Milli-Q ®) to obtain a final volume of 1 mL. The extraction procedure allowed water-soluble chemicals and to some extent fat soluble substances to be extracted. Three solvent blanks were prepared following the same extraction procedure as for the FCMs, but without material.
Bioassays
All extracts were analysed in quadruplicate in the bioassays mentioned in . Each bioassay used an established cell line and comprehensive information of the bioassays are available in Supplementary information (Section SI-1).
Table 2. Summary of bioassays and respective endpoints
The vehicle control was the solvent methanol MeOH/Milli-Q water (1:1), which samples were dissolved in. The standards were dissolved in DMSO due to low solubility in MeOH/MQ water, and therefore DMSO was included as an additional vehicle control. Information on standards for respective assay are shown in . Mitomycin C (MMC), tamoxifen and methoxychlor were used as positive controls for genotoxicity, antioestrogenic activity and agonistic oestrogen activity, respectively.
Data analysis
All sample results and positive controls were normalised to the activity of the vehicle control(s), which was set to 1 for reporter gene assays and 100% for cell viability assays. The standard curves for the nuclear-receptor bioassays were created on a four parameter non-linear regression sigmoidal curve fit using GraphPad Prism 8 Software (San Diego, California USA).
The effect concentration (EC50) and inhibitory concentration (IC50) were calculated from the four parameter regression curve, as previously described by Escher et al.(Citation2018) (Table SI-1).
For the transcription factor Nrf2, the standard curve was fitted by linear regression using GraphPad Prism 8 Software. An effect concentration corresponding to an induction ratio of 1.7 was calculated for Nrf2 activity, as no maximum response exists (Table SI-1) (Escher et al. Citation2014). Micronuclei results were analysed in quadruplicate with GraphPad Prism 8 using one-way ANOVA followed by Dunnett’s multiple comparison test. P-values <0.05 were considered statistically significant.
For all bioassays, the classification of a sample as bioactive was based on a cut-off limit, which was calculated as 1 plus 3 times the standard deviation (SD) of the normalised vehicle control. The cut-off limit for the antioestrogenic and antiandrogenic tests were calculated as 1 minus 3 times the SD of the normalised vehicle control. More information of the cut-off values are presented in Table SI-1. The cut-off value for cytotoxicity was set at 75% of cell viability compared to the vehicle control for all assays, except for the micronuclei test. For this, the cut-off limit of cytotoxicity was set at fourfold increase in EMA-positive events compared to the vehicle control. The bioanalytical equivalent concentration (BEQ) was calculated for the highest concentration for each bioactive extract based on the linear range of the extract’s concentration-response and dose–response curves of the assay-specific standard (Table SI-2).
Results and discussion
Cell viability
FCM extracts were evaluated for cytotoxicity in MCF7 AREc32, HepG2-NFκβ, AR-EcoScreen GRKO M1, DR-EcoScreen and VM7Luc4E2 cell lines at concentrations from 0.3 to 10 mg food packaging material/mL of cell medium, to ensure that specific toxic responses were evaluated under non-cytotoxic conditions (Figure SI-1 – 5). The extract from baking and baking moulds was tested at concentrations from 0.2 to 6 mg food packaging material/mL of cell culture medium because of technical reasons. Samples causing a cell viability of <75% were defined as cytotoxic. Any sample that was cytotoxic is represented by hashed grey-black bars in the graphs for each endpoint. As these samples were cytotoxic, although not severely, the results for those exposure concentrations should be interpreted with care. All other extracts were found to be non-cytotoxic (Figure SI-1 – 5).
Oxidative stress
Oxidative stress was assessed using a stably transfected breast cancer cell line (MCF7 AREc32). This cell line contains a luciferase reporter gene that is under the control of an antioxidant responsive element (ARE), meaning that induction of ARE will result in increased luciferase activity. Activation in ARE triggers an upregulation of genes that code for enzymes and antioxidant proteins involved in the body’s defence against oxidative stress.
Twelve of the FCMs had Nrf2 activity above the cut-off level of 1.7-fold activation at 10 mg/mL (). The extracts showing the highest potency for oxidative stress were cake/pastry boxes/mats, boxes for infant formula/skimmed milk, boxes for cereals and pizza boxes. These samples displayed a 5.1–8.9-fold increase in Nrf2 activity at 10 mg/mL compared to the vehicle control. Most of the extracts that activated Nrf2 at the highest concentration also exerted an activation at the lower concentration 3 mg/mL, in a dose-related manner. tBHQ was used as the standard for the assay (Figure SI-9). The BEQ values, at non-cytotoxic concentrations, ranged from 4.2 to 28 µM tBHQ equivalents (Table SI-2).
Figure 1. Oxidative stress response in MCF7 AREc32 cells exposed to FCM extracts for 24 h (a, b). The graphs represent mean ± SD of quadruplicates (n = 4) from one representative experiment, and the dotted line is the induction ratio 1.7 fold change, which was defined as the cut-off for bioactivity. The hashed grey black bars represent concentrations that were cytotoxic
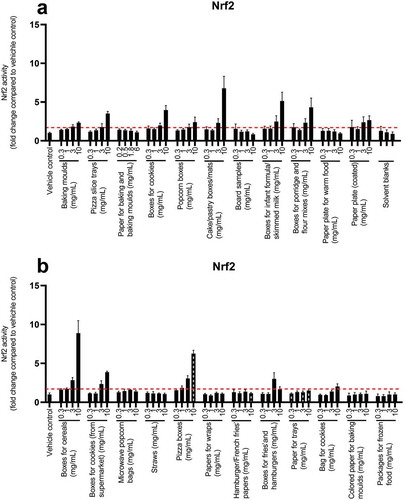
In line with these results, Rosenmai et al. (Citation2017) observed an oxidative stress induction for 16 out of 20 board and paper samples in the Nrf2 CALUX reporter gene bioassay. The highest activities in their study were observed for the cereal box, sausage tray, tomato punnet, imported paperboard, paperboard with water-soluble print and offset print materials.
Based on our results, the four FCMs showing the highest potency for Nrf2 activation (i.e. boxes for cereals, pizza boxes, cake/pastry boxes/mats and boxes for infant formula/skimmed milk) were selected for evaluation of genotoxicity.
Genotoxicity
Based on the results from the Nrf2 activity assay, four samples (boxes for cereals, pizza boxes, cake/pastry boxes/mats and boxes for infant formula/skimmed milk) were tested at 3 and 10 mg/mL in the in vitro micronucleus (MN) test using TK6 cells.
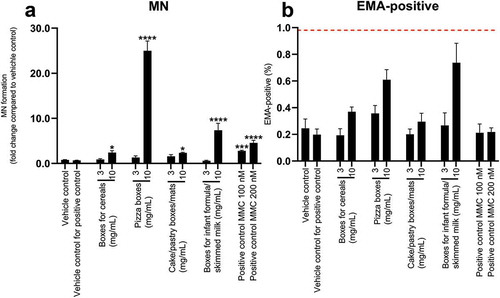
Ethidium monoazide (EMA) was used to measure cytotoxicity, in which a cut-off for cytotoxicity was set as a fourfold increase in EMA-positive events compared to the vehicle control (Bryce et al. Citation2013). None of the samples caused cytotoxicity (). All four samples induced a statistically significant increase in micronucleus events at the highest concentration tested compared to the vehicle control, showing that these samples contain genotoxic compounds ().
Figure 2. Micronuclei formation in TK6 cells after exposure to FCM extracts for 24 h (a) and cytotoxicity test (b) Mitomycin C (MMC) was used as a positive control at concentrations 100 and 200 nM (a,b). The graphs illustrate mean ± SD of quadruplicates (n = 4) from one representative experiment. The dotted line in graph B represent the cut-off limit determined by the manufacturer’s protocol. Data was analysed using one-way ANOVA, followed by Dunnett’s post-hoc test. Results that were statistical significant are indicated by asterisks (* p-value < 0.05, *** p-value < 0.001, **** p-value < 0.0001)
The genotoxicity data were obtained from the human lymphoblastic cell line TK6 that is p53 competent and karyotypically stable, which has proven to produce more reliable results than false-positive prone rodent cell lines (Fowler et al. Citation2012). Compared to the classical chromosomal aberration test, where structural chromosomal damage is studied, the MN test allows detection of both structural and numerical alterations. The usage of TK6 cells has been highlighted to be both highly sensitive and specific (Pinter et al. Citation2020). To our knowledge, this is the first study using a micronucleus test with TK6 cells to investigate paper and board FCMs.
The possible explanation for the high micronucleus events for the pizza box may be due to the fact that cardboard is often manufactured from recycled materials containing inks. Previous studies using DNA repair Rec assay and Comet assay supported the positive genotoxic response for paperboard samples (Ozaki et al. Citation2004, Citation2005). Ozaki et al. (Citation2004, Citation2005) suggested that the genotoxicity might be explained by potential mixture effects, unknown toxicants, paper resins and/or the amount of recycled matter. The recycled material showed a higher induction of DNA damage compared to virgin samples in their study. Still, varying results have been reported for in vitro genotoxicity tests and they appear to be influenced by the extraction method, material, cell type, genotoxic endpoint, dose and metabolic capacity of used cells. Additional research is needed in the use of recycled material in food packages, as there is an increased demand for its use, particularly for circular economies.
Furthermore, the genotoxic responses observed in all other materials in this study may be explained by the extensive usage of coatings or inks (). It is possible that genotoxic and hazardous substances exist in packages in form of ink and coatings, since these are not regulated within the EU, making it difficult to ensure that coatings and inks do not contain hazardous substances.
The ink can be mineral oil based and/or contain photoinitiators that have the ability to generate highly reactive species that covalently can bind to DNA and create DNA adducts (Szeliga and Dipple Citation1998; Tarnow et al. Citation2016). The findings of this study highlight the importance to further evaluate genotoxic substances in FCMs.
Oestrogen receptor activity
Oestrogenic response was assayed in the VM7Luc4E2 cell line, which stably expresses the luciferase gene under control of the oestrogen responsive element (ERE).
Only three extracts out of 23 samples exerted ER agonistic activity above the cut-off value (Figure SI-6). The BEQ for microwave popcorn bags was calculated to 10.6 ρM oestradiol equivalents in 10 mg FCM/mL (Table SI-2).
Antioestrogenic effects were assayed by stimulating VM7Luc4E2 cells with oestradiol in the cell culture medium and measured as a decrease in activity compared to the oestradiol-treated control ().
Figure 3. Antioestrogenic effects in VM7Luc4E2 cells after 24 h of exposure to FCM extracts (a,b) The graphs illustrate mean ± SD of quadruplicates (n = 4) from one representative experiment. Unspiked medium with MeOH/MQ water was used as a control for the assay (1%). The dotted line shows the cut-off limit of 0.7. Samples with an activity below the cut-off were defined as bioactive. The hashed grey black bars represent concentrations that were cytotoxic
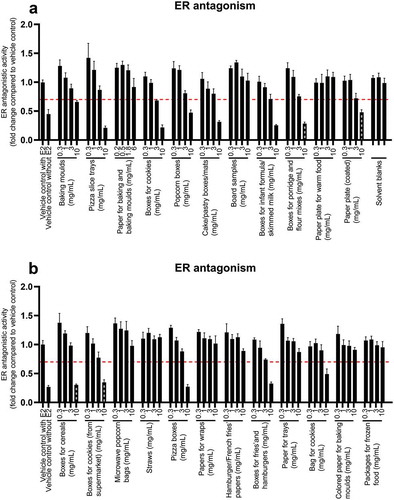
Nine of the samples exerted strong antioestrogenic activity at the highest concentration tested. The effect was dose-related and higher concentrations exerted stronger antioestrogenic effects.
Raloxifen was used as a standard for antioestrogenic activity (Figure SI-10). The BEQ values were 1.9–8.2 nM raloxifen equivalents in 10 FCM mg/mL (Table SI-2). Tamoxifen at a concentration of 3.36 μM was used as a positive control for antioestrogenic activity and had a response between 0.3- and 0.6-fold change compared to the solvent control.
Both agonistic and antioestrogenic receptor effects were seen in the FCM extracts, indicating that chemicals within FCMs have multiple mechanisms of action on the oestrogen receptor. Nevertheless, the antioestrogenic response was observed in more extracts in our experiments. Similarly, activation of ER by paper for household use and food container cardboard has been reported in E-Screen, BG1luc4E2 (renamed VM7Luc4E2) and YES assays (Vinggaard et al. Citation2000; Lopez-Espinosa et al. Citation2007; Rosenmai et al. Citation2017). A possible explanation is the presence of bisphenol A (BPA) and certain phthalates. Exposure to chemicals like BPA and BPA analogues have been linked to impaired ovary function as well as reduced sperm production and quality (Siracusa et al. Citation2018).
Effects on the oestrogen receptor could also be linked to UV-photoinitiators in printing inks that may leach from the outer carton, which previously have been observed with benzophenones (Muncke Citation2010). Studies on the oestrogenicity of benzophenones are however debatable, as oestrogenicity has been observed in vitro using MCF7 cell proliferation and YES assays, while in vivo uterotrophic assay and in vitro human ERα reporter gene assay failed to demonstrate any oestrogenicity (Muncke Citation2010).
Androgen receptor activity
Androgenic effects were examined with the AR-EcoScreen GR-KO M1 assay, which employs stably transfected CHO cells with human androgen receptor elements linked to a luciferase gene (Zwart et al. Citation2018).
No agonistic activity of the androgen receptor was exerted in any of the samples (Figure SI-7).
Antiandrogenic activities were assayed by stimulating AR-EcoScreen GR-KO M1 cells with DHT in the cell culture medium, and effects were measured as decreased activity compared to the DHT-treated control (). Twelve out of 23 FCM extracts induced an antiandrogenic response in a dose-related manner. Hydroxyflutamide was used as a standard for antiandrogenic effects (Figure SI-11). The BEQ values ranged from 0.1 to 3.7 µM hydroxyflutamide equivalents in 10 mg FCM/mL (Table SI-2). The antiandrogenic responses may be explained by chemicals like phenols, phthalates or organotins; the latter is used as a fungicide in paper and pulp (Muncke Citation2010). Phenolic compounds found in coatings and plastic food packaging have been reported to induce antagonistic response of the androgen receptor in the AR CALUX (Krüger et al. Citation2008).
Figure 4. Antiandrogenic response in AR-EcoScreen GR-KO M1 cells after 24 h of exposure to FCM extracts (a,b). Unspiked medium with MeOH/MQ water was used as the control for the assay (1%). The panel shows mean ± SD of quadruplicates (n = 4) from one representative experiment. The dotted line illustrates the cut-off of 0.7. Samples with an activity below the cut-off was defined as bioactive
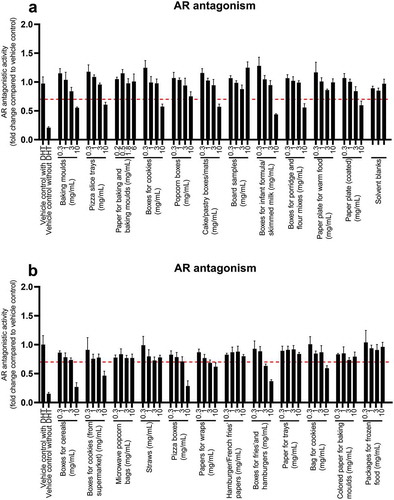
Tests of antiandrogenic effects have been carried out by Mertl et al. (Citation2014) with yeast androgen screen (YAS) and human cell-based AR CALUX bioassays. Two out of three paperboard samples showed antagonistic effects in the YAS reporter gene assay. However, the response was not detected in the antagonistic AR CALUX assay, except in one sample that showed a positive response in both assays. The difference in responses between the two models are not known, but it may be explained by underlying cytotoxicity of the FCM extracts causing false-positive results in the yeast screen assay. Conversely, Rosenmai et al. (Citation2017) also tested antiandrogenic activity, in which nine out of twenty paper and board samples had antiandrogenic activity. It was speculated by the authors that the effect may be explained by the resin acids abietic (AA) and dehydroabietic (DHA) used in paper products, as seen in a study by Rostkowski et al. (Citation2011). Rosenmai et al. (Citation2017) reported antiandrogenic effects for cake tray, baking mould and paper wraps, which is similar to observations in this study. Besides this, other studies have focused on studying chemicals on food packaging materials like inks. Peijnenburg et al. (Citation2010) studied the commonly used ink component photoinitiator 2 isopropylthioxanthone (2-ITX), which was found to have antioestrogenic and antiandrogenic effects in two yeast-based assays.
Aryl hydrocarbon receptor activity
The AhR assay utilises DR-EcoScreen cells, which are mouse hepatoma cells stably transfected with an aryl hydrocarbon responsive element (AhRE) that regulates the expression of the luciferase gene (Anezaki et al. Citation2009).
A dose-related increase in AhR activity was observed for nearly all of the samples (20/23) (). The strongest effects were observed for pizza slice trays, cake/pastry boxes/mats, pizza boxes and bag of cookies.
Figure 5. AhR activity in DR-EcoScreen cells after 24 h of exposure to FCM extracts (a,b). The graphs illustrates mean ± SD of quadruplicates (n = 4) from one representative experiment. The dotted line shows the cut-off limit of 1.5. The hashed grey black bars represent concentrations that were cytotoxic
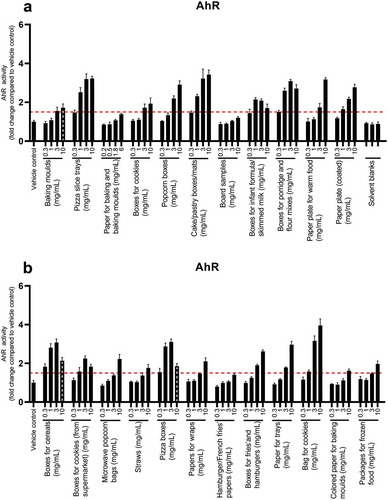
Strong activity was already observed at the lowest concentrations for boxes for porridge and flour mixes, boxes of cereals and pizza boxes. At the highest concentrations, the boxes for infant formula/skimmed milk and boxes for cookies (from the supermarket) showed a slight reduction in AhR activity compared to the lower concentration of 3 mg/mL, indicating cytotoxicity that was not detected in the MTS assay. TCDD was used as a standard (Figure SI-12). The BEQ values, at non-cytotoxic concentrations, ranged from 0.2 to 13 ρM TCDD equivalents (Table SI-2).
The positive response in AhR is supported by previous data on both paper and cardboard using the stably transfected rat hepatoma CALUX-assay (Binderup et al. Citation2002; Bengtström et al. Citation2014; Rosenmai et al. Citation2017). In a recent study by Rosenmai et al. (Citation2017), all samples induced AhR, with the pizza box, tomato punnet, sausage tray and paperboard with offset print showing pronounced inductions of the AhR. The strong response was suggested to be due to additive response caused by the presence of contaminants and/or natural components within the paper and board that have the ability to function as AhR ligands. Furthermore, the photoinitiator 2-ITX in ink have shown to have AhR agonistic activity in the DR CALUX assay, as well as induce the AhR responsive enzyme cytochrome P450 1A1 activity in the EROD assay using the rat hepatoma H4IIE cell line (Peijnenburg et al. Citation2010). Further studies are needed to understand FCMs impact on AhR, as it has vital functions in biotransformation of xenobiotic substances, reproduction, development and intestinal immunological response (Gutiérrez-Vázquez and Quintana Citation2018; Bock Citation2019).
NFκβ activity
NFκβ activity was tested in HepG2-NFκβ cells, which is a stably transfected cell line with a NFκβ responsive element controlling the luciferase gene (Figure SI-8). The transcription factor NFκβ has vital functions in the immune system, and dysfunction has been related to cancer, autoimmune diseases and viral infections (Brasier Citation2006). None of the FCM extracts caused an increased activity in NFκβ, which had the cut-off at 1.5.
The lack of response in the NFκβ reporter gene assay could be explained by several factors, that the extracts did not induce an immune response, potential immunosuppressive effects or lack of cell communication that is critical for proper immunological. Kejlová et al. (Citation2019) also investigated the inflammatory response and observed the induction of cytokine IL-8 in heavy-printing FCM samples in the sophisticated 3D human intestine model EpiIntestinal FT, which suggest that FCMs may affect important functions of leukocytes. Still, few studies have investigated the inflammatory response from FCM and future studies should focus on the potential immunotoxic effects in the gut, since it is the main route of exposure of FCMs.
Future perspective
We have observed activation of oxidative stress, genotoxicity, xenobiotic metabolism and antagonistic effects on the oestrogen as well as androgen receptors. Packages that are of potential concern includes cake/pastry boxes/mats, boxes for infant formula/skimmed milk, pizza boxes, pizza slice trays and bag of cookies. Two materials that were particularly noticeable were cake/pastry boxes/mats and boxes for infant formula/skimmed milk, which suggests that these materials seem to be the most problematic, potentially due to the heavy colouration from the printing inks. These findings are of importance given that substances that causes these effects could migrate into food and thus constitute a health hazard for humans.
One important aspect of the present study is whether the extraction method is representative of realistic migrations from the FCMs to food and subsequently in relation to human exposure. The methanol extraction with microwave treatment at high temperature may exaggerate the migration of water-soluble compounds, although conversely more-lipid-soluble contaminants may not be extracted. Additionally, the extraction procedure was done on the food packaging as a whole product, and single sided extraction of materials having a secondary packaging like infant formula/skimmed milk could have resulted in different results. Nevertheless, the resin acids dehydroabietic (DHA) and abietic (AA) in paper products have shown to migrate under mild extraction procedures, and have been speculated to cause antiandrogenic effects in food package materials (Ozaki et al. Citation2006; Rosenmai et al. Citation2017). In addition, worst-case scenario extractions can be relevant for certain materials that are exposed to high temperatures in their normal use, for example, microwave popcorn bags, and as a screening method to identify potential problematic substances/FCMs.
It is important to keep in mind that volatile substances may seep through cardboard and plastic bag materials, such that dry foods can still be contaminated with chemicals from inks or recycled fibres, particularly after longer storage conditions (Lorenzini et al. Citation2010). Furthermore, it is essential to ensure that observed effects are not from substances present in the food itself.
Several challenges exist when studying food package materials and one of these are NIAS, which currently are not possible to identify and quantitatively determine in targeted chemical analysis. As toxicity can arise from both unknown and known compounds individually and as components in mixtures, it is necessary to base the hazard identification and risk assessment on the material as a whole and not the single known chemicals. An effect-based strategy enables hazard identification; however, there is a need to standardise bioassays in future studies to ensure high-quality performance, reporting, sensitivity, specificity and consistency between laboratories (Groh and Muncke Citation2017).
The results presented here prompt future studies on the presence of hazardous chemicals in paper and board FCMs. Specifically, studies should focus on using more relevant extraction methods and investigate potential alterations in toxicity during passage through the intestinal epithelium in combination with reporter gene bioassays. Finally, the use of effect-based approaches to evaluate the potential effects of such chemicals in food packages should be emphasised, since it cannot be ruled out that the chemicals causing activity in the FCM extracts could migrate and contaminate the food.
Acknowledgements
The study was financially supported by the Swedish University of Agricultural Sciences Early Career Grant awarded to Johan Lundqvist. The authors are grateful to Anders Glynn for reviewing this paper.
Declaration of interest
The authors declare no competing financial interest.
Supplemental Material
Download MS Word (565.5 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Alin J, Hakkarainen M. 2012. Migration from polycarbonate packaging to food simulants during microwave heating. Polym Degrad Stab. 97(8):1387–1395. doi:https://doi.org/10.1016/j.polymdegradstab.2012.05.017
- Anezaki K, Yamaguchi K, Takeuchi S, Iida M, Jin K, Kojima H. 2009. Application of a bioassay using dr-ecoscreen cells to the determination of dioxins in ambient air: a comparative study with HRGC-HRMS analysis. Environ Sci Technol. 43:7478–7483. doi:https://doi.org/10.1021/es901003b
- Bengtström L, Trier X, Granby K, Rosenmai AK, Petersen JH. 2014. Fractionation of extracts from paper and board food contact materials for in vitro screening of toxicity. Food Addit Contam Part A. 31:1291–1300. doi:https://doi.org/10.1080/19440049.2014.912357
- Binderup ML, Pedersen GA, Vinggaard AM, Rasmussen ES, Rosenquist H, Cederberg T. 2002. Toxicity testing and chemical analyses of recycled fibre-based paper for food contact. Food Addit Contam. 19:13–28. doi:https://doi.org/10.1080/02652030110089878
- Bock KW. 2019. Aryl hydrocarbon receptor (AHR): from selected human target genes and crosstalk with transcription factors to multiple AHR functions. Biochem Pharmacol. 168:65–70. doi:https://doi.org/10.1016/j.bcp.2019.06.015
- Brasier AR. 2006. The NF-κB regulatory network. Cardiovasc Toxicol. 6:111–130. doi:https://doi.org/10.1385/CT:6:2:111
- Bryce SM, Avlasevich SL, Bemis JC, Tate M, Walmsley RM, Saad F, Van Dijck K, De Boeck M, Van Goethem F, Lukamowicz-Rajska M, et al. 2013. Flow cytometric 96-well microplate-based in vitro micronucleus assay with human TK6 cells: protocol optimization and transferability assessment. Environ Mol Mutagen. 54(3):180–194. doi:https://doi.org/10.1002/em.21760
- Campanella G, Ghaani M, Quetti G, Farris S. 2015. On the origin of primary aromatic amines in food packaging materials. Trends Food Sci Technol. 46:137–143. doi:https://doi.org/10.1016/j.tifs.2015.09.002
- [EC] European Commission. 2004. Commission Regulation No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC.
- [EC] European Commission. 2011. Commission Regulation No. 10/2011. Off. J. Euro Union, 1–89.
- [EC] European Commission. 2016. Commission Regulation No. 2016/1416. Off. J. Euro Union.
- Escher BI, Allinson M, Altenburger R, Bain PA, Balaguer P, Busch W, Crago J, Denslow ND, Dopp E, Hilscherova K, et al. 2014. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ Sci Technol. 48:1940–1956. doi:https://doi.org/10.1021/es403899t
- Escher BI, Neale PA, Villeneuve DL. 2018. The advantages of linear concentration-response curves for in vitro bioassays with environmental samples: linear CRC. Environ Toxicol Chem. 37:2273–2280. doi:https://doi.org/10.1002/etc.4178
- Escher BI, Van Daele C, Dutt M, Tang JYM, Altenburger R. 2013. Most oxidative stress response in water samples comes from unknown chemicals: the need for effect-based water quality trigger values. Environ Sci Technol. 47:7002–7011. doi:https://doi.org/10.1021/es304793h
- Fowler P, Smith K, Young J, Jeffrey D, Kirkland S, Pfuhler P, Carmichael P. 2012. Reduction of misleading (“false”) positive results in mammalian cell genotoxicity assays. I. Choice of cell type. Choice of cell type. Mutat Res Genetic Toxicol Environ Mutagen. 742:11–25. doi:https://doi.org/10.1016/j.mrgentox.2011.10.014
- Garbin S, Hoekstra E, Simoneau C, Lopes JA, Reina V, Raffael B, Mieth A. 2016. Non-harmonised food contact materials in the EU: regulatory and market situation (No. EUR 28357). doi:https://doi.org/10.2788/234276
- Groh KJ, Muncke J. 2017. In vitro toxicity testing of food contact materials: state-of-the-art and future challenges. Compr Rev Food Sci Food Saf. 16(5):1123–1150. doi:https://doi.org/10.1111/1541-4337.12280
- Gutiérrez-Vázquez C, Quintana FJ. 2018. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 48:19–33. doi:https://doi.org/10.1016/j.immuni.2017.12.012
- Kejlová K, Dvořáková M, Vavrouš A, Ševčík V, Kanďárová H, Letašiová S, Sosnovcová J, Jírová D. 2019. Toxicity of food contact paper evaluated by combined biological and chemical methods. Toxicol In Vitro. 59:26–34. doi:https://doi.org/10.1016/j.tiv.2019.04.001
- [KEMI] Kemikalieinspektionen. 2020. Regeringsuppdraget om kartläggning av farliga ämnen 2017 – 2020. No. 3/20.
- König M, Escher BI, Neale PA, Krauss M, Hilscherová K, Novák J, Teodorović I, Schulze T, Seidensticker S, Kamal Hashmi MA, et al. 2016. Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis. Environ Pollut. 220:1220–1230. doi:https://doi.org/10.1016/j.envpol.2016.11.011
- Koster S, Bani-Estivals MH, Bonuomo M, Bradley E, Chagnon MC, Garcia ML, Godts F, Gude T, Helling R, Paseiro-Losada, et al. 2015. Guidance on best practices on the risk assessment of non intentionally added substances (NIAS) in food contact materials and articles. International Life Sciences Institute (ILSI) Europe. http://ilsi.wpengine.com/europe/wp-content/uploads/sites/3/2016/04/2015-NIAS_version-January-2016.pdf
- Krüger T, Long M, Bonefeld-Jørgensen EC. 2008. Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology. 246(2–3):112–123. doi:https://doi.org/10.1016/j.tox.2007.12.028
- Lopez-Espinosa M-J, Granada A, Araque P, Molina-Molina J-M, Puertollano M-C, Rivas A, Fernández M, Cerrillo I, Olea-Serrano M-F, López C, et al. 2007. Oestrogenicity of paper and cardboard extracts used as food containers. Food Addit Contam. 24:95–102. doi:https://doi.org/10.1080/02652030600936375
- Lorenzini R, Fiselier K, Biedermann M, Barbanera M, Braschi I, Grob K. 2010. Saturated and aromatic mineral oil hydrocarbons from paperboard food packaging: estimation of long-term migration from contents in the paperboard and data on boxes from the market. Food Addit Contam Part A. 27:1765–1774. doi:https://doi.org/10.1080/19440049.2010.517568
- Lundqvist J, Mandava G, Lungu-Mitea S, Lai FY, Ahrens L. 2019. In vitro bioanalytical evaluation of removal efficiency for bioactive chemicals in Swedish wastewater treatment plants. Sci Rep. 9:7166. doi:https://doi.org/10.1038/s41598-019-43671-z
- Melski K, Zabielski J, Kubera H. 2003. Model study on intensified migration of volatile substances from food contacting plastic materials during repeated microwaving. Electron J Pol Agric Univ. 6(1): bwmeta1.element.agro-article-91ef37a0-bd53-4a2d-b16e-867fd0ce6473.
- Mertl J, Kirchnawy C, Osorio V, Grininger A, Richter A, Bergmair J, Pyerin M, Washüttl M, Tacker M. 2014. Characterization of estrogen and androgen activity of food contact materials by different in vitro bioassays (YES, YAS, ERα and AR CALUX) and chromatographic analysis (GC-MS, HPLC-MS). PloS One. 9(7):e100952–e100952. doi:https://doi.org/10.1371/journal.pone.0100952
- Muncke J. 2010. Endocrine disrupting chemicals and other substances of concern in food contact materials: an updated review of exposure, effect and risk assessment. J Steroid Biochem Mol Biol. 127(1–2):118–127. doi:https://doi.org/10.1016/j.jsbmb.2010.10.004
- Neale PA, Braun G, Brack W, Carmona E, Gunold R, König M, Krauss M, Liebmann L, Liess M, Link M, et al. 2020. Assessing the mixture effects in in vitro bioassays of chemicals occurring in small agricultural streams during rain events. Environ Sci Technol. 54(13):8280–8290. doi:https://doi.org/10.1021/acs.est.0c02235
- [OECD] Organisation for Economic Co-operation and Development. 2016a. Test No. 487: In vitro mammalian cell micronucleus test, OECD guidelines for the testing of chemicals. Section 4. OECD Publishing.
- [OECD] Organisation for Economic Co-operation and Development. 2016b. Test No. 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonists. OECD Publishing.
- [OECD] Organisation for Economic Co-operation and Development. 2016c. Test No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals, OECD Guidelines for the Testing of Chemicals. Section 4. OECD Publishing.
- Ozaki A, Ooshima T, Mori Y. 2006. Migration of dehydroabietic and abietic acids from paper and paperboard food packaging into food-simulating solvents and Tenax TA. Food Addit Contam. 23:854–860. doi:https://doi.org/10.1080/02652030600743813
- Ozaki A, Yamaguchi Y, Fujita T, Kuroda K, Endo G. 2004. Chemical analysis and genotoxicological safety assessment of paper and paperboard used for food packaging. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 42:1323–1337. doi:https://doi.org/10.1016/j.fct.2004.03.010
- Ozaki A, Yamaguchi Y, Fujita T, Kuroda K, Endo G. 2005. Safety assessment of paper and board food packaging: chemical analysis and genotoxicity of possible contaminants in packaging. Food Addit Contam. 22:1053–1060. doi:https://doi.org/10.1080/02652030500090885
- Park SR, Park SJ, Jeong MJ, Choi JC, Kim M. 2018. Fast and simple determination and exposure assessment of bisphenol A, phenol, p-tert-butylphenol, and diphenylcarbonate transferred from polycarbonate food-contact materials to food simulants. Chemosphere. 203:300–306. doi:https://doi.org/10.1016/j.chemosphere.2018.03.185
- Peijnenburg A, Riethof-Poortman J, Baykus H, Portier L, Bovee T, Hoogenboom R. 2010. AhR-agonistic, anti-androgenic, and anti-estrogenic potencies of 2-isopropylthioxanthone (ITX) as determined by in vitro bioassays and gene expression profiling. Toxicol In Vitro. 24:1619–1628. doi:https://doi.org/10.1016/j.tiv.2010.06.004
- Peters R, Groeneveld I, Sanchez P, Gebbink W, Gersen A, De Nijs M, Van Leeuwen SPJ. 2019. Review of analytical approaches for the identification of non-intentionally added substances in paper and board food contact materials. Trends Food Sci Technol. 85:44–54. doi:https://doi.org/10.1016/j.tifs.2018.12.010
- Pinter E, Rainer B, Czerny T, Riegel E, Schilter B, Marin-Kuan M, Tacker M. 2020. Evaluation of the suitability of mammalian in vitro assays to assess the genotoxic potential of food contact materials. Foods. 9(2):237. doi:https://doi.org/10.3390/foods9020237
- Rosenmai AK, Bengtström L, Taxvig C, Trier X, Petersen JH, Svingen T, Binderup M-L, Barbara Medea Alice VVL, Dybdahl M, Granby K, et al. 2017. An effect-directed strategy for characterizing emerging chemicals in food contact materials made from paper and board. Food Chem Toxicol. 106:250–259. doi:https://doi.org/10.1016/j.fct.2017.05.061
- Rosenmai AK, Lundqvist J, Le Godec T, Ohlsson Å, Tröger R, Hellman B, Oskarsson A. 2018. In vitro bioanalysis of drinking water from source to tap. Water Res. 139:272–280. doi:https://doi.org/10.1016/j.watres.2018.04.009
- Rostkowski P, Horwood J, Shears JA, Lange A, Oladapo FO, Besselink HT, Tyler CR, Hill EM. 2011. Bioassay-directed identification of novel antiandrogenic compounds in bile of fish exposed to wastewater effluents. Environ Sci Technol. 45:10660–10667. doi:https://doi.org/10.1021/es202966c
- Schweighuber A, Himmelsbach M, Buchberger W, Klampfl CW. 2019. Analysis of polycyclic aromatic hydrocarbons migrating from polystyrene/divinylbenzene-based food contact materials. Monatsh Chem. 150:901–906. doi:https://doi.org/10.1007/s00706-019-2377-1
- Severin I, Souton E, Dahbi L, Chagnon M-C. 2017. Use of bioassays to assess hazard of food contact material extracts: state of the art. Food Chem Toxicol. 105:429–447. doi:https://doi.org/10.1016/j.fct.2017.04.046
- Siracusa JS, Yin L, Measel E, Liang S, Yu X. 2018. Effects of bisphenol A and its analogs on reproductive health: a mini review. Reprod Toxicol Elmsford N. 79:96–123. doi:https://doi.org/10.1016/j.reprotox.2018.06.005
- Szeliga J, Dipple A. 1998. DNA adduct formation by polycyclic aromatic hydrocarbon dihydrodiol epoxides. Chem Res Toxicol. 11:1–11. doi:https://doi.org/10.1021/tx970142f
- Tarnow P, Hutzler C, Grabiger S, Schön K, Tralau T, Luch A. 2016. Estrogenic activity of mineral oil aromatic hydrocarbons used in printing inks. PLOS ONE. 11(1):e0147239. doi:https://doi.org/10.1371/journal.pone.0147239
- Vinggaard AM, Körner W, Lund KH, Bolz U, Petersen JH. 2000. Identification and quantification of estrogenic compounds in recycled and virgin paper for household use as determined by an in vitro yeast estrogen screen and chemical analysis. Chem Res Toxicol. 13:1214–1222. doi:https://doi.org/10.1021/tx000146b
- Zwart N, Nio SL, Houtman CJ, De Boer J, Kool J, Hamers T, Lamoree MH. 2018. High-throughput effect-directed analysis using downscaled in vitro reporter gene assays to identify endocrine disruptors in surface water. Environ Sci Technol. 52:4367–4377. doi:https://doi.org/10.1021/acs.est.7b06604
