?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pentachlorophenol (PCP) is a ubiquitous environmental contaminant commonly existing as its sodium salt (NaPCP), which enters the human body primarily through long term but low-level dietary exposure. PCP contributes to chemical carcinogenesis and teratogenesis. In this study, the probabilistic risk of dietary exposure to PCP in Guangzhou citizens was investigated. In total, 923 food samples in the categories of pork, livestock (beef and lamb), poultry, offal, eggs, and freshwater fish (considered to be relatively susceptible to PCP contamination) were collected from various markets in Guangzhou and tested for PCP. Probabilistic risk assessment model calculations for PCP dietary exposure and margin of exposure (MOE) values were performed using @RISK software, based on a Monte Carlo simulation with 10,000 iterations. The overall detection rate of PCP (above 1 μg kg−1, the detection limit) was 19.9% (184/923), with an average of 7.9 μg kg−1. The highest rate of PCP detection, 28.2%, was in livestock (beef and lamb). The MOE value for dietary PCP exposure in general Guangzhou residents averaged 400, which was far below 5,000 (the borderline for judging a health risk). The lowest MOE value, 190, was observed in the 3- to-6-year old population and indicates a significant risk. In conclusion, this study suggests that PCP exposure in Guangzhou residents is of considerable health risk, especially for the pre-school young children.
Introduction
Pentachlorophenol (PCP) is a representative environmental contaminant that was once a crucial pesticide and fungicide before being banned, and is still ubiquitously present in the global environment (Lin et al. Citation2016; Xu et al. Citation2016; Zheng et al. Citation2011), which has demonstrated detrimental effects in aquaculture and soil systems (Hong et al. Citation2005; Chen et al. Citation2012; Qiao et al. Citation2015). PCP is a potentially harmful chemical to human health, in the form of either organic PCP, or its sodium salt (NaPCP), the latter having enhanced water solubility (Badanthadka and Mehendale Citation2014). Although it has been largely prohibited from general use, its presence in various dietary commodities (including meat, eggs, milk, and honey) remains largely unchanged (Piskorska-Pliszczynska et al. Citation2016; Cui et al. Citation2017).
From 1953, NaPCP was used to manage the snail intermediate vectors of bilharziasis. It had been employed experimentally and under rather stringent supervision up until 1959, when it became more widely accessible through commercial channels, and in farmlands it was employed as a molluscicide, sprayed on rivers and dams, with a number of human fatalities (Blair Citation1961). Up until the middle of the 1980s, PCP was regularly employed as a bug killer, a fungicide and an antimicrobial agent in the household, business, and manufacturing settings in the United States (Carrizo et al. Citation2008; Morgan et al. Citation2015). Nonetheless, in 1987, the U.S. Environmental Protection Agency (U.S. EPA) disallowed almost all uses of PCP, except as a wood preservative for limited industrial applications, after it was classified by the International Agency for Research on Cancer (IARC) as a possible human carcinogen (Group 2B), with observed adverse health effects in exposed mammals (Zheng et al. Citation2012; Morgan et al. Citation2015), and detected in air, soil, food, and hand-wipe samples collected at U.S. homes and childcare centres (Rudel et al. Citation2003; Wilson et al. Citation2003, Citation2007). The U.S. EPA adheres to the Stockholm Convention, an international treaty which was established in 2016, for the strengthened banishment of persistent organic pollutants. Despite the global ban in about 186 countries since the mid-1980s, the United States had continued to manufacture and use this hazardous wood preservative, until the Stockholm Convention voted for the termination of production in the last north American pentachlorophenol plant in Mexico (Zhao Citation2014).
In China, besides its extensive use as a wood preservative prior to the 1980s, the principal intention of NaPCP in China was to eradicate the blood fluke intermediary host snails. Approximately, 60% of the nationwide production was applied for this purpose (Ge et al. Citation2007), while use for wood preservation and other purposes accounted for the other 40% (Ge et al. Citation2007; Zheng et al. Citation2012). From 1950s to 1960s, bilharzia was rampant in 12 provinces of southern China across the Yangtze River and PCP was one of the most effective molluscicides in controlling snails (Zhou et al. Citation2005). Owing to the probable health hazards of PCP, China constrained the use of NaPCP in 1997, however, with the re-emergence of schistosomiasis in habitual endemic areas, the employment of PCP for snail riddance increased again, which led to elevated levels of environmental contamination and increased health risks of PCP in its ubiquitous state (Zheng et al. Citation2012; Satarug et al. Citation2017).
Exposure to high levels of PCP and NaPCP may cause adverse effects on the liver, and damage to the immune, reproductive and developmental systems (Williams Citation1982). Previous investigations have shown that exposure by ingestion and inhalation are the most important pathways of non-occupational PCP absorption into the human body (Wilson et al. Citation2007). According to the announcement from the U.S CDC Agency for Toxic Substances and Disease Registry (ATSDR), where oral exposure is concerned, the length of time of exposure to the chemical may be categorised as acute, intermediate and chronic, with exposure lasting for 1 to 14 days, 15 to 364 days, and ≥364 days, respectively. The Minimum Risk Levels are usually 0.005 and 0.001 mg kg−1 day−1 with end-points indicated as developmental, reproductive and endocrinal effects (Williams Citation1982). The EPA approximates that for a medium-weight adult, exposure to PCP at the level of 0.03 mg kg−1 day−1 may not cause the least adverse health effects (Wilson et al. Citation2007).
Over time, PCP has generated great concern as a persistent organic pollutant, particularly contaminating food in mainland China and other regions (Hong et al. Citation2005). The features of its exposure, including the quantity, period of time, and the coexistence of other toxicants, may influence its health effects (Mather et al. Citation2004). We were interested in assessing PCP levels in foods commercially available in various districts of Guangzhou, particularly in livestock, poultry, eggs and fish. In this study, the levels of PCP in food samples randomly collected during the period between 2016 and 2019 by the Guangzhou Centre for Disease Control and Prevention were examined, and the dietary exposure-related risk was quantitatively assessed. The margin of exposure (MOE) was employed to estimate the residue burdens of PCP from specific food categories, in residents of various age groups ranging from 3 to over 60 years.
Materials and methods
Food sampling
From January 2016 to December 2019, typical PCP-contaminated foodstuffs that included pork, livestock (beef and lamb), poultry, offal, eggs, and freshwater fish were sampled from hypermarkets, agricultural markets, merchandising shops, cafeterias, and family shops by trained agents, from all the eleven districts of Guangzhou. Possibly due to their wide distribution, these commodities were considered to be probable origins of PCP exposure in Guangzhou (Zhang et al. Citation2015, p. 23).
Sampling covered distinct streets and street data were obtained from resident legislative authorities. Three streets were randomly selected and stratified by district and type of street (central or distant) by means of simulated random digits. A total of 33 streets (22 central and 11 peripheral) was designated as food sampling locations. Overall, a total of 923 individual food samples were included in this study.
Analytical technique (GC–MS method)
Sample preparation: pork, chicken, freshwater fish, and other animal tissues were weighed, and 5 g of each sample were minced and placed into a centrifuge tube. 30 μL of 10 μg mL−1 2,4,6-tribromophenol internal standard solution, and 20 mL of 5% trichloroacetic acid solution were added, followed by homogenisation for 1 min.
Extraction was carried out for a further 2 min, then centrifugation at 0 °C, 12,000 rpm for 4 min. The upper organic phase was sucked out and transferred to a test tube, where the extract was dried with nitrogen in a 60 °C water bath, and 2.0 ml acetonitrile was added for ultrasonic dissolution and extraction, followed by separation again through centrifugation at 8000 rpm, heating for 2 min, and collection of the supernatant.
For eggs and other complex matrix samples, a different extraction method was used. Five grams of each sample were added into a 50 mL centrifuge tube, into which 30 μL of 10.0 μg mL−1 2,4,6-tribromophenol internal standard, 10 mL extracting solution (ethyl acetate: n-hexane, 1:9 (V:V)) and 2.5 mL 5% trichloroacetic acid solution were added. The sample was extracted for 5 min, then the tube was centrifuged at 0 °C, 12,000 rpm for 4 min. The upper organic phase was sucked out and transferred to a test tube, to which 8–9 mL acetonitrile were added, then the extraction was dried by nitrogen blowing to a volume of 3 mL in a 60 °C water bath. Then 3 mL of 0.1 mol L−1 sodium hydroxide solution was added into the tube. Further extraction was conducted for 2 min, by centrifugation at 8000 rpm. The upper organic phase was sucked out, during which attention was paid to remove the intermediate suspended solids as much as possible. Then, 0.5 mL of 6 mol L-1 hydrochloric acid solution was added to the aqueous phase, followed by 5 mL n-hexane. After 2 min of extraction and 2 min of centrifugation at 8000 rpm, the upper organic phase was sucked out and put into a test tube with a stopper, dried with nitrogen in a 60 °C water bath, 1.5 mL of acetonitrile added for ultrasonic dissolution, centrifuged at 8000 rpm for 2 min, and the upper acetonitrile phase was collected.
Derivatisation: 0.2 mL of the reagent mixture acetic anhydride-pyridine were added to the above residues, which were then sealed and reacted in a water bath at 60 °C for 15 min, and then cooled out. One mL n-hexane and 0.2 mol/L potassium carbonate solution were added and the solutions were fully mixed and extracted, centrifuged at 8000 rpm, then the organic phase was taken for GC-MS.
GC-MS analysis: DB-5MS capillary column: 30 m × 0.25 mm (inner diameter) × 1.25 μm (film thickness). Injection port temperature: 230 °C. Column temperature: the initial temperature was 80 °C, kept for 2 min, then raised to 250 °C at 10 °C/min, and run for 4 min at 290 °C. Carrier gas: helium, purity ≥99.999%, flow rate 1.0 mL·min−1. Injection volume: 1–2 μL. Ionisation mode: EI source, 70 eV. Ion source temperature: 230 °C. Injection method: no split injection. Selected Ion Monitoring (SIM): pentachlorophenol quantitative ion was 266, qualitative ions were 264, 268, 308. 2,4,6-Tribromophenol quantitative ion was 330, qualitative ions were 328 and 332. shows the standard peak diagram of 10 μg L–1.
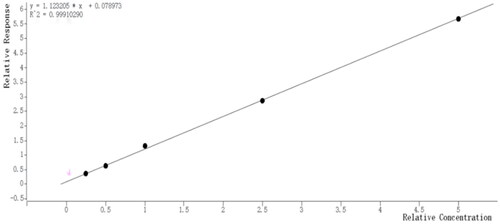
Calculation: 10, 25, 50, 100 and 200 μL of 1.0 μg mL−1 PCP standard solutions were added to 30 μL 10.0 μg mL−1 2,4,6-tribromophenol internal standard solution, 0.5 mL acetonitrile, 0.2 mL derivative reagent, and sealed. The same products were derivatised and extracted together to obtain a 10–200 ng PCP-covering standard curve. The peak area was taken as the ordinate y, and the concentration of PCP was taken as the abscissa x. The working curve was obtained. The linearity of PCP was good, and the correlation coefficient was 0.9991 ().
Quality assurance
In our study, the limit of detection (LOD) was 1.0 μg kg−1, determined from a signal-to-noise ratio of 3:1. PCP standard solutions at low, medium and high concentrations were added to the tissue samples of PCP-negative animals, at standard concentrations of PCP of 10, 50, and 100 μg·kg−1, to test the accuracy and precision of the method. According to the above experimental conditions, extraction, purification and derivatisation were performed and GC-MS/MS was used to determine recoveries. The recoveries were in the range of 65–120%. The limit of quantification (LOQ) was 3.0 μg·kg−1, ascertained at a signal-to-noise ratio greater than 9:1.
Valuation of daily dietary consumption
Data for dietary consumption was derived from a countrywide food intake survey of inner-city and rural populaces of Guangzhou completed in 2011. Data on dietary intake was established from a three-day consecutive 24-h recall questionnaire. The procedure details are obtainable from previous reports (Yang Citation2009; Zhang et al. Citation2017). Totally 2960 inhabitants from 998 homes were investigated in the research (1416 males and 1544 females). Inner-city residents covered 63.8% (1888) of the total, while suburban residents accounted for the rest 36.2% (1072) of the subjects. The ages of the residents ranged from 3 to 86 years, and the average age was 32 years old. The established age groups were 3 to 6, 7 to 17, 18 to 59 years, and 60 years old and above, in the proportion of 6.7% (199), 21.5% (637), 58.6% (1734), and 13.2% (390), respectively (Wang et al. Citation2009; Li et al. Citation2014; Ding et al. Citation2015).
Approximation of daily intake of PCP
The overall dietetic intake of PCP was calculated as an estimated daily intake (EDI) using the following equation (FAO/WHO Citation2010).
EDI is the approximation of diurnal dietary PCP intake (mg·kg−1 body weight day−1). Di is the everyday consumption of each food category in an age group (g person−1 day−1) and Mi is the average concentration of PCP in the food category (μg kg−1). Where PCP was not detected in particular foods, Mi was presumed to be LOD/2 (EFSA Citation2011). W indicates the body mass of each subject (kg). The mean mass of respondents in the various age groups was 20 kg for the 3 to 6 (Lin et al. Citation2016), 40 kg for the 7 to 17 (Song et al. Citation2017), and 62 kg (China Institute of Nutrition and Health Citation2012) for the 18 to 59 and above 60 years age groups. Average daily exposure to PCP was calculated using the @RISK software.
Risk characterisation
PCP is a persistent organic pollutant with genetic toxicity and carcinogenicity, with no detectable genotoxicity threshold. For such carcinogens that do not have a demarcated lower limit, the present Codex Alimentarius Commission (CAC) endorsed the usage of the MOE analysis technology proposed by the European Food Safety Agency (EFSA), for their hazard assessment. A margin of exposure of 10,000 or higher, as regularly predicted on the Bench Mark Dose Lower Confidence Limit (BMDL)10 established from an animal study, and considering overall uncertainties in the interpretation, would generally be of low concern from a public health point of view. The magnitude of a MOE, however, only denotes a level of significance and does not calculate risk. This opinion was expressed by the EFSA Scientific Committee in 2005 and was confirmed in 2022 (EFSA Citation2005). Accordingly, in this study, we utilised MOE analysis to evaluate the dietary exposure to PCP in the Guangzhou population. The lower a MOE value, the higher the threat of the substance to human health, and vice versa.
For a genotoxic carcinogen, the MOE value is the proportion of the 5% confidence interval of its BMDL. Because experimental data in human beings are infrequent, this research considered the lower perimeter of the reference point dose recommended by the Agency for Toxic Substances and Disease Registry (ATSDR), and the BMDL value was projected to be 1 μg·kg−1 per day for chronic oral exposure. Considering the mass variances between adults and children, 20, 40, 62, and 60 kg were used to estimate the exposure per kilogram of body mass and MOE in the different age groups. MOE ranges <5000, 5000 ∼ 500,000, and >500,000 indicate elevated, moderate, and low levels of health hazards, respectively, according to the Health Canada’s MOE evaluation guiding principles for genotoxic carcinogens (Piskorska-Pliszczynska et al. Citation2016).
Statistical analysis
To demonstrate the concentrations of PCP in food samples, the mean ± standard deviation values were created after performing SPSS descriptive analysis. Probabilistic risk valuation standard calculations for PCP dietary exposure and MOE values were accomplished by using @RISK software (Palisade Corporation, 7.6. Industrial, 2018) built on a Monte Carlo simulation with 10,000 reiterations. The outcomes are presented as the mean values and ranges from the 5tht to the 95th percentiles.
Results and discussion
PCP levels in foods
From the 923 food samples of the 6 categories collected from 2016 to 2019, PCP was detected in each class of the food. The overall detection rate of PCP (above 1 μg kg−1, the detection limit) was 19.9% (184/923), with an average value of 7.9 μg kg−1, and the P50 and P95 as not detected (ND) and 12.9 μg kg−1, respectively. The highest rate of PCP detection was in livestock (beef and lamb), with a detection rate of 28.2% (31/110), followed by offal and poultry meat, with detection rates of 26.1% (23/88) and 20.2% (50/247), respectively. The highest average values of PCP were found in livestock (beef and lamb), freshwater fish, and poultry, at 11.7 ± 50, 11.6 ± 61.1, and 9.9 ± 65.4 μg kg−1, respectively ().
Table 1. PCP levels of foods in Guangzhou from 2016 to 2019.
The detection rates of PCP in some food varieties in this study were higher than those in other parts of southern China. For example, the detection rate of PCP in livestock meat in Wenzhou from 2015 to 2016 was 2.3% (1/44) (Cai Citation2017), while that of animal offal sampled in Shenzhen in 2017 was 5.3% (4/75) (Yang et al. Citation2020). Research in the UK found that PCP was detected more frequently in foods such as offal, meat products and eggs, while PCP was detected infrequently, or not at all, in poultry, fish and shellfish, milk and dairy products (Fernandes et al. Citation2019).
Where previously detected levels are concerned, PCP was found in 2 out of 100 samples of pork liver and fish muscle from China, at levels ranging from 0.5 to 2.9 μg·kg−1 (Zhao Citation2014). PCP concentrations in various aquatic species collected from aquaculture farms in China have been reported, ranging from <0.5 to 61 μg kg−1 (Ge et al. Citation2007), while concentrations ranged from <0.01 μg·kg−1 to 1.9 μg·kg−1 in the UK chicken egg samples (Fernandes et al. Citation2019). The detection rate and level of PCP residues in livestock in Guangzhou, as observed in this study, were higher than those in similar studies in other regions, which is a cause for concern and merits further attention. Because of the large-scale use of PCP, predominantly for killing the intermediate host snails of schistosome in China, water bodies have been widely contaminated (Chen et al. Citation2016; Cui et al. Citation2017). PCP is still often used as a chlorinated hydrocarbon herbicide and insecticide, whose residues are widespread in paddy and upland soils where livestock feed on the contaminated vegetation, and drink from polluted water bodies as effluent moves from the soils to the water sources. Its bioaccumulation potential indicates it may pose a risk in certain coastal environments where aquatic products are sourced (Beiras and Tato Citation2018). Through the food chain and diet, humans become exposed to PCP-contaminated livestock, where PCP is suggested to be toxic, bearing carcinogenic and teratogenic effects, and affecting the reproductive system (Huang et al. Citation2019).
Dietary PCP exposure
shows the estimated daily intakes (EDI) of PCP from various foods in different age groups in Guangzhou. The EDI of PCP in each age group was estimated to range from 2.5 ng kg−1 bw day−1 to 5.2 ng kg−1 bw day−1, and the average EDI was estimated to be 2.5 ng kg−1 bw day−1 (the 90% confidence interval extended from 0 to 38.5). Among all age groups, the 3 to 6 years age group had the highest EDI, with a value of 5.20 ng kg−1 bw day−1.
Table 2. PCP EDI in foods in different age groups in Guangzhou.
Pork contributed the most to the entire population’s dietary exposure to PCP, followed by freshwater fish, and eggs, except for the age group older than 60 years. Although the detection value of PCP in pork was not high, its high consumption has caused the population to consume a large amount of PCP through pork. Fish contributed the most to dietary PCP exposure for people over 60 years. Livestock, which showed the highest detection rate and highest average of PCP, were not high in consumption, so their contribution to the dietary PCP exposure in the population is not high.
There are very few studies on the dietary intake of PCP. In the study by Fernandes et al. (Citation2019) in the UK, the adult PCP intake was 0.7 ng kg−1 bw day−1 for mean and 1.4 ng kg−1 bw day−1 for 97.5 percentile consumers. Estimated PCP intake for the 4 to 7-year old children averaged 2.3 ng kg−1 bw day−1, and 4.2 ng kg−1 bw day−1 for 97.5 percentile consumption (Fernandes et al. Citation2019). Although the research methods were different, the dietary intakes for adults and children in this study were higher than that from the study conducted in the UK (Fernandes et al. Citation2019).
Risk characterisation
shows the MOE values of PCP dietary exposure for populations of various age groups in Guangzhou. The MOE value of the total population is 400 (the 90% confidence interval ranged from 0 to 1503.0), and the MOE value of the 3 ∼ 6 years old population is the lowest, at 190 (the 90% confidence interval ranging from 0 to 747.0), indicating that the 3-to 6-year-old children are at relatively high risk of dietary exposure to PCP, probably due to lower body weights and the average dietary intake high enough for body growth and development. The health risks of preschool children’s dietary PCP exposure need to be focused on.
Table 3. Risk characterisation of PCP exposure in different age groups (indicated by MOE) in Guangzhou.
Uncertainty analysis
There are uncertainties in the exposure assessment process. First, the food samples collected in the study only contained six types of food contaminated with PCP, not including all foods that might be contaminated with PCP, or the PCP intake from drinking water and air. Therefore, to some extent, it may have underestimated the consumers’ dietary PCP intake. The second is the vagueness of the baseline dose. As the international community has not yet established a threshold for human PCP exposure as a reference point, this study referred to the Minimal Risk Level of 0.001 mg kg−1 day−1 of chronic oral exposure established by ATSDR as the benchmark dose. The rationality of this data needs to be further verified.
Conclusions
The dietary intake of PCP by Guangzhou residents is relatively high. The inclusive detection rate of PCP in food was 19.9% (184/923), and the detection average was 7.88 μg kg−1. Livestock meat exhibited the highest detection rate of PCP at 28.2% (31/110).
The MOE value for dietary PCP exposure in Guangzhou residents was 395.9, which was below 5,000 (the borderline for judging a health risk), where the lowest MOE value was in the 3-to-6-year-old group (192.4, indicating a heightened threat). Taken together, this study suggests that PCP exposure in the Guangzhou population is extensive, and consequent health hazard is noteworthy, especially where pre-school children are concerned. In this regard, our study is a verification that it is of great significance to have a subsequent study intended for the monitoring of PCP biomarkers in humans, and further assess its health hazards.
Author contributions
Yuhua Zhang: Methodology, Data curation, Writing- Original draft. Florence Mhungu: Writing-Reviewing and Editing. Weiwei Zhang: Conceptualisation, Software. Yanyan Wang: Writing- Original draft preparation, Investigation. Hailin Li: Investigation. Yufei Liu: Validation. Yan Li: Validation. Pingsheng Gan: Experimental detection. Xinhong Pan: Experimental detection. Jie Huang: Investigation. Xianwu Zhong: Data curation. Shaofang Song: Data curation. Yungang Liu: Writing-Reviewing and Editing. Kuncai Chen: Supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Badanthadka M, Mehendale HM. 2014. Chlorophenols. In Wexler P. editor. Encyclopedia of Toxicology 3rd ed. Oxford: Academic Press; p. 896–899
- Beiras R, Tato T. 2018. Marine environmental risk assessment and acute water quality criterion for pentachlorophenol in coastal waters. Ecotoxicology. 27(7):803–808. doi:10.1007/s10646-018-1930-8
- Blair DM. 1961. Dangers in using and handling sodium pentachlorophenate as a molluscicide. Bull World Health Organ. 25(4–5):597–601.
- Cai YY, Lin D, Shan RQ, Wang LL, Gao SH. 2017. Surveillance and analysis of veterinary drug residues in livestock and poultry meat and eggs in Wenzhou. Chin J Health Lab Technol. 27(02):245–246 + 249.
- Carrizo D, Grimalt JO, Ribas-Fito N, Torrent M, Sunyer J. 2008. Pentachlorobenzene, hexachlorobenzene, and pentachlorophenol in children’s serum from industrial and rural populations after restricted use. Ecotoxicol Environ Saf. 71(1):260–266. doi:10.1016/j.ecoenv.2007.08.021
- Chen M, Shih K, Hu M, Li F, Liu C, Wu W, Tong H. 2012. Biostimulation of indigenous microbial communities for anaerobic transformation of pentachlorophenol in paddy soils of southern China. J Agric Food Chem. 60(12):2967–2975. doi:10.1021/jf204134w
- Chen Y, Tao L, Wu K, Wang Y. 2016. Shifts in indigenous microbial communities during the anaerobic degradation of pentachlorophenol in upland and paddy soils from southern China. Environ Sci Pollut Res Int. 23(22):23184–23194. doi:10.1007/s11356-016-7562-8
- China Institute of Nutrition and Health. 2012. China nutrition data yearbook. China: CINH.
- Cui Y, Liang L, Zhong Q, He Q, Shan X, Chen K, Huang F. 2017. The association of cancer risks with pentachlorophenol exposure: focusing on community population in the areas along certain section of Yangtze River in China. Environ Pollut. 224:729–738. doi:10.1016/j.envpol.2016.12.011
- Ding X, Wu L, Li P, Zhang Z, Zhou H, Bai Y, Chen X, Jiang J. 2015. Risk assessment on dietary exposure to Aflatoxin B1 in Post-Harvest Peanuts in the Yangtze River Ecological Region. Toxins. 7(10):4157–4174. doi:10.3390/toxins7104157
- EFSA. 2005. Opinion of the scientific committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. Parma: EFSA.
- EFSA/FAO/WHO. 2011. Towards a harmonised Total Diet Study approach: a guidance document. Geneva (Switzerland): WHO.
- FAO/WHO. 2010. Evaluation of certain food additives. Seventy-first report of the Joint FAO/WHO Expert Committee on Food Additives.
- Fernandes AR, Mortimer D, Rose M, Smith F, Steel Z, Panton S. 2019. Recently listed Stockholm convention POPs: analytical methodology, occurrence in food and dietary exposure. Sci Total Environ. 678:793–800. doi:10.1016/j.scitotenv.2019.04.433
- Ge J, Pan J, Fei Z, Wu G, Giesy JP. 2007. Concentrations of pentachlorophenol (PCP) in fish and shrimp in Jiangsu Province, China. Chemosphere. 69(1):164–169. doi:10.1016/j.chemosphere.2007.04.025
- Hong HC, Zhou HY, Luan TG, Lan CY. 2005. Residue of pentachlorophenol in freshwater sediments and human breast milk collected from the Pearl River Delta, China. Environ Int. 31(5):643–649. doi:10.1016/j.envint.2004.11.002
- Huang X, Han X, Huang Z, Yu M, Zhang Y, Fan Y, Xu B, Zhou K, Song L, Wang X, et al. 2019. Maternal pentachlorophenol exposure induces developmental toxicity mediated by autophagy on pregnancy mice. Ecotoxicol Environ Saf. 169:829–836. doi:10.1016/j.ecoenv.2018.11.073
- Li K, Qiu F, Jiang L, Yang M. 2014. Dietary exposure assessment of aflatoxin of foodstuff and edible oil from Shenzhen residents. Wei Sheng Yan Jiu. 43(4):630–636.
- Lin Z, Bai J, Zhen Z, Lao S-j, Li W, Wu Z, Li Y, Spiro B, Zhang D. 2016. Enhancing pentachlorophenol degradation by vermicomposting associated bioremediation. Ecol Eng. 87:288–294. doi:10.1016/j.ecoleng.2015.12.004
- Mather FJ, White LE, Langlois EC, Shorter CF, Swalm CM, Shaffer JG, Hartley WR. 2004. Statistical methods for linking health, exposure, and hazards. Environ Health Perspect. 112(14):1440–1445. doi:10.1289/ehp.7145
- Morgan M, Jones P, Sobus J. 2015. Short-term variability and predictors of urinary pentachlorophenol levels in Ohio preschool children. Int J Environ Res Public Health. 12(1):800–815. doi:10.3390/ijerph120100800
- Piskorska-Pliszczynska J, Strucinski P, Mikolajczyk S, Maszewski S, Rachubik J, Pajurek M. 2016. Pentachlorophenol from an old henhouse as a dioxin source in eggs and related human exposure. Environ Pollut. 208(Pt B):404–412. doi:10.1016/j.envpol.2015.10.007
- Qiao M, Wang GP, Zhang C, Roelofs D, van Straalen NM, Zhu YG. 2015. Transcriptional profiling of the soil invertebrate Folsomia candida in pentachlorophenol-contaminated soil. Environ Toxicol Chem. 34(6):1362–1368. doi:10.1002/etc.2930
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. 2003. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 37(20):4543–4553. doi:10.1021/es0264596
- Satarug S, Vesey DA, Gobe GC. 2017. Current health risk assessment practice for dietary cadmium: data from different countries. Food Chem Toxicol. 106(Pt A):430–445. doi:10.1016/j.fct.2017.06.013
- Song Y, Wang Y, Mao W, Sui H, Yong L, Yang D, Jiang D, Zhang L, Gong Y. 2017. Dietary cadmium exposure assessment among the Chinese population. PLoS One. 12(5): e0177978. doi:10.1371/journal.pone.0177978
- Wang J, Liu XM, Zhang ZQ. 2009. Exposure assessment of liver cancer attributed to dietary aflatoxins exposure in Chinese residents. Zhonghua Yu Fang Yi Xue Za Zhi. 43(6):478–481.
- Williams PL. 1982. Pentachlorophenol, an assessment of the occupational hazard. Am Ind Hyg Assoc J. 43(11):799–810. doi:10.1080/15298668291410602
- Wilson NK, Chuang JC, Lyu C, Menton R, Morgan MK. 2003. Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J Expo Anal Environ Epidemiol. 13(3):187–202. doi:10.1038/sj.jea.7500270
- Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. 2007. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res. 103(1):9–20. doi:10.1016/j.envres.2006.04.006
- Xu T, Zhao J, Xu Z, Pan R, Yin D. 2016. The developmental effects of pentachlorophenol on zebrafish embryos during segmentation: a systematic view. Sci Rep. 6:25929. doi:10.1038/srep25929
- Yang DY, Wang Z, Yang L-Q. 2020. Analysis of monitoring results of veterinary drug residues in poultry food in Shenzhen in 2017–2018. Chin J Lab Technol. 30(22):2786–2788 + 2792.
- Yang YX. 2009. China food composition. Beijing: Peking University Medical Press. (In Chinese)
- Zhang WW, He J, Li Y, Yu C, Lin X, Liang B. 2015. Analysis on contamination of aflatoxinB1 in food and oil in Guangzhou from 2009 to 2013(In Chinese). Chin J Food Hygiene. 27(3):291–294.
- Zhang Y, L, , Y, He J, Liang, B, Yu C, Zhang W, Huang J. 2017. Food consumption and nutrients intake among residents in Guangzhou city. Chin J Public Health. 33:4.
- Zhao D. 2014. Determination of pentachlorophenol residue in meat and fish by gas chromatography-electron capture detection and gas chromatography–mass spectrometry with accelerated solvent extraction. J Chromatogr Sci. 52(5):429–435. doi:10.1093/chromsci/bmt054
- Zheng W, Wang X, Yu H, Tao X, Zhou Y, Qu W. 2011. Global trends and diversity in pentachlorophenol levels in the environment and in humans: a meta-analysis. Environ Sci Technol. 45(11):4668–4675. doi:10.1021/es1043563
- Zheng W, Yu H, Wang X, Qu W. 2012. Systematic review of pentachlorophenol occurrence in the environment and in humans in China: not a negligible health risk due to the re-emergence of schistosomiasis. Environ Int. 42:105–116. doi:10.1016/j.envint.2011.04.014
- Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, Zheng J, Utzinger J. 2005. The public health significance and control of schistosomiasis in China – then and now. Acta Trop. 96(2–3):97–105. doi:10.1016/j.actatropica.2005.07.005