Abstract
This study aimed to assess the spatiotemporal bioavailability of polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs) and dioxin-like polychlorinated biphenyls (dl-PCBs) in wild adult mussels, Mytilus galloprovincialis, collected along the Portuguese Atlantic coast between 2009 and 2020. The work is part of a national environmental monitoring program. The purpose was to evaluate the dioxins’ temporal trends, the human and ecological risks, and the correlation between mussels’ location and the main pollutant sources in Portugal. The levels and congener patterns of the most toxicity-relevant 17 PCDD/Fs and 12 dl-PCBs were determined, with the dl-PCBs dominant. The sum of Σ17PCDD/Fs and Σ12dl-PCBs values ranged from 2.0 to 4.0 ng WHO-TEQ kg−1, (wet weight basis), below the limits established by the European Commission for contamination in fish and fishery products (6.5 ng per kg−1). The study included five years – 2009, 2010, 2016, 2018 and 2020 –, allowing to verify the impact of European Directive 2013/39/UE against the pollution of water in Portugal; it was observed that dioxin levels in mussels diminished over the time. Statistical analysis allowed verification of different spatial trends of dl-PCB profiles along the Portuguese Atlantic coast. The mono-ortho dl-PCB pentachlorinated congener IUPAC #118 prevailed in 2009 and 2018 in all sampling sites, and it was predominant in 2010, 2016 and 2020, followed by the congeners IUPAC #105, #156 and #167. The IUPAC #167, #169 and #123 were the most abundant hexachlorinated congeners, and the IUPAC #77 the most abundant tetrachlorinated congener. This work emphasises the importance of monitoring dioxins and mapping the congeners in Atlantic coastal ecosystems, to contribute to their elimination.
Graphical Abstract
Introduction
The Stockholm Convention provided for the elimination of polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs) and dioxin-like polychlorinated biphenyls (dl-PCBs) by 2028 (Stockholm Convention Citation2003). However, the compounds are still discarded into the environment daily all over the world, continuing to be a global environmental issue due to their toxicity, bioaccumulation, persistence, and mobility throughout the ecosystems (Weber et al. Citation2018). Most of dioxins are released into the atmosphere from combustion processes, such as secondary metallurgy industry, commercial, municipal, or medical waste incineration, from burning fuels (wood, coal, oil), burning of household trash, and burning of any material that contains chlorine, such as plastics and wood treated with pentachlorophenol (PCP) (Stieglitz et al. Citation1997; Ba et al. Citation2009; Ansari and Matondkar Citation2014). Low concentrations have been detected in home-heating systems, exhaust from cars and cigarette smoke. Natural emission sources such as forest fires and volcanic eruptions create dioxins, too. In addition, chlorine bleaching of pulp and paper, certain types of chemical manufacturing and industrial processing, and pesticide waste have been also responsible for these pollutants. Once released into the air, PCDDs, PCDFs and PCBs disperse and deposit from the atmosphere onto land, surface water and vegetation. The compounds present a wide variability in their physicochemical properties, namely, the vapour pressure, water solubility and partition coefficients, which decide their behaviour and mobility within the different environmental compartments. They have been transported across international boundaries far from their sources, even to regions where they have never been used or produced (Adlard et al. Citation2018).
Pollutants diminish the quality of water, producing a decrease in natural resources. Marine environments have been contaminated through several anthropogenic activities (Borja et al. Citation2011; Ansari and Matondkar Citation2014). Dioxins’ accumulation in marine organisms exerts damaging effects at different levels, from organisms themselves to communities and ecosystems. For some time, the consumption of marine organisms has been considered the main way of human exposure to PCDDs, PCDFs and PCBs (Pompa et al. Citation2003). These are the reasons why, in Europe, Directive 2013/39/UE (Citation2013) of the European Parliament and of the Council supports and encourages the investigation and assessment of chemical pollution in marine waters.
This study belongs to an integrated pollution monitoring program of the Environmental Portuguese Agency (APA – Agência Portuguesa do Ambiente). After having studied the main atmospheric emissions in the country (Antunes et al. Citation2012), this work aimed to assess the spatiotemporal trends of PCDD/Fs and dl-PCBs in the Portuguese Atlantic coast. Wild Mytilus galloprovincialis were used as environmental sentinels of these lipophilic contaminants due to their ecological and food-chain importance (Cajaraville et al. Citation2000; Brooks et al. Citation2009) and because they are edible organisms that Portuguese citizens appreciate. Mussels were among the first animals to be used by researchers for assessing the environmental quality of seawater (Beyer et al. Citation2017). The European Commission had set limits for foodstuff contamination by PCDD/Fs and dl-PCBs (Commission Regulation (EC) No. 199/Citation2006). In fish and fishery products, the maximum concentrations for dioxins are 3.5 pg WHO-PCDD/Fs-TEQ and 6.5 pg WHO-PCDD/Fs-PCBs-TEQ per kilogram, wet weight.
The aim of this study was to determine the spatiotemporal distribution of PCDDs, PCDFs and dl-PCB levels, the congener profiles, and their relevance in mussels from Portuguese Atlantic coast, to evaluate the pollutants’ bioavailability, the sanitary risk of molluscs’ consumption, and the quality of marine water. The study involved five years – 2009, 2010, 2016, 2018 and 2020, a timespan that makes it possible to verify the influence of European Directive 2013/39/UE (Citation2013) on the pollution of water in Portugal.
Materials and methods
Standards and reagents
PCDD/Fs precision and recovery stock solution with native compounds (EPA 1613 PAR), PCDD/Fs labelled compound stock solution for extraction (EPA 1613 LCS), PCDD/PCDFs internal standard spiking solution for injection (EPA 1613 ISS) and PCDD/Fs calibration and verification standards (EPA 1613 CSL, CS1–CS5), all in nonane solution, were purchased from Wellington Laboratories (Ontario, Canada). The dl-PCBs native solution for precision and recovery (WP-STK), dl-PCBs surrogate spiking solution for extraction (WP–LCS), dl-PCBs internal standard solution for injection standards (WP–ISS) and dl-PCBs calibration and verification standards (WP–CS1–CS7), all in nonane solution, were also purchased from Wellington Laboratories (Ontario, Canada). All internal standards were 13C12-labelled compounds. All solvents were from Merck (pesticide grade; Darmstad, Germany). Soxhlet cellulose extraction thimbles (30 mm × 80 mm) were from Schleicher & Schuell (Dassel, Germany). Power-prep columns were from Power Prep (Fluid Management System, Waltham, MA, USA); silica (19 cm), basic alumina (11 g, 19 cm) and carbon/Celite (0.34 g, 4 cm) columns were used.
Method validation
Two Certified Reference Materials, WMS-01 (Reference Lake Sediment) from National Water Research Institute, Canada, and SS-06 (Sewage sludge SS-06) from Metranal (Analytika Ltd. Czech Republic) were used in method validation. The water content, according to certificate values, were residual (<5%). Participation in proficiency laboratorial testing by Sigma-Aldrich and RTC Dioxins and Furans – Soils, LPTP14-S2 (2014), LPTP16-S3 (2016) and LPTP18-S3 (2018) – SPE016-10G – were used to confirm the method performance. The method accuracy and precision were determined by replicate analysis using CRMs, blanks, and samples fortified with PCDDs, PCDFs and dl-PCBs. Blank and control samples were analysed daily.
Sampling sites
Wild adult Mytilus galloprovincialis of 3–6 cm, collected in 2009, 2010, 2016, 2018 and 2020, were used to ensure dioxins availability along the whole Portuguese Atlantic coast. Fifty (50) mussels were collected in eight selected points described in , in each studied year.
Table 1. Mussels’ sampling sites: location and industrial activities nearby.
Samples and sample preparation
The mussels were placed in an aquarium filled with sub-surface sea water collected from the same site as the samples and subjected to depuration for 24 h. After this period, the mussel shells were shucked live and opened to avoid minimum tissue damage. Then the mussels were inverted for 15 min to allow draining the excess of water. The soft tissues of mussels were removed, and the samples were frozen to −20 °C, during a maximum period of two weeks. Then, they were lyophilised and reduced to fine powder in a jaw mill. After homogenisation, the powder was extracted. Soxhlet apparatus were used with a cellulose thimble containing approximately 5 g sample of dry mussels. Extractions were performed following USEPA Method 1613 for PCDD/Fs and USEPA Method 1688 for dl-PCBs. The methods had similar procedures; the difference was the internal standards with the 13C-labelled compounds added in the extraction and injection phases (EPA 1613 LCS, EPA 1613 ISS, WP-LCS and WP-ISS). Toluene was used as solvent, ca. 200 mL, and the extraction was performed during 24 h. Then, the extract was concentrated and later submitted to clean up procedures. These included treatment with sulphuric acid to remove lipids. The acid treatment was done by mixing the toluene extract with three successive aliquots of 50 mL concentrated sulphuric acid and let stand for 24 h period (first acid aliquot) and 3 h for the second and third aliquots. After decantation, the organic phase was submitted to multi-layered silica/alumina/active carbon columns (Power Prep/FMS apparatus). A gradient elution, using an automatic program, was done. PCDDs and PCDFs were kept in the active carbon column and dl-PCBs were retained in the other columns (silica and alumina, respectively). PCDDs and PCDFs were eluted using toluene from the opposite flow direction and the dl-PCBs were eluted using mixtures of dichloromethane/hexane. The PCDDs and PCDFs fraction was concentrated to dryness using a rotary evaporator and a nitrogen stream, and after it was diluted, 5 µL of nonane and 5 µL of internal standards (EPA 1613 ISS) solution were added. The dl-PCBs fraction (with non- and mono-ortho-PCBs) was concentrated to dryness using a rotary evaporator and a nitrogen stream, and then the internal standard solution was added (WP-ISS solution, 20 µL).
Chemical analysis
PCDDs, PCDFs and dl-PCBs congener separation and identification were done by GC/HRMS (Agilent 6890 chromatograph coupled with a high resolution AutoSpec Ultima Micromass spectrometer; EI+ source, SIM mode). A VF-5MS column (60 m x 0.25 mm i.d. x 0.25 µm; Varian, Mideelburg, The Netherlands) was used. Helium was the carrier gas and the interface temperature was set at 280 °C. The resolving power was kept higher than 10 000. The electron energy and source temperature were 35 eV and 250 °C, respectively. TEQ concentrations for the 17 PCDD/Fs and 12 dl-PCBs were determined by multiplying the analytical result of each congener by the corresponding WHO-TEF.
Statistical analysis
Multivariate analyses were performed by means of the free software PAST: Paleontological Statistics Software Package for Education and Data Analysis (version 4.11) (Hammer et al. Citation2001). Variables were chosen from selected chromatographic peaks, considered as the most significant in profile definition by visual observation and comparison of chromatographic profiles. The universe of objects is the sampling points P1 to P8. Data sets are shown in Supplementary Material Tables 1S and 2S. Principal component analyses (PCA) were performed on non-rotated factors and missing values filled by mean substitution.
Results
Method validation
To ensure the identity of PCDDs, PCDFs and dl-PCBs, ion ratio abundance was evaluated for both native and labelled compounds, in accordance with the QC limits defined in USEPA Method 1613 (PCDDs and PCDFs) and Method 1688 (dl-PCBs). Recoveries of 13C-labelled internal standards added at the extraction phase were evaluated, and the values were between 40 and 160%. For CRM tests, recoveries of native congeners were also evaluated, with results (observed value/certified value) in the range of 80–120%. Finally, proficiency testing results obtained in LPTP14-S2 (year 2014), LPTP16-S3 (year 2016) and LPTP18-S3 (year 2018) from Sigma-Aldrich and RTC were all satisfactory with Z-scores lower than 2, proving the validity of methodology.
Method performance agreed with the requirements of the analytical methodology used to determine PCDD/Fs and PCBs in foodstuff (European Union Citation2017), and it was confirmed in the already mentioned proficiency tests.
PCDD/Fs and dl-PCBs levels in wild mussels
The sum of toxic equivalent (WHO-TEQ) concentrations of the 17 most toxic Σ17PCDD/Fs and 12 Σ12dl-PCBs in Portuguese mussels (from all sampling sites) are given in ; the data are reported on a wet weight (w.w.) basis.
Table 2. Spatiotemporal WHO-TEQ bioavailability levels (ng kg−1) of Σ17PCDD/Fs and Σ12PCBs in wild mussels from the Portuguese Atlantic coast.
The sum of Σ17PCDD/Fs plus Σ12dl-PCBs WHO-TEQ levels are shown in . As can be seen, the bioavailable concentrations in the wild mussels analysed during the period 2009–2020 are about half of the established European limits for fish and fishery products (3.5 pg WHO-PCDD/Fs-TEQ kg−1 ww).
Figure 1. Spatial trends of PCDD/Fs and PCBs (sum of WHO-TEQ values) in wild mussels collected along the Portuguese Atlantic coast (sampling sites (P1 to P8, described in ).
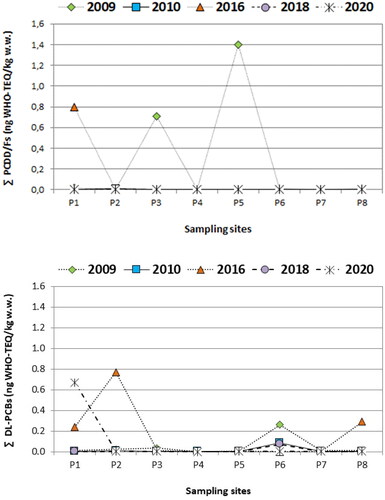
As can be seen in and , after 2009 the mussels showed PCB levels higher than PCDD/Fs levels, the maximum values ranging from 0.01 to 0.67 ng WHO-TEQ kg−1. These results agree with the literature data: PCBs generally contribute the major proportion to the overall TEQ concentrations of dioxins and dioxin-like chemicals in marine biota (Vetter et al. Citation2001; Gaus et al. Citation2004). However, the sum of Σdl-PCBs-TEQ concentration levels in Portuguese mussels was lower than those reported from the Spanish Atlantic coast (Carro et al. Citation2010; Belas et al. Citation2011; Giannico et al. Citation2022).
Organohalogen fingerprints in wild mussels
An important aspect in any PCDD/Fs and PCBs inventory is the assignment of the congener profiles, or fingerprints, because congeners are substances with a wide variability in their physicochemical properties such as vapour pressure, water solubility and partition coefficients, which determine their behaviour and mobility within any environmental compartment. Congeners also have different toxicity (TEQs). The knowledge of congener patterns may help to identify the contamination sources and pathways (Weber and Watson Citation2011; Torres et al. Citation2013) and are crucial to evaluate the health impacts and the necessity of public health intervention. For example, the exposure to some PCBs is associated with melanoma (sufficient evidence), non-Hodgkin lymphoma and breast cancer (limited evidence) (International Agency for Research on Cancer Citation2016). The use of individual exposure in isolation may lead to the underestimation of the true health impact of dioxin chemical mixtures. In this study, the 17 most toxic PCDD/Fs and 12 PCB congeners were monitored for eight beaches during all five years.
PCDD/Fs profiles
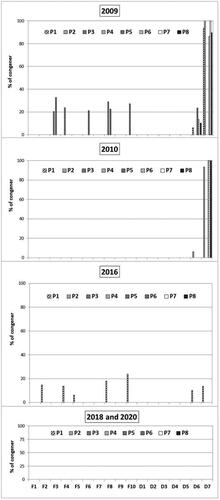
While combustion is identified as the most important source of PCDD/Fs, the metal industry is also a major source, in particular the secondary metal industry such as copper smelting. The emissions of the main Portuguese units were monitored by our research team (Antunes et al. Citation2012). Regarding mussels, the profiles of PCDD/Fs showed some analogy in 2009 and 2010, but not in 2016, 2018 and 2020, as illustrated in . As can be seen, octachlorodibenzo-p-dioxin, OCDD, was the predominant congener in 2009 and 2010 in all sampling points (P1–P8), and in 2016 it was only detected in mussels from one location in the north of Portugal: Caminha (P1). All the congeners became vestigial (below LOD) in 2018 and 2020.
PCBs profiles
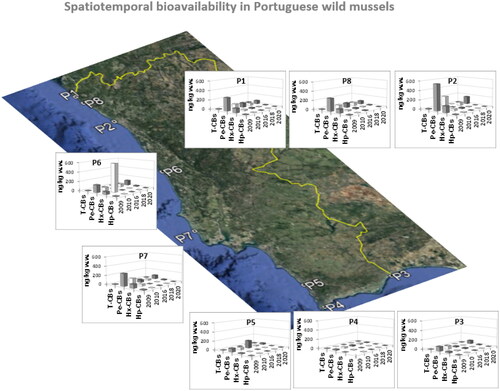
According to the literature, PCBs in the marine environment originate solely from human activity (Sánchez et al. Citation1993). The dl-PCBs were dominant in Portuguese M. galloprovincialis samples (). Congener profiles are in . The temporal and spatial trends are described in Supplementary Material Figure 1S and 2S. The highest concentration levels were from the mono-ortho pentachlorinated IUPAC #118 and IUPAC #105, the former being dominant in all samples in 2009 and 2018 (from 52 to 191 ng/kg, and from 2.1 to 119 ng/kg, respectively). Most samples showed them also dominant in 2010, 2016 and 2020. The mono-ortho hexachlorinated IUPAC #167 and IUPAC #156 were also present in moderate levels (below LOD to 51 ng/kg and below LOD to 65 ng/kg, respectively). The IUPAC #167 congener appeared in high levels in 2010 and 2016 in two sampling sites: Buarcos (P6) (572 ng/kg) and Vila Real (P3) (18 ng/kg). The mono-ortho pentachlorinated IUPAC #105 was found at Vila do Bispo (P4) (8.7 ng/kg), in 2020. The second most toxic congener, the non-ortho hexachlorinated IUPAC#169 showed to be dominant in Matosinhos (P2) (26 ng/kg), in 2016 (Supplementary Table 5S).
The sum of concentration levels of dl-PCB isomer classes revealed that pentachlorobiphenyls were the most bioavailable in mussels from the Portuguese Atlantic coast (). Consistent with the present study, this selective accumulation of penta- and also hexachlorinated PCBs has been previously observed in mussels (and fish) from the Mediterranean coast, the Baltic Sea, the North Sea and also the Spanish Atlantic coast (Belas et al. Citation2011; Di Leo et al. Citation2014).
Discussion
Mussels are an important food of the Portuguese diet, especially during summertime. Because they are filter-feeding organisms with very low metabolism, the accumulation of POPs, in particular PCDD/Fs and dl-PCBs, is determined primarily by the compounds’ equilibrium partitioning with marine water (Livingstone Citation1992; Widdows and Donkin Citation1992; Lee et al. Citation1996; Beyer et al. Citation2017). So, the contamination uptake depends on the chemicals dissolved in the surrounding seawater and food particles (mainly phytoplankton) (Beyer et al. Citation2017). PCDDs and PCDFs have been introduced into the marine environment mostly by atmospheric deposition and riverine and surface run-off (Porte and Albaigés Citation1993), while PCBs mainly originate from industrial products and materials at their end-of-life (Vetter et al. Citation2001). In Portugal, steel works are the highest emitters of PCDD/Fs (Antunes et al. Citation2012). Their flue gas contains higher amounts of PCDFs than PCDDs. All are semi-volatile and poorly water-soluble compounds; however, their octanol–water coefficients (Kow) vary from the mono-PCDD/Fs (Kow∼7–8) to the octa-PCDD/Fs (Kow∼11–12) (Lohmann and Jones Citation1998). Their atmospheric transport and behaviour were described as influenced by the gas–particle partition. When PCDD/Fs are introduced into the atmosphere, the compounds are first diluted with the cleaner air, they are transported through the atmosphere and start to age, undergoing transformations (Eitzer and Hites Citation1989; Tysklind et al. Citation1993); then they are deposited leading to homologue profiles of sediments, air and rainwater, showing an enhancement of octachlorodioxin (OCDD) (Lohmann and Jones Citation1998). Less-chlorinated congeners have been found to a greater extent in the vapour phase. In our study, OCDD was the predominant PCDD congener in wild mussels, in 2009 and 2010, in all sampling points (P1–P8), but in 2016, it was only detected in the molluscs from North of Portugal: Caminha (P1). Taking into consideration the PCDD/Fs profiles found in the main Portuguese gaseous emissions (Antunes et al. Citation2012), and the congener profiles found in mussels, , although the molluscs’ levels were very low, they support the contribution of transformations during the transport of PCDD/Fs in the Atlantic environment, with an enhanced level of OCDD in mussels. The clear decline of PCDD/Fs content in mussels observed after 2016 is surely due to emission abatement actions in Portugal.
Regarding dl-PCBs, a multiplicity of parameters controls their geochemistry and transport, and the analytical results have been very different. The literature data is highly diversified, and sometimes difficult to interpret. Our results showed that among mono-ortho dl-PCBs, IUPAC congener #118 dominates (10% to 89%), followed by congeners #105, #167 and #156. PCB #77 was the highest non-ortho dl-PCB, followed by PCB #126. The abundance of PCB #118 in Portuguese wild mussels is consistent with the literature data reported for marine organisms, and it is likely to be explained by its high concentration in commercial PCBs mixtures, such as AROCLOR 1254–1260, and by its molecular structure and high lipophilicity, which facilitate stability and persistence. The Portuguese pattern resembles profiles on mussels from other marine areas (Kurt and Boke Ozkoc Citation2004; Di Leo et al. Citation2014). The congeners PCB #105, PCB #167 and PCB #169 have been used in equipment such as electrical transformers, gas transmission turbines, electrical capacitors, and vacuum pumps. The pollutants had been discharged into the environment from these sources in surface runoff, landfill, and incineration of sludge (Sánchez et al. Citation1993). The most toxic congener PCB #169 has also been released into the environment through emission gases of Al and Cu metallurgies, appearing in mussels via atmospheric deposition (Kaya et al. Citation2018). The congeners PCB #118 and PCB #105 may also originate from land-based agricultural and industrial activities via wastewater, river, and/or surface runoff (Llobet et al. Citation2003; Kaya et al. Citation2018). In Portugal, pesticides (including organochlorine pesticides (OCPs)) use per hectare doubled between 1990 and 2014 (from 3.04 to 6.84 kg/ha), being among the European countries with highest consumption (Amaro da Costa and Santos Citation2021). Regarding sales of herbicides (active substances) in Portugal, the known official data are from 2010 and they are given in Supplementary Table 3S (Portuguese DGADR Report n° 173/Citation2011). As can be seen, the organochloride pesticides that have been used are 2,4-dichlorophenoxyacetic acid (2,4D), 2-methyl-4-chlorophenoxyacetic acid (MCPA), (3,5,6-trichloro-2-pyridinyl)oxy acetic acid (triclopyr), N′-(3-chloro-4-methylphenyl)-N,N-dimethylurea (chlortoluron), N-(3,4-dichlorophenyl)propanamide (propanil), 2-N-tert-butyl-6-chloro-4-N-ethyl-1,3,5-triazine-2,4-diamine (terbuthylazine) and methyl-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate (diclofop-methyl). Pollution reaches the Atlantic coast mainly through the main river systems such as the Minho, Lima, Cávado and Ave, Douro, Vouga, Mondego, Zêzere and Tagus, Sado, Mira and Guadiana, which transport considerable quantities of agricultural and industrial wastes (Ferreira et al. Citation2003; Carvalho et al. Citation2009). The prevalence of PCB #118 is associated with the molecular arrangement of the congener, which hinders its degradation and facilitates its biomagnification (Storelli et al. Citation2007). So, the presence of PCBs in wild mussels, although below the European limits, may be attributed to illegal waste dumping, runoff from urban, industrial, and agricultural areas, and industrial discharges, too. This research data agrees with previous published works (Eljarrat et al. Citation2002; Ferreira et al. Citation2003; Carvalho et al. Citation2009; Di Leo et al. Citation2014; Giannico, et al. Citation2022).
Because dietary intake is a very important pathway of human exposure to PCBs, the European Commission established the maximum limits for dl-PCBs in fish and fishery products as 6.5 pg WHO-PCDD/Fs-PCBs-TEQ per gram ww (Commission Regulation (EC) No. 199/Citation2006). For (total) PCBs, in 2003 the WHO derived a tolerable daily intake (TDI) of 20 ng PCB/kg bw per day (WHO 2003). The contamination levels found in mussels are directly correlated with the compounds’ contents in the marine water where they live. Previous work (Kampire et al. Citation2016) demonstrated that PCB concentrations are higher in mussels than in water, because bivalves are filter-feeding organisms who concentrate the contaminants to levels well above those present in water. The bioconcentration factors of various PCBs were determined experimentally in aquatic species, ranging from 200 up to 70,000 or higher (WHO 1992). So, the results obtained in this work demonstrate the good quality of Portuguese Atlantic water.
Statistical analysis
In Portugal it rains much more in the north than in the centre and south of the country: in average, it rains twice the amount in the north than in the centre and about one third in the south (Supplementary Table 4S). The country also has different characteristics: the north of Portugal has more and diverse industries, and it is more populated than the south. The capital (and suburbs), Lisbon, located in the littoral centre, has the highest population pressure (10% of citizens) but it has less industries and they are more dispersed. The south of Portugal is dominated by agricultural activities. PCA analysis showed a good correlation between the sum of dl-PCBs levels in sampling stations in these three regions of the country during the time, as illustrated in and .
Figure 5. PCA analysis: sum of dl-PCBs by congener isomer classes concentration (ng/kg) versus year of monitoring (data sets in Supplementary Table 1S).
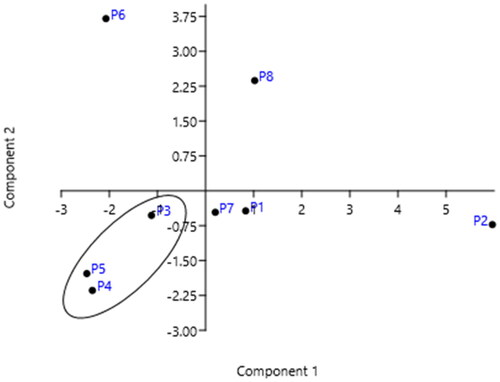
Figure 6. PCA analysis: sum of dl-PCBs concentration (ng/kg) at each sampling station versus year of monitoring (data sets in Supplementary Table 2S).
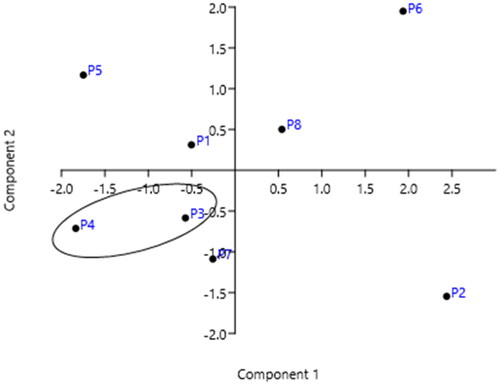
Conclusion
The spatial distribution and trends of PCDD/Fs and dl-PCBs in mussels from all the Portuguese Atlantic coast from 2009 to 2020 were determined. Dioxins toxicity and nonbiodegradability, high bioaccumulation potential, persistence and long-range atmospheric transport, and ecological risk, continue to attract worldwide attention. Intake from drinking water is negligible, and food is the main source of human intake of PCBs. Except for 2009, wild mussels showed total PCB levels higher than PCDD/Fs levels. The sum of PCDD/Fs-PCBs-TEQ showed levels significantly below the limits established by the European Union. The research data demonstrated the good quality of Portuguese marine water and the importance of Directive 2013/39/UE within the European Union.
| Abbreviations | ||
| CRM | = | Certified Reference Material |
| DGADR | = | Direcção-Geral de Agricultura e Desenvolvimento Rural |
| dl-PCBs | = | dioxin-like polychlorinated biphenyls |
| EI+ | = | Electronic Impact/Ionization |
| EPA | = | Environmental Protection Agency |
| GC-HRMS | = | gas chromatography/high-resolution mass spectrometry |
| IUPAC | = | International Union of Pure and Applied Chemistry |
| LOD | = | Limit of Detection |
| OCDD | = | octachlorodibenzo-p-dioxi |
| PCA | = | Principal Component Analysis |
| PCDDs | = | polychlorinated dibenzo-p-dioxins |
| PCDFs | = | polychlorinated dibenzofurans |
| POPs | = | Persistent Organic Pollutants |
| QC | = | Quality Control |
| TEQ | = | Toxic Equivalency Factor |
| UE | = | European Union |
| USEPA | = | United States Environmental Protection Agency |
| WHO | = | World Health Organization |
Supplemental Material
Download Zip (722.3 KB)Acknowledgement
The findings and conclusions in this publication are those of the authors and do not necessarily represent the official position of the Reference Laboratory of Environmental Portuguese Agency, or the Department of Chemistry, Faculty of Science and Technology, New University of Lisbon.
Disclosure statement
No potential conflict of interest was reported by author(s).
References
- Adlard B, Donaldson SG, Odland JO, Weihe P, Berner J, Carlsen A, Bonefeld-Jorgensen EC, Dudarev AA, Gibson JC, Krümmel EM, et al. 2018. Future directions for monitoring and human health research for the Arctic Monitoring and Assessment Programme. Glob Health Action. 11(1):1480084. doi:10.1080/16549716.2018.1480084
- Amaro da Costa C, Santos, JL. 2021. What is the best policy to reduce the use of pesticides in Portugal. Public Policy Port J. 6 (2):209–230. [accessed 2022 Jun 3]. https://repositorio.ipv.pt/bitstream/10400.19/6953/1/Policy%20paper.pdf.
- Ansari ZA, Matondkar SGP. 2014. Anthropogenic activities including pollution and contamination of coastal marine environment. J Ecophysiol Occup Health. 14(1-2):71–78. doi:10.15512/joeoh/2014/v14i1-2/50743
- Antunes P, Viana P, Vinhas T, Rivera J, Gaspar EMSM. 2012. Emission profiles of polychlorinated dibenzodioxins, polychlorinated dibenzofurans (PCDD/Fs), dioxin-like PCBs and hexachlorobenzene (HCB) from secondary metallurgy industries in Portugal. Chemosphere. 88(11):1332–1339. doi:10.1016/j.chemosphere.2012.05.032
- Ba T, Zheng M, Zhang B, Liu W, Xiao K, Zhang L. 2009. Estimation and characterization of PCDD/Fs and dioxin-like PCBs from secondary copper and aluminum metallurgies in China. Chemosphere. 75(9):1173–1178. doi:10.1016/j.chemosphere.2009.02.052
- Belas J, González-Quijano A, Vaamonde A, Fumega J, Soriano JA, González JJ. 2011. PCBs in wild mussels (Mytilus galloprovincialis) from the N–NW Spanish coast: current levels and long-term trends during the period 1991–2009. Chemosphere. 85:533–541. doi:10.1016/j.chemosphere.2011.08.017
- Beyer J, Green NW, Brooks S, Allan IJ, Ruus A, Gomes T, Bråte ILN, Schøyen M. 2017. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: a review. Mar Environ Res. 130:338–365. doi:10.1016/j.marenvres.2017.07.024
- Borja A, Belzunce MJ, Garmendia JM, Rodríguez JG, Solaun O, Zoritya I. 2011. Impact of pollutants on coastal and benthic marine communities. In: Sánchez-Bayo F, van den Brink PJ and Mann RM, editors. Ecological impacts of toxic chemicals. The Netherlands: Bentham Science Publishers Ltd; p. 165–186. eISBN:978-1-60805-121-2.
- Brooks S, Lyons B, Goodsir F, Bignell J, Thain J. 2009. Biomarker responses in mussels, an integrated approach to biological effects measurements. J Toxicol Environ Health Part A. 72(3-4):196–208. doi:10.1080/15287390802539038
- Cajaraville MP, Bebianno MJ, Blasco J, Porte C, Sarasquete C, Viarengo A, et al. 2000. The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: a practical approach. Sci Total Environ. 247(2-3):295–311. doi:10.1016/s0048-9697(99)00499-4
- Carro N, García I, Ignacio M, Mouteira A. 2010. Spatial and temporal trends of PCBs (polychlorinated biphenyls) in mussel from Galician coast (1998–2008). Environ Int. 36(8):873–879. doi:10.1016/j.envint.2010.04.002
- Carvalho PN, Rodrigues PNR, Basto MCP, Vasconcelos MTSD. 2009. Organochlorine pesticides levels in Portuguese coastal areas. Chemosphere. 75 (5):595–600. doi:10.1016/j.chemosphere.2009.01.060
- Commission Regulation (EC) No. 199/ 2006 of 3 February 2006. Official Journal of the European Union 2006; L 32/34-38. Amending Regulation (EC) No 466/2001 setting maximum levels for certain contaminants in foodstuffs as regards dioxins and dioxin-like PCBs. [accessed 2022 Sep 16]. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R0199&rid=4.
- Di Leo A, Annicchiarico C, Cardellicchio N, Giandomenico S, Conversano M, Castellano G, Basile F, Martinelli W, Scortichini G, Spada L, et al. 2014. Monitoring of PCDD/Fs and dioxin-like PCBs and seasonal variations in mussels from the Mar Grande and the Mar Piccolo of Taranto (Ionian Sea, Southern Italy). Environ Sci Pollut Res Int. 21(23):13196–13207. doi:10.1007/s11356-014-2495-6 [PMC] l
- Directive 2013/39/UE. 2013. Directive /39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. [accessed 2022 Feb 2]. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32013L0039.
- Eljarrat E, Monjonell A, Caixach J, Rivera J. 2002. Toxic potency of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and polychlorinated biphenyls in food samples from Catalonia (Spain). J Agric Food Chem. 50(5):1161–1167. − doi:10.1021/jf011021v
- Eitzer B, Hites R. 1989. Atmospheric transport and deposition of polychlorinated dibenzo-p-dioxins and dibenzofurans. Environ Sci Technol. 23, 11:1396–1401. doi:10.1021/es00069a011
- European Union (EU). 2017. Commission Regulation (EC) No 644/2017 of 5 April 2017. Laying down methods of sampling and analysis for the control of levels of dioxins, dioxin-like PCBs and non-dioxin-like PCBs in certain foodstuffs and repealing Regulation (EU) No 589/2014. [accessed 2022 Jan 11]. https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32017R0644.
- Ferreira AM, Martins M, Vale C. 2003. Infuence of difuse sources on levels and distribution of polychlorinated biphenyls in the Guadiana River estuary, Portugal. Mar Chem. 83(3-4):175–184. doi:10.1016/S0304-4203(03)00111-7
- Gaus C, Donohue MO, Connell D, Mueller J, Haynes D, Paepke O. 2004. Exposure and potential risks of dioxins to the marine mammal Dugong. Organohalogen Compd. 66:1559–1566. [accessed 2022 Feb 2]. https://www.osti.gov/etdeweb/biblio/20828195.
- Giannico OV, Baldacci S, Desiante F, Basile FC, Franco E, Fragnelli GR, Diletti G, Conversano M. 2022. PCDD/Fs and PCBs in Mytilus galloprovincialis from a contaminated area in Italy: the role of mussel size, temperature and meteorological factors. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 39(6):1123–1135. doi:10.1080/19440049.2022.2059108
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron. 4(1):9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
- International Agency for Research on Cancer (IARC). 2016. Polychlorinated biphenyls and polybrominated biphenyls. Monographs. 107:7. [accessed 2022 Jul 7]. https://www.iarc.who.int/news-events/iarc-monographs-volume-107-polychlorinated-biphenyls-and-polybrominated-biphenyls/.
- Kampire E, Rubidge G, Adams JB, Human L. 2016. Congener profiles of polychlorinated biphenyls and the effect on marine mussels at an outfall site, Port Elizabeth, South Africa. WSA. 42 (3):496–504. doi:10.4314/wsa.v42i3.16
- Kaya D, Imamoglu I, Sanin FD, Sowers KR. 2018. A comparative evaluation of anaerobic dechlorination of PCB-118 and Aroclor 1254 in sediment microcosms from three PCB-impacted environments. J Hazard Mater. 341:328–335. doi:10.1016/j.jhazmat.2017.08.005
- Kurt PB, Boke Ozkoc H. 2004. A survey to determine levels of chlorinated pesticides and PCBs in mussel and seawater from the Mid-Black sea coastal of Turkey. Mar Poll Bull. 48(11-12):1076–1083. doi:10.1016/j.marpolbul.2003.12.013
- Lee KM, Kruse H, Wassermann O. 1996. The pattern of organochlorines in mussels Mytilus edulis L. from the south west Baltic Sea. Arch Environ Contam Toxicol. 31(1):68–76. doi:10.1007/BF00203909
- Livingstone P. 1992. Mussels and environmental contaminants: molecular and cellular aspects. In: Gosling EM, editor. The mussel Mytilus: ecology, physiology, genetics and culture. Amsterdam: Elsevier; p. 425–464.
- Llobet JM, Bocio A, Domingo JL, Teixidó A, Casas C, Müller L. 2003. Levels of polychlorinated biphenyls in foods from Catalonia, Spain: estimated dietary intake. J Food Prot. 66(3):479–484.
- Lohmann R, Jones KC. 1998. Dioxins and furans in air and deposition: a review of levels, behaviour and processes. Sci Total Environ. 219 (1):53–81. doi:10.1016/S0048-9697(98)00237-X
- Pompa G, Caloni F, Fracchiolla ML. 2003. Dioxin and PCB contamination of fish and shellfish: assessment of human exposure. Vet Res Commun. 27:159–167. doi:10.1023/B:VERC.0000014134.23782.10
- Porte C, Albaigés J. 1993. Bioaccumulation patterns of hydrocarbons and polychlorinated biphenyls in bivalves, crustaceans, and fishes. Arch Environ Contam Toxicol. 26:273–281. doi:10.1007/BF00203552
- Portuguese Direção-Geral de Agricultura e Desenvolvimento Rural (DGADR) Report n° 173/ 2011. [accessed 2023 Feb 6]. https://www.drapc.gov.pt/base/documentos/vendafitofarm2010.pdf.
- Sánchez J, Solé M, Albaigés J. 1993. A comparison of distribution of PCB congeners and other chlorinated compounds in fishes from coastal areas and remote lakes. Int J Environ Anal Chem. 50(4):269–284. doi:10.1080/03067319308027603
- Stieglitz L, Bautz H, Roth W, Zwick G. 1997. Investigation of precursor reactions in the de-novo-synthesis of PCDD/PCDF on fly ash. Chemosphere. 34(5-7):1083–1090. doi:10.1016/S0045-6535(97)00410-4
- Stockholm Convention. 2003. [accessed 2022 Jan 6]. http://chm.pops.int
- Storelli MM, Barone G, Marcotrigiano GO. 2007. Residues of polychlorinated biphenyls in edible fish of the Adriatic Sea: assessment of human exposure. J Food Science. 72 (4):C183–C187. doi:10.1111/j.1750-3841.2007.00348.x
- Torres JPM, Leite C, Krauss T, Weber R. 2013. Landfll mining from a deposit of the chlorine/organochlorine industry as source of dioxin contamination of animal feed and assessment of the responsible processes. Environ Sci Pollut Res. 20(4):1958–1965. doi:10.1007/s11356-012-1073-z
- Tysklind M, Faengmark I, Marklund S, Lindskog A, Thaning L, Rappe C. 1993. Atmospheric transport and transformation of polychlorinated dibenzo-p-dioxins and dibenzofurans. Environ. Sci. Technol. 27(10):2190–2197. doi:10.1021/es00047a028
- Vetter W, Scholz E, Gaus C, Müller JF, Haynes D. 2001. Anthropogenic and Natural Organohalogen Compounds in Blubber of Dolphins and Dugongs (Dugong dugon) from Northeastern Australia. Arch Environ Contam Toxicol. 41:221–231. doi:10.1007/s002440010241
- Weber R, Watson A. 2011. Assessment of the PCDD/PCDF fingerprint of the dioxin food scandal from biodiesel in Germany and possible PCDD/F sources. Oganohalogen Compd. 73:400–403.
- Weber R, Herold C, Hollert H, Kamphues J, Blepp M, Ballschmiter K. 2018. Reviewing the relevance of dioxin and PCB sources for food from animal origin and the need for their inventory, control and management. Environ Sci Eur. 30(1):1–42. doi:10.1186/s12302-018-0166-9
- [WHO] World Health Organzation. 1992. Polychlorinated biphenyls (PCBs) and polychlorinated terphenyls (PCTs). IPCS International Program on Chemical Safety. Health and Safety Guide No. 68. [accessed 2022 Feb 4]. https://inchem.org/documents/hsg/hsg/hsg68.htm.
- [WHO] World Health Organization. 2003. Polychlorinated biphenyls: human health aspects Concise International Chemical Assessment Document 55. [accessed 2023 Mar 13]. https://apps.who.int/iris/bitstream/handle/10665/42640/9241530553.pdf.
- Widdows J, Donkin P. 1992. Mussels and environmental contaminants: bioaccumulation and physiological aspects. In: Gosling E, editor. The mussel mytilus: ecology, physiology, genetics and culture. Amsterdam: Elsevier Science; p. 383–424.