Abstract
Lupin seeds have a high potential as an alternative for animal proteins in feed and food. However, the possible presence of alkaloids hinders the usage of lupins in human diets. This review aims to identify the main factors that influence the presence of alkaloids in lupins. A literature study covering English-published scientific papers in Scopus from 1980 to 2022 was performed. Biotic, abiotic, and genotypic factors influence the production of these toxic secondary metabolites by lupines. In particular, sweet cultivars with high 13-hydroxylupanine and 13-tigloyloxylupanine concentrations, abundant light exposure and standard diurnal cycles, well-watering procedures, relatively cold environment, N-deficient fertilizer with 240 mg K kg−1 and 60 mg P kg−1, high soil pH, and organic growing system conditions, are the best options to avoid high global alkaloid content. Results of this study can be used to develop predictive mechanistic models, although there is still the necessity to collect additional data by performing multi-variate studies.
Introduction
Lupin seeds are gaining attention as high nutritional alternatives to animal protein and gluten-containing products (Otterbach et al. Citation2019) for their substantial protein and carbohydrates content (40% each), absence of (or low in) gluten (10%), and presence of numerous minerals, vitamins, and other beneficial substances (Vishnyakova et al. Citation2020). To date, only four lupin species have been domesticated worldwide: L. angustifolius, also called blue lupin or narrow-leaved lupin (NLL); L. luteus, most commonly known as yellow lupin; L. albus or white lupin; and L. mutabilis, also named pearl lupin or Andean or South American lupin (Schrenk et al. Citation2019).
Lupins are seen as an alternative to soybean since they are pulse legumes that possess a high adaptability rate to temperate and cold climates, low-fertile soils, high altitudes, and harsh conditions, while actively enriching the soil with nitrogen (Gulisano et al. Citation2019). Despite all their benefits, the actual lupin production by European farmers is generally lower than that of several other soybean alternatives, such as fava beans and peas (Otterbach et al. Citation2019). The main underlying reason is the presence of alkaloids in lupins, especially in their seeds, which makes them less attractive (Hama and Strobel Citation2020; Griffiths et al. Citation2021).
Alkaloids are one of the largest classes of secondary metabolites, which are substances present in many plants, some of which can act as attractors of pollinators, defence against bacterial, viral, and fungal diseases, as well as deter herbivores. They can be broadly divided into different classes, such as quinolizidine, indole, pyrrolizidine, and tropane alkaloids. Quinolizidine alkaloids (QAs) are the most abundant in the Fabaceae family, particularly in the Lupinus genus (Griffiths et al. Citation2021). Several lupin species, such as yellow lupin, can also contain indole alkaloids (IA), for instance, gramine.
Alkaloids are remarkably soluble substances (3–32 g L−1 at 25 °C), which are very persistent in the environment. Hence, they are classified as persistent and mobile organic compounds (PMOCs). This characteristic implies that in the event that a lupin crop has high alkaloid levels, the toxicity might transfer to the nearby environment. Such a trait is problematic, since the current lupin seeds harvesting practice only removes half of the plant material, while the residues of stems, leaves, and roots remain in the field as green manure. Thus, 30% of the alkaloid content is left in the field. Nearly half of this content is exported to the drainage water (0.4–18 µg L−1) during high flow events via macropores. The half-life of alkaloids in natural water ranges from 36 to 60 days, which allows the toxic metabolites to reach new crops watered with this water, as well as nearby soils and topsoils, or even animals drinking from that contaminated water. In fact, traces of alkaloids have been detected in topsoils at depths of 0–5 cm (0.1–10 µg g−1 DW), and 15–30 cm (0.2–8.5 µg g−1 DW), as well as in soil pore water (0.2–7.5 µg L−1) (Hansen et al. Citation2021; Hama et al. Citation2022).
The QAs present in the Lupinus genus, e.g. lupanine, lupinine, sparteine, an- gustifoline, multiflorine, aphylline, anagyrine, and cytisine, can make up to 5% of the plant’s dry weight (DW) (Otterbach et al. Citation2019; Griffiths et al. Citation2021). More than 170 different QAs have been identified in the range of lupin species, with a high variable QA pattern among species. They are responsible for the plant’s defence against pathogens and predators, and their accumulation and production depends on genotype, presence of disease-causing agents, climatic and environmental conditions, and management techniques. QAs are biosynthesized in green tissues of the plant, transported via the phloem, and stored in all organs of the plant, including seeds (Boschin and Resta Citation2013).
QA are toxic to both humans and animals (Ku et al. Citation2020; Vishnyakova et al. Citation2020). Therefore, two general thresholds for the presence of QA in lupin-based foods have been established in Australia (Australia New Zealand Food Authority Citation2001) and some European countries such as France (Direction générale de la santé Citation1998) or Great Britain (ACNFP Annual Report Citation1996): being 0.02% DW for animal feed and 0.01% DW for human consumption (Frick et al. Citation2018; Otterbach et al. Citation2019). These limits are often exceeded, which implies that the particular lupin batch cannot be used or is downgraded, leading to economic losses and food waste (Philippi et al. Citation2015; Frick et al. Citation2017).
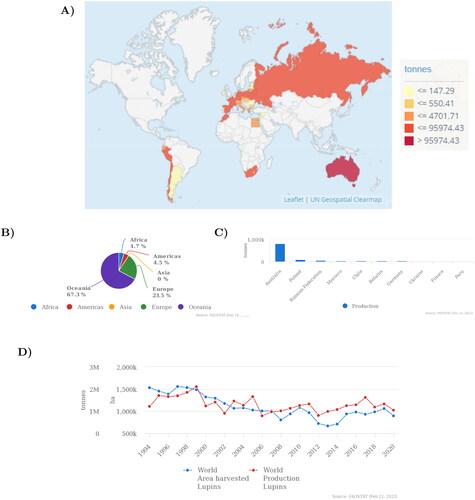
To date, Australia (67.3%) and Europe (23.5%) are by far the largest lupin producers worldwide (FAO Citation2020) (). Lupin domestication started in the twentieth century when they were used as green manure and animal feed (Vishnyakova et al. Citation2020). Global production increased gradually over the 90s but started to decrease in the new century until it stabilized around 1 M tones (FAO Citation2020) (). Nowadays, the production of lupins is spread all over the World, emphasizing their adaptability to different climates. However, regional climates have a direct implication on QA contamination of lupins. For instance, Mediterranean climates result in higher QA contamination in the case of L. albus and L. angustifolius, as compared to subcontinental climatic conditions (Boschin et al. Citation2008).
Figure 1. (A) Production quantities of lupins by country (FAO Citation2020). (B) Production share of lupins by region (FAO Citation2020). (C) Top 10 lupins producers (mean values from 2000 to 2020) (FAO Citation2020). (D) Mean production and yield quantities of lupins in the World, from 1961 until 2020 (FAO Citation2020).
Recently, several mitigation measures have been suggested to either obtain low-alkaloid lupin crops or debitter the collected seeds, to reduce the QA levels in lupin seeds. The first approach is difficult to implement successfully due to natural selection, susceptibility to herbivores, and climate variability, which makes the QA content hard to influence and unpredictable across different harvest years and locations (Cowling and Tarr Citation2004). Unfortunately, the debittering approach is cumbersome and removes from the seeds not only the QAs but also a large portion of soluble proteins, minerals, flavonoids, monosaccharides, and sucrose (Gulisano et al. Citation2019; Otterbach et al. Citation2019).
This study aims to bring together the state-of-the-art knowledge of both abiotic and biotic environmental factors, as well as genotypic characteristics linked to the presence of QAs in lupins. Motivated by the scattered data available, it also intends to detect the knowledge gaps.
Methodology
A systematic review approach (Boland et al. Citation2017) was performed in order to obtain a complete summary and synthesis of the literature. The selection of literature was based on three phases: (1) an identification phase of possible relevant papers in which English scientific papers were obtained from the Scopus scientific database, as well as relevant cited papers from these hits, (2) a screening and eligibility phase, in which records were removed when not fitting the study aims based on reading their abstracts. This resulted in papers considered relevant and possibly relevant, and (3) a final inclusion phase where all remaining records were read in full to determine their final relevance. For the identification phase, a specific search was carried out, as indicated in the Supplementary Annex, considering: (a) QAs names and (b) QAs influence factors. Only relevant papers published between 2000 and 2022 (December) were considered. However, since their citations were also included, older papers were covered as well.
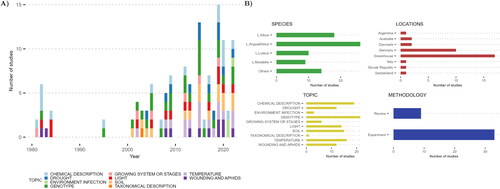
Once the identification stage (phase 1) was complete and duplicates were removed, 67 hits remained. After the whole screening stage process (phase 2 and 3), a selected set of 42 papers were read in full, and information of each presented study was coded in a database for a number of topics and with a brief summary. Ten topics were considered, being chemical description, growing system or stages, temperature, drought, light, wounding and aphids, environment infection, soil, genotype, and taxonomical description. In the same line, were recorded for each paper the target lupin species, the methodology used, the location of the experiments (in case it was not a review), and the publication year ().
Figure 2. (A) Total number of studies and topics per year (from 1981 to 2022). (B) An overview of the studies reviewed. The numbers represent the number of studies, the total can be greater than 42 because one study can be focused on multiple species, use different crop locations, and investigate different topics.
Genotype and lupin varieties
Not all lupins produce and accumulate the same amount of QAs. In particular, two different types can be distinguished: the so-called bitter and sweet lupins (Tirdiľová et al. Citation2022). The first ones accumulate more than 500 mg kg−1 DW of QAs in seeds, while the last ones accumulate less than 500 mg kg−1 DW (Hama and Strobel Citation2020). Although sweet lupins might be more attractive for food and feed consumption due to their lower QA levels, they have poorer resistance to diseases and predators as compared to bitter lupins (note this is one of the main QAs functions). Therefore, efforts are ongoing for the development of a bitter-sweet variety, with high QA concentration in the leaves to protect the plant, and low QA concentration in the seeds to meet the legal limits (Otterbach et al. Citation2019).
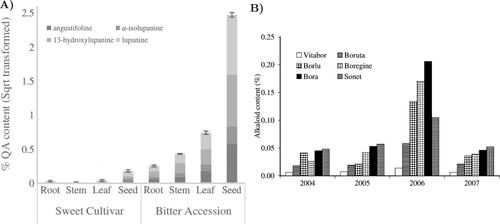
The bitter and sweet varieties have some differences in their QA biosynthetic pathways to lower toxicity. The most significant is the low quantity in sweet lupins of enzymes involved in the formation of lupanine, which diminishes the final concentration of QAs. Generally speaking, QAs derived from L-lysine, and their biosynthetic pathways are catalysed by lysine decarboxylase enzyme, while 17-oxosparteine synthase plays also an important role. Since this last enzyme is only present in the leaves, specifically in chloroplasts, QAs are biosynthesized in the stroma of leaf chloroplasts following a light-regulated cycle. Then, they are transported via the phloem and finally stored in all organs of the plant, but mostly in epidermal and subepidermal tissues of stems and leaves, as well as in seeds, which are especially rich in QAs (). In particular, 50% of the seed’s QAs content is synthesized in situ, while the other 50% is translocated principally by the phloem. The concentrations of QAs in the different plant organs evolve with time of day (Section Light), as well as with the variety (Section Genotype and lupin varieties), the growing stage and the presence of pests (Section Crop stages and abiotic wounding), and the environmental factors (Section Climatic factors). In broad terms, the concentration of QAs is higher in seeds, followed by pods, flowers, leaves, stems, and roots (De Cortes Sánchez et al. Citation2005; Lee et al. Citation2007; Boschin and Resta Citation2013; Frick et al. Citation2018; Hama et al. Citation2022) ().
Figure 3. (A) Specific and total QAs mean content in different plant tissues of sweet and bitter lupins (Frick et al. Citation2018). (B) Mean alkaloid content of different six L. angustifolius genotypes (Jansen et al. Citation2009).
QAs composition differs between species and studies. For instance, for L. angustifolius, lupanine (40%) is the predominant QA, followed by 13-hydroxylupanine (20%) and angustifoline (20%) (Wink et al. Citation1995). But other in vitro studies showed 13-hydroxylupanine (44.5%) to be the QA that was present in the highest amount, accompanied by angustifoline (13.6%) and lupanine (8.1%) (Philippi et al. Citation2016). Nevertheless, lupanine and sparteine are the most toxic QAs to both humans and animals; and the first one is the precursor for a large number of QAs (Gremigni et al. Citation2001; Lee et al. Citation2007; Frick et al. Citation2017; Cely-Veloza et al. Citation2022). The total QA composition, which is initially determined by the genotype, plays an important role in defence against aphids (Philippi et al. Citation2016) (Section 3.3).
Almost all studies investigating the impact of the genotype on QA levels focus on sweet L. angustifolius, which is the lupin species whose cultivars present the greatest variation of QA content (Boschin et al. Citation2008). In particular, ‘Danja’, ‘Boregine’, and ‘Borlu’ cultivars are linked to higher QA content, whereas ‘Vitabor’, ‘Bo083521AR’, and ‘Yorrel’ contain lower QA levels (Cowling and Tarr Citation2004; Jansen et al. Citation2012) (). Although the effect of genotype on the total QA content of seeds is lower than the impact of location and year, its effect is undeniable (Cowling and Tarr Citation2004; Boschin et al. Citation2008; Beyer et al. Citation2015; Jansen et al. Citation2015).
Sweetness is a recessive genetic factor. Thus, with seed multiplication there is the risk of a genetic shift towards higher QA content due to pollen flow from bitter material, which increases over generations as a consequence of natural selection (Boschin and Resta Citation2013).
Climatic factors
Climate has a direct effect on lupin development, which in turn affects QAs production and accumulation. The most relevant climatic factors are temperature, light, and drought stress (Frick et al. Citation2017; Tirdiľová et al. Citation2022). These factors are also subject to climate change, which makes the quantification of their impact even more important.
Light
As QA synthesis takes place in the chloroplasts, it follows a diurnal light- regulated cycle with a stimulated period during the day and low values during the night (Boschin and Resta Citation2013). The main underlying reason is the light-regulated cycle of the involved enzymes: on the one hand, the synthesis of lysine is directly enhanced by light. On the other hand, the pH of the chloroplast stroma changes from pH 7 in the dark to pH 8 in the light. Since lysine decarboxylase and other relevant enzymes have an optimum pH of 8 and are considerably less active at pH 7, light again favours QA biosynthesis. Finally, lysine decarboxylase is activated by reduced thioredoxin, which becomes less present in the light (Wink and Hartmann Citation1984).
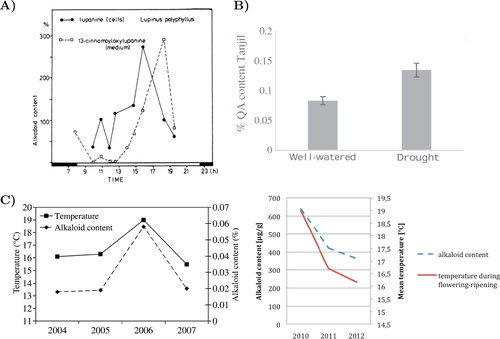
From monitoring the QA level fluctuation over a 36 h period, it could be stated that QA contents reach their maximum at noon or early afternoon, and their minimum during night. Precisely, setting the accumulated early morning QA level as the reference, it can be seen that it increases at noon up to 300–470%, depending on the lupins species and cultivar, and decreases to 60% at night (Wink and Witte Citation1984) ().
Figure 4. (A) Diurnal fluctuation in alkaloid contents of cell suspension cultures of L. polyphyllus (Wink and Witte Citation1984). (B) Grain QA (average) content in ‘Tanjil’ cultivar (L. angustifolius) under constant drought stress (Frick et al. Citation2018). (C) Relationship between the growing mean temperature and the alkaloid content of L. angustifolius according to several studies (Jansen et al. Citation2015, Citation2009).
Besides the direct impacts of climatic factors on QA levels, several lupin growth stages, such as germination, are more effective in the presence of light, as compared to no-light conditions (De Cortes Sánchez et al. Citation2005). In the same way, the required growing degree-days are decreased with longer day lengths (Christiansen and Jornsgard Citation2002).
Temperature
Numerous studies have focused on the impact of temperature on the QA content of different lupin species. As a rule, the total QA content increases with rising temperatures, whose effect starts at the beginning of flowering and lasts up to pod ripening. However, the impact can vary across different cultivars (Frick et al. Citation2018).
An increase of 3 °C in the mean temperature results in a rise of the QA content of 3% (Jansen et al. Citation2015, Citation2009) (). Temperature does not only affect the QA accumulation and production, it can also alter the phenological stages of Lupinus. For instance, no pods are developed at a constant ambient temperature higher than 30 °C (Jansen et al. Citation2009; Podleśny and Podlesna Citation2012), or lower than −2 °C or −9 °C, depending on the cultivar (Gulisano et al. Citation2019; Vishnyakova et al. Citation2020). If the plants are exposed to very high temperatures at flowering, some morphological changes might occur, such as smaller height and less leaf area (Podleśny and Podlesna Citation2012). Nevertheless, at low temperatures (10 °C), all varieties form fewer leaves than at slightly higher temperatures (18 °C) (Christiansen and Jornsgard Citation2002). Moreover, the total number of days from sowing to flowering is inversely related to temperature (Christiansen and Jornsgard Citation2002).
The vernalisation period has also an important effect on the flowering time, although, to the best of our knowledge, no study linked it to the QAs levels. All mid- and late-season lupin cultivars of the four domesticated lupin species are responsive to the vernalisation time. The flowering time reduces from 3 to 5 days for one vernalisation week and it can increase up to 2 weeks if 4 weeks of vernalisation are present. L. mutabilis and L. luteus cultivars are especially susceptible. What is also common in all species is the reduction of leaf nodes in late-flowering lines. It can be from 3 or 4 nodes, up to 20 in some very late-season L. luteus cultivars such as ‘P28716’ (Adhikari et al. Citation2012).
Rain and drought
Initially, it was thought that rain was not strongly associated with QA seed content (Frick et al. Citation2018). However, some new studies suggest the contrary, highlighting rain and drought stresses as important abiotic factors that can modify the morphology and QA levels of lupins. From germination to maturity, some accumulated precipitation is required, which varies across species and cultivars. For instance, L. mutabilis needs accumulated rainfall between 350 and 800 mm, whereas L. angustifolius requires only between 200 and 250 mm (Gulisano et al. Citation2019; Vishnyakova et al. Citation2020).
From our review results, there are no studies relating the QA concentration to excessive rain. On the other hand, it is quite clear that if the plant suffers early drought stress, the QA content rises gradually (Frick et al. Citation2018) (). Some studies also point out that under terminal drought stress, the QA content might decrease (Boschin et al. Citation2008).
Environmental factor interactions
Not all lupin species and cultivars react in the same way under the mentioned climatic factors. For instance, ‘Danja’ or ‘Tanjil’ cultivars (L. angustifolius) are almost not affected by drought or temperature stress respectively. The ‘Tallerack’ cultivar (L. angustifolius) is an even more extreme case since its QA levels remain nearly constant under both drought and temperature stress.
Morphological stages are regulated by the combination of different abiotic factors. For instance, the thermal time to flowering is decreased from 1100 to 900 °C at 10 h day-length, to 750–500 °C at 18–24 h day-length (Christiansen and Jornsgard Citation2002). In the same line, it is the combination of high temperature, drought stress, and light exposure that favors QA biosynthesis the most. Even in the most stable crops, the impact can be noticed (Frick et al. Citation2018). This knowledge could guide the breeding of more climate-resistant lupin crops, considering the IPCC climate scenarios, that warn of more than 1.5 °C temperature increase at the end of the century and the steady repetition of drought events especially in the south of Europe (Shukla et al. Citation2022), which will make the production of lupins a major challenge.
Crop stages and abiotic wounding
The total QA content fluctuates during lupin germination, increases after anthesis, and decreases in the maturation and ripening stages. However, if we look closely at different QAs, the concentration of some of them increases, while in the same period, the concentration of others decreases. This can be explained by biochemical reactions that transform some QAs into others, as well as the mobilization of alkaloidal nitrogen (De Cortes Sánchez et al. Citation2005; Otterbach et al. Citation2019; Hama and Strobel Citation2020).
According to De Cortés-Sánchez et al., QAs of L. angustifolius and L. albine are present starting 3 days after the onset of germination, whereafter the concentrations increase progressively but return to the initial concentrations at the end of the germination period ().
Figure 5. (A) Total alkaloid content in lupin seeds during germination (De Cortes Sánchez et al. Citation2005). (B) Patterns of accumulation of individual QAs in seeds and pods of bitter L. angustifolius throughout development as analyzed by LC- MS (Otterbach et al. Citation2019). (C) Wounding-induced alkaloid accumulation (mean) of L. polyphyllus (Wink Citation1983). (D) Aphis cytisorum resistance in relation to alkaloid content of Lupin plants (leaflets) (Wink et al. Citation1982).
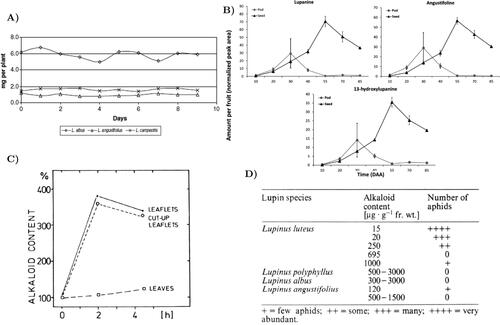
Only 3 days are necessary to ensure that more than 60% of seeds germinate, reaching 100% germination rate after 3 more days in the L. albus case, while more days are required for the germination of all or almost all L. angustifolius seeds.
For the anthesis stage, there is high evidence that in L. angustifolius, QAs accumulate in seeds up to 55 days after anthesis (DAA), when their concentrations peak (0.2 mg of lupanine) and start decreasing to around 50% of their maximum levels. However, in the pods, the maximum concentration is reached after 30 DAA (0.08 mg of lupanine), returning to the background concentrations at 55 DAA (0.002 mg of lupanine) (Otterbach et al. Citation2019) ().
After the plant matures, the amount of accumulated QAs starts to diminish. At ripening, lupins tend to transfer QAs to the seeds since they are the reproductive organs of the plants. Specifically, setting the QA accumulation of mature lupins as the reference, it is possible to state that it decreases to 75% in pre-senescent (still green) plants, to 25% in senescent (yellow) specimens, and to 12% in post-senescent (dry, dead) ones (Wink and Hartmann Citation1981). The length and time of the growing season can vary across different lupin species and regions, as well as due to the climate and cultivar variability. For example, L. angustifolius’ growing season lasts from 70 to 120 days, whereas the growing days of L. mutabilis are from 240 to 300 (Gulisano et al. Citation2019; Vishnyakova et al. Citation2020), which means that the QAs increasing and decreasing periods differ also in length and time of the year.
External factors might also modify the QA levels, such as the presence of pathogens and mechanical wounding. Like many secondary metabolites, QAs constitute a chemical defence of the plant against pest and herbivore actions. Bitter lupins have higher QA levels, which makes them less susceptible to wounding and infections. Since their QA concentrations are already high, under biomass removal they increase their QA content by only 32.8%; whereas sweet varieties of L. albus and L. angustifolius increase at most by 67.9% of their QA content trying to reach a QA level to defend themselves against predators (Chludil et al. Citation2009).
About 10% of broom plants are heavily infested by aphids, and lupins are similar. However, if lupin QA levels are high enough, there is a good probability that aphids’ multiplication can be prevented. Infested plants usually contain 50% or less alkaloids than aphid-free plants (Wink et al. Citation1982) (). Nevertheless, there are some cultivars that still have relatively low overall QAs levels, while still presenting a significant resistance against aphids. This is the case of ‘Kalya’, ‘Bora’, and ‘Borlu’ (L. angustifolius). It turns out that 13-hydroxylupanine and 13-tigloyloxylupanine are especially involved in the reduced aphid multiplication rate; their concentrations might be determined by the genotype (Philippi et al. Citation2016).
In the same line, different aphid species react differently to QA content. For instance, the well-adapted lupin aphid Macrosiphum albifrons is not affected by QAs, whereas Aphis fabae, A. pisum, M. persicae, and A. craccivora present a negative correlation between aphid multiplication and QA content (Philippi et al. Citation2016).
QA production under biotic stress takes place both in the light and in the dark, and even at 4 °C, in contrast to diurnal QA formation. Depending on the part of the plant that is suffering from the infestation, the QA levels will increase differently: the leaflets and the cut-up leaflets are the most susceptible to wounding-induced QA (with rises up to 400%), whereas the QA content of the leaves does not increase abruptly (). Moreover, the leaf QA content seems to be influenced by the QA content of the neighbouring leaflet (Wink Citation1983).
Management factors
Soil characteristics are determinants for lupins QA level (Frick et al. Citation2017). They can be modified by the usage of fertilizers, which can enhance the resistance and lower the QAs production of the cultivars. It is worth remarking that lupins control soil erosion and improve soil properties since they fix air nitrogen to the soil (Jansen et al. Citation2015; Hama et al. Citation2022). Therefore, throughout history, their use has been linked to crop rotation and soil quality amendment (Griffiths et al. Citation2021; Tirdiľová et al. Citation2022).
Lupin species differ concerning their demands for optimal growth, but in general, commercial cultivars of lupins grow poorly on alkaline or neutral soils. The higher the soil pH is, the lower the lupin QA level is, as well as the lower kernel yield and protein content. For instance, L. angustifolius almost doubles its QAs content when grown on low pH soils (pH between 5.3 and 5.8), compared to high pH soils (pH between 6.7 and 7.1) (Jansen et al. Citation2012) ().
Figure 6. (A) Influence of the soil pH on the main alkaloid content (annual mean) of different L. angustifolius cultivars (Jansen et al. Citation2012). (B) Effect of various nitrogen N-forms on total alkaloid content in seeds of ‘Butan’ (sweet) and ‘Bac’ (bitter) L. albus cultivars (mean and standard deviation). Data from (Ciesiolka et al. Citation2005). (C) Total QAs levels under K concentrations for a bitter variety (‘Fest’) and three sweet L. angustifolius varieties (Gremigni et al. Citation2001). (D) Total QAs levels under P and K concentrations for ‘Danja’, a sweet L. angustifolius variety (Gremigni et al. Citation2003). (E) Effect of the growing system on L. angustifolius cultivars (annual mean) (Jansen et al. Citation2015).
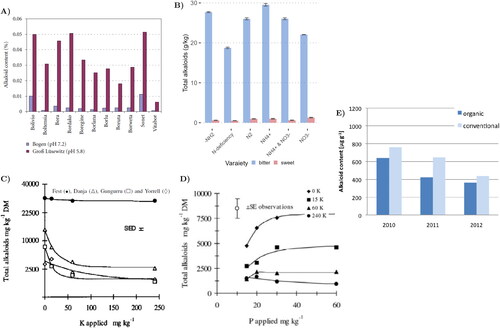
At the same time, genotypic differences still play a very important role. Briefly, L. angustifolius and especially L. luteus are less sensitive to calcareous soils than L. albus (Jansen et al. Citation2012). Moreover, as can be seen in , some cultivars, such as ‘Vitabor’ present a lower QA content, no matter the soil pH conditions to which they are exposed. In general, bitter cultivars are less susceptible to soil differences than sweeter ones (Gremigni et al. Citation2003, Citation2001).
The presence or deficiency of potassium (K), phosphorus (P), and nitrogen (N) are also linked to the seed’s QAs concentration (Boschin and Resta Citation2013). Under K deficiency, the seed QA content of sweet L. angustifolius increases significantly, even up to an 8-fold increase under severe deficiency in sweet varieties (Gremigni et al. Citation2003). This deficiency affects the seed yield as well, which reduces exponentially under lack of K (Gremigni et al. Citation2001). In fact, the seed yield reaches its maximum at 60 mg K kg−1. In a K deficiency context, the predominant QA in sweet varieties is lupanine, while in bitter varieties, it is 13-hydroxylupanine. Conversely, bitter varieties of L. angustifolius such as ‘Fest’ are not responsive to the K soil content (Gremigni et al. Citation2003, Citation2001).
On the other hand, P deficiency reduces seed QA concentrations in sweet lupins, but not in bitter varieties. Under P deficiency, the QA profile in harvested seeds of sweet varieties mimics that of the bitter ones, with 13-hydroxylupanine dominating over lupanine, whereas with adequate or abundant P, lupanine is also the predominant QA in sweet varieties (Gremigni et al. Citation2003). Similarly to what happens under K abundance, the seed QA content increases exponentially reaching its maximum at 30 mg P kg−1 (Gremigni et al. Citation2003). However, the inhibitory effects of P deficiency appear only under K deficiency, which results in the lowest seed QA concentration under abundant K (240 mg K kg−1) and P (60 mg P kg−1). The interaction between K and P is well-established (Gremigni et al. Citation2003).
Regarding the form of N applied as fertiliser, there is a wide range of compounds that lupins can utilise. For both sweet and bitter L. albus varieties, the lowest QA content is found under N deficiency. In the case of ‘Butan’, a sweet L. albus variety, the N forms that give rise to the highest total QA content are N2, NH+, and NO3- whereas − NH2 and NH+ are best for bitter cultivars such as ‘Bac’ (). The protein content of the seeds of both types increases with N2 and in the sweet variety case, decreases also with the combination of [NH+ and NO3−] (Ciesiolka et al. Citation2005).
The growing system has also a small effect on grain QAs content, with organic conditions resulting in lower QAs content than conventional conditions (Frick et al. Citation2017) (). However, comparing the QA concentrations by year, location, genotype, and the growing system, all the factors previously mentioned are far more determinant than the growing system (Cowling and Tarr Citation2004; Jansen et al. Citation2015). Furthermore, a study into the possible interaction of the growing system with the genotype shows there is almost no connection (Jansen et al. Citation2015).
Conclusions and recommendations
QAs are a large family of molecules, whose impact on lupins is influenced by three main factors: biotic, abiotic, and genetic. The studies that tested the importance of these generic factors and their interaction demonstrate that QA levels are affected by the interaction of the biotic and abiotic regimes. With the aim to grow low-alkaloid cultivars and to reduce as much as possible QA accumulation the following should be considered:
To choose a sweet lupin variety with high 13-hydroxylupanine and 13-tigloyloxylupanine concentrations to enhance protection against pathogens, such as aphids, while considering low-alkaloid cultivars.
To consider an N-deficient fertilizer with 240 mg K kg−1 and 60 mg P kg−1, together with a relatively high soil pH (≈ 7.2).
To provide a relatively cold environment, with abundant light and standard diurnal cycles, as well as to follow an effective plant irrigation procedure.
To consider an organic growing system.
To date, there is a lack of multi-variate studies that link the different influencing factors on the QAs production in lupins. It is not easy to compare current studies on the same topic, since the ambient conditions are different, and thus, the quantitative difference between the QA levels does not have a clear source.
Some factors that influence QAs formation depend directly on farm management, such as the growing system, the fertilizer use, or the genotype choice. However, other determinant factors like light exposure, ambient temperature, or drought stress, are usually out of human control. In view of this fact, we should enhance the positive choices among the controllable factors, and consider different climate change scenarios to understand how lupin crops will develop in the near future. In this way, we could not only prevent human and animal poisoning from lupin ingestion but also protect other crops and habitats that could indirectly suffer, while avoiding food loss and enhancing food security.
Supplemental Material
Download MS Word (31.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adhikari K, Buirchell B, Sweetingham M. 2012. Length of vernalization period affects flowering time in three lupin species. Plant Breed. 131(5):631–636.
- Australia New Zealand Food Authority. 2001. Lupin alkaloids in food a toxicological review and risk assessment technical report series no. 3. www.foodstandards.gov.ausrc.filestr3.pdf.
- Beyer H, Schmalenberg AK, Jansen G, Jürgens HU, Uptmoor R, Broer I, Huckauf J, Dietrich R, Michel V, Zenk A, et al. 2015. Evaluation of variability, heritability and environmental stability of seed quality and yield parameters of L. angustifolius. Field Crops Res. 174:40–47.
- Boland A, Cherry G, Dickson R. 2017. Doing a systematic review: a student’s guide. 2nd ed. Los Angeles: SAGE.
- Boschin G, Annicchiarico P, Resta D, D'Agostina A, Arnoldi A. 2008. Quinolizidine alkaloids in seeds of lupin genotypes of different origins. J Agric Food Chem. 56(10):3657–3663.
- Boschin G, Resta D. 2013. Alkaloids derived from lysine: quinolizidine (a focus on lupin alkaloids). In: Ramawat KG, Merillon JM, editors. Natural products: phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Berlin (Heidelberg): Springer; p. 381–403.
- Cely-Veloza W, Quiroga D, Coy-Barrera E. 2022. Quinolizidine-based variations and antifungal activity of eight lupinus species grown under greenhouse conditions. Molecules. 27(1):305.
- Chludil H, Vilariño M, Franco M, Leicach S. 2009. Changes in Lupinus albus and Lupinus angustifolius alkaloid profiles in response to mechanical damage. J Agric Food Chem. 57(14):6107–6113.
- Christiansen JL, Jornsgard B. 2002. Influence of day length and temperature on number of main stem leaves and time to flowering in lupin. Ann Appl Biol. 140(1):29–35.
- Ciesiolka D, Muzquiz M, Burbano C, Altares P, Pedrosa MM, Wysocki W, Folkman W, Popenda M, Gulewicz K. 2005. An effect of various nitrogen forms used as fertilizer on Lupinus albus L. yield and protein, alkaloid and α-galactosides content. J Agron Crop Sci. 191(6):458–463.
- Cowling WA, Tarr A. 2004. Effect of genotype and environment on seed quality in sweet narrow-leafed lupin (Lupinus angustifolius L.). Aust J Agric Res. 55(7):745–751.
- De Cortes Sánchez M, Altares P, Pedrosa MM, Burbano C, Cuadrado C, Goyoaga C, Muzquiz M, Jiménez-Martínez C, Dávila-Ortiz G. 2005. Alkaloid variation during germination in different lupin species. Food Chem. 90(3):347–355.
- Direction générale de la santé. 1998. Bulletin Officiel n° 98/27 du Conseil superieurd’hygiene publique de France. Paris (France): Bernard Kouchner, Journal officiel de la République française.
- FAO. 2020. Crops and livestock products. License: CC BY-NC-SA 3.0 IGO; [accessed 2022 Dec 14]. https://www.fao.org/faostat/en/#data/QCL/visualize.
- Frick KM, Foley RC, Kamphuis LG, Siddique KHM, Garg G, Singh KB. 2018. Characterization of the genetic factors affecting quinolizidine alkaloid biosynthesis and its response to abiotic stress in narrow-leafed lupin (Lupinus angustifolius L.). Plant Cell Environ. 41(9):2155–2168.
- Frick KM, Kamphuis LG, Siddique KHM, Singh KB, Foley RC. 2017. Quinolizidine alkaloid biosynthesis in lupins and prospects for grain quality improvement. Front Plant Sci. 8:87.
- ACNFP Annual Report. 1996. Appendix IX. ACNFP report on seeds from the narrow leaved lupin (Lupinus angustifolius). Ministry of Agriculture, Fisheries and Food and Department of Health; p. 14–15, 107–123.
- Gremigni P, Hamblin J, Harris D, Cowling WA. 2003. The interaction of phosphorus and potassium with seed alkaloid concentrations, yield and mineral content in narrow-leafed lupin (Lupinus angustifolius L.). Plant Soil. 253(2):413–427.
- Gremigni P, Wong MTF, Edwards NK, Harris D, Hamblin J. 2001. Potassium nutrition effects on seed alkaloid concentrations, yield and mineral content of lupins (Lupinus angustifolius). Plant Soil. 234(1):131–142.
- Griffiths MR, Strobel BW, Hama JR, Cedergreen N. 2021. Toxicity and risk of plant-produced alkaloids to daphnia magna. Environ Sci Eur. 33(1):10.
- Gulisano A, Alves S, Martins JN, Trindade LM. 2019. Genetics and breeding of Lupinus mutabilis: an emerging protein crop. Front Plant Sci. 10:1385.
- Hama JR, Jorgensen DBG, Diamantopoulos E, Bucheli TD, Hansen HCB, Strobel BW. 2022. Indole and quinolizidine alkaloids from blue lupin leach to agricultural drainage water. Sci Total Environ. 834:155283.
- Hama JR, Strobel BW. 2020. Natural alkaloids from narrow-leaf and yellow lupins transfer to soil and soil solution in agricultural fields. Environ Sci Eur. 32(1):26
- Hansen HCB, Hilscherova K, Bucheli TD. 2021. Natural toxins: environmental contaminants calling for attention. Environ Sci Eur. 33(1):. 12
- Jansen G, Aguilar OM, Jürgens HU, Schliephake E, Ordon F. 2012. Effect of the soil pH on the alkaloid content of Lupinus angustifolius. Int J Agron. 2012:1–5.
- Jansen G, Jürgens HU, Ordon F. 2009. Effects of temperature on the alkaloid content of seeds of Lupinus angustifolius cultivars. J Agron Crop Sci. 195(3):172–177.
- Jansen G, Jürgens HU, Schliephake E, Seddig S, Ordon F. 2015. Effects of growing system and season on the alkaloid content and yield of different sweet L. angustifolius genotypes. J Appl Bot Food Qual. 88:1–4.
- Ku YS, Contador CA, Ng MS, Yu J, Chung G, Lam HM. 2020. The effects of domestication on secondary metabolite composition in legumes. Front Genet. 11:581357.
- Lee MJ, Pate JS, Harris DJ, Atkins CA. 2007. Synthesis, transport and accumulation of quinolizidine alkaloids in Lupinus albus L. and L. angustifolius L. J Exp Bot. 58(5):935–946.
- Otterbach SL, Yang T, Kato L, Janfelt C, Geu-Flores F. 2019. Quinolizidine alkaloids are transported to seeds of bitter narrow-leafed lupin. J Exp Bot. 70(20):5799–5808.
- Philippi J, Schliephake E, Jürgens HU, Jansen G, Ordon F. 2016. Correlation of the alkaloid content and composition of narrow-leafed lupins (Lupinus angustifolius L.) to aphid susceptibility. J Pest Sci. 89(2):359–373.
- Philippi J, Schliephake E, Jürgens HU, Jansen G, Ordon F. 2015. Feeding behavior of aphids on narrow-leafed lupin (Lupinus angustifolius) genotypes varying in the content of quinolizidine alkaloids. Entomol Exp Appl. 156(1):37–51.
- Podleśny J, Podlesna A. 2012. The effect of high temperature during flowering on growth, development and yielding of blue lupine – barley mixture. J Food Agric Environ. 10:500–504.
- Schrenk D, Bodin L, Chipman JK, del Mazo J, Grasl-Kraupp B, Hogstrand C, Hoogenboom L, Leblanc JC, Nebbia CS, Nielsen E, et al. 2019. Scientific opinion on the risks for animal and human health related to the presence of quinolizidine alkaloids in feed and food, in particular in lupins and lupin-derived products. EFSA J. 17:e05860.
- Shukla PR, Skea J, Slade R, Khourdajie AA, van Diemen R, McCollum D, Pathak M, Some S, Vyas P, Fradera R, Belkacemi M, Hasija A, Lisboa G, Luz S, Malley J, editors. 2022. IPCC, 2022: Climate change 2022: mitigation of climate change. Contribution of working group III to the sixth assessment report of the intergovernmental panel on climate change. Cambridge (UK); New York (NY): Cambridge University Press.
- Tirdiľová I, Vollmannová A, Čéryová S, Obtulovič P, Árvay J, Zetochová E. 2022. Impact of 3-year period as a factor on the content of biologically valuable substances in seeds of white lupin. Plants. 11(16):2087.
- Vishnyakova MA, Kushnareva AV, Shelenga TV, Egorova GP. 2020. Alkaloids of narrow-leaved lupine as a factor determining alternative ways of the crop’s utilization and breeding. Vavilovskii Zhurnal Genet Selektsii. 24(6):625–635.
- Wink M. 1983. Wounding-induced increase of quinolizidine alkaloid accumulation in lupin leaves. Zeitschrift für Naturforschung C. 38(11–12):905–909.
- Wink M, Hartmann T. 1984. Enzymology of quinolizidine alkaloid biosynthesis. In: Zalewski RI, Skolik JJ, editors. Natural Products Chemistry 1984: A Collection of Invited Section and Colloquium Lectures Presented at the 14th IUPAC International Symposium on the Chemistry of Natural Products, Poznan, Poland; 1984 Jul 9–14. Amsterdam: Elsevier.
- Wink M, Hartmann T. 1981. Sites of enzymatic synthesis of quinolizidine alkaloids and their accumulation in lupinus polyphyllus. Zeitschrift für Pflanzenphysiol. 102(4):337–344.
- Wink M, Hartmann T, Rheinheimer J, Ludger W. 1982. Interrelationship between Quinolizidine alkaloid producing legumes and infesting insects: exploitation of the alkaloid- containing phloem sap of Cytisus scoparius by the broom aphid aphis cytisorum. Zeitschrift für Naturforschung C. 37(11-12):1081–1086.
- Wink M, Meißner C, Witte L. 1995. Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry. 38(1):139–153.
- Wink M, Witte L. 1984. Turnover and transport of quinolizidine alkaloids. Diurnal fluctuations of lupanine in the phloem sap, leaves and fruits of Lupinus albus L. Planta. 161(6):519–524.
