Abstract
This study explores the implementation of the One Sample Strategy (OSS), a co-regulation program aimed at managing mycotoxin risk in Texas maize. Fumonisin-contaminated cereals and oilseeds that contain greater than 5 mg kg−1 of the toxin (B1, B2, and B3) are a risk for equids and rabbits, and levels greater than 60 mg kg−1 are a risk to ruminants. The OSS, previously successful in managing aflatoxin risk in Texas maize, was evaluated for its effectiveness in handling fumonisin risk in maize, specifically as it relates to ruminants. In 2017, 25 analysts across seven firms qualified to participate in the program. To ensure greater accuracy in testing, working control samples were provided to the participating OSS firms with the requirement that their results fall within +/− 20% of the target concentration. Ninety-four percent of the working controls met this specification. The capability to grind maize to the OSS prescribed particle size was met by 100% of participants. To verify testing accuracy, file samples collected from each OSS firm were analysed by UPLC–MS/MS. The 177 fumonisin verification samples analysed by Office of the Texas State Chemist (OTSC) were correlated (r = 0.93) with co-regulation laboratories. Results were plotted in an operating curve to depict type I and type II errors. Error analysis revealed a type I error rate of 13% and type II error rate of 2% for the 5 mg kg−1 guidance level, and 6% and 8%, respectively, for the 60 mg kg−1 guidance level. For 2017, 994 official reports of analysis for fumonisin in whole maize in the Texas High Plains were issued by the seven laboratories that employed 25 OTSC-credentialed analysts. The OSS co-regulation program, supported by a quality systems approach and government regulations, has proven effective in managing fumonisin risk in Texas maize, enhancing both market confidence and livestock safety.
Introduction
Co-regulation is a collaborative regulatory approach between government and private sectors, using government-endorsed codes of practice and action plans (Garcia Martinez et al. 2007; Institute of Medicine and National Research Council of the National Academies Citation2010). It allows firms to play a legitimate role in public health improvement (Sebillotte Citation2019). Co-regulation can be viewed from two perspectives: a single entity, where the government is the problem-owner, or a multiple entity, where responsibility is shared among all parties involved (van der Voort Citation2015). This inclusion approach fosters community engagement in regulatory processes.
In Texas, the One Sample Strategy (OSS) has been effective in managing aflatoxin risk in maize through a multiple entity co-regulation model. The Texas Feed and Fertilizer Control Service (FFCS), including the Office of the Texas State Chemist (OTSC) and an ISO/IEC 17025 accredited laboratory, regulates feed and feed ingredients to ensure they are free from contaminants. The Texas Commercial Feed Control Act (TAC §141.148) grants FFCS authority to regulate contaminated feed and grain.
The Texas Commercial Feed Control Act specifies, “a person commits an offense if the person distributes, conspires to distribute, or causes another person to distribute commercial feed that contains or bears a poisonous or deleterious substance that may render it injurious to animals under ordinary conditions of use (TAC §141.148 (6)). This legal position is further supported by TAC §141.002 under the definition of commercial feed, as detailed in TAC §141.002 (C). It states, “the following are not commercial feeds subject to this Chapter: (2) whole grain or whole seed not containing toxins or chemical adulterants”. Therefore, whole grain or whole seed, which contain mycotoxins, would be considered contaminated and can be regulated. Examples of these mycotoxins include aflatoxin and fumonisin. Further, the State Chemist is tasked with preparing rules and minimum standards as defined in TAC 141.004. Texas first promulgated rules for fumonisin in 1989 prior to any guidance issued from the United States Food and Drug Administration (FDA). However, in 1990 FDA provided guidance for the US industry involving fumonisin contamination that was updated in 1991 (FDA Citation2001).
Fumonisin, a toxin that chemically contaminates grain and feed, poses significant risks to both human and animal health (FDA Citation2001; FAO Citation2019). It is a sphingolipid inhibitor (Merrill et al. Citation2001) that has also been associated with esophageal cancer in humans (Reddy et al. Citation2010) and neural tube defects in the fetus that lead to spina bifida in children (Marasas et al. Citation2004). With regard to livestock, horses, swine, and rabbits are highly sensitive to fumonisins. Cattle and poultry are less sensitive, but there are guidance levels for total fumonisins in animal feed for these livestock as well (FDA Citation2001). Maize is susceptible to fumonisin, and Texas is a major producer of maize utilized for animal feed. Nearly 98% of maize produced in Texas is used for livestock feed (Dorsett Citation2019). A primary market for maize is the cattle feeding industry, so it follows that management of this mycotoxin is important to the health and well-being of livestock as well as to the health and well-being of the public.
A fumonisin management strategy employed by FFCS involved blending of contaminated grain to lower contamination levels, specifically for feeding cattle in confinement. Under Texas statue (TAC Citation1995), The State Chemist promulgated rules for blending grain containing high levels of fumonisin, permitting intra-state commercial use without violating the Federal Food, Drug, and Cosmetic Act (FDCA) section 402(a)(1) titled “Adulterated Food”. It is under this same state authority that OTSC launched a mycotoxin risk management plan that involved co-regulation. The program was the One Sample Strategy (OSS). In this program, the State Chemist established criteria under which firms that conform to United States Department of Agriculture (USDA)-Federal Grain Inspection Service (FGIS) could perform official tests on behalf of OTSC. The program included sufficient rigor, as outlined in the One Sample Strategy Handbook, to provide confidence to both the State Chemist and the Texas maize market that results were accurate and sufficient for trade and regulation (OTSC Citation2023). The overall concept of the program is to test once and use the results for multiple purposes.
Texas has a history of collaboration with USDA agencies like FGIS and Risk Management Agency (RMA). Leveraging these relationships, the State Chemist introduced OSS to RMA, who approved it for crop insurance indemnification for aflatoxin in 2011 and expanded it to all mycotoxins by 2017 (USDA and RMA Citation2017). Fumonisin rules, which aligned with FDA guidance, appeared no longer necessary and were repealed in 2018. In this process, OTSC pivoted to regulating fumonisin using FDA guidance, which while non-binding, provide authority for both FDA and FFCS to take regulatory action under authority defined in the statute as described earlier (FDA Citation2001). However, the State Chemist also deemed that he no longer possessed the authority to approve blending plans for fumonisin, which was specified in the fumonisin rule, even though blending can provide substantial benefit to the grain industry during years when fumonisin levels are high. High levels of fumonisin have occurred in years 2008, 2016, 2017, and most recently, 2022.
Implementation of an OSS for aflatoxin was preceded by an evaluation of the Texas grain industry’s testing capability and the validation of rapid test kits to perform accurate testing to 300 ng g−1 and beyond (Herrman et al. Citation2014). A similar approach was taken in expanding the OSS program to fumonisin. Prior to 2017, FGIS had validated kits to 30 mg kg−1 for fumonisin, however, the need exists in Texas for fumonisin rapid test kits to measure up to 100 mg kg−1. At the time of program implementation, only the Charm WETS5 kit was validated as capable of measuring at these high levels. Since this time, EnviroLogix Inc. has produced a kit (TotalTox Fumonisin) that possesses the capability of measuring fumonisin at levels sufficient for the OSS program (FGIS Citation2023). Similar to the OSS program for aflatoxin, participants need to implement a quality systems approach as outlined in the OSS handbook including ensuring representative sample collection, proper sample preparation, recordkeeping, and verification of test results by the OTSC ISO/IEC 17025(2017) accredited lab (OTSC Citation2023). The alignment of risk communication strategies between grain elevators, OTSC, FGIS, and RMA is also critical.
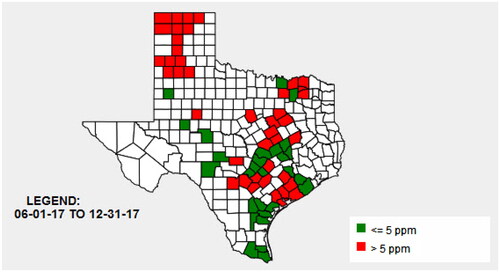
Samples collected during the 2017 harvest () revealed that 27% of the harvested maize was above 5 mg kg−1 (based on result average within a county, 70 Texas counties, n = 915). Of those 70 counties, 42 had at least one result with a level exceeding 5 mg kg−1. At the time, maize containing greater than 60 mg kg−1 was subject to a blend plan. Guidance levels for 60 mg kg−1 fumonisin-contaminated maize state that it can only be fed to ruminants being raised for slaughter, under the age of 3-months old (not to exceed 50% of diet) (FDA Citation2001). In 2017, 10 counties had a least one result with a level exceeding 60 mg kg−1, and all of those counties were in the Texas High Plains. Because of the prevalence of fumonisin, seven facilities in the Texas High Plains opted in to the OSS program for fumonisin. OTSC field investigators inspected the seven participating establishments and 23 grain elevators to ensure accurate sampling and testing techniques. Implementation of the OSS was able to take place within a two-week period. RMA’s expansion of the program to include all mycotoxins (covering aflatoxin, DON, and fumonisin) occurred with expediency, in part, due to the past success of the program for aflatoxin (USDA and RMA Citation2017). The quick adoption of the OSS program for fumonisin highlights the need to evaluate the implementation and effectiveness of this co-regulation program to improve fumonisin risk management in Texas.
Materials and methods
Seven laboratories in the Texas High Plains participated in the program during the 2017 harvest, servicing 23 grain elevators. The implementation of the co-regulation program included an evaluation of the firms’ food safety plans, onsite qualification of the firms’ analysts, monitoring the firms’ performance during harvest, and verification of the results of samples from the participating firms’ satellite laboratories by the OTSC ISO/IEC 17025 accredited laboratory in College Station, Texas (OTSC Citation2023).
Participants in the OSS contacted OTSC to express their interest. This contact was followed by providing them the OSS handbook and instructing them in how to complete a food safety plan. Elements in the plan included:
Firms acquired the necessary tools to comply with the program including sample probes, grinders, sieves, check weight, and validated fumonisin test kits.
Analyst background checks conducted on all proposed participants.
Analysts trained in accurate testing procedures and then to perform each test to qualify as OSS analysts.
Firms were equipped with control material (working control) that was used daily. Results from the control material were recorded and were required to be within the upper and lower control limits prior to performing official tests.
All official tests were recorded and retained samples were kept for verification.
Verification samples were collected frequently, but no less than once per week and shipped to OTSC for verification that was performed within 5-days of receipt.
Verification results were returned to grain elevators weekly.
All results from the OSS participants were submitted to OTSC on Friday afternoon,
Any discrepancies between OSS and OTSC verification results were investigated and corrective actions taken.
All results were submitted to RMA at the end of harvest.
The senior manager of Quality Assurance at OTSC provided technical assistance upon request.
Field performance evaluation
Field performance evaluation assesses the firms’ implementation of USDA-FGIS methodology, as contained in the OSS handbook (OTSC Citation2023). A firm submits a plan that is reviewed and approved by the FFCS field operations manager, and then field investigators are sent to verify equipment and employees. Grinding performance is evaluated by measuring particle size using a 20-mesh sieve. The OSS target amount of ground maize passing through the sieve is 70% (% fines). OSS participants are required to analyze control samples (working controls), and results must fall within specified duplication limits. For fumonisin, the acceptable range is ±20%. Firms analyze samples using an approved fumonisin test kit from USDA-FGIS (formerly, USDA Grain Inspection, Packers and Stockyards Administration or GIPSA) (FGIS Citation2023). For a test kit to be on the approved USDA-FGIS list, it is required to meet the design criteria and performance specifications set forth by this entity (USDA-AMS-FGIS Citation2023). Accuracy must be demonstrated at targeted concentrations of 0.50, 2.0, 5.0, and 30 mg kg−1 with an option for an extended range of conformance. At least 95% of the results (20 out of 21) acquired by three analysts for seven separate samples for each concentration level must be within the acceptable ranges. Acceptable range is determined by two times the maximum relative standard deviation (RSDmax) for the particular concentration level. Concentrations of 0.50, 2.0, 5.0, and 30 mg kg−1 have RSDmax = 18%, 14%, 13%, and 13%, respectively. Concentration above 30 mg kg−1 have an RSDmax = 13%. The kit used was the Charm Sciences Inc. ROSA WETS5 Fumonisin Quantitative Kit (FGIS Part Number: LF-FUMQ-WETS5). Proficiency of the analyst is determined with FFCS investigator oversight and includes activities such as balance checks, sample preparation, and sample analysis. A credentialed analyst is one who has successfully completed the testing portion on the “Mycotoxin Risk Management Qualification Checklist” (OTSC Citation2023). During harvest, the credentialed personnel are responsible for sampling, grinding, and analyzing maize samples for fumonisin.
Control material
The control material provided to the OSS facilities was produced in-house by OTSC. OTSC received ISO 17034 (2016) accreditation for the production of aflatoxin, fumonisin, and deoxynivalenol in ground maize reference materials (A2LA Citation2023). Naturally-contaminated maize was ground through a 0.75 micron screen on a Retsch SR300 and blended for 120 min in a commercial mixer (Multiquip, Model MC94PE) to produce the reference material with the desired fumonisin levels. These levels were confirmed using UPLC-MS/MS as a standard testing approach. Homogeneity and stability of material were established by measuring 12 samples in duplicate. Statistical design was taken from ISO 13528 for homogeneity testing (ISO Citation2022). The acceptable testing variances were set forth in the USDA-AMS-FGIS mycotoxin test kit specifications (USDA-AMS-FGIS Citation2023).
Sample results verification
Verification samples
Licensed FFCS investigators collected the retained verification samples from the facilities and delivered and/or shipped those samples (under the required chain-of-custody) to the OTSC lab. The same samples analyzed by the facilities using the approved test kit were then subjected to fumonisin determination by OTSC using UPLC-MS/MS.
Analysis of fumonisin by UPLC–MS/MS
For all fumonisin determination by the OTSC laboratory, an ISO/IEC 17025 accredited in-house method was used for analyses. This method is based on the method developed at OTSC (Li et al. Citation2010). Modifications to the UPLC-MS/MS procedure included the following: extraction of a 50 gram sample with 250 ml of 70:30 methanol:water; shaker time of 60 min instead of 15 min, and filtration of extract through Whatman #1 filter instead of centrifugation. Fumonisin certified analytical standards and isotope-labeled standard were purchased from Biopure-Romer Lab, Inc, Tulln, Austria (FB1, 50 µg/mL, Cat. No. 002003; FB2, 50 µg/mL, Cat. No. 002004; and FB3, 50 µg/mL, Cat. No. S02007; fumonisin internal standard (U-[13C34]-FB1), 25 µg/mL, Cat. No. ILM003). All commercial standards were solutions prepared in acetonitrile-water (50:50). Analysis was performed on a Waters Quattro Premier XE mass spectrometer with Waters Acquity UPLC (column type – Acquity UPLC BEH C18 and UPLC BEH C18 VanGuard pre-column).
The validation of the in-house method for fumonisin determination included analysis of blank samples (cornmeal as matrix) fortified at different levels, regulatory samples, and working control samples previously tested by another in-house method (Protocol 16701 – “Determination of Fumonisin in Feed by HPLC using NDA”). Accuracy and precision target limits were taken from AOAC (AOAC Citation2016; Horwitz and Albert Citation2006)
Data analysis
Verification data, which includes the OTSC UPLC–MS/MS fumonisin result and the corresponding facility test kit result, were used to determine acceptance probability at two violation levels, 5 mg kg−1 and 60 mg kg−1. To construct the performance curve, acceptance probability is plotted against fumonisin concentration and line of best fit is determined. Acceptance probability was determined by the facility’s kit result relative to the OTSC UPLC–MS/MS result. The OTSC result with the corresponding facility test kit result was sorted based on the OTSC result. This allowed for the creation of concentration bins. Count was determined within each bin (0–1 mg kg−1, 1–3 mg kg−1, 3–5 mg kg−1, 5–7 mg kg−1, 7–9 mg kg−1, 9–13 mg kg−1, 13–15 mg kg−1, >15 mg kg−1). For the first three bins, facility results >5 mg kg−1 were counted. This count was divided by the total count for that bin. The acceptance probability was 1 – [count >5/Total count]. The same process was applied to the remaining bins except that facility results ≤5 mg kg−1 were counted. With 5 mg kg−1 set as the violation criteria, the acceptance probability was adjusted for these bins (1-probability). A similar procedure was used in creating the performance curve for 60 mg kg−1. The bins were 0–1 mg kg−1, 1–3 mg kg−1, 3–10 mg kg−1, 10–20 mg kg−1, 20–40 mg kg−1, 40–60 mg kg−1, 60–70 mg kg−1, 70–90 mg kg−1, and > 90 mg kg−1. Determination of acceptance probability was done in the same manner as for 5 mg kg−1 but with the 60 mg kg−1 limit.
Using JMP®, a matched pairs t-test was performed to compare the facilities’ test kit results to the OTSC UPLC-MS/MS results and evaluate the deviation in fumonisin test results of the retained samples between the OSS firm (facility) and the OTSC lab. Regression analysis compared fumonisin results from verification samples across all firms with the OTSC lab.
Results and discussion
For the UPLC–MS/MS validation results, the intra-day and inter-day reproducibility were evaluated at 200, 500 and 1000 ng g−1 for FB1 and FB2, and 100, 250 and 500 ng g−1 for FB3. In animal feed cornmeal, matrix recoveries of FB1 ranged from 93% to 98% (RSD from 5% to 8%). Recoveries of FB2 ranged from 104% to 107% (RSD from 2% to 6%). Recoveries of FB3 ranged from 94 to 96% (RSD from 2% to 5%).
Regulatory samples had concentrations of total fumonisin of 2, 13, and 64 mg kg−1. Working controls had a concentration of around 6 mg kg−1. Recoveries of FB1 ranged from 95% to 114% (RSD from 2% to 8%). Recoveries of FB2 ranged from 102% to 107% (RSD from 3% to 7%). Recoveries of FB3 ranged from 93 to 106% (RSD from 5% to 8%). By the application of an isotope-labeled internal standard, the matrix effect in UPLC-MS/MS analysis was effectively eliminated, and the performance of quantification method met the requirements outlined in AOAC methodology as well as FDA and EU regulation criteria (Li Citation2009).
Evaluation of grinding performance showed that all facilities were able to achieve the particle size of greater than 70% (% fines) through a 20-mesh sieve (). All facilities analyzed control samples at the beginning and end of the day and compared their results with upper and lower control limits (duplication limits) to ensure testing accuracy. All facilities performed well when analyzing fumonisin control samples, with 94% of the controls meeting the acceptance range for the assigned concentration (). These results suggest that both the facility’s analyst and test kit were performing satisfactorily and producing accurate results.
Table 1. OSS laboratory performance: Controls and particle size.
Further evidence of fumonisin testing accuracy was obtained through a co-lab verification between the participating OSS facilities and OTSC. In a matched pairs evaluation comparing the fumonisin test kit results from the facilities to the UPLC–MS/MS results from OTSC, the mean fumonisin concentration measured by the facility was 42.0 mg kg−1, while the OTSC mean was 31.7 mg kg−1. The mean difference between the two methods was 10.3 mg kg−1, with a standard error of 1.294 mg kg−1. The difference was statistically significant (p < .0001), indicating a consistent discrepancy between the two measurement methods. Ideally, matched pairs should show no significant difference between the methods; however, it is expected that the test kit may not perfectly align with a high-end instrument method. The matched pairs evaluation does highlight the kit’s bias, indicating that the kit results tend to be higher.
A log transformation was applied to the verification data due to the wide range of concentrations (<1 mg kg−1 to >200 mg kg−1), which improved the model’s assumptions and fit. The 177 fumonisin verification samples analyzed by OTSC showed a strong positive correlation (R = 0.90) with the co-regulation laboratories’ results (). This relationship explained 81% of the variation in the data (R2 = 0.81). The verification of sample testing accuracy enables OTSC to monitor testing performance, characterized in operating curves with maximum levels (ML) of 5 mg kg−1 and 60 mg kg−1 ( and ).
Figure 2. Comparison of facility fumonisin result by test kit to OTSC result by UPLC-MS/MS. Log transformation performed on original units of mg kg−1.
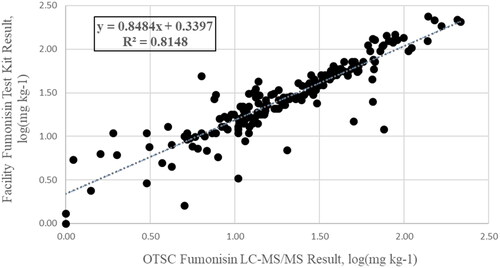
Figure 3. Acceptance probability of fumonisin at 5 mg kg−1 regulatory limit by One Sample Strategy labs compared to the Texas State Chemist laboratory.
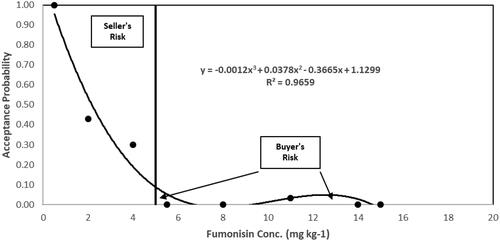
The operating curve in intersects the 5 mg kg−1 vertical axis at the 9% acceptance level (calculated using the equation). The area above the operating curve and less than the 5 mg kg−1 ML represents the portion of samples categorized by the OSS facilities as adulterated (>5 mg kg−1 fumonisin) that OTSC found to contain fumonisin at or below the ML. This area is referred to as seller’s risk, false positive or type I error. The area below the operating curve and greater than or equal to the ML represents the portion of samples categorized by the OSS facility as not adulterated (≤5 mg kg−1 fumonisin) where OTSC analysis results were greater than the ML. This area of the operating curve is referred to as buyer’s risk, false negative, or type II error. The cumulative percentage of samples categorized as type I was 13% and the type II error represented 2% of the cumulative percentage of samples greater than 5 mg kg−1. The third order polynomial model yielded a coefficient of determination (R2) of 0.97 indicating that it explains 97% of the variability in analytical variation between OSS laboratories and OTSC.
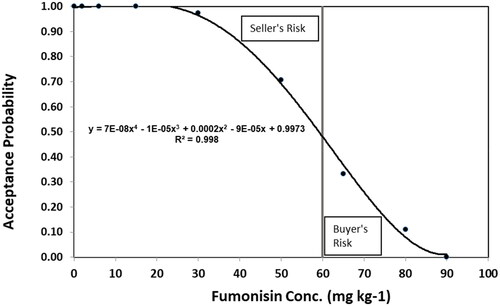
The operating curve in intersects the 60 mg kg−1 vertical axis at the 46% acceptance level (calculated using the equation). In this situation, the area above the operating curve and less than the 60 mg kg−1 ML represents the portion of samples categorized by the OSS facilities as >60 mg kg−1 fumonisin that OTSC found to contain fumonisin below the ML. This area is referred to as seller’s risk, false positive or type I error. This would lead a firm to implement a blending plan when not required. The area below the operating curve and greater than the 60 mg kg−1 ML represents the portion of sample categorized by the OSS facility as not requiring a blend plan (<60 mg kg−1 fumonisin) for OTSC results that were greater than the 60 mg kg−1 ML (requiring a blend plan). This area of the operating curve is referred to as buyer’s risk, false negative, or type II error. The probability of type I and type II error in is around 14% (6.2% for type I and 7.5% for type II). The 4th order polynomial model yielded a coefficient of determination (R2) of 0.998 indicating that it explains 99.8% of the variability in analytical variation between OSS laboratories and OTSC.
Figure 4. Acceptance probability of fumonisin at 60 mg kg−1 regulatory limit by One Sample Strategy labs compared to the Texas State Chemist laboratory.
These performance curves resemble those characterizing aflatoxin testing performance in Texas through the One Sample Strategy aflatoxin risk management program and theoretical performance curves established through experimental data (Sasser et al. Citation2018). The high R2 values for both models indicate robust predictive capabilities, but the slight differences in polynomial order suggest that higher thresholds may require more complex modelling to capture the variability accurately. The error rates should inform regulatory policies, with potential adjustments to the acceptable limits or additional safety margins based on the observed discrepancies. These results highlight the differing risks and accuracies associated with the two fumonisin thresholds (guidance levels), suggesting that while lower thresholds ensure conservative safety margins, higher thresholds require more rigorous verification to maintain reliability. Further studies could explore ways to reduce both type I and type II errors through improved testing methodologies, better alignment of test kits, and enhanced training for analysts.
Conclusions
In 2017, the harvested acreage for maize was 2.2 million acres (140 bushels/acre) with a value of $1.2 billion (Texas Almanac Citation2022). In the High Plains, an estimated 723,900 planted acres yielded 98,998,700 bushels of maize (Herrman et al. Citation2018). One hundred twenty-two commercial grain elevators with an estimated 71 million bushel storage capacity operate in the Texas High Plains (Herrman et al. Citation2018). There were a total of 116 cattle feedlots (Herrman et al. Citation2018). With the fumonisin guidelines for ruminants set at less than 60 mg kg−1, the relevance at the time to manage maize with levels of greater than 60 mg kg−1 of fumonisin was apparent.
The implementation of co-regulation within a quality system-based code of practice successfully managed fumonisin risk in Texas using co-regulation as a governance option. The public-private partnership between RMA of USDA, FFCS, and the grain elevators appears to have an overall benefit on regulating the market. The market would have been at a standstill had the fumonisin-testing lab capability not increased 7-fold through the OSS program (which, in turn, allowed for official reports of analysis to be issued). For the 2017 crop year, maize that tested above 2.1 mg kg−1 was eligible with the default discount factor (DF) of 0.500, even if the elevator did not discount a single load of grain. Farmers benefited with net revenues of over $52 million (Brown Citation2023). The OSS program also exposed the need for an increased measurement range for fumonisin test kits. As a result of Texas’s need for test kit capabilities greater than 30 mg kg−1, the Charm test kit was validated to 74 mg kg−1 in the previous year, and thus enabled test kit capabilities to shift from a limit of 30 mg kg−1 to as high as 100 mg kg−1 in 2017. Not a single grain elevator seizure or regulatory action was required due to management of the maize at the facility level. Sharing of this kit validation (and previous kit validation work) with FGIS technical center initiated a change by FGIS to create a more robust kit validation process. The use of a single laboratory analysis for multiple purposes (purchasing, crop insurance and regulatory risk management) is a major benefit of the OSS program and improves market certainty. However, the study further highlights the necessity of a quality system to ensure defensible results.
Understandably, one can point out that issues with fumonisin are of a regional nature, but the scalable properties of co-regulation allow for expansion of the program beyond the High Plains and borders of Texas. With the adoption of OSS principles, improved management of mycotoxins in the United States is certainly feasible. The program can improve market liquidity and provide legal certainty on a much larger scale and be applied to testing of other commodities that pose risks, such as aflatoxin in peanuts or deoxynivalenol in wheat. A success story related to the adoption of techniques used in the OSS program can be told with the Kenya maize milling industry – where 80% of the maize milling sector participate in aflatoxin proficiency testing and control in Africa (APTECA) (Herrman Citation2022).
The OSS program works because it utilizes the concepts of a quality management system (QMS) to ensure accurate, defensible, and timely official results. Having a QMS decreases the risk of measurement inaccuracy and improves certification accuracy. With the availability of matrix-matched reference materials, the OSS facilities can better manage risk associated with the test kit. Verification of the facilities’ results provides feedback and the opportunity to make corrections.
Some of the challenges at the time the OSS program was introduced for aflatoxin risk management included: creation of reference material, capability to verify a large number of samples in an accredited laboratory, and industry buy-in. The challenge of reference material production has been addressed by OTSC. In Texas, with a steady stream of naturally-contaminated material available, OTSC is an ISO-accredited reference material producer for aflatoxin, fumonisin, and deoxynivalenol in ground maize (A2LA Certificate 3915.02). Regarding the verification of a large number of samples, OTSC can easily meet the needs of the regulated community. However, other states and regulatory entities may lack the resources (people and instrumentation) to manage such a program. Concerning industry buy-in, OTSC maintains a relationship with its stakeholders, and it was partly through this relationship that the program evolved. There was a clear frustration within the supply chain (farmers, producers, grain mills, RMA) regarding discrepancies in testing results and speed at which those results were available. Industry must recognize the benefits because the program is still in place after 12 years (6 years for fumonisin).
The OSS strategy provides tremendous value within the industry to manage risk and support commerce of fumonisin-contaminated maize by providing market confidence, facilitating crop insurance indemnification, and channeling fumonisin-contaminated maize to the appropriate end use.
References
- [A2LA] American Association for Laboratory Accreditation 2023. A2LA Directory of Accredited Organizations. [accessed 2024 June 7] https://customer.a2la.org/index.cfm?event=directory.detail&labPID=ED8A68B0-BBDD-459C-98C3-380AD93BACA2.
- [AOAC] Association of Official Analytical Chemists 2016. Appendix F: Guidelines for Standard Method Performance Requirements. Gaithersburg, MD: AOAC International.
- [FAO] Food and Agricultural Organization. 2019. General standard for contaminants and toxins in food and feed CXS 193-1995. World Health Organization; p. 38–42.
- [FDA] Food and Drug Administration 2001. Guidance for industry: fumonisin levels in human foods and animal feeds. [accessed 2024 Apr 2] https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-fumonisin-levels-human-foods-and-animal-feeds.
- [FGIS] Federal Grain Inspection Service 2023. FGIS approved mycotoxin rapid test kits [accessed 2024 Apr 2] https://www.ams.usda.gov/sites/default/files/media/FGISApprovedMycotoxinRapidTestKits.pdf.
- [ISO] International Organization for Standardization 2022. ISO 13528:2022(E) Statistical methods for use in proficiency testing laboratory comparison, in Annex B: homogeneity and stability of proficiency test items.
- [OTSC] Office of the Texas State Chemist 2023. One sample strategy for mycotoxin risk management in Texas - 2023 Handbook. [accessed 2024 Apr 2] https://otsc.qualtraxcloud.com/Showdocument.aspx?ID=7186.
- [TAC] Texas Administrative Code 1995. Texas commercial feed control act - Texas agriculture code (1981), Chapter 141. [accessed 2024 Apr 2] https://statutes.capitol.texas.gov/Docs/AG/htm/AG.141.htm.
- [USDA and RMA] United States Department of Agriculture and Risk Management Agency 2017. Managers bulletin: MGR-17-015 - one sample strategy for mycotoxins in Texas. [accessed 2024 Apr 2] https://legacy.rma.usda.gov/bulletins/managers/2017/mgr-17-015.pdf.
- [USDA] United States Department of Agriculture 2017. Frequently Asked Questions Fumonisin. [accessed 2024 Apr 2] https://www.rma.usda.gov/en/News-Room/Frequently-Asked-Questions/Fumonisin.
- [USDA-AMS-FGIS] United States Department of Agriculture, Agriculture Marketing Service, and Federal Grain Inspection Service 2023. Design Criteria and Test Performance Specifications for Quantitative Fumonisin Test Kits. [accessed 2024 June 6] https://www.ams.usda.gov/sites/default/files/media/fumonisincriteria.pdf.
- Brown AA. 2023. Improvement of feed and nutrient security for sulfur and fumonisin in Texas feedlot cattle rations [Doctoral dissertation]. Texas A&M University.
- Dorsett J. 2019. Transformation Tuesday: Corn. Texas Farm Beareu. [accessed 2024 Apr 2] https://tabletop.texasfarmbureau.org/2019/10/transformation-tuesday-corn/.
- Garcia Martinez M, Fearne A, Caswell JA, Henson S. 2007. Co-regulation as a possible model for food safety governance: opportunities for public–private partnerships. Food Policy. 32(3):299–314. doi: 10.1016/j.foodpol.2006.07.005.
- Herrman TJ, Lee KM, Jones B, McCormick C. 2014. Aflatoxin sampling and testing proficiency in the Texas grain industry. Reg Sci. 2(1):7–13. doi: 10.21423/JRS.REGSCI.2112.
- Herrman TJ, Sasser M, Rooney M. 2018. White paper on fumonisin risk management and communication in the high plains. [accessed 2024 Jul 3]. https://otscweb.tamu.edu/Risk/Fumonisin%20White%20Paper.pdf.
- Herrman TJ. 2022. APTECA, a quality systems approach to improve profitability and food protection in the grain processing sector of Eastern and Southern Africa. [accessed 2024 Apr 2] https://apteca.tamu.edu/Videos/herrman_presentation/tim_herrman_presentation.mp4.
- Horwitz W, Albert R. 2006. The Horwitz ratio (horrat): a useful index of method performance with respect to precision. J AOAC Int. 89(4):1095–1109.
- Institute of Medicine and National Research Council of the National Academies. 2010. Enhancing Food Safety: The Role of the Food and Drug Administration. In: Oria M, Wallace, RB, editors. National Academies Press; [accessed 2024 Jul 3]. ProQuest Ebook Central, https://ebookcentral.proquest.com/lib/tamucs/detail.action?docID=3378695.
- Li W. 2009. In-house document: validation of Fumonisin in Feed SOP 16711 LC/MS/MS. Office of the Texas State Chemist. October 13, 2009. Available from: Office of the Texas State Chemist, College Station, TX; Doc ID: 15165.
- Li W, Herrman TJ, Dai SY. 2010. Rapid determination of fumonisins in corn-based products by liquid chromatography/tandem mass spectrometry. J AOAC Int. 93(5):1472–1481. doi: 10.1093/jaoac/93.5.1472.
- Marasas WF, Riley RT, Hendricks KA, Stevens VL, Sadler TW, Gelineau-van Waes J, Missmer SA, Cabrera J, Torres O, Gelderblom WC, et al. 2004. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J Nutr. 134(4):711–716. Jr. doi: 10.1093/jn/134.4.711.
- Merrill AH, Jr, Sullards MC, Wang E, Voss KA, Riley RT. 2001. Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins. Environ Health Perspect. 109 Suppl 2(Suppl 2):283–289. doi: 10.1289/ehp.01109s2283.
- Reddy K, Salleh B, Saad B, Abbas HK, Abel CA, Shier KRN. 2010. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Reviews. 29(1):3–26. doi: 10.3109/15569541003598553.
- Sasser M, Herrman TJ, Lee KM. 2018. Evaluation of coregulation as a governance option to manage aflatoxin risk in Texas maize. J Food Prot. 81(4):554–560. doi: 10.4315/0362-028X.JFP-17-312.
- Sebillotte C. 2019. Efficiency of public-private co-regulation in the food sector: the French voluntary agreements for nutritional improvements. OCL. 26:34. doi: 10.1051/ocl/2019029.
- Sustainable Crop Insurance Services 2017. Fumonisin Update #3. [accessed 2024 Apr 2] http://www.sustainablecropins.com/fumonisin-update-3/.
- Texas Almanac 2022. Principal crops in Texas. [accessed 2024 Apr 2] https://www.texasalmanac.com/articles/principal-crops-in-texas.
- van der Voort H. 2015. Co-regulatory failure in the food industry: explaining regulatory failure by means of two contrasting interpretations of governance. Eur j Risk Regul. 6(4):502–511. doi: 10.1017/S1867299X00005067.