Abstract
This work investigated the adsorption ability of unmodified and carbon-modified TiO2 nanoparticles for the removal of methyl orange (MO) from aqueous solution. Carbon–TiO2 was obtained by carbonization of ethanol vapors at three different temperatures (200, 300, and 400°C), and their adsorption was compared with unmodified TiO2 nanoparticles. The Freundlich adsorption model was found to fit for TiO2 and C–TiO2-200, while carbon modification of TiO2 at a high temperature fitted the Langmuir–Freundlich model (C–TiO2-300 and C–TiO2-400). Generally, the carbonization of C–TiO2 increased the adsorption capacity of TiO2 nanoparticles, however the BET surface of modified and pristine TiO2 was almost the same. The zeta potential of modified TiO2 is higher than unmodified TiO2, which leads to efficient adsorption of MO onto modified TiO2.
1. Introduction
Environmental protection is the practice of protecting the natural environment, which is also of benefit to humans. Azo dyes are among the thousands of industrial dyes that break down to toxic products harmful to ecosystems and human health [Citation1]. Thus, the efficient removal of these dye pollutants from wastewaters is very important.
Many types of biological and chemical process including ozonation [Citation2], flocculation [Citation3], coagulation [Citation3,4], and adsorption [Citation5–7] have been used to remove dye pollutants. Adsorption is simple, low cost, and in many cases the most effective of these.
Although activated carbon is one of the most efficient adsorbents for different types of pollutant, it is expensive, usually time-consuming, and complicated to prepare. Thus, many attempts have been made to find economical yet effective adsorbents. Nano-adsorbents are highly efficient and have been extensively used for dye removal. The very high surface area to volume ratio of many nanomaterials makes them extremely advantageous as adsorbents. For example, Raveendra et al. [Citation8] synthesized nano ZnTiO3 ceramic for the removal of azo dyes from aqueous solutions. Iron oxide nanospheres were synthesized by Khosravi et al. and used to remove anionic dyes from water [Citation9]. Wu et al. used a hydrothermal procedure to prepare magnetic Fe3O4@C nanoparticles for dye adsorption [Citation10].
Nanostructured titanium dioxide (TiO2), which is a semiconductor material, is nontoxic, inexpensive, active in reactions, and chemically stable [Citation11–14]. Therefore, it has many applications, including its use as an adsorbent [Citation7,15,16]. TiO2 has also been applied to remove anionic dye pollutants from wastewaters. The main issue in applying TiO2 as an adsorbent is the problem of separation from an aqueous medium. Separation techniques, such as membrane filtration [Citation17] are expensive. Yet commercial TiO2 does not show a very high adsorption capacity for dye removal compared to activated carbons [Citation6]. Doping the other materials to TiO2 is an effective method to improve its adsorbent and photocatalytic character, also enhancing the separation efficiency.
The modification of TiO2 with carbon creates a synergetic effect established by the adsorption capacity of activated carbon and the photoactivity of TiO2 [Citation11,18,19]. Carbon-modified TiO2 has been used to adsorb azo dyes, such as reactive red 198 and direct green 99, [Citation1] or reactive black 5 diazo dye [Citation20]. These studies show that the combination of activated carbons and TiO2 nanoparticles can produce a proper adsorbent which has both high adsorption capacity and excellent photocatalytic activity.
In the present study, carbon-modified TiO2 (C–TiO2) was prepared by ethanol carbonization and used for the removal of methyl orange (MO) dye pollutant from aqueous solution. The characterization of the prepared adsorbents was carried out by SEM images, N2 adsorption/desorption isotherm, and XRD patterns. UV–vis spectroscopy was used to follow the adsorption process. The adsorption equilibrium, adsorption kinetics, and the effect of pH on dye removal efficiency were investigated. The effect of carbonization temperature on the zeta potential (ZP) and removal efficiency of MO by the prepared adsorbents was also studied. The ability to recover an alternative adsorbent is important from both an economic and a practical perspective. Thus, desorption studies were also performed.
2. Experimental
2.1. Materials
MO sodium salt dye was supplied by J.T. Baker and used without further purification. TiO2 (nanopowder avg size = 25 nm with a chemical purity of ≥99.5) was purchased from Sigma–Aldrich and used as a precursor for the modification of TiO2 (C–TiO2).
2.2. Instruments
MO concentration was analyzed by UV–vis spectroscopy using a Jasco V-670 spectrophotometer (Jasco, Japan). The X-ray diffraction (XRD) patterns of modified TiO2 were obtained with a PANalytical X́Pert PRO alpha 1 diffractometer. Cu Kα1 radiation (1.5406 Å; 45 kV, 30 mA) was used, generated by primary beam Johansson monochromator. The Hitachi S-4800 (Hitachi, Japan) Ultra-High Resolution Scanning Electron Microscope (SEM) was used to analyze the morphology and size of the adsorbents.
N2 adsorption–desorption isotherms were obtained with an ASAP2010 (Micromeritics). The samples were outgassed at 100°C for 12 h, before nitrogen adsorption. The pore volume distributions as a function of pore size was calculated based on the Dubinin–Radushkevich equation using adsorption branches of the measured isotherm.
FTIR type Vertex 70 by Bruker Optics (Germany) was used to identify the surface functional groups of the prepared adsorbents. The FTIR spectra were recorded at 4 cm−1 resolution from 400 to 4,000 cm−1 and 100 scans per sample. The surface charge and point of zero charge of the modified and unmodified TiO2 were determined by isoelectric point titration as a function of pH using a Zetasizer Nano ZS (ZEN3500, Malvern). The determination of carbon was obtained using an Organic Elemental Analyzer Flash 2000 (Thermo Scientific, Germany).
2.3. Preparation of carbon-modified TiO2
The carbon-modified TiO2 was prepared using the method described by Janus et al. [Citation21]. The installation setup used for adsorption preparation is schematically shown in Fig. .
Fig. 1. Schematic presentation of installation setup for the synthesis of C–TiO2 [Citation21].
Notes: (1) Gas cylinder with argon, (2) controller, (3) Dreschel bottle with ethanol, (4) pipe furnace, (5) combustion boat with powder sample, and (6) scrubber.
![Fig. 1. Schematic presentation of installation setup for the synthesis of C–TiO2 [Citation21].Notes: (1) Gas cylinder with argon, (2) controller, (3) Dreschel bottle with ethanol, (4) pipe furnace, (5) combustion boat with powder sample, and (6) scrubber.](/cms/asset/e37d8c12-da6f-420c-ae45-0c4fa30e1d61/tdwt_a_1094678_f0001_oc.gif)
Briefly, 1 g of TiO2 in a porcelain boat was modified with carbon by heating up to the specified temperature with a pipe furnace under ethanol vapor for 40 min and maintaining that temperature for 1 h. The ethanol vapor was prepared by bubbling pure argon through ethanol at room temperature.
Commercial TiO2 consists of a mixture of anatase/rutile phases [Citation22]. Previously, we have shown that up to 600°C the phase ratio of anatase to rutile remained nearly constant and phase transition was not observed [Citation6]. Thus, to investigate only the influence of carbonization temperature on the removal efficiency of C–TiO2 adsorbents, these were synthesized at temperatures of 200, 300, and 400°C. The C–TiO2 samples were identified as C–TiO2-200, C–TiO2-300, and C–TiO2-400, based on their carbonization temperatures.
2.4. Batch adsorption experiments
2.4.1. Adsorption kinetics and equilibrium
In order to evaluate the adsorption characterization of the synthesized adsorbents and to determine their adsorption capacity, adsorption kinetic and equilibrium studies were performed.
For the kinetic adsorption study 10 mL of a solution of 10 mg/L MO with 0.02 g of each C–TiO2 adsorbents was placed in a shaker (200 rpm and 25°C) and the residual MO concentration at the proper time intervals, Ct, was determined at 473 nm. The amount of adsorbed MO per unit mass of the adsorbents at time t, qt, was calculated by Eq. (Equation1(1) ):
(1)
where C0 (mg/L) is the initial concentration of the dye, V (L) is the solution volume, and m (g) is the mass of the adsorbent.
For the equilibrium experiments, a series of solutions with different initial concentrations (4–18 mg/L) were prepared, then 10 mL of each solution with 0.02 g of the desired adsorbent was placed in a shaker (200 rpm and 25°C) for 24 h. The equilibrium concentration of MO and Ce was measured at 473 nm, and the amount of adsorbed dye per unit mass of the adsorbent at equilibrium, qe, was calculated using Eq. (Equation2(2) ):
(2)
2.5. Effect of pH
To investigate the adsorption efficiency of C–TiO2 adsorbents at different pH, a series of MO solutions (6 mg/L) with different initial pH (2–10) were prepared, and 10 mL of each solution with 0.02 g of the desired adsorbent was shaken overnight. The removal percentage of MO was calculated by Eq. (Equation3(3) ):
(3)
where A0 and A are the initial and equilibrium absorbance of MO at 473 nm, respectively.
2.6. Zeta potential
ZP is the electrical potential in the double layer at the interface between a particle, which moves in an electrical field and the surrounding liquid. Measuring ZP is also a method of characterizing the particle surface in the processes running on this surface. In this work, triton was used as a dispersing agent to pre-disperse the adsorbents in deionized water. To determine the ZP, 0.053 g of adsorbent and 0.25 mL of triton were added to 25 mL of deionized water and stirred for 10 min. This was then filled up to 50 mL with deionized water and subsequently mixed for 10 min. Next, the solution with a dilution ratio of 1:50 was stirred for 20 min.
2.7. Desorption experiments
Five adsorption/desorption cycles were performed to evaluate C–TiO2 adsorbent efficiency after recovery.
Firstly, 0.2 g of the desired adsorbent was loaded onto 50 mL of the MO solution with an initial concentration of 200 (mg/L), overnight. Then the adsorbent was separated and washed several times to remove unadsorbed MO traces. The adsorbent was dried in an oven at 80°C overnight. The dried adsorbent was then washed with NaOH solution (1 M) several times until the elute pH became neutral. The adsorbent was washed with distilled water again and dried in an oven (80°C, overnight). In order to evaluate the removal efficiency of the recovered adsorbent, the above procedure was repeated another four times. Since an amount of adsorbent is lost during elution, the ratio between the mass of the adsorbent and the volume of the MO solution was kept at 4 (). After each cycle, the removal percentage of MO by recovered C–TiO2 adsorbents was calculated by Eq. (Equation4
(4) ):
(4)
where A0 and A are the initial and equilibrium absorbance of MO at 473 nm in each cycle.
3. Results and discussion
3.1. Characterization of C–TiO2 adsorbents
The surface structures of the C–TiO2 adsorbents were studied by SEM images. It can be seen from Fig. that, all the C–TiO2 samples consist of nanometer-size particles, like the TiO2 precursor (Fig. (a)). The particle sizes of the TiO2, C–TiO2-200, and C–TiO2-300 samples are about 30 and 50 nm (most particles are sized > 50 nm), respectively, and C–TiO2-400 adsorbent (Fig. (d)) consists of particles with various sizes (20–60 nm). Thus, increasing the carbonization temperature to 300°C increased the particle size (Fig. (b–d)).
Fig. 2. SEM of (a) unmodified TiO2, (b) C–TiO2-200, (c) C–TiO2-300, and (d) C–TiO2-400. The scale bar represents 500 nm.
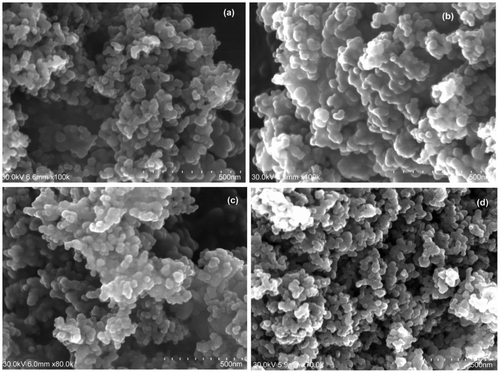
Fig. shows the nitrogen adsorption/desorption isotherm of the obtained adsorbents. The type IV isotherm and textural properties of C–TiO2 samples listed in Table reveal that the obtained adsorbents have a mesoporous structure. Table also shows that the total surface area was slightly increased by increasing the carbonization temperature. These data correspond to the SEM images.
Fig. 3. Nitrogen adsorption/desorption isotherm of (a) TiO2, (b) C–TiO2-200, (c) C–TiO2-300, and (d) C–TiO2-400.
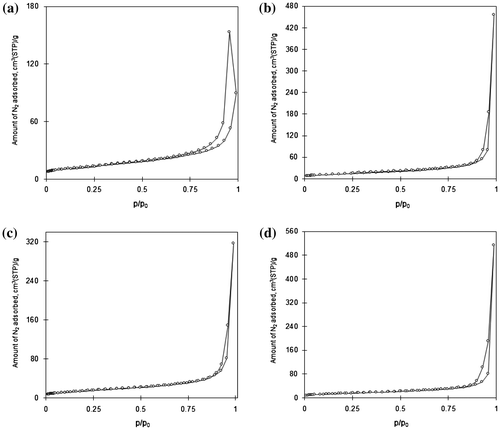
Table 1 Textual properties of TiO2 and C–TiO2 adsorbents
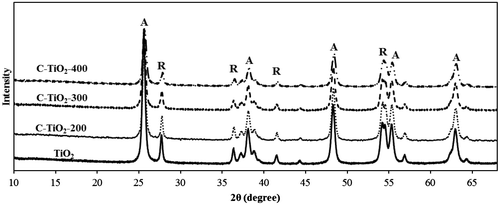
The effect of carbonization temperature on the crystalline phase of C–TiO2 samples was studied by XRD technique. Fig. shows the XRD patterns of the prepared adsorbents and TiO2 precursor. It can be concluded that when TiO2 nanoparticles were heated up to 400°C, no phase transition occurred, which is in agreement with our previous work [Citation6].
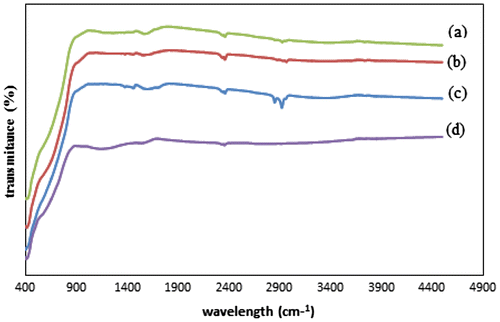
The FTIR spectra of the modified and unmodified TiO2 are presented in Fig. . It can be observed that with increasing temperature, a new peak appeared in the range of 2,850–3,000 cm−1 assigned to the methyl group. This indicates not only the presence of ethanol in carbonization, but also that temperature can be related to the increased amount of carbon on modified TiO2 nanoparticles. This finding is in agreement with the obtained elemental analysis results: The highest carbon content was found in C–TiO2-400 (6.3%), compared to 0.12 and 4.53% for C–TiO2-200 and C–TiO2-300, respectively. This marked increase in carbon content also corresponds to the introduction of a methyl group on the TiO2 surface.
3.2. Kinetic and equilibrium studies
A high adsorption rate is very desirable in water purification applications.
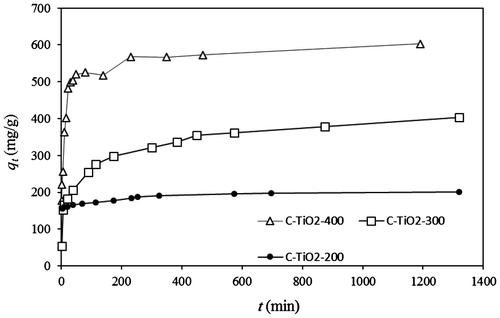
Fig. shows the time dependency of the adsorption of MO by C–TiO2 adsorbents. In each case, the dye removal was achieved within less than 60 min. The adsorption rate increased further with rising carbonization temperature. This result corresponds to the results of the nitrogen adsorption/desorption isotherm and SEM images. Indeed, the increase in adsorbent surface area with rising carbonization temperature leads to the increase in adsorption rate.
Fig. 6. Time dependency of the adsorption of MO by C–TiO2 adsorbents. Solid and dashed lines represent the predicted qt values by PSO model.
The kinetic data were analyzed with pseudo-first-order (PFO) and pseudo-second-order rate equations [Citation6]. The integrated form of the PFO model can be expressed as:(5)
where qt and qe are the amounts of the dye adsorbed at time t and at equilibrium e, respectively, and k1 is the PFO rate coefficient.
The following equation shows the pseudo-second-order (PSO) model [Citation6]:(6)
where k2 is the pseudo-second-order rate coefficient.
According to the obtained correlation coefficients, the experimental kinetic data were best fitted to the pseudo-second-order equation for the adsorption of MO onto the C–TiO2 adsorbents. The fitting results are listed in Table . The solid lines in Fig. show the predicted values of qt based on the PSO model as a function of time.
Table 2 Obtained constants of PFO and PSO rate equations for the adsorption of MO onto C–TiO2 adsorbents
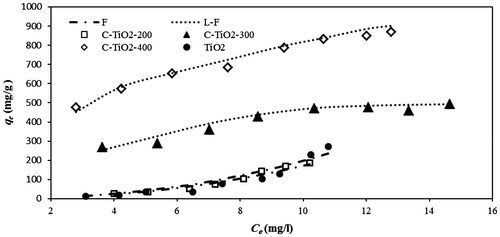
Fig. shows the equilibrium data of MO adsorption onto the prepared adsorbents. These data show that while carbonization at 300 and 400°C increases the adsorption capacity of C–TiO2-300 and C–TiO2-400 in comparison to unmodified TiO2, carbonization at 200°C has no significant effect on C–TiO2-200 adsorption capacity. Thus, it can be concluded that, although, carbon modification of TiO2 at 200°C increases its surface area (Table ), which could also improve the adsorption efficiency, a large surface area does not always indicate efficient adsorption. The surface charge of adsorbents also plays a significant role in the adsorption process, and seems more important for high adsorption capacity, as discussed below.
Langmuir, Freundlich, Langmuir–Freundlich, and Temkin isotherms were used to analyze the experimental equilibrium data, and to obtain the maximum adsorption capacity of synthesized C-TiO2 adsorbents for MO dye removal.
The Langmuir isotherm can be expressed as follows [Citation23]:(7)
where qm (mg/g) and KL (L/mg) are the maximum adsorption capacity and Langmuir constant, respectively.
The following equation shows the Freundlich isotherm which described the multisite adsorption [Citation24]:(8)
where KF (mg/g) (L/mg)1/n is the Freundlich constant, and the parameter n describes the system heterogeneity.
The Langmuir–Freundlich (Sips) isotherm, which is a combination of the Langmuir and Freundlich isotherms, can be expressed as follows [Citation25]:(9)
where Ks (l/mg)1/n is the adsorption constant.
The Temkin isotherm, [Citation26] which is a two-parameter adsorption isotherm, can be expressed as below:(10)
where α and β are the isotherm constants.
The results of fitting to the adsorption isotherm models are listed in Table . According to the obtained correlation coefficients, the experimental equilibrium data for the adsorption of MO onto TiO2 and C–TiO2-200 were fitted to the Freundlich isotherm and those for adsorption onto C–TiO2-300 and C–TiO2-400 were best fitted to the Langmuir–Freundlich isotherm. The predicted values of qe based on the Langmuir–Freundlich equation are presented in Fig. as solid lines.
Table 3 Obtained isotherm constants for adsorption of MO onto TiO2 and C–TiO2 adsorbents
3.3. Effect of pH and ZP
The pH of the solution is an effective function in dye removal efficiency, especially in the case of semiconductor adsorbents. Thus, the removal percentages of MO by C–TiO2 adsorbents were investigated at different pH.
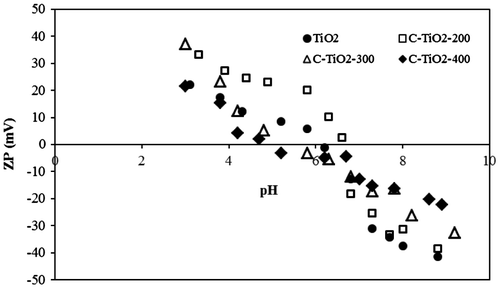
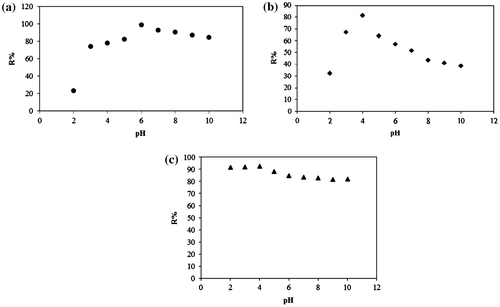
Fig. (a–c) show the removal percentage of MO by carbon-modified TiO2 adsorbents at different pH. The maximum removal efficiency was observed at pH 6 and 4 for C–TiO2-200 and C–TiO2-300, respectively. In the case of C–TiO2-400, the maximum removal percentage was achieved in an acidic medium up to pH 4, and then decreased slightly. For C–TiO2-200, C–TiO2-300, and C–TiO2-400, the point of zero charge (pHPZC) was found to be at pH 6.53, 5.19, and 4.48, respectively, while the pHPZC for unmodified TiO2 was around 6.13 (data not shown). A change in pHPZC after modification was caused by the presence of methyl groups on the surface of the modified TiO2.
Fig. 8. Removal percentage of MO by (a) C–TiO2-200 (b) C–TiO2-300 and (c) C–TiO2-400 at different pH.
The adsorbent surface has a negative charge at solution pH higher than pHPZC, while the surface charge is positive at pH lower than pHPZC [Citation27].
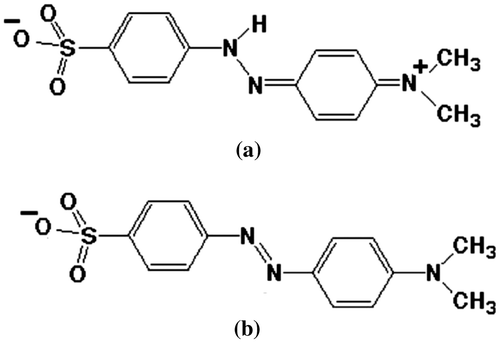
Fig. shows the chemical structure of MO in acidic and basic media. In the case of C–TiO2-200 and C–TiO2-300, high removal efficiency was reached in an acidic medium, while basic pH decreased the removal percentages. Thus, in acidic media (lower than pHpzc of 6.53 and 5.19 for C–TiO2-200 and C–TiO2-300, respectively), the strong attractive interaction between the – group of MO and positive surface of the mentioned adsorbents causes maximum removal efficiency. At basic pH, the repulsion interaction between the negative end of the MO molecules and negative surface of the C–TiO2-200 and C–TiO2-300 decreased the removal percentages.
As shown in Fig. (c), the removal percentage of MO by C–TiO2-400 almost remains unchanged at acidic and basic pH. This suggests that MO molecules are not adsorbed through the ionic – group [Citation5]. It seems that MO adsorption is due to the interaction between the central nitrogen or benzene rings and the surface of C–TiO2-400.
ZP was measured to illustrate the mechanism of the pH effect on MO removal efficiency by C–TiO2 adsorbents. It was very difficult to make this type of modified TiO2 nanoparticle disperse homogeneously into the aqueous solution, especially as dispersion takes a long time. Triton was therefore used to pre-disperse the adsorbents in deionized water. As can be seen from Fig. , the TiO2 surface has a highly negative ZP in suspension, leading to repulsion between particles. In contrast, the modified TiO2 had a significantly lower ZP (the ZP of TiO2, C–TiO2-200, C–TiO2-300, and C-TiO2-400 are −32.2, −23.3, −15.2, and −10.8 mV, respectively). Thus, the decline in negative ZP not only leads to flocculation [Citation1], but also causes better adsorption of MO onto modified TiO2 than unmodified TiO2. Furthermore, carbonization at 200°C led to a slight decrease in the ZP of C–TiO2-200 as compared to unmodified TiO2, while adsorbents prepared at 300 and 400°C demonstrated a large change in ZP. Thus, the adsorption capacity of unmodified TiO2 and C–TiO2-200 is almost the same (Fig. ). The much lower ZP of C–TiO2-300 and C–TiO2-400 is due to their higher adsorption capacity. This may suggest that the electrostatic interaction between the dye-(C–TiO2) complex and negatively charged surface of heat-treated TiO2 becomes dominant. In addition, the interaction mechanism of dye molecules and the sample calcined at different temperatures are different; the interaction is stronger at high temperatures.
3.4. Desorption and regeneration studies
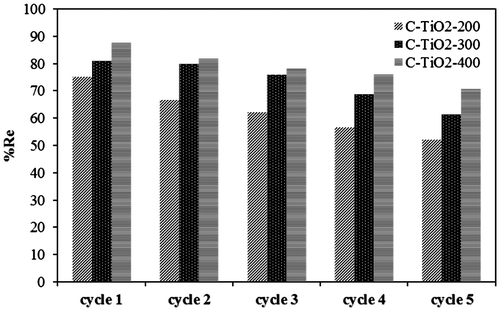
The results of the adsorption/desorption experiments are shown in Fig. . These results approve the electrostatic interaction between MO and C–TiO2 adsorbents, since this interaction is limited by NaOH solution.
Although the removal efficiency decreased by 30, 24, and 19% after five cycles for C–TiO2-200, C–TiO2-300, and C–TiO2-400, respectively, it remained above 50% for all C–TiO2 adsorbents. Thus, all prepared adsorbents, especially C–TiO2-400 (with its 19% efficiency decrease), could be reused at least five times, which is both highly economical and practical.
3.5. Adsorption mechanism
The Weber–Morris intraparticle diffusion model was applied to identify the adsorption mechanism of MO adsorption onto the modified TiO2. This model, which includes terms to account for both intraparticle diffusion and boundary layer diffusion, is expressed as follows:(11)
where Kdif (mg/g min−0.5) is the rate constant of intraparticle diffusion and Ci (mg/g) stands for thickness of the boundary layer.
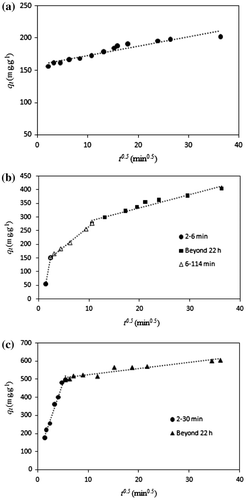
The plot of qt vs. t0.5 for MO adsorption on C–TiO2-200, C–TiO2-300, and C–TiO2-400 are illustrated in Fig. . It can be seen from Fig. (a) that qt was plotted against t0.5 in a straight line with an intercept (Ci). This indicates that the intraparticle diffusion was important, but not the only rate-determining step in the adsorption process. Moreover, the obtained intercept value (Ci = 1.579 mg/g) is positive, indicating rapid adsorption in a short period of time. Furthermore, the rate constant (Kdif = 0.145 mg/g min0.5) indicates a rapid diffusion process. Fig. (b) and (c) shows the intraparticle diffusion plots for MO adsorption onto C–TiO2-300 and C–TiO2-400, which reveal the same general features as the multi-linear qt vs. t0.5 plot. The whole adsorption process for C–TiO2-400 and C–TiO2-300 consist of two and three steps, respectively. In C–TiO2-300, the initial sharply sloped portion of 2–6 min may occur due to the small particle size noted above, indicating external surface adsorption. The second step is the gradual adsorption stage from 6 to 114 min, during which intraparticle diffusion may be the rate-limiting step. The third and final equilibrium stage beyond 22 h corresponds to the slowing down of intraparticle diffusion due to low solute concentrations in the solution. When adsorption has reached saturation on the exterior surface, the MO ions may have entered the pore surface, which is hydrated in liquid phase. In C–TiO2-400 (Fig. (c)) an initial curved portion representing increased sorption is followed by a linear portion as equilibrium is attained. The primary linear portion at 2–30 min may be attributed to the external surface, and the second curved portion (beyond 22 h) may be ascribed to the pore diffusion or intraparticle diffusion. Two distinct linear regions of differing slopes are visible on the graph, indicating that intraparticle diffusion is involved in the adsorption process, but is not the only rate-limiting mechanism. Other mechanisms may also simultaneously control the rate of MO adsorption onto C–TiO2-400; further investigation is required in order to describe these.
4. Conclusions
Carbon-modified TiO2 adsorbents were prepared at different carbonization temperatures (200–400°C), and used for the adsorptive removal of MO dye pollutant from aqueous solution. Our results show that MO dye pollutant was rapidly removed by all C–TiO2 adsorbents; the adsorption process took less than 1 h. It was revealed that the MO adsorption capacity of TiO2 increases by carbon modification. Changing the carbonization temperature had no significant effect on the surface area of TiO2, but did enhance maximum adsorption capacity, which might be due to the increase in adsorbent ZP. The Freundlich adsorption model was found to fit well for TiO2 and C–TiO2-200, while carbon-modified TiO2 fitted the Langmuir–Freundlich model at higher temperatures. All the prepared adsorbents could be regenerated at least five times and used as economical adsorbents in purification applications.
References
- M. Janus, E. Kusiak, J. Choina, J. Ziebro, A.W. Morawski, Enhanced adsorption of two azo dyes produced by carbon modification of TiO2, Desalination 249 (2009) 359–363.10.1016/j.desal.2009.04.013
- J. Chen, L. Zhu, Catalytic degradation of Orange II by UV-Fenton with hydroxyl-Fe-pillared bentonite in water, Chemosphere 65 (2006) 1249–1255.10.1016/j.chemosphere.2006.04.016
- E. Guibal, J. Roussy, Coagulation and flocculation of dye-containing solutions using a biopolymer (Chitosan), React. Funct. Polym. 67 (2007) 33–42.10.1016/j.reactfunctpolym.2006.08.008
- I. Kabdasli, M. Gürel, O. Tünay, Characterization and treatment of textile printing wastewaters, Environ. Technol. 21 (2000) 1147–1155.10.1080/09593330.2000.9619001
- B. Yahyaei, S. Azizian, Rapid adsorption of anionic dyes by ordered nanoporous alumina, Chem. Eng. J. 209 (2012) 589–596.10.1016/j.cej.2012.08.055
- S. Jafari, S. Azizian, B. Jaleh, Adsorption kinetics of methyl violet onto TiO2 nanoparticles with different phases, Colloids Surf., A: Physicochem. Eng. Aspects 384 (2011) 618–623.10.1016/j.colsurfa.2011.05.030
- B. Tanhaei, A. Ayati, M. Lahtinen, M. Sillanpää, Preparation and characterization of a novel chitosan/Al2O3/magnetite nanoparticles composite adsorbent for kinetic, thermodynamic and isotherm studies of Methyl Orange adsorption, Chem. Eng. J. 259 (2015) 1–10.10.1016/j.cej.2014.07.109
- R.S. Raveendra, P.A. Prashanth, R. Hari Krishna, N.P. Bhagya, B.M. Nagabhushana, H. Raja Naika, K. Lingaraju, H. Nagabhushana, B. Daruka Prasad, Synthesis, structural characterization of nano ZnTiO3 ceramic: An effective azo dye adsorbent and antibacterial agent, J. Asian Ceram. Soc. 2 (2014) 357–365, doi: 10.1016/j.jascer.2014.07.008.
- M. Khosravi, S. Azizian, Adsorption of anionic dyes from aqueous solution by iron oxide nanospheres, J. Ind. Eng. Chem. 20 (2014) 2561–2567.10.1016/j.jiec.2013.10.040
- R. Wu, J.-H. Liu, L. Zhao, X. Zhang, J. Xie, B. Yu, X. Ma, S.-T. Yang, H. Wang, Y. Liu, Hydrothermal preparation of magnetic Fe3O4@C nanoparticles for dye adsorption, J. Environ. Chem. Eng. 2 (2014) 907–913.10.1016/j.jece.2014.02.005
- K. Bubacz, B. Tryba, A.W. Morawski, The role of adsorption in decomposition of dyes on TiO2 and N-modified TiO2 photocatalysts under UV and visible light irradiations, Mater. Res. Bull. 47 (2012) 3697–3703.10.1016/j.materresbull.2012.06.038
- A. Di Paola, G. Cufalo, M. Addamo, M. Bellardita, R. Campostrini, M. Ischia, R. Ceccato, L. Palmisano, Photocatalytic activity of nanocrystalline TiO2 (brookite, rutile and brookite-based) powders prepared by thermohydrolysis of TiCl4 in aqueous chloride solutions, Colloids Surf., A: Physicochem. Eng. Aspects 317 (2008) 366–376.10.1016/j.colsurfa.2007.11.005
- B. Chládková, E. Evgenidou, L. Kvítek, A. Panáček, R. Zbořil, P. Kovář, D. Lambropoulou, Adsorption and photocatalysis of nanocrystalline TiO2 particles for Reactive Red 195 removal: effect of humic acids, anions and scavengers, Environ. Sci. Pollut. Res. (2015) 1–11, doi: 10.1007/s11356-015-4806-y.
- S. Sabar, M.A. Nawi, W.S.W. Ngah, Photocatalytic removal of Reactive Red 4 dye by immobilised layer-by-layer TiO2/cross-linked chitosan derivatives system, Desalin. Water Treat. 1–7, doi: 10.1080/19443994.2015.1004113(2015).
- S. Helali, E. Puzenat, N. Perol, M.-J. Safi, C. Guillard, Methylamine and dimethylamine photocatalytic degradation—Adsorption isotherms and kinetics, Appl. Catal. A: Gen. 402 (2011) 201–207.10.1016/j.apcata.2011.06.004
- A.A. Vega, G.E. Imoberdorf, M. Mohseni, Photocatalytic degradation of 2,4-dichlorophenoxyacetic acid in a fluidized bed photoreactor with composite template-free TiO2 photocatalyst, Appl. Catal. A: Gen. 405 (2011) 120–128.10.1016/j.apcata.2011.07.033
- H. Han, R. Bai, Effect of thickness of photocatalyst film immobilized on a buoyant substrate on the degradation of methyl orange dye in aqueous solutions under different light irradiations, Ind. Eng. Chem. Res. 50 (2011) 11922–11929.10.1021/ie200787j
- J. Matos, J. Laine, J.M. Herrmann, Association of activated carbons of different origins with titania in the photocatalytic purification of water, Carbon 37 (1999) 1870–1872.10.1016/S0008-6223(99)00198-0
- J. Matos, J. Laine, J.M. Herrmann, Effect of the type of activated carbons on the photocatalytic degradation of aqueous organic pollutants by UV-irradiated Titania, J. Catal. 200 (2001) 10–20.10.1006/jcat.2001.3191
- M. Janus, E. Kusiak, A. Morawski, Carbon modified TiO2 photocatalyst with enhanced adsorptivity for dyes from water, Catal. Lett. 131 (2009) 506–511.10.1007/s10562-009-9932-z
- M. Janus, M. Inagaki, B. Tryba, M. Toyoda, A. Morawski, Carbon-modified TiO2 photocatalyst by ethanol carbonisation, Appl. Catal. B 63 (2006) 272–276.10.1016/j.apcatb.2005.10.005
- M.-H. Baek, J.-W. Yoon, J.-S. Hong, J.-K. Suh, Application of TiO2-containing mesoporous spherical activated carbon in a fluidized bed photoreactor—Adsorption and photocatalytic activity, Appl. Catal. A: Gen. 450 (2013) 222–229.10.1016/j.apcata.2012.10.018
- I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum, J. Am. Chem. Soc. 40 (1918) 1361–1403.10.1021/ja02242a004
- H. Freundlich, Over the adsorption in solution, J. Phys. Chem. 57 (1906) 385–470.
- R. Sips, On the structure of a catalyst surface, J. Chem. Phys. 16 (1948) 490–495.10.1063/1.1746922
- B. Saha, C. Orvig, Biosorbents for hexavalent chromium elimination from industrial and municipal effluents, Coord. Chem. Rev. 254 (2010) 2959–2972.10.1016/j.ccr.2010.06.005
- N. Fiol, I. Villaescusa, Determination of sorbent point zero charge: usefulness in sorption studies, Environ. Chem. Lett. 7 (2009) 79–84.10.1007/s10311-008-0139-0