Abstract
Membrane systems are commonly used in the water industry to produce potable water and for advanced wastewater treatment. One of the major drawbacks of membrane systems is biofilm formation (biofouling), which results in an unacceptable decline in membrane performance. Three novel in situ biofouling characterization techniques were assessed: (i) optical coherence tomography (OCT), (ii) planar optodes, and (iii) nuclear magnetic resonance (NMR). The first two techniques were assessed using a biofilm grown on the surface of nanofiltration (NF) membranes using a transparent membrane fouling simulator that accurately simulates spiral wound modules, modified for in situ biofilm imaging. For the NMR study, a spiral wound reverse osmosis membrane module was used. Results show that these techniques can provide information to reconstruct the biofilm accurately, either with 2-D (OCT, planar optodes and NMR), or 3-D (OCT and NMR) scans. These non-destructive tools can elucidate the interaction of hydrodynamics and mass transport on biofilm accumulation in membrane systems. Oxygen distribution in the biofilm can be mapped and linked to water flow and substrate characteristics; insights on the effect of crossflow velocity, flow stagnation, and feed spacer presence can be obtained, and in situ information on biofilm structure, thickness, and spatial distribution can be quantitatively assessed. The combination of these novel non-destructive in situ biofilm characterization techniques can provide real-time observation of biofilm formation at the mesoscale. The information obtained with these tools could potentially be used for further improvement in the design of membrane systems and operational parameters to reduce impact of biofouling on membrane performance.
1. Introduction
Membrane systems for fresh water production have seen a rapid increase in number of plants and plant size in recent years fueled by growing fresh water demands by increasing human population, industrial and agricultural activity, economic growth and urbanization [Citation1]. Whenever micro-organisms are present in a membrane system, biofilm formation (i.e. bacterial deposition and growth) takes place due to the availability of biodegradable nutrients in a continuous flow of water through the system [Citation2]. Biofouling refers to biofilms that affect membrane systems causing operational problems such as an unacceptable pressure drop increase, permeate flux reduction, or salt passage increase [Citation3].
Most of the commonly applied methods to analyze materials accumulated on membrane and spacer surfaces are destructive, including both the removal of the membrane from the system and the subsequent removal of the accumulated material from the membrane surface for analysis for composition and amount. Biofouling in membrane-based water treatment systems is usually detected using measurements such as pressure drop increase along the feed channel or reduction in permeate flux; biofouling diagnosis is done by membrane autopsy involving destructive opening and inspection of the membrane module. This approach has limitations as the disruptions can cause the biofilm sample to be damaged, contaminated, and even result in structural changes. Another important disadvantage of ex-situ and destructive methods to analyze a biofilm is the variability of small-scale samples when compared to the behavior of the biofilm over time [Citation4].
The growth dynamics and complex structural heterogeneity of biofilms has been studied in detail, especially through the application of various microscopic techniques [Citation5]. 1-D and 2-D microscopic characterization are in most cases inadequate to assess the actual spatial distribution and dynamics of a biofilm. The problem with 3-D analysis at a microscopic scale is that biofilm structures are not relevant in a complex hydrodynamic system like a feed spacer based membrane system. A comprehensive 3-D mesoscale (millimeter–centimeter scale) analysis of a biofilm is necessary for a detailed spatial analysis, while a fundamental continuous non-destructive monitoring of the biofilm processes over time is required to fully assess the biofilm dynamics.
Non-destructive in situ biofilm characterization techniques are gaining attention due to the qualitative and quantitative information that can be obtained from biofilms formed in membrane systems under operating conditions. Three non-destructive in situ imaging methods were assessed in this study: (i) optical coherence tomography (OCT), (ii) oxygen imaging with planar optodes, and (iii) nuclear magnetic resonance (NMR).
OCT is a novel technique to characterize biofouling in water treatment applications. OCT offers possibilities to investigate biofilms without addition of stains or signal enhancers that may affect the biofilm. OCT is a contact-free and non-invasive optical method capable of capturing micrometer-resolution biofilm images from within optical scattering media [Citation6]. OCT-related techniques enable detailed visualization of biofilm structures, as well as determining the impact of hydrodynamics on biofilm formation and behavior in a membrane module [Citation7]. Several studies have shown that OCT can be used to characterize the mesoscale biofilm structure, filling the gap between micro and macroscale [Citation8–15].
The introduction of planar optodes for mapping 2-D oxygen (O2) concentrations in natural systems [Citation16] was a significant step forward to study the heterogeneity of O2 distribution and dynamics in sediments and biofilms [Citation17–19]. Planar optodes use luminescent O2 indicators immobilized in an O2 permeable polymeric matrix, which can be coated on foils or glass surfaces. The principle is based on measuring the dynamic collisional quenching of the indicator luminescence by O2 [Citation20]. A further application of this technology is the use of a planar optode in conjunction with a camera recording luminescence intensities to yield 2-D O2 distributions at the sediment–water interface [Citation16,19,21–23]. Conventional digital cameras with simple normalized luminescence intensity imaging can yield images of 2-D O2 distributions with a high signal-to-noise ratio and spatial resolution [Citation24]. These are comparable or even surpass those obtained with expensive and sophisticated luminescence lifetime imaging systems [Citation23,25].
To investigate the mesoscale biofilm structure, NMR has been used to show the biofilm structure in the millimeter range while allowing for simultaneous imaging of large sample volumes of up to several mm3 [Citation26–30]. This non-destructive in situ characterization technique was successfully applied for non-invasive online monitoring of biofilm development, sloughing, forced detachment, and chemical cleaning. NMR allows visualization and quantification of the development of biofilms and interaction with the surrounding fluid at the mesoscale [Citation31]. Earth’s field (EF) NMR uses comparably weaker magnetic field gradients than high-field NMR systems, minimizing the signal loss due to diffusion. EF NMR offers an interesting option due to its portability, low cost, and the comparative homogeneity of the detection field [Citation32].
The objective of this study is to assess and determine the merits of these three non-destructive in situ characterization techniques for the study of biofilm development on membrane surfaces under similar operating conditions (e.g. hydrodynamic and nutrient concentration conditions). Complementary structural distribution and behavioral information of the biofilm can be obtained combining these methods.
2. Material and methods
2.1. Monitor and membranes
A transparent flat sheet membrane fouling simulator (MFS) for in situ biofilm measurements was used for this study. Details of the monitor system can be found elsewhere [Citation33–35]. A nanofiltration (NF) membrane (4040-TS80-TSF, Trisep Corporation, USA) was used for the OCT and planar optodes studies. For the NMR study, a spiral wound reverse osmosis (RO) membrane module (Dow FILMTEC™ XLE-2521, USA) was used.
Biofouling was promoted by feeding the units a nutrient solution containing sodium acetate, sodium nitrate, and sodium dihydrogen orthophosphate in the mass ratio for C:N:P of 100:20:10, increasing the feed water carbon concentration by 1 mg L−1. Nutrients were dosed to the feed water to accelerate biofilm accumulation. Table shows the experimental conditions under which the biofilm was grown in the different membrane cells used for each characterization technique. The monitors were operated at a linear flow velocity of 0.16 m s−1 (16 L h−1), representative for linear velocities as applied in spiral wound membrane systems in practice [Citation36]. The thickness of a feed spacer is reported in mil (1 mil = 25.4 μm; 31 mil equals 787 μm).
Table 1 Experimental conditions used for biofilm growth for each non-destructive biofilm characterization technique
2.2. Optical coherence tomography (OCT)
A spectral domain optical coherence tomograph (Thorlabs Ganymede OCT System) was used. The OCT was fitted with a 5× telecentric scan lens. 2-D and 3-D scans were taken using the instrument software (ThorImage 4.2.4). The imaged area was 3.83 mm × 1.69 mm, at a resolution of 1,026 × 626 pixels (2-D), and 6 mm × 6 mm × 1.08 mm (total final volume in 3-D), at a resolution of 545 × 545 × 401 pixels corresponding to a voxel size of 11 μm × 11 μm × 2.7 μm.
2.3. Planar optodes
The planar optode used in this study was based on the dye PtTPTBPF (platinum(II) meso-tetra (4-fluorophenyl) tetrabenzoporphyrins excitation 595 nm, emission 775 nm) immobilized in a polystyrene matrix (4% w/w PE/chloroform) [Citation37]. Oxygen imaging was done with an Apogee Imaging Systems Ascent A285 CCD camera equipped with a monochrome Sony ICX-285 Interline CCD Sensor (1,392 × 1,040 pixels). The oxygen sensing dye was excited by amber light emitting diodes (LEDs lumiled type, 595 nm) placed around the camera lens.
2.4. Nuclear magnetic resonance (NMR)
A Magritek Terranova-MRI Earth’s field (EF NMR) system was used with a 7.6 cm inner diameter horizontal bore (and 84 mm 1H radiofrequency (RF) solenoid coil) operating at effectively 0.05 mT with an 18.8 mT prepolarizing field and maximum gradient strengths in the x, y, and z directions of 85 μT m−1. A spiral wound RO membrane module was placed in the bore.
3. Results and discussion
Three in situ characterization techniques were used to analyze biofilm formation under practical operating hydrodynamic conditions and biodegradable nutrient concentration dosed to the feed water. Each technique has its own merits. 2-D and 3-D biofilm reconstructions were completed using OCT scans, biomass spatial distribution was obtained through the analysis of oxygen distribution measured with planar optodes, and signal moment determination using an Earth’s magnetic field NMR provided information on biofilm formation.
3.1. OCT 2-D reconstruction
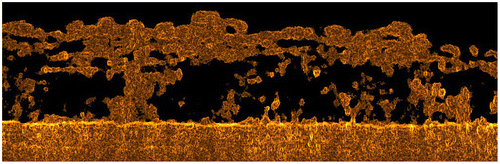
Fig. shows an example of a 2-D image obtained from OCT scans which were analyzed based on a tomography scan processing method used in previous publications [Citation11,12,38] and further processed using edge recognition scripts based on image processing and analysis in Java (ImageJ/FIJI open source software). Void areas can be identified through the biofilm, as well as a change in the density of the biomass depending on the localization in the scan section. Moreover, a variation in the refractive index of the biofilm may suggest a modification in the internal structure of the biofilm. These 2-D images can subsequently be used to evaluate biofilm structural properties such as surface coverage, average thickness, and internal biofilm structures, e.g. porosities.
3.2. OCT 3-D reconstruction
For a membrane system with feed spacer, a 3-D reconstruction is necessary to assess the biomass distribution over the membrane surface and between the feed spacer filaments.
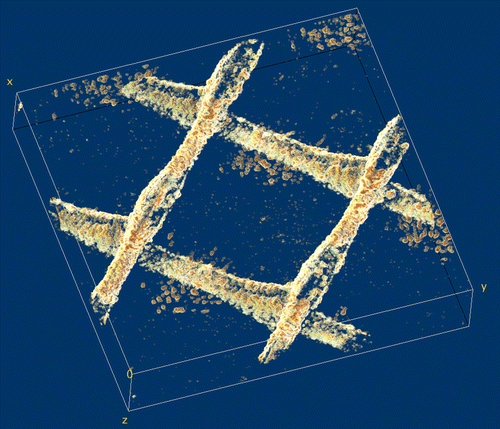
Measured 3-D OCT scans were rendered to filter noise, resulting in a 3-D reconstruction of biomass distribution. Fig. shows an example of the 3-D reconstruction of the grown biofilm in the flow cell containing a feed spacer and membrane. Biofilm started to accumulate mainly on the feed spacer. The biofilm distribution can be observed and quantified based on its spatial location (i.e. membrane, spacer). From this type of analysis, information on the amount of biomass can be quantified spatially resolved. This non-invasive approach enables time-series analysis, providing information on how operational parameters (e.g. water flux through the membrane, hydraulic retention time, biological activity) affect the biofilm formation, structure, and adaptation. These insights in biofouling evolution will eventually allow optimization of membrane system performance.
Fig. 2. 3-D reconstruction of a biofilm grown on the surface of a membrane and feed spacer in a flow cell; the image was obtained after processing OCT 3-D scans in an area of 6 mm × 6 mm × 1.08 mm. Spacer filaments contained most of the biomass detected.
OCT can provide information on how feed water biodegradable nutrient concentration and salt concentration may affect internal biofilm regions (i.e. concentration polarization) [Citation8]. In addition, 3-D simulations can benefit from the structural properties obtained by OCT and related to different hydrodynamic conditions (i.e. varying crossflow velocity, spacer channel thickness, feed spacer geometry).
3.3. Biomass spatial distribution based on O2 distribution
The optode technique can measure spatial oxygen distribution, oxygen dynamics in time as well as oxygen consumption rates thereby giving insights in the spatial distribution of microbial activity. Application of transparent planar optodes allows alignment of O2 distribution at the optode surface with other imaged structures like biomass. A simple camera system can be used at the same time to directly image biomass in a flow cell and image O2 distribution using sensing optodes.
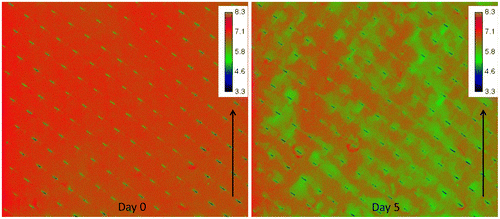
Fig. shows an example of the spatial distribution of oxygen concentration (mg L−1) in a flow cell containing a sheet of membrane and feed spacer at two points in time. Day 0 represents the start of the experiment where no biofilm growth occurred yet. As such, the day 0 image clearly shows a very uniform oxygen distribution throughout the imaged area. On day 5 patches of lower oxygen concentration can be identified, signaling biofilm development.
Fig. 3. Spatial distribution of oxygen concentration (mg L−1) at the inlet side of the MFS on day 0 and after 5 days of biofilm development. The arrow indicates the water flow direction. Scale bar represents oxygen concentration (mg L−1). The imaged area is 4.0 mm × 3.5 cm. Biofilm accumulation started on the feed spacer.
The non-invasive O2 measurement technique can show the spatial development of O2 consumption rates, flow channels and stagnant areas [Citation24]. This information can be used for studies on concentration polarization, i.e. salt accumulation at the membrane surface resulting in increased salt passage and reduced water flux. The new optode-based O2 imaging technique applied to MFS allows non-destructive and spatially resolved quantitative biological activity measurements for on-site biofouling diagnosis and laboratory studies.
3.4. NMR signal moment determination to detect fouling
The NMR imaging technique allows the quantification of spatial biofilm distribution in spiral wound membrane modules, as well as determining velocity fields and their evolution as biofouling develops. EF NMR produces coarser resolution images compared to a high-field NMR. This is mainly due to the much lower total NMR signal (S). Nevertheless, via an analysis of the complex signal in the vicinity of the center of k-space (reciprocal image space), the first three moments of the signal spatial distribution can be obtained. These moments, ‹x›, σ2, and γ3 (mean, variance and skew) are determined by the imposed magnetic field gradient via measurement of the signal phase and magnitude (|S(k)/Smax|) [Citation32].
The progression of the biofilm formation in a spiral wound RO module was followed with the EF NMR. Fig. demonstrates that the technique is more sensitive and can identify biofilm formation at an earlier stage compared to feed channel pressure drop measurements typically used.
Fig. 4. The second moment (σ2) of the fouling RO membrane module acquired using EF NMR compared to the feed channel pressure drop as a function of fouling time. The equation to calculate the second moment (σ2) through the signal intensity (S) and magnitude (|S(k)/Smax|) in k-space is shown (adapted from Fridjonsson et al. [Citation32]). NMR detection of biofouling is at an earlier stage than the pressure drop increase.
![Fig. 4. The second moment (σ2) of the fouling RO membrane module acquired using EF NMR compared to the feed channel pressure drop as a function of fouling time. The equation to calculate the second moment (σ2) through the signal intensity (S) and magnitude (|S(k)/Smax|) in k-space is shown (adapted from Fridjonsson et al. [Citation32]). NMR detection of biofouling is at an earlier stage than the pressure drop increase.](/cms/asset/bea34ada-6c78-4651-adbf-721419d1ce65/tdwt_a_1180483_f0004_b.gif)
The non-invasive unit can be used in real-time during plant operation. EF NMR can further be coupled with NMR microscopy techniques [Citation29] to fully characterize the evolution of biofouling in terms of the spatial distribution, velocity field, and flow displacement. Combined with OCT and optode-based O2 imaging techniques, these non-invasive tools can give detailed insights into the behavior and response of biofouling formation under operating conditions. This knowledge is relevant for the design of low fouling spiral wound membrane modules, changes in feed spacer geometry, and operational parameters in NF/RO systems to reduce the impact of biofouling on membrane performance.
4. Conclusions
There is a substantial need for novel measurement techniques that enable non-destructive, real-time, and spatially resolved observation of biofilm formation and structures in membrane systems. OCT can be beneficial to improve the understanding of structure/function relationships as the tool enables detailed visualization at the mesoscale. However, for comprehensive and fundamental understanding of biofilm development, combination of different methods describing the biofilm structure is required. The merits of the techniques assessed in this study include: (i) a non-invasive quantitative measurement of membrane biofouling, (ii) study the effect of hydrodynamics on biofouling development, (iii) determination of oxygen conditions in biofilms which exhibit dynamic changes in response to flow and substrate conditions, (iv) determine the effect of crossflow velocity, flow stagnation, stop-start intervals of crossflow, and feed spacer presence, and (v) obtaining in situ information on biofilm structure, thickness, spatial distribution, and how this may impact membrane system performance. This non-destructive in situ information under representative conditions for practice will lead to a more detailed and better understanding of the development and evolution of biofouling in NF/RO systems, which can result in improved biofouling control and mitigation, thereby improving the overall performance of these water treatment processes.
Acknowledgments
The research reported in this paper was supported by King Abdullah University of Science and Technology (KAUST), Saudi Arabia. The authors acknowledge funding for the Earth’s field NMR instrument from the ARC via LE110100189.
Notes
Presented at EuroMed 2015: Desalination for Clean Water and Energy Palermo, Italy, 10–14 May 2015. Organized by the European Desalination Society.
References
- Z.-Y. Li, V. Yangali-Quintanilla, R. Valladares-Linares, Q. Li, T. Zhan, G. Amy, Flux patterns and membrane fouling propensity during desalination of seawater by forward osmosis, Water Res. 46(1) (2012) 195–204.10.1016/j.watres.2011.10.051
- H.C. Flemming, G. Schaule, T. Griebe, J. Schmitt, A. Tamachkiarowa, Biofouling—The Achilles heel of membrane processes, Desalination 113 (1997) 215–225.10.1016/S0011-9164(97)00132-X
- W.G. Characklis, K.C. Marshall, Biofilms, John Wiley & Sons, New York, NY, 1990.
- D. Nivens, R. Palmer Jr., D. White, Continuous nondestructive monitoring of microbial biofilms: A review of analytical techniques, J. Ind. Microbiol. 15(4) (1995) 263–276.10.1007/BF01569979
- T.R. Neu, B. Manz, F. Volke, J.J. Dynes, A.P. Hitchcock, J.R. Lawrence, Advanced imaging techniques for assessment of structure, composition and function in biofilm systems, FEMS Microbiol. Ecol. 72(1) (2010) 1–21.10.1111/fem.2010.72.issue-1
- A.F. Fercher, W. Drexler, C.K. Hitzenberger, T. Lasser, Optical coherence tomography-principles and applications, Rep. Prog. Phys. 66(2) (2003) 239–303.10.1088/0034-4885/66/2/204
- C. Xi, D. Marks, S. Schlachter, W. Luo, S.A. Boppart, High-resolution three-dimensional imaging of biofilm development using optical coherence tomography, BIOMEDO 11(3) (2006) 034001-1–034001-6.
- M. Wagner, D. Taherzadeh, C. Haisch, H. Horn, Investigation of the mesoscale structure and volumetric features of biofilms using optical coherence tomography, Biotechnol. Bioeng. 107(5) (2010) 844–853.10.1002/bit.22864
- Y. Gao, S. Haavisto, W. Li, C.Y. Tang, J. Salmela, A.G. Fane, Novel approach to characterizing the growth of a fouling layer during membrane filtration via optical coherence tomography, Environ. Sci., Technol. 48(24) (2014) 14273–14281.
- C. Haisch, R. Niessner, Visualisation of transient processes in biofilms by optical coherence tomography, Water Res. 41(11) (2007) 2467–2472.10.1016/j.watres.2007.03.017
- C. Dreszer, A.D. Wexler, S. Drusová, T. Overdijk, A. Zwijnenburg, H.C. Flemming, J.C. Kruithof, J.S. Vrouwenvelder, In-situ biofilm characterization in membrane systems using Optical Coherence Tomography: Formation, structure, detachment and impact of flux change, Water Res. 67 (2014) 243–254.10.1016/j.watres.2014.09.006
- N. Derlon, N. Koch, B. Eugster, T. Posch, J. Pernthaler, W. Pronk, E. Morgenroth, Activity of metazoa governs biofilm structure formation and enhances permeate flux during Gravity-Driven Membrane (GDM) filtration, Water Res. 47(6) (2013) 2085–2095.10.1016/j.watres.2013.01.033
- R. Valladares Linares, A.D. Wexler, S.S. Bucs, C. Dreszer, A. Zwijnenburg, H.C. Flemming, J.C. Kruithof, J.S. Vrouwenvelder, Compaction and relaxation of biofilms, Desalin. Water Treat. 57 (2015) 1–13.
- S. West, M. Wagner, C. Engelke, H. Horn, Optical coherence tomography for the in situ three-dimensional visualization and quantification of feed spacer channel fouling in reverse osmosis membrane modules, J. Membr. Sci. 498 (2016) 345–352.10.1016/j.memsci.2015.09.047
- F. Blauert, H. Horn, M. Wagner, Time-resolved biofilm deformation measurements using optical coherence tomography, Biotechnol. Bioeng. 112(9) (2015) 1893–1905.10.1002/bit.25590
- R. Glud, N. Ramsing, J. Gundersen, I. Klimant, Planar optodes: A new tool for fine scale measurements of two-dimensional O2 distribution in benthic communities, Mar. Ecol. Prog. Ser. 140 (1996) 217–226.10.3354/meps140217
- M.S. Frederiksen, R.N. Glud, Oxygen dynamics in the rhizosphere of Zostera marina: A two-dimensional planar optode study, Limnol. Oceanogr. 51(2) (2006) 1072–1083.10.4319/lo.2006.51.2.1072
- M. Larsen, S.M. Borisov, B. Grunwald, I. Klimant, R.N. Glud, A simple and inexpensive high resolution color ratiometric planar optode imaging approach: Application to oxygen and pH sensing, Limnol. Oceanogr.: Methods 9(9) (2011) 348–360.10.4319/lom.2011.9.348
- M. Kühl, L.F. Rickelt, R. Thar, Combined imaging of bacteria and oxygen in biofilms, Appl. Environ. Microbiol. 73(19) (2007) 6289–6295.10.1128/AEM.01574-07
- B. DeGraff, J. Demas, Luminescence-based oxygen sensors. In Reviews in Fluorescence 2005, Springer, New York, NY, 2005, pp. 125–151.
- G. Holst, B. Grunwald, Luminescence lifetime imaging with transparent oxygen optodes, Sens. Actuators, B 74(1–3) (2001) 78–90.10.1016/S0925-4005(00)00715-2
- M. Kuhl, L. Polerecky, Functional and structural imaging of phototrophic microbial communities and symbioses, Aquat. Microb. Ecol. 53(1) (2008) 99–118.10.3354/ame01224
- M. Staal, E.I. Prest, J.S. Vrouwenvelder, L.F. Rickelt, M. Kühl, A simple optode based method for imaging O2 distribution and dynamics in tap water biofilms, Water Res. 45(16) (2011) 5027–5037.10.1016/j.watres.2011.07.007
- N.M. Farhat, M. Staal, A. Siddiqui, S.M. Borisov, S.S. Bucs, J.S. Vrouwenvelder, Early non-destructive biofouling detection and spatial distribution: Application of oxygen sensing optodes, Water Res. 83 (2015) 10–20.10.1016/j.watres.2015.06.015
- E.I. Prest, M. Staal, M. Kühl, M.C.M. van Loosdrecht, J.S. Vrouwenvelder, Quantitative measurement and visualization of biofilm O2 consumption rates in membrane filtration systems, J. Membr. Sci. 392–393 (2012) 66–75.10.1016/j.memsci.2011.12.003
- B. Manz, F. Volke, D. Goll, H. Horn, Measuring local flow velocities and biofilm structure in biofilm systems with magnetic resonance imaging (MRI), Biotechnol. Bioeng. 84(4) (2003) 424–432.10.1002/(ISSN)1097-0290
- B. Manz, F. Volke, D. Goll, H. Horn, Investigation of biofilm structure, flow patterns and detachment with magnetic resonance imaging, Water Sci. Technol. 52(7) (2005) 1–6.
- J.D. Seymour, S.L. Codd, E.L. Gjersing, P.S. Stewart, Magnetic resonance microscopy of biofilm structure and impact on transport in a capillary bioreactor, J. Magn. Reson. 167(2) (2004) 322–327.10.1016/j.jmr.2004.01.009
- D.A. Graf Von Der Schulenburg, J.S. Vrouwenvelder, S.A. Creber, M.C.M. Van Loosdrecht, M.L. Johns, Nuclear magnetic resonance microscopy studies of membrane biofouling. J. Membr. Sci. 323 (2008) 37–44.10.1016/j.memsci.2008.06.012
- S.A. Creber, J.S. Vrouwenvelder, M.C.M. van Loosdrecht, M.L. Johns, Chemical cleaning of biofouling in reverse osmosis membranes evaluated using magnetic resonance imaging, J. Membr. Sci. 362 (2010) 202–210.10.1016/j.memsci.2010.06.052
- M. Wagner, B. Manz, F. Volke, T.R. Neu, H. Horn, Online assessment of biofilm development, sloughing and forced detachment in tube reactor by means of magnetic resonance microscopy, Biotechnol. Bioeng. 107(1) (2010) 172–181.10.1002/bit.v107:1
- E.O. Fridjonsson, S.A. Creber, J.S. Vrouwenvelder, M.L. Johns, Magnetic resonance signal moment determination using the Earth’s magnetic field, J. Magn. Reson. 252 (2015) 145–150.10.1016/j.jmr.2015.01.013
- J.S. Vrouwenvelder, J.A.M. Van Paassen, L.P. Wessels, A.F. Van Dam, S.M. Bakker, The membrane fouling simulator: A practical tool for fouling prediction and control, J. Membr. Sci. 281 (2006) 316–324.10.1016/j.memsci.2006.03.046
- J.S. Vrouwenvelder, S.M. Bakker, L.P. Wessels, J.A.M. van Paassen, The membrane fouling simulator as a new tool for biofouling control of spiral-wound membranes, Desalination 204 (2007) 170–174.10.1016/j.desal.2006.04.028
- S.S. Bucs, N. Farhat, A. Siddiqui, R. Valladares Linares, A. Radu, J.C. Kruithof, J.S. Vrouwenvelder, Development of a setup to enable stable and accurate flow conditions for membrane biofouling studies, Desalin. Water Treat. 57(28) (2016) 12893–12901.10.1080/19443994.2015.1057037
- J.S. Vrouwenvelder, C. Hinrichs, W.G.J. Van der Meer, M.C.M. Van Loosdrecht, J.C. Kruithof, Pressure drop increase by biofilm accumulation in spiral wound RO and NF membrane systems: Role of substrate concentration, flow velocity, substrate load and flow direction, Biofouling 25(6) (2009) 543–555.10.1080/08927010902972225
- F. Niedermair, S.M. Borisov, G. Zenkl, O.T. Hofmann, H. Weber, R. Saf, I. Klimant, Tunable phosphorescent NIR oxygen indicators based on mixed benzo- and naphthoporphyrin complexes, Inorg. Chem. 49(20) (2010) 9333–9342.10.1021/ic100955z
- N. Derlon, M. Peter-Varbanets, A. Scheidegger, W. Pronk, E. Morgenroth, Predation influences the structure of biofilm developed on ultrafiltration membranes, Water Res. 46(10) (2012) 3323–3333.10.1016/j.watres.2012.03.031