Abstract
The existence of excess sodium dodecyl sulfate (SDS) molecules in a single-walled carbon nanotube (SWNT) solution dispersed by hybridization with SDS leads to unstable atomic force microscopy (AFM) imaging. In this study, we demonstrate sequential dialysis against pure water in order to remove excess SDS molecules from an SDS-SWNT hybrid dispersion. A 1:102 volume ratio of SDS-SWNT dispersion to water in the dialysis was found to be effective in realizing stable AFM observations of the SDS-SWNT hybrids despite imperfect filtering of SDS via dialysis. On the other hand, the SDS-SWNT hybrids were stable even when this volume ratio was 1:106. Further, the SDS-SWNT hybrids were present in the solution even when the dialyzed samples were stored for 14 days. Our results reveal that dialysis under optimal conditions enables improved handling of SDS-SWNT hybrids, particularly for stable AFM observations.
1.Introduction
Since carbon nanotubes (CNTs) were first fabricated, many researchers have been attracted by the extraordinary properties of CNTs, such as their mechanical strength and unique optical absorbance [Citation1–4 Citation Citation Citation4]. On the other hand, the insolubility of CNTs in most solutions has posed a challenge in certain CNT-based biological applications such as biosensors [Citation5–8 Citation Citation Citation8].
In order to disperse CNTs in aqueous solution, various methods of wrapping CNTs with organic molecules including DNA and proteins have been proposed. In particular, sodium dodecyl sulfate (SDS) is one of the most popularly used surfactants in order to solubilize CNTs [Citation9–14 Citation Citation Citation Citation Citation14]. Because SDS molecules exhibit negative charge in neutral-pH aqueous solutions, the SDS-wrapped CNT molecules are separated from each other in solution [Citation10]. Previous studies have thus far employed 0.5–10% of SDS solutions to disperse CNTs. Although CNTs can be uniformly dispersed using SDS, the extra SDS molecules present in the dispersed CNT solution can cause complications when used in certain applications. For example, when the dispersed CNTs are observed by atomic force microscopy (AFM), the existence of the excess SDS molecules prohibits stable imaging. In fact, very few studies have reported AFM observations of SDS-wrapped CNTs [Citation15–17 Citation Citation17]. In these studies, the sample preparation procedures for AFM observation were not simple, although dropping a sample solution onto a flat substrate is sufficient to prepare AFM samples in general. For example, Campbell et al. heated SDS-CNT solutions and mixed them with similarly heated MgCl2 solution prior to depositing the solution onto a freshly cleaved ruby mica substrate for AFM observations.
In this light, one solution is to remove excess SDS molecules from the SDS-CNT dispersion. It is well known that dialysis treatment is a useful method to remove excess molecules from certain solutions in general. In fact, several studies have reported the removal of SDS molecules from CNT dispersions via dialysis [Citation10,Citation18–20 Citation Citation20]. Richard et al. and Chou et al. have reported transmission electron microscope (TEM) observations of dialyzed SDS-CNT hybrids to evaluate the structural change of the hybrids due to dialysis [Citation10,Citation18]. Wei et al. characterized dialyzed SDS-CNT samples using photoluminescence spectroscopy [Citation19]. Further, Boghossian et al. measured dialyzed samples by single particle tracking [Citation20]. However, AFM observations of the dialyzed samples have not thus far been reported. When compared with TEM and spectroscopy, AFM imaging should be strongly affected by excess SDS molecules because an AFM probe directly traces the sample surfaces.
In this study, we focus on the widely used hybrids of SDS and single-walled CNTs (SWNTs) – SDS-SWNT hybrids. We examine the dialysis of SDS-SWNT hybrids to determine the optimized conditions for dialysis, and we characterize the dialyzed samples by AFM imaging to directly evaluate the effects of removal of excess SDS on AFM images. It is possible to completely remove SDS molecules if the SWNT dispersion (including SDS) is dialyzed against large quantities of water or other solvents; however, this may negatively affect the stability of SDS-SWNT hybrids because SDS molecules can be detached from the SWNT when the extra SDS molecules are completely removed. For this reason, we examine both the mild dialysis and standard dialysis conditions in our study.
2. Materials and methods
2.1. Preparation of SDS-SWNT hybrids
Ten milligrams of SWNT powder (super purified HiPco single-walled carbon nanotubes, Unidym, Inc., CA) was mixed with 20 ml of 1% SDS aqueous solution. The mixture was sonicated using a tip-sonicator (VCX-130, Sonics & Materials, Inc., CT) at 20 W for 1 h. Subsequently, a clear top layer of the mixture was collected after centrifugation at 14,000 × g for 6 h (Mini Spin Plus, Eppendorf, Hamburg, Germany).
2.2. Dialysis of SDS-SWNT dispersion
The dispersion was dialyzed with a 5-nm-pore-sized membrane (cellulose tube 8/32, Sanko Junyaku Co. Ltd., Tokyo, Japan) against pure water. The membrane can pass molecules that have molecular weight (MW) of about 14,000 while MW of SDS is 288 [Citation21]. The volume ratios of the dispersion to water were 1:102, 1:103, and 1:106. The dilution period was 24 h for the volume ratios of 1:102 and 1:103. For the volume ratio of 1:106, dialysis with 1:103 volume ratio was repeated twice. Decrease of SDS concentration was confirmed by UV–vis spectra.
2.3. AFM observations
The samples were diluted 100 times with pure water, and subsequently, 20 μl solutions of each of the samples were dropped onto a mica surface. The mica surface was functionalized with 1% 3-aminopropyltriethoxysilane before use (AP-mica) [Citation22]. After 10 min of incubation, the samples were dried or rinsed/dried. While the dispersion was used for AFM observation immediately after the dialysis, the remained dispersion before dilution with water was stored for 14 days to study the aging effects. AC-mode AFM observations were performed to observe the dialyzed SDS-SWNT hybrids under ambient conditions (JSPM-5200, JEOL Ltd., Tokyo, Japan). Free amplitude and setpoint voltage were around –5.6 and −4.5 V for the imaging, respectively. Although there was fluctuation of the setpoint voltage during the scanning, we confirmed that the fluctuation was negligible for accurate height measurements. The diameters of the SWNTs were measured from the cross sections of the AFM images.
3. Results and discussions
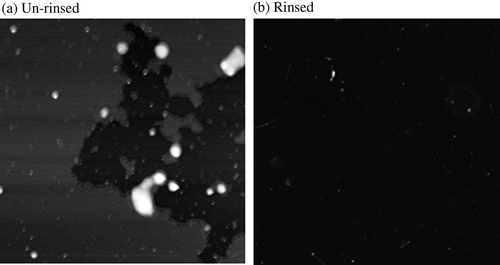
The primary obstacle to using SDS-SWNT hybrids in various applications is the existence of excess SDS. Although we attempted to observe SDS-SWNT hybrids via AFM without dialyzing the solution, our results were unsatisfactory. When the AFM sample of the non-dialyzed hybrid dispersion was prepared without rinsing after dropping the dispersion onto an AP-mica surface, the resulting AFM imaging was very unstable, and the AFM probe was easily damaged. Although the imaging was unstable, (a) showed an occasionally obtained AFM image of non-dialyzed SDS-SWNT sample without rinsing. The sample surface was partially covered with film-like structures. Probably the surface was covered with SDS molecules. After all, it was impossible to observe SDS-SWNT hybrids. This suggests that the excess SDS molecules on the AP-mica surface prevented stable imaging. When the sample was rinsed with water, although the AFM imaging itself became stable, an almost flat surface was observed without the presence of hybrids (Figure1(b)). In all probability, most of the SDS-SWNT hybrids were removed by the rinsing treatment. In order to address this problem, we used dialysis to remove excess SDS.
Figure 1. AFM images of non-dialyzed SDS-SWNT hybrids on AP-mica. (a) An un-rinsed sample. (b) A rinsed sample. Scan size of the images was 2 μm × 2 μm.
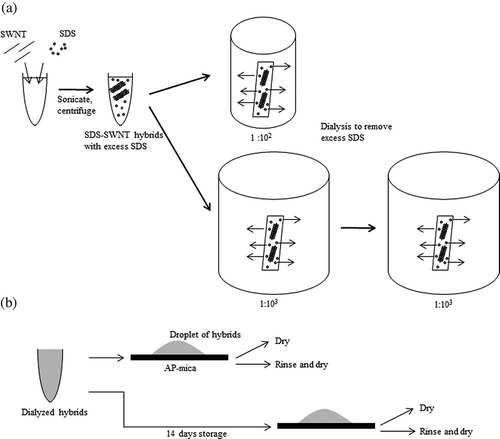
A schematic representation of our experiment is shown in SDS-SWNT hybrids were prepared by sonication and centrifugation of a mixture of SDS and SWNTs. Subsequently, the samples were dialyzed against pure water to remove excess SDS molecules, as shown in (a). The volume ratios of dispersion to water were 1:102, 1:103, and 1:106. Next, we prepared four types of AFM samples for each of the three above-mentioned dialyzed SDS-SWNT hybrid samples ((b)). After the dialysis, for one set of samples, the dispersions were dropped onto an AP-mica surface and subsequently dried or rinsed/dried. Another set of samples was prepared after storing the dialyzed dispersion for 14 days.
Figure 2. Schematic views of experiment. (a) Dialysis procedure. (b) Sample preparation for AFM observations.
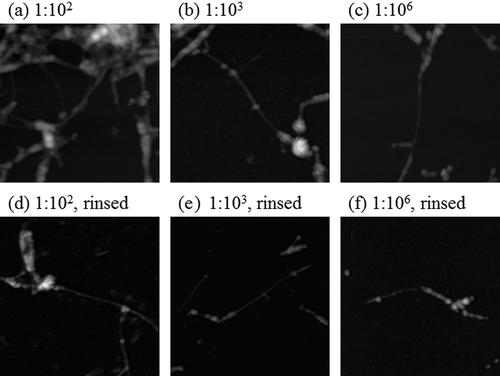
shows the AFM images of the dialyzed SDS-SWNT hybrids on an AP-mica surface functionalized by 1% 3-aminopropyltriethoxysilane. In the case of the dialyzed samples, the resulting AFM imaging was stable, and the dispersed SWNT molecules were clearly observed in all cases.
Figure 3. AFM images of dialyzed SDS-SWNT hybrids on AP-mica. (a, d) The volume ratio of dispersion to water in dialysis was 1:102. (b, e) The volume ratio of dispersion to water in dialysis was 1:103. (c, f) The volume ratio of dispersion to water in dialysis was 1:106. Cases (a) to (c) and (d) to (f) were not rinsed and rinsed with water before drying, respectively. Lines in figures indicate cross sections of SDS-SWNT hybrids. Scan size of the images was 2 μm × 2 μm.
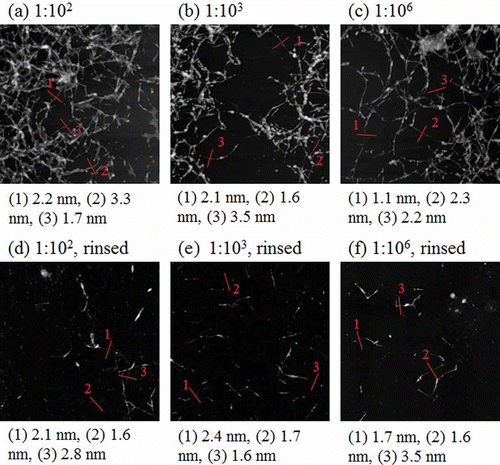
When the samples were dried without rinsing, SDS-SWNT hybrids were observed on the surfaces ((a), (b), and (c)). The distribution of the hybrids on the surface was not uniform, and therefore, it was difficult to quantify the number of the hybrids observed. Consequently, there was no significant difference between the samples shown in (a), (b), and (c).
Our results reveal two important facts. First, even in the volume ratio of 1:102, excess SDS molecules were removed in sufficient quantity. Such a sample may be applicable for nanodevices wherein the effects of excess SDS are negligible. Second, clear AFM images of SDS-SWNT hybrids were obtained even in the volume ratio of 1:106; in this case, the excess SDS was almost completely removed. This suggests that the SDS molecules attached to SWNTs remained on the SWNT surfaces even in the absence of excess SDS molecules. The detachment of SDS molecules from SWNT surfaces may be a slow process, occurring over a duration of longer than 24 h.
When the samples were rinsed with water before drying, the number of hybrids on the AP-mica surface dramatically decreased ((d), (e), and (f)). The distribution of hybrids was not uniform; however, not many aggregates were observed. This suggests that the SDS-SWNT hybrids did not adhere strongly to the AP-mica surface. On the other hand, in the case of the non-dialyzed samples, most of the hybrids were removed from the surface when the samples were rinsed with water. The excess SDS molecules, which act as typical surfactants, probably inhibit the adsorption of the SDS-SWNT hybrids onto the AP-mica surface. The results reveal that the removal of excess SDS via dialysis is effective despite imperfect filtering.
The lines in indicate a few representative cross sections of the SDS-SWNT hybrids. Although aggregates were observed particularly for the cases shown in (a), (b), and (c), we selected the middle positions of certain isolated hybrids as representatives. The values under each image indicate the height of each hybrid as estimated from the cross sections. The cross-sectional heights varied from 1.1 to 3.5 nm for the selected isolated hybrids. There was no significant difference between the samples shown in (a)–(f). The data suggested that the heights of the hybrids were not uniform, and that the height was not significantly affected by the volume ratios of dialysis (1:102, 1:103, or 1:106). shows magnified AFM images of the same samples. The images indicated that the surfaces of the SDS-SWNT hybrids were uniform, although some parts had small lumps. The data suggested that the morphology of the SDS-SWNT hybrids was not drastically changed after the dialysis.
Figure 4. Magnified AFM images of dialyzed SDS-SWNT hybrids on AP-mica. (a, d) The volume ratio of dispersion to water in dialysis was 1:102. (b, e) The volume ratio of dispersion to water in dialysis was 1:103. (c, f) The volume ratio of dispersion to water in dialysis was 1:106. Cases (a) to (c) and (d) to (f) were not rinsed and rinsed with water before drying, respectively. Scan size of the images was 500 nm × 500 nm.
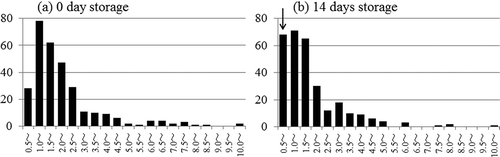
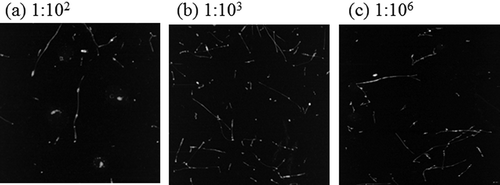
We examined the stored SDS-SWNT hybrids for 14 days after dialysis in order to evaluate their stability (). After storage, several SDS-SWNT hybrids were still observed even in the sample that was dialyzed with the volume ratio of 1:106. The data clearly suggested that the attached SDS molecules can remain on the SWNT surfaces even in the absence of excess SDS molecules for at least 14 days. On the other hand, it is noteworthy that almost no aggregates were observed after the storage interval. This indicates that some of the hybrids could not be maintained in the dispersed state.
Figure 5. AFM images of SDS-SWNT hybrids stored for 14 days after dialysis. (a), (b), and (c) The volume ratios of dispersion to water in dialysis were 1:102, 1:103, and 1:106, respectively. Scan size of the images was 2 μm × 2 μm.
shows the diameter distributions of the SDS-SWNT hybrids as estimated by cross-sectional analysis of the AFM images. In a manner similar to that for the samples shown in , the heights of the isolated hybrids were analyzed. Upon comparing fresh samples ((a)) with the stored ones ((b)), we observed almost similar diameter distributions. The only difference was that the stored samples were richly populated by slender hybrids of less than 1 nm in diameter (as indicated by the arrow in (b)). When we checked the reproducibility of this phenomenon with independent samples, similar results were obtained. The appearance of these slender hybrids is a phenomenon that requires further investigation.
4. Conclusion
Our results enabled us to determine the optimal conditions for dialysis of SDS-SWNTs in order to remove excess SDS. The optimal conditions were verified by sequential dialysis experiments, and consequently, stable AFM imaging of SDS-SWNT hybrids was realized. Although the adhesion of SDS and SWNT is based on physisorption, the hybrids were stable after dialysis even after 14 days of storage. In particular, it is noteworthy that even a rough dialysis with volume ratio of 1:102 is effective in ensuring stable AFM imaging. The clear AFM images of the hybrids obtained were comparable with those obtained with a volume ratio of 1:106. The SDS-SWNT hybrids with reduced excess SDS molecules are potentially useful for various industrial applications.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (23540479) of Japan Society for the Promotion of Science (JSPS).
References
- Iijima , A. and Ichihashi , T. 1993 . Single-shell carbon nanotubes of 1-nm diameter . Nature , 363 : 603 – 605 .
- Ruoff , R.S. and Lorents , D.C. 1995 . Mechanical and thermal properties of carbon nanotubes . Carbon , 33 : 925 – 930 .
- Baughman , R.H. , Zakhidov , A.A. and de Heer , W.A. 2002 . Carbon nanotubes – The route toward applications . Science , 297 : 787 – 792 .
- Tans , S.J. , Verschueren , A.R.M. and Dekker , C. 1998 . Room-temperature transistor based on a single carbon nanotube . Nature , 393 : 49 – 52 .
- Elgrabli , D. , Abella-Gallart , S. , Aguerre-Chariol , O. , Robidel , F. , Rogerieux , F. , Boczkowski , J. and Lacroix , G. 2007 . Effect of BSA on carbon nanotube dispersion for in vivo and in vitro studies . Nanotoxicology , 1 : 266 – 278 .
- Pantarotto , D. , Partidos , C.D. , Graff , R. , Hoebeke , J. , Briand , J.P. , Prato , M. and Bianco , A. 2003 . Synthesis, structural characterization, and immunological properties of carbon nanotubes functionalized with peptides . J. Am. Chem. Soc. , 125 : 6160 – 6164 .
- Wu , Z. , Xu , Y. , Zhang , X. , Shen , G. and Yu , R. 2007 . Microwave plasma treated carbon nanotubes and their electrochemical biosensing application . Talanta , 72 : 1336 – 1341 .
- Koh , B. , Park , J.B. , Hou , X. and Cheng , W. 2011 . Comparative dispersion studies of single-walled carbon nanotubes in Aqueous Solution . J. Phys. Chem. B. , 115 : 2627 – 2633 .
- Bachilo , S.M. , Strano , M.S. , Kittrell , C. , Hauge , R.H. , Smalley , R.E. and Weisman , R.B. 2002 . Structure-assigned optical spectra of single-walled carbon nanotubes . Science , 298 : 2361 – 2366 .
- Richard , C. , Balavoine , F. , Schultz , P. , Ebbesen , T.W. and Mioskowski , C. 2003 . Supramolecular self-assembly of lipid derivatives on carbon nanotubes . Science , 300 : 775 – 778 .
- Jiang , L.Q. , Gao , L. and Sun , J. 2003 . Production of aqueous colloidal dispersions of carbon nanotubes . J. Colloid Interface Sci. , 260 : 89 – 94 .
- Moore , V.C. , Strano , M.S. , Haroz , E.H. , Hauge , R.H. , Smalley , R.E. , Schmidt , J. and Talmon , Y. 2003 . Individually suspended single-walled carbon nanotubes in various surfactants . Nano Lett. , 3 : 1379 – 1382 .
- Benedict , B. , Pehrsson , P.E. and Zhao , W. 2005 . Optically sensing additional sonication effects on dispersed HiPco nanotubes in aerated water . J. Phys. Chem. B. , 109 : 7778 – 7780 .
- Sun , Z. , Nicolosi , V. , Rickard , D. , Bergin , S.D. , Aherne , D. and Coleman , J.N. 2008 . Quantitative evaluation of surfactant-stabilized single-walled carbon nanotubes: Dispersion quality and its correlation with zeta potential . J. Phys. Chem. C. , 112 : pp. 10692 – 10699 .
- ’Connell , M.J. O , Bachilo , S.M. , Huffman , C.B. , Moore , V.C. , Strano , M.S. , Haroz , E.H. , Rialon , K.L. , Boul , P.J. , Noon , W.H. , Kittrell , C. , Ma , J. , Hauge , R.H. , Weisman , R.B. and Smalley , R.E. 2002 . Band gap fluorescence from individual single-walled carbon nanotubes . Science , 297 : 593 – 596 .
- Campbell , J.F. , Tessmer , I. , Thorp , H.H. and Erie , D.A. 2008 . Atomic force microscopy studies of DNA-wrapped carbon nanotube structure and binding to quantum dots . J. Am. Chem. Soc. , 130 : 10648 – 10655 .
- Angelikopoulos , P. , Gromov , A. , Leen , A. , Nerushev , O. , Bock , H. and Campbell , E.E.B. 2010 . Dispersing individual single-wall carbon nanotubes in aqueous surfactant solution below the cmc . J. Phys. Chem. C. , 114 : 2 – 9 .
- Chou , A. , Bocking , T. , Liu , R. , Singh , N.K. , Moran , G. and Gooding , J.J. 2008 . Effect of dialysis on the electrochemical properties of acid-oxidized single-walled carbon nanotubes . J. Phys. Chem. C. , 112 : 14131 – 14138 .
- Wei , L. , Li , L.-J. , Chan-Park , M.B. , Yang , Y. and Chen , Y. 2010 . Aggregation-dependent photoluminescence sidebands in single-walled carbon nanotube . J. Phys. Chem. C. , 114 : 6704 – 6711 .
- Boghossian , A.A. , Choi , J.H. , Ham , M.-H. and Strano , M.S. 2011 . Dynamic and reversible self-assembly of photoelectrochemical complexes based on lipid bilayer disks, photosynthetic reaction centers, and single-walled carbon nanotubes . Langmuir , 27 : 1599 – 1609 .
- Fishman , M.L. and Eirich , F.R. 1971 . Interactions of aqueous poly(N-vinylpyrrolidone) with sodium dodecyl sulfate. I. Equilibrium dialysis measurements . J. Phys. Chem. , 75 : 3135 – 3140 .
- Umemura , K. , Ishikawa , M. and Kuroda , R. 2001 . Controlled immobilization of DNA molecules using chemical modification of mica surfaces for atomic force microscopy: Characterization in air . Anal. Biochem. , 290 : 232 – 237 .