Abstract
One prominent method of modifying the properties of dielectric elastomers (DEs) is by adding suitable metal oxide fillers. However, almost all commercially available silicone elastomers are already heavily filled with silica to reinforce the otherwise rather weak silicone network and the resulting metal oxide filled elastomer may contain too much filler. We therefore explore the replacement of silica with titanium dioxide to ensure a relatively low concentration of filler. Liquid silicone rubber (LSR) has relatively low viscosity, which is favorable for loading inorganic fillers. In the present study, four commercial LSRs with varying loadings of silica and one benchmark room-temperature vulcanizable rubber (RTV) were investigated. The resulting elastomers were evaluated with respect to their dielectric permittivity, tear and tensile strengths, electrical breakdown, thermal stability and dynamic viscosity. Filled silicone elastomers with high loadings of nano-sized titanium dioxide (TiO2) particles were also studied. The best overall performing formulation had 35 wt.% TiO2 nanoparticles in the POWERSIL® XLR LSR, where the excellent ensemble of relative dielectric permittivity of 4.9 at 0.1 Hz, breakdown strength of 160 V µm−1, tear strength of 5.3 MPa, elongation at break of 190%, a Young’s modulus of 0.85 MPa and a 10% strain response (simple tension) in a 50 V μm−1 electric field was obtained.
1. Introduction
Polydimethylsiloxanes (PDMSs) and other silicone-based elastomers are widely used in dielectric elastomer (DE) formulation due to their favorable electro-mechanical properties.[Citation1] Dielectric elastomers which consist of an elastomer film with deposited electrodes on both sides have lately gained increased interest as materials for actuators, generators, and sensors. Several promising materials have been developed based on silicone elastomers, and approaches are currently limited to two categories of elastomers, namely 1) composites with semiconductive metal oxides or screened conductive particles and 2) molecular modified elastomers.[Citation2,Citation3,Citation4]. We here explore the most common approach of composites with high permittivity metal oxides as this method by far results in the cheapest elastomers due to the relatively low cost of e.g. TiO2 and the ease of processing.
There are two types of silylation-cure based commercial silicone elastomer formulations which are based on curing temperatures, namely 1) high-temperature vulcanizable (HTV) and 2) room-temperature vulcanisable (RTV) rubbers. HTV rubbers can be further classified as 1) liquid silicone rubbers (LSRs) and 2) millable silicone rubbers, depending on the degree of polymerization.[Citation5]
LSRs are a family of tough and versatile silicones, enabling fast-cured, high-precision injection molding for high-performance parts such as transducer devices.[Citation6] They can be molded into everything from o-rings to intricate geometries with thin walls, and they remain elastic without tearing.[Citation7] Since they are cured by addition reaction, the formulations enable a high degree of control over the network topology – and thus on the mechanical properties – of the final material.[Citation8] LSRs may be clear or translucent and can be manufactured to have unique properties such as solvent resistance, increased thermal stability or low outgassing.[Citation9] Furthermore, they can incorporate additives and unique fillers, such as pigments and active pharmaceutical ingredients, with minimal changes to the key characteristics of the cured elastomer, i.e. toughness and elasticity.[Citation10]
In most cases LSRs use a platinum catalyzed addition cure system and require a curing temperature above 80ºC.[Citation11] They typically comprise ~ 75 wt.% linear silicone polymers and ~ 23 wt.% fumed silica, with the remainder consisting of a combination of curing additives such as hydride crosslinkers and inhibitors.[Citation12] The majority of commercially available LSRs are formulated as two-part systems: 1:1 mix ratio silicones with viscosities ranging from 50,000 to >1,000,000 cps.[Citation13] Part A contains a platinum catalyst and Part B contains a crosslinker and an inhibitor. Fumed silica improves the ultimate mechanical properties of the silicone and has the ability to interact non-covalently with the polymer, thereby allowing stress relief when a shear is applied in an uncured or a cured state. Moreover, fumed silica also increases the tear growth resistance strongly. Semi-volatile inhibitors control the reaction rate, in order to provide an acceptable liquid elastomer pot life until heat is applied.[Citation14] The combination of medium-high viscosity polymers and silica gives uncured LSRs a creamy consistency similar to petroleum jelly.[Citation15] Processing LSRs successfully depends on using equipment designed to mix and pump thick materials without introducing air.[Citation16] For thin films this requirement becomes even more important.
The dielectric and mechanical properties of LSR elastomers are dependent on many factors due to the molecular ‘architecture’ formed by the polymers in the elastomer and their interaction with additives.[Citation17] Polymer viscosity, filler type and filler quantity will affect the tensile strength, maximum elongation and toughness of the cured silicone. From a transducer point of view LSRs should possess high dielectric permittivity, little dielectric loss, high tear strength, large elongation at the break, a low Young’s modulus (in the case of an actuator), little viscous dissipation and the films should be perfectly homogeneous.[Citation18] Crosslink density can be optimized by adjusting the ratio of vinyl and hydride groups in the LSR formulation, which offers the advantage of optimizing hardness from very soft (Shore hardness 40 ‘00’) to hard (Shore hardness 90 ‘A’) with minimal changes in the uncured viscosity. The filled soft materials with high permittivity and low dielectric loss could also be beneficial to the electromechanical coupling studies, such as microwave applications.[Citation19] It was shown in our previous study that titanium dioxide (TiO2) nanoparticles increase both the dielectric permittivity and breakdown strength of LSR formulations.[Citation20] The rutile TiO2 exhibits a higher dielectric constant (εr ~110) than the anatase TiO2 (εr ~45), which therefore makes it a good candidate for achieving higher nanocomposite elastomer permittivity.[Citation21] Several other studies on similar systems have been conducted but without varying the silica content as the commercial elastomers do not usually provide such handle. Amongst this large ensemble of work the pioneering work of Carpi and Rossi [Citation21] on titanium dioxide in silicone for dielectric elastomer application as well as more recent work of Wang et al [Citation22] on more complex titanates can be mentioned. In the present study Wacker Chemie has provided commercial elastomers with varying silica content. XLR 630 is the commercially available elastomer and MJK 5/13 and 4/13 contain reduced amounts of silica. Thereby it is possible to replace silica with titanate without varying significantly on crosslinking density, type of silica, catalyst etc.
Four commercial LSRs, with different viscosities, and one RTV were chosen in the present work, in which the dielectric and mechanical properties of pure materials and hydrophobic rutile TiO2 composite formulations are reported. The elastomers were evaluated based on two figures of merit, namely with respect to actuation Fom(DEA) [Citation23] and to generation Fom(DEG).[Citation24] The benchmark normalization material used in this instance was the pure RTV ELASTOSIL® RT 625, from Wacker Chemie, such that a value of 1 or higher corresponded to improved actuation/generation properties.[Citation25]
2. Experimental section
2.1. Materials
Four liquid silicone rubbers (LSRs) were supplied by Wacker Chemie AG, Germany. The LSRs were LR3043/30 (denoted LR3043) and 3 types of XLR (extra liquid rubber), namely XLR 630 (denoted XLR) and its two derivates MJK 5/13 and MJK 4/13 with respectively 25 wt.% and 50 wt.% less silica compared to the XLR. All other constituents in the elastomer formulations remained identical. The mixing ratios of parts A and B were 1:1.
Additionally, one room-temperature vulcanizable rubber (RTV) RT 625 was supplied by Wacker Chemie AG, Germany. The mixing ratio of parts A and B was 9:1.
The dynamic viscosity η (Pa·s) with shear rate 10 s−1 at 23°C of these four LSRs and one RTV mixtures are 430 (LR 3043), 12 (XLR), 6.5 (MJK 5/13), 2.9 (MJK 4/13), and 12 (RT 625), respectively.
The two utilized hydrophobic TiO2 nanofillers were:
SACHTLEBEN® R420: Rutile TiO2 was supplied by Sachtleben Chemie GmbH, Germany. Primary particle size was 250–290 nm.
AEROXIDE® T805: Anatase/rutile TiO2 was supplied by Evonik Industries AG, Germany. Primary particle size was 25 nm. We denote both types of filler as nanofillers. The size of R420 is, however, on the upper limit for being regarded as a nanofiller.[Citation26]
Solvent OS-20 (an ozone-safe volatile methylsiloxane (VMS) fluid) was purchased from Dow Corning, USA. OS-20 was not added to the three pure LSR (XLR, MJK 5/13 and 4/13) or RT 625 formulations, since their viscosities allow for coating without solvent. The mixing ratio of LR3043 premix A and B and OS-20 was 5:5:7 by mass in the following, while OS-20 addition in the XLR–TiO2 formulations was 50 wt.% of the TiO2 loading.
R420 TiO2 (250–290 nm) fillers were added into RT625, LR3043 and XLR, respectively. The increased viscosity of the mixtures constitutes a problem when small nanoscale fillers such as T805 TiO2 (25 nm) are applied. Therefore this type of filler was solely added to the LR with the lowest viscosity, namely MJK 4/13.
2.2. Sample preparation
Nanofillers were mixed into the silicone premix A using a Speedmixer (DAC 150FVZ, Hauschild Co., Germany) for 2 minutes at 3000 rpm. Premix B and the OS-20 solvent were then mixed in to the first mixture for another 2 minutes at 3000 rpm with the Speedmixer. The films were coated with a film applicator (3540 bird, Elcometer, Germany) on a glass substrate and cured in an oven at 110°C for 8 minutes. The thickness of the film was approximately 40–80 μm.
2.3. Characterization
2.3.1. Thermal analyses
The thermal stability of the films was evaluated by thermal gravimetric analysis (TGA Q500, TA Instruments, USA), and measurements were carried out under a nitrogen atmosphere at a heating rate of 10°C min−1 from room temperature to 900°C.
2.3.2. Tear test
The tear strength of the elastomers was measured using a material tester (Zwick/Roell Zmart.pro, Zwick GmbH & Co. KG, Germany). The sample of 110 mm length and 10 mm width with a 0.5 mm nick at its middle edge was placed between two clamps and initially separated by a distance of 33 ± 2 mm. The test specimen was elongated at 500 ± 50 mm min−1 (i.e. strain rate of 25%/s) until failure along the nick. Each sample was subjected to four tear measurements and then averaged.
2.3.3. Tensile stress–strain
The tensile stress–strain of the elastomers was measured using a material tester (Zwick/Roell Zmart.pro, Zwick GmbH & Co. KG, Germany). The sample of 115 mm length and 6 mm width was placed between two clamps and initially separated by a distance of 33 ± 2 mm. The test specimen was elongated uniaxially at 500 ± 50 mm min−1 with respect to length and forced throughout the test to a level of accuracy within ±2% until sample failure at the middle part. Each composition was subjected to four tensile measurements which were then averaged.
2.3.4. Breakdown strength test
Electrical breakdown tests were performed on an in-house-built device based on international standards (IEC 60,243–1 (1998) and IEC 60,243–2 (2001)) with DC voltage output power. Film thicknesses were measured through the microscopy of cross-sectional cuts, and the distance between the spherical electrodes was set accordingly with a micrometer stage and gauge. An indent of less than 5% of sample thickness was added, to ensure that the spheres were in contact with the sample. The polymer film was slid between the two spherical electrodes (radius of 20 mm), and the breakdown was measured at the point of contact with a stepwise increasing voltage applied (50–100 V step−1) at a rate of 0.5–1 steps s−1. Each sample was subjected to 12 breakdown measurements, and an average of these values was given as the breakdown strength of the sample.
2.3.5. Viscosity measurements
The dynamic viscosities of mixtures were tested by the flow sweep of an ARES-G2 rheometer (TA Instruments, USA) with a shear rate ranging from 10–3 to 10 s−1 at 23°C in an ambient atmosphere using a parallel-plate geometry of 25 mm in diameter.
2.3.6. Dielectric characterization
Dielectric relaxation spectroscopy (DRS) was performed on a Novocontrol Alpha-A high-performance frequency analyzer (Novocontrol Technologies GmbH & Co. KG, Germany) operating in the frequency range 10–1–106 Hz at 23°C. The sample diameters tested were 25 mm, while thickness was approximately 0.5–1.0 mm.
2.3.7. Actuation investigation
The actuation performance of films of thickness 60–80 μm was measured by the actuation set-up, which has been described in previous work.[Citation20] Both surfaces of films were metallized with silver 100 nm-thick electrodes (width 30 mm × length 90 mm). Conductive electrode tapes were attached to make a connection with the high voltage amplifier. The top side of the film was fixed with a clamp on a stage. While the bottom side of the film was hung with a certain weight to get 10% pre-stretch. The voltage was applied to the films in steps of 500 V, starting from 0 V up to 9 kV. The film length under different voltage was recorded by a camera. The thickness strain Sz is calculated by the decrease of film length (under different voltage) divided by the electrode length (90 mm). Tensile stress–strain, breakdown strength and actuation measurements are shown in .
2.3.8. Morphology observation
The morphology of the fillers and film surfaces was examined by a scanning electron microscopy (SEM) (FEI Inspect S, USA). All samples were coated with gold under vacuum before testing.
3. Results and discussion
In the following, the properties of the LSRs and the benchmark RTV are discussed, followed by a discussion on LSR composites.
3.1. LSR and RTV formulations with no additional fillers
In the following sections four different commercially available LSRs, with varying amounts of silica fillers, are investigated and discussed, with a focus on properties relevant to DEs.
3.1.1. Thermal analysis
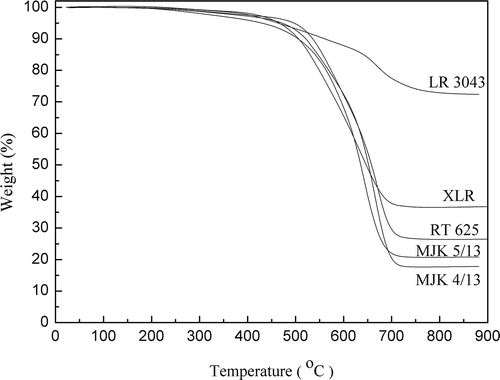
Thermogravimetric analysis (under nitrogen atmosphere) of the silicone films enables the determination of filler content.[Citation27] In purely commercial silicone formulations, the majority of residue at 900°C corresponds to the initial silica addition.[Citation6] presents the degradation curves under N2 of the four LSRs (LR3043, XLR, MJK 5/13 and MJK 4/13) and one RTV (RT625) film. All formulations behave similarly, with no significant degradation taking place before 250°C, followed by slow degradation up to around 500°C, where weight losses of 5–8% are detected. LR3043 produces the most residue (72 wt.%), while XLR and RT625 present 37 wt.% and 27 wt.% residues, respectively, and MJK 5/13 and MJK 4/13 contain the least amount of inorganic residues at 21 wt.% and 18 wt.%. This agrees well with the viscosities from section 2.1.
A summary of the data obtained from TGA is shown in . Key values are defined here as temperatures at 2% weight loss (Td2%) and the temperature (Tmax) at which the fastest derivative weight (%°C−1) was observed, as well as the final inorganic residue at 900°C.[Citation28] The temperature of Td2% is especially important with respect to production scenarios. One serious drawback with a low Td2% would be bubble formation during production or the elastomer curing weakening the produced materials or causing premature electrical breakdowns.[Citation27] The overall thermal stability of films increases in line with an increase in silica content – as expected.
Table 1. Thermal properties of the LSR and RTV films.
3.1.2. Breakdown strength
Electrical breakdown might be caused by several factors, such as intrinsic breakdown, thermal breakdown, electromechanical breakdown and partial discharge breakdown.[Citation29] In thin dielectric films, when the heat generated within the materials cannot be dissipated sufficiently, a critical voltage will be reached when the applied voltage is increased, termed ‘the maximum thermal voltage,’ i.e. the voltage before the thermal runway occurs.[Citation30] Breakdown strength reveals – besides true material properties – the homogeneity of samples as well as dielectric losses present in the materials. Imperfections may lead to premature treeing [Citation31] and local heating, due to variations in thermal conductivity.[Citation30]
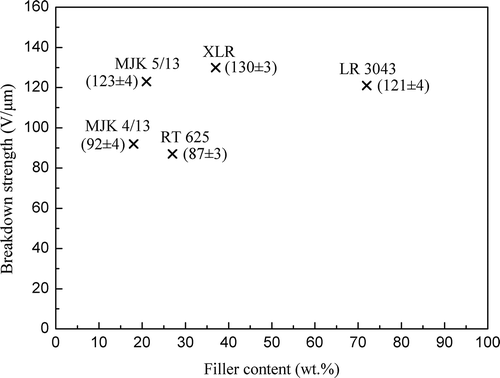
The relationship between breakdown strength and silica content in the investigated silicone rubbers is displayed in . The breakdown strength of the LSR films is generally much higher than that of the reference RTV. Moreover, breakdown strength increases with increased filler content for the three LSRs. However, for even higher filler loading, such as LR3043 (72 wt.% as determined from TGA), the films are probably less homogeneous due to the high viscosity of the reaction mixture. Thus the electrical breakdown strength reaches a maximum at a certain loading where homogeneity is lost partly and therefore a decrease in the electrical breakdown strength is observed.
Figure 3. Breakdown strength versus filler content as determined from the TGA of LSR and RTV films. All films are of similar thickness (60–80 μm).
In order to get more insight into the electrical stability as well as the homogeneity of the samples as Weibull analysis was performed on the breakdown strength data. The data is fitted to the Weibull cumulative distribution:
where P is the cumulative distribution at a given electrical field E, β refers to shape parameter, and η refers to scale parameter.[Citation32,Citation33]
The fitted data is shown in . For the elastomers the XLR is by far the best performing elastomer with respect to electrical reliability. Both shape and scale parameters of the XLR are the highest. The scale parameter is closely related to the determined breakdown strengths. The shape parameter is somewhat more complicatedly connected to the standard deviations but from the Weibull analysis important information can be obtained. The steeper the Weibull plot, the higher βand the narrower probability distribution of breakdown, thus a more homogeneous material. As the silica content is dropped 25% (MJK 5/13) (compared to the commercial elastomer) both parameters drop significantly. For the MJK 4/13 where the silica content is lowered 50% compared to XLR is clear that both Weibull parameters are even further decreased. It is therefore clear that it is not trivial to decrease the silica content while maintaining product performance.
Table 2. Weibull analysis parameters for LSR and RTV samples.
The RT625 elastomer shows relatively good homogeneity (large β) but lower η. The latter is most likely due to the decreased Young’s modulus of this type of formulation. The LR3043 behaves comparable to the XLR with 25% reduction of silica (MJK 5/13). Data for all samples agree very well with the Weibull distribution with no indication of bimodality in the distributions.
3.1.3. Mechanical properties
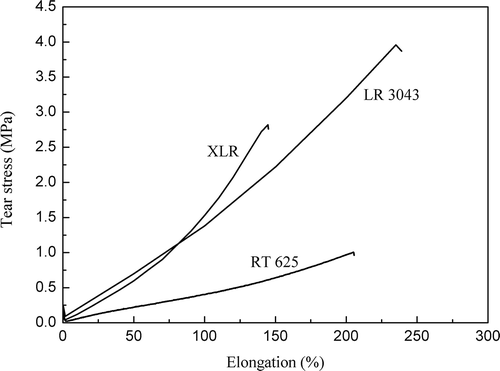
Tear strengths of MJK 5/13 and MJK 4/13 elastomers are 64% and 49%, respectively, of the XLR elastomer, according to product information from the supplier. The XLR exhibits good tear characteristics for thin film production, but poorer properties cannot be accepted for the production of micro-structured thin films. Therefore, these two LSRs were discarded for mechanical testing and the other three silicone formulations (LR3043, XLR, and RT625) were chosen in the following studies to compare mechanical performances.
Tear strength and elongation at the break point of various silicone films are displayed in . LSR elastomer tear strengths are much higher than RT625 and increase in line with any increase in silica content, while elongation at the break point of XLR is slightly lower than that seen for RT625 and LR3043. Both tear strength and elongation at the break point in the LR3043 tear experiment are the highest amongst the investigated elastomers, which is in agreement with the good inter-particle interaction of silica particles with the silicone matrix.[Citation34]
The tensile and tear results are summarized in . High inorganic silica loading results in high tensile strength, high tear strength and a high Young’s modulus. Moreover, the breakdown results shown in suggest that the resistance of the elastomers to dielectric breakdown is enhanced by an increase in the Young’s modulus (as seen in ), which has been reported in previous work on polyethylenes [Citation24] and LSR elastomers.[Citation20]
Table 3. The mechanical properties of LSR and RTV films.
3.2. LSR–TiO2 formulations
LSR materials enable a fast curing process, and the resulting elastomers possess favorable dielectric strengths and mechanical properties – as seen from studies in this work and in previous work.[Citation20] XLR with the overall best properties for mixing in particles is chosen as a matrix to investigate the TiO2 filled elastomers, in order to further improve the properties of the elastomers with respect to energy densities. High-permittivity TiO2 particles are blended into the LSRs to improve the dielectric constants of the composites. While previous results indicate that low TiO2 content cannot increase permittivity significantly [Citation20], too high TiO2 loading results in aggregates, and thus other properties deteriorate sharply due to poor film formation properties.[Citation35]
3.2.1. Viscosity and thermal stability
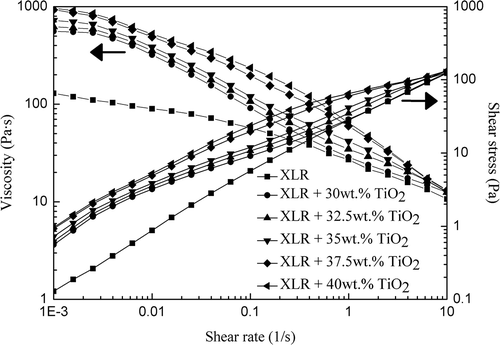
The dynamic viscosity of pre-mixtures plays an important role in the processing of thin films in relation to coating ability and resulting film thickness. In addition, the viscosity of mixtures is an important factor when fillers aim at being homogeneously distributed in the matrix.
The dynamic viscosity profiles of pure XLR and XLR–TiO2 mixtures at the shear rate of 10–3–10 s−1 are shown in . OS-20 solvent is needed in XLR–TiO2 mixtures to allow for the coating of thin films, and the mixing ratio of TiO2 and OS-20 is 2:1 by mass in all XLR–TiO2 mixtures. A large viscosity increase appears at low shear rates in line with increased filler addition (30–40 wt.%) at low shear rates. However, the difference decreases in line with increasing shear rate, and curves of 30–35 wt.% especially are very closed to pure XLR after 0.1 s−1. Finally, all of the viscosity and shear stress curves coincide at 10 s−1, which is considered the shear rate of a conventional coating process.[Citation36] The results indicate that the filler content of 30–40 wt.% chosen in this work does not cause evident limitations in processing.
3.2.2. Dielectric permittivities
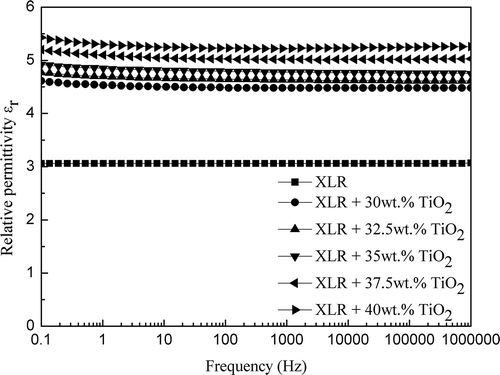
Dielectric permittivity variations, as a function of frequency for both pure XLR and XLR–TiO2 elastomers, are shown in . The results show a clear increase in the relative permittivity of the composite films in line with increased TiO2 loading within the measured frequency range. It is well-known that the TiO2 filler has much higher permittivity (εr ~ 110) compared to that of pure silicone (εr ~ 2.8).[Citation37] The influence of the TiO2 filler’s inherent high permittivity on the overall permittivities of the composites increases as loading concentration increases. On the other hand, the relative permittivity of the filled elastomer is found to be governed by the polarization associated with silicone and particles as well, as it is strongly influenced by interfacial polarization at the interface between PDMS and the TiO2 nanoparticles.[Citation38] The presence of more interfacial polarization at the interface between PDMS and TiO2 results in high permittivity at low frequencies, as TiO2 loading concentration increases.
Figure 6. Frequency-dependent relative permittivity spectra of the pure XLR and XLR–TiO2 elastomers at 23°C.
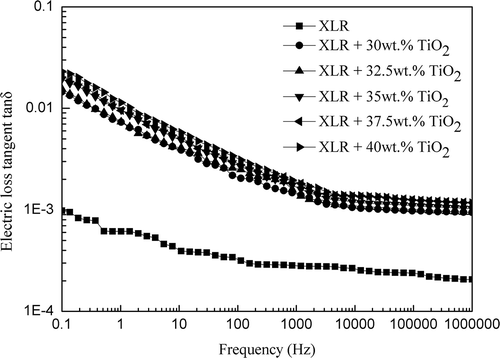
Variations in dielectric losses, expressed as tan delta, in both pure XLR and XLR–TiO2 films, are presented in . It can be observed that the tanδ value of pure XLR is almost frequency-independent, while the tanδ values of composite films increase in line with decreasing frequency. Therefore, the presence of TiO2 fillers has a stronger influence over the electric loss tangent of composites at lower frequency ranges (< 100 Hz), whereas losses are negligible (i.e. comparable to pure silicone) above 100 Hz. The level of the dielectric losses is, however, still acceptable.
3.2.3. Mechanical properties
It is well-known that polymer molecular weight (chain length), particle loading and specific particle characteristics (specific surface area and morphology) affect the mechanical performances of composites.[Citation39] Active particles interact with the polymer matrix through the adsorption of polymer molecules on their surface (immobilization) and with each other. However, the deterioration of the mechanical strength of composites by fillers was also observed, which is mainly due to agglomerates. Most often, reinforcing particles are mechanically blended into PDMS polymers prior to composite curing, thereby resulting in loss of control over the degree of particle dispersion that leads to large agglomerates.[Citation40]
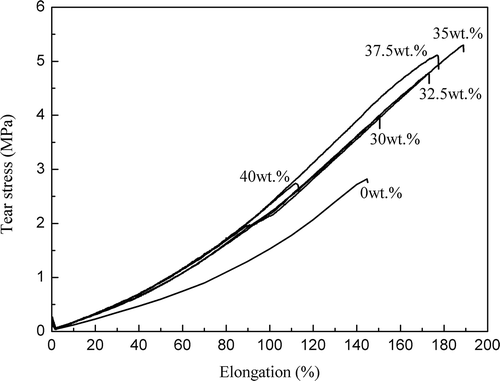
Tear strength curves for the pure XLR and XLR–TiO2 composite films are shown in , while displays the relation between tear strength and elongation on breaking, respectively, of the films and TiO2 content in the mixtures. Both tear strength and elongation at the break point increase in line with increased filler concentration for loadings of TiO2 < 35 wt.%. Once the particle content increases to more than 35 wt.%, neither stress nor elongation at breaking decreases. This can be attributed to two factors: softening effects due to the interference of cross-linking (major effect for TiO2 concentrations lower than 35 wt.%) and hardening effects due to the intrinsic high elastic modulus of TiO2 (major effect for TiO2 concentrations higher than 35 wt.%). At 35 wt.% of filler content, the two effects balance; therefore, the composite film exhibits excellent tear strength and elasticity. In SEM images of R420 TiO2 filler, pure XLR film and XLR film with 35 wt.% TiO2 (R420) are shown. It is clear that with the composite XLR there are some non-homogeneities but the size of these remain rather low with the example given here showing one of the more non-homogeneous areas.
Figure 9. Stress and elongation at breaking in the tear experiment on the pure XLR and XLR–TiO2 elastomers.
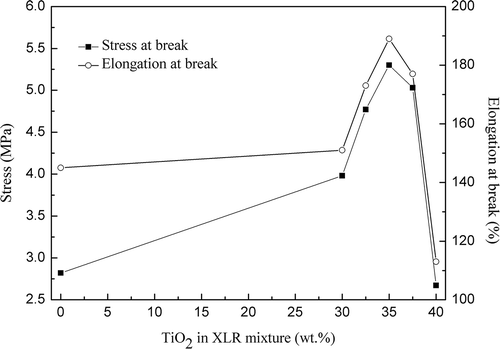
Figure 10. SEM images of R420 TiO2 filler (a), pure XLR film (b) and XLR+35 wt.% R420 TiO2 film (c).

The mechanical and breakdown performances of the pure XLR and XLR–TiO2 films are listed in . The Young’s modulus stated here is the ratio of stress to strain measured at 5% strain. It is clear that at 35 wt.% TiO2 concentration, the composite system shows the highest strength and maximal elasticity, as well as outstanding breakdown strength and a favorable Young’s modulus. Therefore, 35 wt.% TiO2 content is also chosen to be added into both high viscous LSR LR3043 and RTV RT625 formulations, in order to compare the effect of the elastomer matrix.
Table 4. Mechanical properties and breakdown strength of the XLR–TiO2 films. The optimum of a given property is marked in gray.
3.2.4. Relation between dielectric and mechanical properties
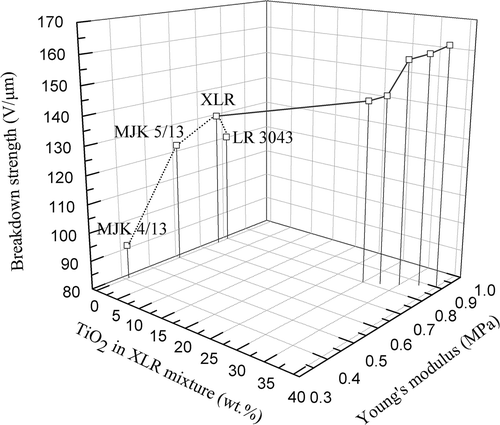
shows relations between TiO2 content in the XLR system, the Young’s modulus and the breakdown strength of the film. It is evident that the addition of TiO2 fillers enhances the dielectric breakdown strength of the investigated XLR composites, partly due to well-dispersed nanofillers leading to larger field enhancement. The variation in data is partly due to the fact that not all films have identical thicknesses due to very different coating behavior. A recent study showed the opposite phenomenon that the concentration of charge carriers was strongly increased upon addition of fillers.[Citation41] However, as the commercial silicone elastomers are complex formulations, one formulation may differ significantly from another due to addition of various stabilizers and additives. It can also be seen in that the Young’s modulus of the film plays a signification role in breakdown strength. The introduction of additional inorganic fillers enhances the rigidity of the composites and consequently increases the Young’s modulus of the film. The results from indicate that the resistance of the elastomers to dielectric breakdown is indeed enhanced by the increase in Young’s modulus (Y) for the given composites. The data that the electrical breakdown strength increases progressively with the increase in Young’s modulus is also reported by Kollosche and Kofod as well as a previous study of ours.[Citation20,Citation42]
Figure 11. 3D plot of breakdown strength as a function of the Young’s modulus and filler amount for the XLR–TiO2 (R420) films and the pure LSRs.
Films with high Y have more compactly structured chains which can trap charges. This is most likely due to lower mobility of trapped charges, which then require a higher electric field in order to introduce electron avalanches.[Citation20] Also electromechanical instability may be reduced upon increasing the Young’s modulus. However, it is not possible to increase Y unlimitedly for a given material, since the formulations start to become inhomogeneous, with the result that the opposite effect (deterioration) is encountered.[Citation24,Citation43]
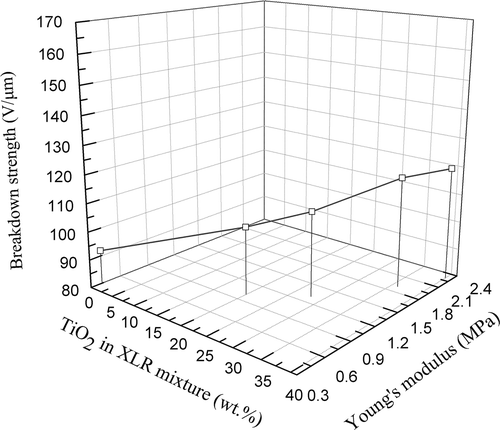
MJK 4/13 formulation is furthermore sought, strengthened by strongly reinforcing TiO2 particles (T805) [Citation44], to evaluate whether or not the silica content in a traditionally formulated LSR can be replaced with TiO2, in order to obtain simultaneously acceptable mechanical properties and improved dielectric performances. shows the 3D plot for MJK 4/13-TiO2 (T805) films. The relation between TiO2 content and breakdown (BD) and Young’s modulus (Y) is similar to that of the XLR–TiO2 (R420) formulations. A much smaller type of filler is applied here (T805 TiO2 (25 nm) compared to R420 TiO2 (250–290 nm)), since MJK 4/13 allows for small and strongly reinforcing particles. The more specific surface area of smaller T805 TiO2 causes greater van der Waals forces between the fillers, resulting in the higher viscosity of the mixtures and larger modulus of the films. From it is clear that the Young’s moduli of MJK 4/13-TiO2 films are 1.0–2.3 MPa, with 20–40 wt.% TiO2 (T805) loading, which is unfavorable for actuators, while the Young’s modulus of XLR film with 40 wt.% TiO2 (R420) is 0.9 MPa (as seen in ). Additionally, more agglomerates are formed in the highly viscous mixture, which might negatively affect other properties such as lifetime. Also other effects may play a role such as the photo-catalytic properties of TiO2 that can destroy the polymer chains [Citation45] as well as the lower OH-group surface density of TiO2 compared with SiO2.[Citation46] It is, however, clear no matter the effect that the replacement of silica with TiO2 is not straight forward when the requirements to the elastomer remain so fierce.
Figure 12. 3D plot of breakdown strength as a function of the Young’s modulus and filler amount for the MJK 4/13-TiO2 (T805) films.
The Weibull analysis of the data () clearly reveals that the shape parameter is increased steadily upon increased TiO2 loading. This is in good agreement with the increase in the Young’s modulus. However, it seems that there is a local minimum in the scale parameter. The data at 35 wt.% loading furthermore – as the only sample – show a tendency toward fitting an S-shape (bimodal breakdown distribution) and this may be an experimental artifact due to insufficient mixing.
Table 5. Weibull analysis parameters for XLR–TiO2 and RTV–TiO2 samples.
In the data for the 3 types of elastomers with 35 wt.% TiO2 is shown. From that it can easily be observed that XLR by far accommodates 35 wt.% TiO2 the best. This results show that obtaining good performance of the composite relies on finding the best performing elastomer in the pristine state.
3.3. LSR–TiO2 and RTV–TiO2 formulations
LSR and RTV composites, as well as pure RT625 elastomers, are evaluated based on two figures of merit, namely with respect to actuation Fom(DEA) and to generation Fom(DEG). The theory and derivation of Fom, to assess elastomer performance as a dielectric elastomer actuator (DEA), have been performed by Sommer-Larsen and Larsen [Citation23] and based on this Fom for a dielectric elastomer generator (DEG) presented by McKay et al. [Citation24]
where Ebreakdown (V μm−1) is the electrical field at which electrical breakdown occurs, εr the relative dielectric permittivity, ε0 the permittivity of free space (8.85 × 10–12 F m−1) and φ the strain energy function of the elastomer, which is assumed to be equal for each silicone formulation.
The figure of merit provides important information on the performance quality of the dielectric elastomer material, since the figure takes into account not only actuation (breakdown strength) but also maximum voltage. Transducers are usually operated at significantly lower voltages than their breakdown strengths. Fom depends upon the dielectric constant, dielectric breakdown strength and the elastic modulus of the elastomer material, which highlights the most important properties for dielectric materials.[Citation25] Maximum elongation, in theory, should also be considered, but due to the high maximum elongations of the investigated elastomers in this study, electrical breakdown strength is the limiting factor.
lists the mechanical and dielectric properties of the LSR and RTV composite films, as well as the figures of merit. The figures of merit of the TiO2-filled formulations all exceed that of the unfilled RTV RT625 formulation.
Table 6. Dielectric and mechanical performances and figures of merit (Fom) of the LSR–TiO2 and RTV–TiO2 formulations. Optimal properties are marked in gray.
However, it can be seen from that both the breakdown strength and the relative permittivity of LR3043-TiO2 are lower than the XLR–TiO2 formulation. One possibility for such a phenomenon is that the reduction of TiO2 dipolar group mobility within the high viscous LR3043 reduces polarization within the composite. When a large amount of filler is loaded into LSR, more immobile nanolayers form and the mobility of the chain decreases continuously, resulting in a reduction in the composite’s permittivity.[Citation41] On the other hand, more particles in LR3043 act to suppress surface erosion caused by partial discharges, which are also possible sources of breakdown.[Citation47]
Furthermore, it is clear that LSR composite formulations have much better dielectric and mechanical performances than the RTV composite. Thus, both the Fom(DEA) and Fom(DEG) of LSR films are greater than RT625-TiO2, and XLR–TiO2 is certainly the best candidate due to the significantly higher Fom than its counterparts.
3.4. Electromechanical properties
In any given electrical field (E), the mechanical response (strain, p) of the elastomer can be expressed by [Citation10]:
where ε0 is the permittivity of free space (8.85 × 10–12 F m−1), εr the relative dielectric permittivity, Y the Young’s modulus (Pa), U the voltage (V), and d (m) the original thickness of the elastomer.
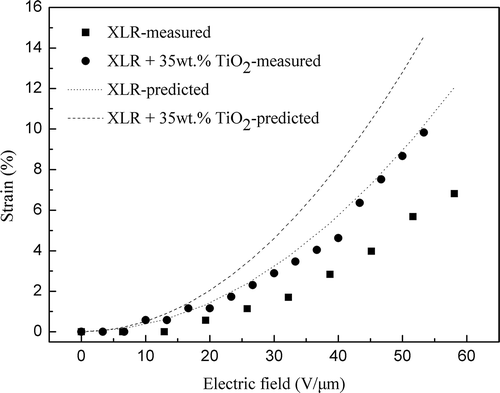
Pure XLR (film thickness = 78 μm, Y = 0.76 MPa, εr = [email protected] Hz) and XLR+35 wt.% TiO2 (film thickness = 75 μm, Y = 0.85 MPa and εr = [email protected] Hz) films were tested for electromechanical strain response. The electric field, as a function of the strain, is illustrated in , which highlights, as expected, that the strain increases faster for XLR films with 35 wt.%TiO2 in line with increasing electric field compared to the pure XLR film, e.g. XLR-35 wt.%TiO2 with 9.8% strain @ 53 V μm−1 and pure XLR with 6.8% strain @ 58 V μm−1.
The predicted strain is calculated from the measured thickness, Young’s modulus and relative permittivity using Equation (4). shows that the actual measured strains are not consistent with the predicted measurements, one likely reason for which is that dielectric loss dissipates as heat rather than being stored in the form of elastic energy.[Citation20] The samples tested for actuation are furthermore laminated by two single films, and air voids at the interfaces may be introduced upon stretching, thereby leading to the slightly lower relative permittivity of the non-ideal laminate. However, it is obvious that actuation is greatly enhanced by the addition of 35 wt.% titanium dioxide particles (almost a factor of two).
4. Conclusions
Liquid silicone rubbers (LSRs) exhibit better thermal, dielectric and mechanical properties than room-temperature vulcanizable (RTV) rubbers when applied in DE transduction. The low intrinsic viscosity of XLR LSR formulation enables high TiO2 loading of up to 35 wt.%, with minimal destruction of mechanical properties and the best elastomers were obtained with this elastomer as basis. However, too high TiO2 loading (>35 wt.%) results in aggregates with the current processing equipment, and thus other properties of the elastomers deteriorate accordingly. The resulting 35 wt.% loading composite exhibits the highest figures of merit for both actuation Fom(DEA) and generation Fom(DEG) in all studied formulations. The XLR-35 wt.%TiO2 elastomer possesses superior overall performance, with a maximum tear strength of 5.3 MPa and a relative permittivity of 4.92 @ 0.1 Hz, dielectric breakdown of 158 V μm−1 and a favorable Young’s modulus of 0.85 MPa, as well as a 10% strain response in a 50 V μm−1 electric field.
The study furthermore showed that replacing silica with TiO2 in commercial elastomers was not straight-forward as the resulting elastomer suffered from several shortcomings with the major problem being that the tear strength was largely reduced already in the original non-filled elastomers. Also it was found that in order to obtain the most reliable elastomer with respect to electrical properties, the elastomer without permittivity-enhancing fillers should be very reliable as the increased Young’s modulus from the filler loading cannot compensate for a poor base material. This may be due to the photo-catalytic properties of TiO2 that can destroy the polymer chains as well as the lower OH-group surface density of TiO2 compared with SiO2.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- A.L. Larsen, K.K. Hansen, P. Sommer-Larsen, O. Hassager, A. Bach, S. Ndoni, and M. Jørgensen, Elastic properties of nonstoichiometric reacted PDMS networks, Macromolecules 36 (2003), pp. 10063–10070. doi:10.1021/ma034355p
- F.B. Madsen, A.E. Daugaard, S. Hvilsted, M.Y. Benslimane, and A.L. Skov, Dipolar cross-linkers for PDMS networks with enhanced dielectric permittivity and low dielectric loss, Smart Mater. Struct. 22 (2013), pp. 104002. doi:10.1088/0964-1726/22/10/104002
- F.B. Madsen, L. Yu, A.E. Daugaard, S. Hvilsted, M.Y. Benslimane, and A.L. Skov, A new soft dielectric silicone elastomer matrix with high mechanical integrity and low losses, RSC Adv. 5 (2015), pp. 10254–10259. doi:10.1039/C4RA13511C
- B. Kussmaul, H. Krueger, S. Risse, and G. Kofod, Synergistic improvement of actuation properties with compatibilized high permittivity filler, Adv. Funct. Mater. 22 (2012), pp. 3958–3962. doi:10.1002/adfm.201200320
- S. Vudayagiri, L. Yu, and A.L. Skov, Techniques for hot embossing microstructures on liquid silicone rubbers with fillers, J. Elastomers Plast. (2014). doi:10.1177/0095244314526743
- E. Delebecq and F. Ganachaud, Looking over liquid silicone rubbers: (1) Network topology vs chemical formulations, ACS Appl. Mater. Interfaces. 4 (2012), pp. 3340–3352. doi:10.1021/am300502r
- C. Hopmann, C. Behmenburg, U. Recht, and K. Zeuner, Injection molding of superhydrophobic liquid silicone rubber surfaces, Silicon 6 (2014), pp. 35–43. doi:10.1007/s12633-013-9164-0
- E. Delebecq, N. Hermeline, A. Flers, and F. Ganachaud, Looking over liquid silicone rubbers: (2) Mechanical properties vs network topology, ACS Appl. Mater. Interfaces. 4 (2012), pp. 3353–3363. doi:10.1021/am300503j
- W.L. Wu and J.Y. Cai, Study on short basalt fiber reinforced silicone rubber composites, Advanced Mater. Res. 800 (2013), pp. 383–386. doi:10.4028/www.scientific.net/AMR.800
- A.L. Skov, A.G. Bejenariu, J. Bøgelund, M. Benslimane, and A.D. Egede, Influence of micro- and nanofillers on electro-mechanical performance of silicone EAPs, SPIE Proceedings 8340, SPIE, San Diego, CA, 2012, pp. 83400M-1–83400M-10.
- S.S. Hassouneh, A.E. Daugaard, and A.L. Skov, Design of elastomer structure to facilitate incorporation of expanded graphite in silicones without compromising electromechanical integrity, Macromol. Mater. Eng. 300 (2015), pp. 542–550. doi:10.1002/mame.201400383
- G. Rajesh, P.K. Maji, M. Bhattacharya, A. Choudhury, N. Roy, A. Saxena, and A.K. Bhowmick, Liquid silicone rubber vulcanizates: Network structure – property relationship and cure kinetics, Polym. Polym. Compos. 18 (2010), pp. 477–487.
- E. Haberstroh, W. Michaeli, and E. Henze, Simulation of the filling and curing phase in injection molding of liquid silicone rubber (LSR), J. Reinf. Plast. Compos. 21 (2002), pp. 461–471. doi:10.1177/0731684402021005476
- Z. Chen, D. Li, A.J. Shi, Y. Li, and S.X. Wu, Properties characteristics of silicone inhibitors, Advanced Mater. Res. 2527 (2013), pp. 43–46.
- L.M. Lopez, A.B. Cosgrove, J.P. Hernandez-Ortiz, and T.A. Osswald, Modeling the vulcanization reaction of silicone rubber, Polymer Eng. Sci. 47 (2007), pp. 675–683. doi:10.1002/(ISSN)1548-2634
- M. Benslimane, H.E. Kiil, and M.J. Tryson, Electromechanical properties of novel large strain PolyPower film and laminate components for DEAP actuator and sensor applications. SPIE Proceedings 7642, SPIE, San Diego, CA, 2010, pp. 764231-1–764231-11.
- F.B. Madsen, I. Dimitrov, A.E. Daugaard, S. Hvilsted, and A.L. Skov, Novel cross-linkers for PDMS networks for controlled and well distributed grafting of functionalities by click chemistry, Polym Chem. 4 (2013), pp. 1700–1707. doi:10.1039/c2py20966g
- L. Yu and A.L. Skov, Monolithic growth of partly cured polydimethylsiloxane thin film layers, Polymer J. 46 (2014), pp. 123–129. doi:10.1038/pj.2013.72
- C. Brosseau and P. Talbot, Instrumentation for microwave frequency-domain spectroscopy of filled polymers under uniaxial tension, Meas. Sci. Technol. 16 (2005), pp. 1823–1832. doi:10.1088/0957-0233/16/9/015
- S. Vudayagiri, S. Zakaria, L. Yu, S.S. Hassouneh, M. Benslimane, and A.L. Skov, High breakdown-strength composites from liquid silicone rubbers, Smart Mater. Struct. 23 (2014), pp. 105017. doi:10.1088/0964-1726/23/10/105017
- F. Carpi and D.D. Rossi, Dielectrics and Electrical Insulation, IEEE Trans. Dielectr. Electr.l Insul. 12 (2005), pp. 835–843. doi:10.1109/TDEI.2005.1511110
- G.L. Wang, Y.Y. Zhang, L. Duan, K.H. Ding, Z.F. Wang, and M. Zhang, Property reinforcement of silicone dielectric elastomers filled with self-prepared calcium copper titanate particles, J. Appl. Polym. Sci. (2015). doi:10.1002/APP.42613
- P. Sommer-Larsen and A.L. Larsen, Materials for dielectric elastomer actuators, SPIE Proc. 5385 (2004), pp. 68–77.
- T.G. McKay, E. Calius, and I.A. Anderson, Dielectric constant of 3M VHB: A parameter in dispute. SPIE Proceedings 7287, SPIE, San Diego, CA, 2009, pp. 72870P-1–72870P-10.
- L. Yu, S. Vudayagiri, S.B. Zakaria, and M.Y. Benslimane, Filled liquid silicone rubbers: Possibilities and challenges. SPIE Proceedings 9056, SPIE, San Diego, CA, 2014, pp. 90560S-1–90560S-9.
- M. Hosokawa, K. Nogi, M. Naito, and T. Yokoyama, Nanoparticle Technology Handbook, M. Hosokawa, ed., Elsevier, Amsterdam, 2007. pp. 5.
- L. Yu, L.B. Gonzalez, S. Hvilsted, and A.L. Skov, Soft silicone based interpenetrating networks as materials for actuators. SPIE Proceedings 9056, SPIE, San Diego, CA, 2014, pp. 90560C-1–90560C-9.
- L. González, A.L. Skov, and S. Hvilsted, Ionic networks derived from the protonation of dendritic amines with carboxylic acid end-functionalized PEGs, Polym Chem. 51 (2013), pp. 1359–1371. doi:10.1002/pola.26503
- L.A. Dissado and J.C. Fothergill, Electrical Degradation and Breakdown in Polymers, Peter Peregrinus Publisher, London, UK, 1992, pp. 63–65.
- S.B. Zakaria, P.H.F. Morshuis, M.Y. Benslimane, V.G. Krist, and A.L. Skov, The electrical breakdown of thin dielectric elastomers: Thermal effects. SPIE Proceedings 9056, SPIE, San Diego, CA, 2014, pp. 90562V-1–90562V-12.
- M.H. Ahmad, H. Ahmad, N. Bashir, Y.Z. Arief, Z. Abdul-Malek, R. Kurnianto, and F. Yusof, A new statistical approach for analysis of tree inception voltage of silicone rubber and epoxy resin under AC ramp voltage, Int. J. Electrical Eng. Inform. 4 (2012), pp. 27–39. doi:10.15676/ijeei
- S.B. Zakaria, P.H.F. Morshuis, M.Y. Benslimane, L. Yu, and A.L. Skov, The electrical breakdown strength of pre-stretched elastomers, with and without sample volume conservation, Smart Mater. Struct. 24 (2015), pp. 055009. doi:10.1088/0964-1726/24/5/055009
- R. Kochetov, I.A. Tsekmes, and P.H.F. Morshuis, Electrical conductivity, dielectric response and space charge dynamics of an electroactive polymer with and without nanofiller reinforcement, Smart Mater. Struct. 24 (2015), pp. 075019. doi:10.1088/0964-1726/24/7/075019
- A. Camenzind, T. Schweizer, M. Sztucki, and S.E. Pratsinis, Structure & strength of silica-PDMS nanocomposites, Polymer 51 (2010), pp. 1796–1804. doi:10.1016/j.polymer.2010.02.030
- V.P. Silva, M.P. Paschoalino, M.C. Gonçalves, M.I. Felisberti, W.F. Jardim, and I.V.P. Yoshida, Silicone rubbers filled with TiO2: Characterization and photocatalytic activity, Mater. Chem. Phys. 113 (2009), pp. 395–400. doi:10.1016/j.matchemphys.2008.07.104
- U. Eriksson, G. Engstrom, and M. Rigdahl, Viscosity of some clay-based coating colors at high shear rates, Rheol. Acta 29 (1990), pp. 352–359. doi:10.1007/BF01339890
- B. Hudec, K. Husekova, E. Dobrocka, T. Lalinsky, J. Aarik, and K. Frohlich, High-permittivity metal-insulator-metal capacitors with TiO2 rutile dielectric and RuO2 bottom electrode, Mater. Sci. Eng. 8 (2010), pp. 012024–012027.
- G.Z. Liu, C. Wang, C.C. Wang, J. Qiu, M. He, J. Xing, K.J. Jin, H.B. Lu, and G.Z. Yang, Effects of interfacial polarisation on the dielectric properties of BiFeO3 thin film capacitors, Appl. Phys. Lett. 92 (2008), pp. 122903-1–122903-3. doi:10.1063/1.2900989
- H. Liu, L. Zhang, D. Yang, Y. Yu, L. Yao, and M. Tian, Mechanical, dielectric and actuated strain of silicone elastomer filled with various types of TiO2, Soft Mater. 11 (2013), pp. 363–370. doi:10.1080/1539445X.2012.661821
- D. Tan, Y. Cao, E. Tuncer, and P. Irwin, Nanofiller dispersion in polymer dielectrics, Mater. Sci. Appl. 4 (2013), pp. 6–15. doi:10.4236/msa.2013.44A002
- Q. Wang and G. Chen, Effect of nanofillers on the dielectric properties of epoxy nanocomposites, Adv. Mater. Res. 1 (2012), pp. 93–107. doi:10.12989/amr.2012.1.1.093
- M. Kollosche and G. Kofod, Electrical failure in blends of chemically identical, soft thermoplastic elastomers with different elastic stiffness, Appl. Phys. Lett. 96 (2010), pp. 071904–1. doi:10.1063/1.3319513
- T. He, X. Zhao, and Z. Suo, Dielectric elastomer membranes undergoing inhomogeneous deformation, J. Appl. Phys. 106 (2009), pp. 083522. doi:10.1063/1.3253322
- J.L. Yang, Z. Zhang, A.K. Schlarb, and K. Friedrich, On the characterization of tensile creep resistance of polyamide 66 nanocomposites. Part I. Experimental results and general discussions, Polymer 47 (2006), pp. 2791–2801. doi:10.1016/j.polymer.2006.02.065
- L.H. Lin, H.J. Liu, J.J. Hwang, K.M. Chen, and J.C. Chao, Photocatalytic effects and surface morphologies of modified silicone-TiO2 polymer composites, Mater. Chem. Phys. 127 (2011), pp. 248–252. doi:10.1016/j.matchemphys.2011.01.069
- P. Paoprasert, S. Kandala, D.P. Sweat, R. Ruther, and P. Gopalan, Versatile grafting chemistry for creation of stable molecular layers on oxides, J. Mater. Chem. 22 (2012), pp. 1046–1053. doi:10.1039/C1JM13293H
- Z. Li, K. Okamoto, Y. Ohki, and T. Tanaka, Effects of nano-filler addition on partial discharge resistance and dielectric breakdown strength of micro-Al2O3/epoxy composite, IEEE Trans. Dielectr. Electr.l Insul. 17 (2010), pp. 653–661. doi:10.1109/TDEI.2010.5492235