ABSTRACT
This article focuses on the hybridization of thermoplastic polymer matrices with conducting polymers and graphene derivatives. Polypropylene (PP), polymethylmethacrylate (PMMA) and polyoxymethylene (POM) were used as primary polymer matrices and polypyrrole (PPY) and polyaniline (PANI) as secondary conducting polymers. Highly conductive-reduced graphene oxide (rGO) and graphene (G) have been used as reinforcements. A Taguchi analysis has been performed for the blends to find the optimal combination of the blends with respect to electrical conductivity (σ) and mechanical properties. Both electrical and mechanical properties were improved by the hybridization process. The maximum electrical conductivity of 0.85 S.cm−1 has been acquired with POM/PPY/G blend with 3 wt.% and 5 wt.% of PPY and graphene loading, respectively. The mechanical properties have been found to improve with all the blends but, PP/PPY/G blend with 3 wt.% and 6 wt.% of PPY and graphene loading displays overall better properties in comparison with other blends.
1. Introduction
Polymer nanocomposites represent a new alternative to conventionally filled polymers. Nanocomposites are a broad range of materials consisting of two or more components, with at least one component having dimensions in the nm range (between 1 and 100 nm). Because of their nanometer sizes, filler-dispersed nanocomposites exhibit markedly improved properties when compared with the pure polymers or their traditional composites. Nanocomposites may possess improved mechanical, physical, and electronic properties. The properties of nanocomposite materials depend not only on the properties of their individual parent materials but also on their morphology and interfacial characteristics [Citation1]. They have very good reinforcement in the nanometer scale and have a surface-to-volume ratio that is very high. Because of these improved properties, nanocomposite materials are being used in an increasing number of industrial applications including transportation, automotive, aerospace, defense, sporting goods, energy, and infrastructure sectors [Citation2].
Graphene is an exciting nanomaterial being widely investigated today for its potential applications in a wide range of areas, such as flexible electronic devises, shape memory polymers, sensors photovoltaic devices, conductive nanofibers, antibacterial composites, energy conversion, and storage [Citation3,Citation4]. It exhibits attractive mechanical, electrical, physical, and chemical properties, which can be utilized in polymers to enhance their properties. Though numerous challenges remain in developing a fundamental understanding of graphene-based materials and their polymer composites [Citation5] would lead to potentially useful new products [Citation6]. Some major findings from literature have been described in the paragraphs that follow. Applications of graphene-based materials to specific fields are presented in .
In many previous studies, it has been reported that graphene materials can be intercalated by various monomers and subsequent polymerization was carried out, in which incorporation of graphene materials as fillers can significantly reduce gas permeability. As specific examples, graphene oxide (GO), nano/microstructured polypyrrole (PPY) and polyaniline (PANI) are some typical important functional materials, which have many applications in lithium-ion batteries with high energy levels, super-capacitors, catalysts, solar cells, nano-devices, chemical sensors, and biosensors. There has been a significant amount of research carried out on graphene and polymer composites toward incorporating or improving electrical conductivity (). Different polymer matrices, ranging from thermoplastic to thermoset, with different weight and volume percentage addition of graphene materials, have been attempted through different manufacturing techniques and interesting results have been reported [Citation9–Citation16].
Table 1. Literature review on electrical conductivity of nanocomposites of different polymers and graphene derivatives.
The common observations are:
Electrical conductivity can be incorporated by addition of conductive particles through techniques, such as melt blending, solution casting, in situ processing, and chemical vapor deposition (for sensor-related applications) are used, but it is in the range of 1 × 10–8 S.cm−1.
Electrical conductivity can be improved with the addition of graphene materials along with a secondary polymer or filler [Citation28]. Dispersion of graphene particles in a polymer matrix is difficult and there exists a restriction on the addition of graphene above a certain level (maximum of 10 wt.%), due to the formation of agglomerates. This results in poor conductivity.
In prior work, simple composite blends have been attempted and most of the researchers did not consider complex blends or hybridization to overcome the limitation of adding graphene material above a certain level. This would have allowed the addition or use of other possible materials to add along with graphene to improve electrical and mechanical properties.
The mechanical properties and thermal stability of the composites have improved considerably and this versatility of graphene/polymer nanocomposites points to their potential application in automotive, aerospace, electronics, and packaging [Citation29]. Furthermore, electrical conductivity can be improved with the addition of graphene materials along with a secondary polymer or filler [Citation28].
Mechanical properties give an important measure of product quality and tensile testing can be used to assess the performance of carbon nanotubes-reinforced composites. A significant number of investigations have been carried out in improving mechanical properties of graphene/polymer nanocomposites. These properties depend mostly on the following factors [Citation30]:
Type of polymer matrix,
Type of fabrication method,
Functionalization or functionalities of graphene derivative,
Dispersibility of graphene derivatives in the matrix,
Stress transfer between graphene materials and the polymer matrix to the transfer of the load to the filler, and
Amount of graphene materials in the matrix.
Generally, incorporating graphene materials through any fabrication method improves the mechanical properties to some extent, though tensile strength decreases due to agglomeration [Citation31]. The tensile strengths of different graphene-based composites have been listed in . Furthermore, from this literature search, it was noticed that many investigations/developments of graphene–polymer composites were done by both melt blending and in situ processes with a focus on improving electrical conductivity. However, higher electrical conductivities were achieved with the composites prepared by in situ techniques.
Table 2. Literature review on tensile strength of nanocomposites of different polymers and graphene derivatives.
This is a major limitation for large-scale production when compared with melt blending process. In order to ensure the practical application of our results, in this work, we have utilized melt blending process for the preparation of nanocomposites with graphene materials and different polymer matrices. The investigation primarily focused on hybridizing the nanocomposites with the primary polymer (thermoplastics), secondary polymers (conducting polymers), and graphene materials and analyzing their mechanical properties.
From the literature, it has been noticed that the two main factors that contribute to improve any properties in a reinforced composite material are dispersion and aspect ratio. With particulate composites, the aspect ratio cannot be considered as it is applies only for fiber-reinforced composites. Hence, the dispersion can only be major contributing factors in graphene-based composites that can be considered and improved. It has been well reported by many others that it is highly difficult to avoid formation of agglomeration in particulate composites and this causes disconnected particles in the composite system. These disconnected particles could cause poor electrical and mechanical properties in the composites. This problem could be avoided or overcome by adding suitable potential additional component. No attempt has been made to hybridize graphene with two possible polymers together. The primary polymer being a thermoplastic, the secondary polymer could be conducting polymer which potentially ‘bridges’ graphene particles that are separated through agglomeration. This led us to develop three component hybrid composite systems to understand their effects on final targeted properties.
2. Materials and methods
Graphite flakes, PPY with powder conductivity of 30 S.cm−1 and hydroiodic acid (HI) were purchased from Sigma-Aldrich, New Zealand. Hydrochloric acid (HCl) and sulphuric acid (H2SO4) were supplied by Macron Chemicals, U.S.A. Potassium permanganate (KMnO4) and hydrogen peroxide (H2O2) were obtained from J.T. Baker, Netherlands and ECP Chemicals, New Zealand, respectively. Graphene was bought from EMFUTUR Technologies, Spain. LLDPE (Rotathene) and PP (H5300) were purchased from Vanglobe, New Zealand and Honam, Korea, respectively. PMMA (CM207) and POM (Celcon) were bought from and Chi Mei, Taiwan, and Celanese, China, respectively. These particular polymers have been selected in order to
understand how the electrical and mechanical properties of the nanocomposite are dependent on the differing blends of conducting polymers and highly conducting fillers,
assess the compatibility of graphene with polymers that have different level of polarity and effects of polarity, and
identify ideal combinations of hybrid blends of graphene derivative, conducting polymer, and thermoplastic polymer.
3. Experimental details
The objective of the design of experiments (DOE) is to find the best factor-level combination that will give the optimum formulation/parameters. The Taguchi method of experimental design is commonly used to investigate the influence of process variables on a selected response [Citation46]. For the studied nanocomposites, the properties of most interest were electrical conductivity and ultimate tensile strength (UTS). Once the choice of factors and levels were selected, the levels were decided followed by construction of orthogonal array. The factors chosen were:
Reduced graphene oxide or graphene content
Secondary polymer content (PPY or PANI)
Type of primary polymers (PP, PMMA, POM)
The above three parameters were varied at three different levels, and a number of combinations of the factors at these levels were attempted to arrive at an optimum combination. MINITAB 17 was used for creating Taguchi analysis. Four types of hybrid nanocomposites were prepared (shown in ) and hence, four Taguchi analyses were developed. shows the levels of factors and experimental combinations. Electrical conductivity and UTS were main properties of interest and Taguchi analysis discusses both properties.
Table 3. (a) Experimental design and (b) experimental combinations (hybrids of rGO/GO, PPY/PANI, and polymers).
These upper and lower limits for factors A and B have been chosen for following reasons.
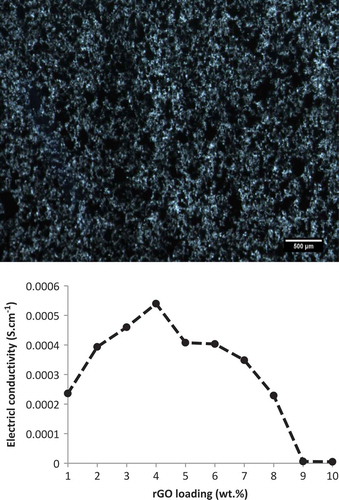
First, preliminary experiments on rGO/LLDPE nanocomposites prepared (melt blending followed by compression molding to form sheets) using highly conductive hydroiodic acid-reduced graphene oxide (HI-rGO) did not reach the expected electrical conductivity of 0.1 S.cm−1 but it helped predict the upper and lower limits for graphene derivatives to design Taguchi analysis for a proper statistical analysis. The conductivity values for rGO/LLDPE were too low due to agglomeration of rGO particles, as shown in . In particular, the conductivity did not improve beyond 4 wt.% loading of rGO. Therefore, there was a restriction on the component proportions which did not allow exploration of the entire mixture space.
Second, it was hard to perform melt blending with more than 3 wt.% of PPY and PANI as these two powders being highly voluminous, made handling difficult, and the composites also became brittle over 3 wt.%. Third, it is ideal to use lower amounts of graphene materials and conducting polymers for improving required properties for a cost-effective process. These polymers are reasonably stable above 200°C, for melt blending, which was assessed by thermogravimetric analysis.
The analysis involved third-order interactions as (A*B*C), where [A = rGO/G content (wt.%), B = PPY/PANI content (wt.%), and C = type of polymer/polarity (wt.%)]. The experiments were carried out according to the L9-OA The electrical conductivity (averaged from five replicates) was considered as Taguchi array response and analyzed with different statistical factors. After conducting the experiments, the results were converted into signal-to-noise (S/N) ratio (as a measure of robustness used to identify control factors that reduce variability in a product or process.
3.1. Electrical conductivity (from Taguchi analysis): a comparison
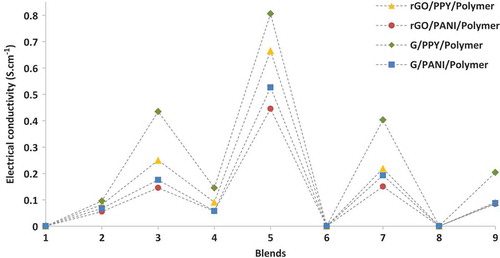
A desired composite formulation can be determined by peak points of a linear or nonlinear trend of factors involved or considered. Thus, the preferable combination to get high electrical conductivity is 2 wt.% rGO/G (A2), 4 wt.% PPY/PANI (B2) and POM (C3). For electrical conductivity, PPY/PANI content and POM type had the same nonlinear trend as electrical conductivity. Therefore, a composite including 4 wt.% of rGO/G, 2 wt.% of PPY/PANI and POM can become a desired composite formulation for electrical conductivity improvement. The top three values belong to POM hybrids (3, 5, and 7); the second top values are for PMMA hybrids (2, 4, and 9) the lowest ones are PP hybrids (1, 6, and 8). The final L9-OA displaying response values are shown in (the hybrids are denoted with numbers assigned in Taguchi design).
A desired material formulation of the composites to enhance the electrical conductivity can be determined by the sum of S/N ratios. shows main effects plots of S/N ratios for electrical conductivity, from which, it can be observed that electrical conductivity increases at 2 wt.% addition of rGO/G and PPY/PANI but at 4 wt.% addition it decreases. This is believed to be due to the formation of more noncondensable gaseous/volatile fractions by voids and agglomeration as visualized from scanning electron microscopy (SEM) images (). Similar behavior was noticed by Tchoudakov et al. [Citation47]. Electrical conductivity greatly depends on the polymer chosen, with POM giving the highest conductivity, and a general trend of increasing conductivity with increasing polymer polarity [Citation47].
Figure 5. Main effects plots for all the hybrids (electrical conductivity) – (a) rGO/PPY/polymers, (b) G/PPY/polymer, (c) rGO/PANI/polymers, and (d) G/PANI/polymers.
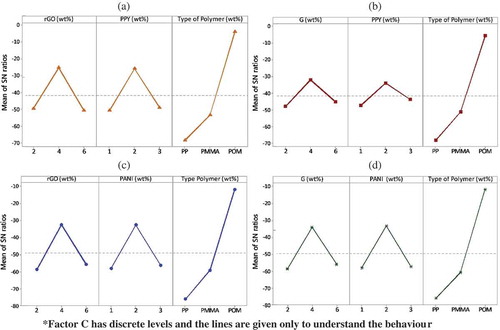
Figure 7. Main effects plots for all the hybrids (ultimate tensile strength) – (a) rGO/PPY/polymers, (b) rGO/PANI/polymers, (c) G/PPY/polymer, and (d) G/PANI/polymers.
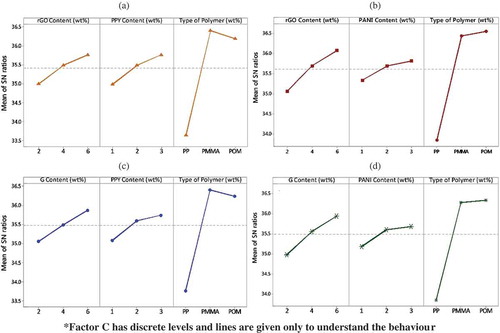
Figure 8. Percentage increase of UTS in all four hybrid nanocomposites calculated from their neat polymers.
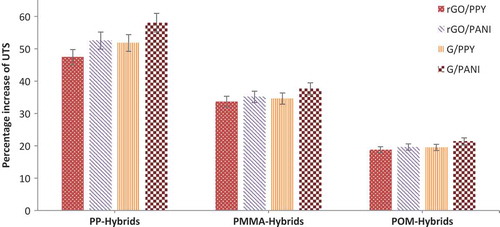
Figure 11. SEM images of the top hybrids that give highest electrical conductivity in their corresponding thermoplastic matrices (inside – 2 µm).
Whether interactions between factors exist or not can be shown by plotting a matrix of interaction plot. Parallel lines in an interaction plot indicate no interaction. However, the interaction plot does not tell if the interaction is statistically significant [Citation48]. An analysis of variance (ANOVA) table [Citation49–Citation51] breaks down the effect of each factor, the experimental error, and the possible interactions of the factors. In this process, it was seen that the significant factors are the type of polymer (C), rGO/G content, and PPY/PANI content (A*B). The final ANOVA table () gives the percentage contribution (exact value in percent) of each of the significant factors affecting the response value. The main conclusion that can be drawn from ANOVA is that the choice of polymer plays the major role in affecting conductivity, comparatively with graphene/rGO and conducting polymers.
Table 4. ANOVA table for all the hybrids (electrical conductivity).
A general regression for the complete model was performed using MINITAB 17 software and the final the regression values are given in . The R2 values are all near 99%, indicating that the empirical model derived from the experimental data can predict the response with high accuracy. Normal probability plots for means of this process showed that the model meets the assumptions of the analysis. Residuals (not shown) were found to be scattered and without any definite pattern that proved systematic shortcomings of the model.
Table 5. Regression values of hybrids from S/N ratios (electrical conductivity).
3.2. Tensile properties of hybrids (from Taguchi analysis): a comparison
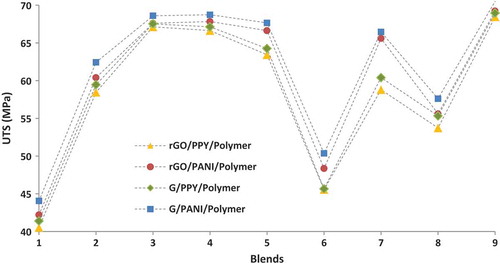
Tensile properties (ultimate tensile strength, UTS) of all the hybrids have been measured (averaged from five replicates) to assess their mechanical behavior, a property of great interest for commercial applications. The response from UTS measurements has been shown in . For PP and PMMA hybrids, the UTS increases with increasing addition of graphene derivatives and conducting polymers but, by contrast, the UTS of POM hybrids decreases. This could be due to mechanical percolation threshold occurrence at 2 wt.% of graphene materials and 1 wt.% conducting polymers. A final observation is that using rGO or G gives composites with similar tensile strengths. Both rGO/G and PPY/PANI properties increase in a very similar way with the differences mainly in the magnitude of the increase. For POM hybrids, the UTS decreases slightly with the addition of both graphene derivatives and conducting polymers. This deterioration could be due to a number of causes, including:
The percolation threshold/critical loading of conducting polymer and/or filler reaching its maximum point. Particle–particle contact has to be avoided for enhancement of mechanical properties (discussed in SEM section).
The hydrogen bonding may get weakened due to the fully packed structure [Citation24].
More speculatively, it is possible that the peculiar mechanical behavior of the POM hybrids is due to a plasticizing effect, which in turn is related to weak interactions between the graphene derivatives, conducting polymer, and thermoplastic polymer [Citation52].
The main effect plots for UTS from Taguchi analysis have been given in . Different from electrical conductivity ones, it can be seen that UTS was improved with increased addition of graphene derivatives and conducting polymers. The desired parametric combination has been found with POM hybrid composites with 6 wt.% graphene derivative and 3 wt.% conducting polymer additions. The tensile strengths of neat PP, PMMA, and POM are 36.45, 51.23, and 56.51 MPa. The tensile strength is enhanced considerably by the hybridization mechanism as shown in . The highest percentage increase comes with PP followed by PMMA and POM hybrid nanocomposites. These enhancements are attributed to the effective load transfer from the matrix to graphene due to their strong interfacial adhesion. Effective reinforcement can be achieved while further loading will cause the stacking of graphene nanosheets due to the strong van der Waals force between the nanosheets. However, the dynamics of these enhancements reduce after 4 wt.% and 2 wt.% additions of graphene derivatives and conducting polymers, respectively, the assumed point of mechanical percolation.
In terms of tensile strength, all interactions are very significant except polypropylene-based hybrids. This is believed to be due to fundamental incompatibility of the polymer with the graphene derivatives/conducting polymers, with SEM images showing substantial voids and agglomeration even at low loading. In this evaluation of UTS performance, it is difficult to enhance the global composite properties in the presence of contradictory interaction effects. However, it is assessed to understand the similarities and differences between four different types of hybrid composites developed.
The ANOVA table for tensile properties of hybrid nanocomposites has been given in . It can be observed that from the % contribution, the effects of all three factors are similar as observed for the electrical conductivity results. Thermoplastic polymer ranks first in terms of increasing tensile properties, followed by graphene materials and conducting polymers. Having described the importance of regression analysis previously, the same analyses have been done for tensile properties of hybrid nanocomposites as well. The regression values of all four hybrid nanocomposites have been listed in . All the R2 values are around 99%, which indicates that the model can predict the response with high accuracy.
Table 6. ANOVA table for all the hybrids (ultimate tensile strength).
Table 7. Regression values of hybrids from S/N ratios (ultimate tensile strength).
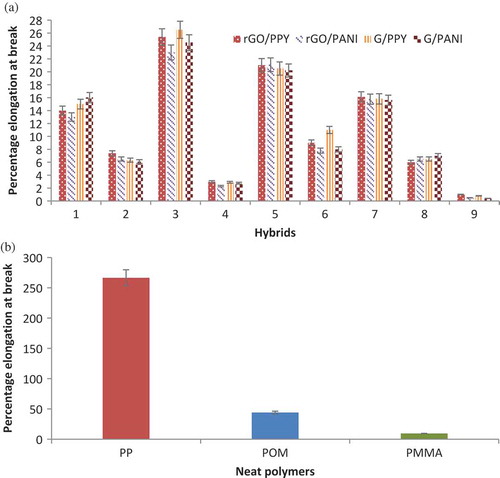
Considering the effect of density of polymers used (shown in )), despite PPY and PANI having similar high density, they contribute little to improving the tensile strength. This indicates that density of the individual material does not affect the properties when composites are prepared.
3.2.1. Percentage elongation at break
Percentage elongation of all the hybrids has been given in which shows the effect of filler loading on elongation at break of all the hybrids composites. The composite without graphene indicates the value of elongation at break is 266.5%, 44.13%, and 9.32% for PP, POM, and PMMA, respectively. Composites with graphene derivatives exhibited brittle fracture and the tensile strength and elongation at break decreased with increasing filler loading. This is attributed to the effect of filler–matrix adhesion and decreasing crystallinity. The high surface area of G (710.7 m2.g−1) and HI-rGO (677.4 m2.g−1) could also be a significant factor for these changes and improvements in the mechanical properties of the nanocomposites [Citation53]. In particular, for PP hybrids, the percentage elongation decreased significantly due to the formation of agglomerates of graphene nanoparticles. This could be seen on SEM images (Section 3.4).
In a general comparison with the existing literature ( and ), improvement in both electrical and mechanical properties is significant due to hybridization process. A similar electrical conductivity with our highest outcome has been reported by Wu et al. [Citation23] as 0.1 S.cm−1 with PMMA reinforced by graphene. However, a higher graphene loading of 5 wt.% (1 wt.% higher) was used by them compared to this work. The mechanical properties vary depending upon and subject to a variety of factors such as type of polymer matrix (as well grades), type of reinforcement, and processing method. Particularly from the literature, tensile properties were found to be improved by reinforcing any graphene derivative into polymer matrices which has been noticed in this work as well. However, a comparison of improvement of both electrical and mechanical properties of the same nanocomposites is missing from the literature, which is the significance of this work.
3.3. Crystallinity and microstructural characterization
X-ray diffraction (XRD) and microstructural (SEM) analyses were performed to get more information about the state of dispersion of the components of the nanocomposite/crystallinity, changes in functional elements and microstructure, respectively.
3.3.1. X-ray diffraction
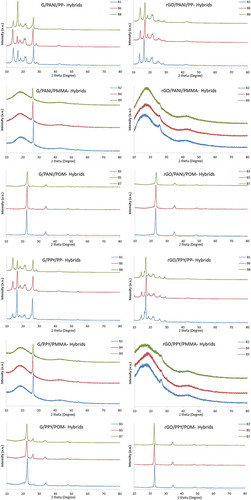
shows XRD patterns of neat polymers and graphene derivatives. Compared to graphene, rGO shows a much smaller peak centered around 26°. This indicates that with higher d-spacing nanosheets occurred after chemical reduction. In addition, the XRD patterns of PP show characteristic diffraction peaks at 2θ = 13.8°, 16.6°, 18.4°, 21.1°, and 21.8°, which correspond to the (110, 040, 130, 111), and (131) + (041) crystalline planes, respectively [Citation54]. Pure PMMA shows a predominant and comparatively broad peak with a maximum at 17.5° indicating the amorphous nature of the polymer [Citation55]. For POM, the positions of the narrow peaks are around 23°, 34.6°, 48.4°, and 54.1° for diffraction planes having Miller indices (100, 105, 115), and (205), respectively [Citation56], revealing the highly crystalline structure. XRD patterns of hybrids are shown in . From different peak levels and intensities of PP hybrids, it is proposed that d-spacing changes with different loading conditions of graphene derivatives [Citation39].
With POM hybrid nanocomposites (), it can also be seen that rGO/G polymer nanocomposite sheets exhibited sharp peaks, indicating that the nanosheets were packed into an ordered structure, as also suggested by the corresponding SEM images in . While the lack of a diffraction peak in the XRD pattern does not necessarily suggest the absence of stacked platelets [Citation57], in the XRD profiles of rGO/POM and G/POM composites, the broad diffraction peak corresponding to the aggregation of rGO/G nanosheets cannot be detected. This suggests that the presence of the POM efficiently prevented the aggregation of nanoparticles.
Surprisingly, the intersheet spacings for rGO/G in the PMMA hybrid nanocomposites were significantly larger than those seen in PP and POM hybrids with similar graphene oxide/polymer mass ratios. This observation may be explained by the distinctly different interactions that PP/POM and PMMA would have with graphene derivatives and conducting polymers. The polar amine groups of PPY and PANI would interact strongly with the hydrophilic groups in graphene derivatives, leading to ‘full’ interaction between both components and resulting in large changes of intersheet spacing with a small modification of the composition.
Contrariwise, interactions between the hydrophobic methacrylate groups of PMMA and graphene derivatives/conducting polymers would be weak. As such, PMMA chains would likely remain in looped conformations, which would more efficiently pack within the intersheet boundary, fitting well into the waving structures of the nanosheets of graphene derivative [Citation58,Citation59]. Such behavior would account for the relatively small increases in intersheet spacing for graphene derivatives-PMMA hybrid nanocomposites even at high polymer concentrations [Citation60]. As PP and POM are both highly crystalline polymers yet apparently give two extreme results in terms of electrical conductivity, an analysis on the effect of crystallinity has been done using XRD. However, the change is not large as observed from the XRD peaks and decreases with increasing concentrations of graphene materials and conducting polymers. Hence, a direct correlation between increasing content of graphene derivatives and decreasing intersheet spacing was observed for all the hybrids manufactured.
3.4. Morphological characterizaisation
To clarify the reinforcement mechanism and load transfer from the polymer matrix to the reinforcement, scanning electron micrographs were used to observe the morphology of the fracture surface of tensile samples. It can be seen from that PP hybrids have large agglomerated particles, while PMMA hybrids have pores and disconnected layers. Though POM hybrids show some crazing, the particles are found to be dispersed and to have high miscibility, which should lead to increased electrical conductivity.
3.4.1. Mechanism of hybridization
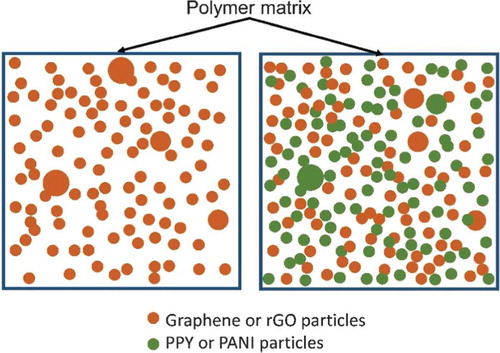
Both graphene materials and conductive polymers are conjugated systems. They are compatible with each other. Depending on the suitability of base polymer (thermoplastic matrix), conducting polymers and graphene materials transfer electrons freely. Even though maximum percolation threshold is identified with just graphene particulate composites, formation of agglomeration forms ‘gaps’ between particles forming ‘bridges.’ This leads to a decrease in electrical conductivity. From morphological analysis, these gaps would have been potentially filled by secondary conducting polymer, enhancing the conductive network in the composite. Except PP hybrid composites, this hypothesis applies to PP and POM ones, based on the SEM images showed in the previous section. proposes the possible hybridization mechanism in the composites developed which leads to improved electrical conductivity and mechanical properties.
4. Conclusion
Applying a variety of experimental and analytical techniques, we have been able to tailor a hybrid nanocomposite that is comparatively inexpensive, electrically conducting, and 20% stronger than the base polymer matrix. Comparing electrical conductivity of thermoplastic polymer reinforced (0.0001 S.cm−1) with graphene derivatives, electrical conductivity of hybrid composites developed in this study is 1000 times higher (0.9 S.cm−1). The same techniques have also given a broad overview of the combination space and properties that arise from it. With these experiments, we upgraded our existing knowledge about the influence of the different process parameters on the electrical conductivity. This study has shown the application of Taguchi method in the performance evaluation of a hybridization process for blending thermoplastics, conducting polymer and graphene materials. Regression modeling has helped us generate an equation to describe the statistical relationship between the process’s parameters and the response variable (electrical conductivity) and to predict new observations. The difference in the performance of rGO and G are likely due to factors such as:
percolation rates across particle-to-particle interfaces,
cross-linked connections giving a highly conductive network, and
charge transfer across the sample via ionic channels, compared with graphene.
These results also show how close the structure of rGO is to that of graphene, despite having defects, as the two materials have similar effects on the electrical and tensile properties of the hybrid composites. The hybrid nanocomposites of G/POM prepared by melt blending have the maximum conductivity of 0.85 S.cm−1 at the G/rGO loading of 3% and the conductivity drops over 4% loading due to the formation of agglomeration of graphene particles.
Acknowledgments
This work was supported by the Ministry of Business, Innovation, and Employment, New Zealand under Sustainable Research Grant. Thanks to CACM technicians for their technical support. We also thank Dr. Reuben Brown and Prof. Stoyko Fakirov for their help and assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Velram Balaji Mohan
Velram Balaji Mohan received a BTech in Polymer Technology from Anna University, India and an ME (Hons) in Materials and Process Engineering from the University of Waikato, New Zealand. Currently, he is working toward a PhD in the Centre for Advanced Composite Materials (CACM) at the University of Auckland, New Zealand on the development of functional graphene/polymer nanocomposites.
Krishnan Jayaraman
Krishnan Jayaraman received his bachelor’s degree in Mechanical Engineering with Honors from the University of Madras, India in 1984. He graduated with a master’s degree in Mechanical Engineering from Howard University, U.S.A. in 1986 and a PhD in Engineering Mechanics from Virginia Polytechnic Institute & State University, U.S.A. in 1992. He was a research associate in the Materials Response Group of Virginia Polytechnic Institute & State University from 1992 to 1993 and a postdoctoral fellow in the Pulp and Paper Centre of the University of Toronto, Canada from 1993 to 1995. He became a lecturer in the Department of Mechanical Engineering at the University of Auckland in 1995 and was promoted to the position of associate professor in 2013.
Debes Bhattacharyya
Debes Bhattacharyya is a distinguished professor (holds a Personal Chair) in the Department of Mechanical Engineering and has been the founding Director of the Centre for Advanced Composite Materials (CACM) at the University of Auckland, since 2004. In 2010, he was also appointed the Director of Plastics Centre of Excellence to synergize the research between the two centers. He also holds an adjunct professor position at Washington State University, Pullman, U.S.A. Professor Bhattacharyya was the Head of Mechanical Engineering Department from 1999 to early 2005 when he introduced a new degree in Mechatronics and successfully led the Department (was placed within top 5%) through the first Performance-Based Research Funding exercise.
References
- P. Ajayan, L. Schadler, P. Braun, and T. Russell, Library-nanocomposite science and technology, MRS Bull. 29 (2004), pp. 975.
- T. Pinnavaia and G. Beall, Polymer-Clay Nanocomposites, John Wiley & Sons, New York, NY, 2000.
- V.B. Mohan, D. Liu, K. Jayaraman, M. Stamm, and D. Bhattacharyya, Improvements in electronic structure and properties of graphene derivatives, Adv. Mater. Lett. 7 (2016), pp. 421–429. doi:10.5185/amlett.2016.6123
- X. An, H. Ma, B. Liu, and J. Wang, Graphene oxide reinforced polylactic acid/polyurethane antibacterial composites, J. Nanomater. (2013), article number 18.
- J.R. Potts, D.R. Dreyer, C.W. Bielawski, and R.S. Ruoff, Graphene-based polymer nanocomposites, Polymer 52 (2011), pp. 5–25. doi:10.1016/j.polymer.2010.11.042
- H.-B. Zhang, W.-G. Zheng, Q. Yan, Y. Yang, J.-W. Wang, Z.-H. Lu, G.-Y. Ji, and Z.-Z. Yu, Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding, Polymer 51 (2010), pp. 1191–1196. doi:10.1016/j.polymer.2010.01.027
- T.K. Das and S. Prusty, Graphene-based polymer composites and their applications, Polym.-Plast. Technol. 52 (2013), pp. 319–331. doi:10.1080/03602559.2012.751410
- W. Choi and J.-W. Lee, Graphene Synthesis and Applications, CRC Press, Boca Raton, FL, 2012.
- V.B. Mohan, R. Brown, K. Jayaraman, and D. Bhattacharyya, Characterisation of reduced graphene oxide: effects of reduction variables on electrical conductivity, Mater. Sci. Eng. B 193 (2015), pp. 49–60. doi:10.1016/j.mseb.2014.11.002
- R.J. Young, I.A. Kinloch, L. Gong, and K.S. Novoselov, The mechanics of graphene nanocomposites: a review, Compos. Sci. Technol. 72 (2012), pp. 1459–1476. doi:10.1016/j.compscitech.2012.05.005
- H. Tang, G.J. Ehlert, Y. Lin, and H.A. Sodano, Highly efficient synthesis of graphene nanocomposites, Nano Lett. 12 (2011), pp. 84–90. doi:10.1021/nl203023k
- H. Kim, Y. Miura, and C.W. Macosko, Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity, Chem. Mater. 22 (2010), pp. 3441–3450. doi:10.1021/cm100477v
- W. Qin, F. Vautard, L.T. Drzal, and J. Yu, Mechanical and electrical properties of carbon fiber composites with incorporation of graphene nanoplatelets at the fiber–matrix interphase, Compos. Part B 69 (2015), pp. 335–341. doi:10.1016/j.compositesb.2014.10.014
- A. Almajid, L. Sorochynska, K. Friedrich, and B. Wetzel, Effects of graphene and CNT on mechanical, thermal, electrical and corrosion properties of vinylester based nanocomposites, Plast. Rub. Comp. 44 (2015), pp. 50–62. doi:10.1179/1743289814Y.0000000117
- H. Aguilar-Bolados, M.A. Lopez-Manchado, J. Brasero, F. Avilés, and M. Yazdani-Pedram, Effect of the morphology of thermally reduced graphite oxide on the mechanical and electrical properties of natural rubber nanocomposites, Compos. Part B 87 (2016), pp. 350–356. doi:10.1016/j.compositesb.2015.08.079
- M. Saafi, L. Tang, J. Fung, M. Rahman, and J. Liggat, Enhanced properties of graphene/fly ash geopolymeric composite cement, Cem. Concr. Res. 67 (2015), pp. 292–299. doi:10.1016/j.cemconres.2014.08.011
- T. Kuilla, S. Bhadra, D. Yao, N.H. Kim, S. Bose, and J.H. Lee, Recent advances in graphene based polymer composites, Prog. Polym. Sci. 75 (2010), pp. 1350–1375. doi:10.1016/j.progpolymsci.2010.07.005
- N.D. Luong, N. Pahimanolis, U. Hippi, J.T. Korhonen, J. Ruokolainen, L.-S. Johansson, J.-D. Nam, and J. Seppälä, Graphene/cellulose nanocomposite paper with high electrical and mechanical performances, J. Mater. Chem. 21 (2011), pp. 13991–13998. doi:10.1039/c1jm12134k
- S. Bose, T. Kuila, M.E. Uddin, N.H. Kim, A.K.T. Lau, and J.H. Lee, In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites, Polymer 51 (2010), pp. 5921–5928. doi:10.1016/j.polymer.2010.10.014
- D. Galpaya, M. Wang, M. Liu, N. Motta, E. Waclawik, and C. Yan, Recent advances in fabrication and characterization of graphene-polymer nanocomposites, Graphene 1 (2012), pp. 30–49. doi:10.4236/graphene.2012.12005
- M. Hernández, M. Bernal, R. Verdejo, T.A. Ezquerra, and M.A. López-Manchado, Overall performance of natural rubber/graphene nanocomposites, Compos. Sci. Technol. 73 (2012), pp. 40–46. doi:10.1016/j.compscitech.2012.08.012
- P. Kun, O. Tapasztó, F. Wéber, and C. Balázsi, Determination of structural and mechanical properties of multilayer graphene added silicon nitride-based composites, Ceram. Int. 38 (2012), pp. 211–216. doi:10.1016/j.ceramint.2011.06.051
- C. Wu, X. Huang, G. Wang, L. Lv, G. Chen, G. Li, and P. Jiang, Highly conductive nanocomposites with three-dimensional, compactly interconnected graphene networks via a self-assembly process, Adv. Funct. Mater. 23 (2013), pp. 506–513. doi:10.1002/adfm.201201231
- H. Kim, A.A. Abdala, and C.W. Macosko, Graphene/polymer nanocomposites, Macromolecules 43 (2010), pp. 6515–6530. doi:10.1021/ma100572e
- S. Stankovich, D.A. Dikin, G.H.B. Dommett, K.M. Kohlhaas, E.J. Zimney, E.A. Stach, R.D. Piner, S.T. Nguyen, and R.S. Ruoff, Graphene-based composite materials, Nature 442 (2006), pp. 282–286. doi:10.1038/nature04969
- P. Steurer, R. Wissert, R. Thomann, and R. Mülhaupt, Functionalized graphenes and thermoplastic nanocomposites based upon expanded graphite oxide, Macromol. Rapid Commun. 30 (2009), pp. 316–327. doi:10.1002/marc.200800754
- S. Ansari and E.P. Giannelis, Functionalized graphene sheet-poly(vinylidene fluoride) conductive nanocomposites, J. Polym. Sci. Part B: Polym. Phys. 47 (2009), pp. 888–897. doi:10.1002/polb.21695
- R. Taipalus, T. Harmia, M.Q. Zhang, and K. Friedrich, The electrical conductivity of carbon-fibre-reinforced polypropylene/polyaniline complex-blends: experimental characterisation and modelling, Compos. Sci. Technol. 61 (2001), pp. 801–814. doi:10.1016/S0266-3538(00)00183-4
- K. Hyunwoo, A.A. Ahmed, and W.M. Christopher, Graphene/Polymer Nanocomposites. Graphite, Graphene, and Their Polymer Nanocomposites, CRC Press, Boca Raton, FL, 2012, pp. 513–556.
- C. Sellam, Graphene based nanocomposites for mechanical reinforcement, Ph.D. diss., Queen Mary University of London; 2015.
- M.F. Ashby, Engineering Materials 1: An Introduction to Properties, Applications, and Design, 4th ed., Butterworth-Heinemann, Boston, MA, 2012.
- M. Martin-Gallego, M.M. Bernal, M. Hernandez, R. Verdejo, and M.A. Lopez-Manchado, Comparison of filler percolation and mechanical properties in graphene and carbon nanotubes filled epoxy nanocomposites, Eur. Polym. J. 49 (2013), pp. 1347–1353. doi:10.1016/j.eurpolymj.2013.02.033
- M.A. Rafiee, J. Rafiee, Z. Wang, H. Song, Z.-Z. Yu, and N. Koratkar, Enhanced mechanical properties of nanocomposites at low graphene content, ACS Nano 3 (2009), pp. 3884–3890. doi:10.1021/nn9010472
- L.-C. Tang, Y.-J. Wan, D. Yan, Y.-B. Pei, L. Zhao, Y.-B. Li, L.-B. Wu, J.-X. Jiang, and G.-Q. Lai, The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites, Carbon 60 (2013), pp. 16–27. doi:10.1016/j.carbon.2013.03.050
- A. Satti, P. Larpent, and Y. Gun’ko, Improvement of mechanical properties of graphene oxide/poly(allylamine) composites by chemical crosslinking, Carbon 48 (2010), pp. 3376–3381. doi:10.1016/j.carbon.2010.05.030
- X. Wang, H. Yang, L. Song, Y. Hu, W. Xing, and H. Lu, Morphology, mechanical and thermal properties of graphene-reinforced poly(butylene succinate) nanocomposites, Compos. Sci. Technol. 72 (2011), pp. 1–6. doi:10.1016/j.compscitech.2011.05.007
- A.V. Raghu, Y.R. Lee, H.M. Jeong, and C.M. Shin, Preparation and physical properties of waterborne polyurethane/functionalized graphene sheet nanocomposites, Macromol. Chem. Phys. 209 (2008), pp. 2487–2493. doi:10.1002/macp.200800395
- P. Song, Z. Cao, Y. Cai, L. Zhao, Z. Fang, and S. Fu, Fabrication of exfoliated graphene-based polypropylene nanocomposites with enhanced mechanical and thermal properties, Polymer 52 (2011), pp. 4001–4010. doi:10.1016/j.polymer.2011.06.045
- M. El Achaby, F.-E. Arrakhiz, S. Vaudreuil, A. El Kacem Qaiss, M. Bousmina, and O. Fassi-Fehri, Mechanical, thermal, and rheological properties of grapheme-based polypropylene nanocomposites prepared by melt mixing, Polym. Compos. 33 (2012), pp. 733–744. doi:10.1002/pc.22198
- M. Fang, K. Wang, H. Lu, Y. Yang, and S. Nutt, Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites, J. Mater. Chem. 19 (2009), pp. 7098–7105. doi:10.1039/b908220d
- D. Cai, J. Jin, K. Yusoh, R. Rafiq, and M. Song, High performance polyurethane/functionalized graphene nanocomposites with improved mechanical and thermal properties, Compos. Sci. Technol. 72 (2012), pp. 702–707. doi:10.1016/j.compscitech.2012.01.020
- J. Wang, X. Wang, C. Xu, M. Zhang, and X. Shang, Preparation of graphene/poly(vinyl alcohol) nanocomposites with enhanced mechanical properties and water resistance, Polym. Int. 60 (2011), pp. 816–822. doi:10.1002/pi.3025
- J. Liang, Y. Huang, L. Zhang, Y. Wang, Y. Ma, T. Guo, and Y. Chen, Molecular-level dispersion of graphene into poly (vinyl alcohol) and effective reinforcement of their nanocomposites, Adv. Funct. Mater. 19 (2009), pp. 2297–2302. doi:10.1002/adfm.200801776
- X. Yang, L. Li, S. Shang, and X.-M. Tao, Synthesis and characterization of layer-aligned poly(vinyl alcohol)/graphene nanocomposites, Polymer 51 (2010), pp. 3431–3435. doi:10.1016/j.polymer.2010.05.034
- R.K. Layek, S. Samanta, D.P. Chatterjee, and A.K. Nandi, Physical and mechanical properties of poly(methyl methacrylate)-functionalized graphene/poly(vinylidine fluoride) nanocomposites: piezoelectric β polymorph formation, Polymer 51 (2010), pp. 5846–5856. doi:10.1016/j.polymer.2010.09.067
- D.R. Cox, The Theory of the Design of Experiments, Chapman & Hall, Boca Raton, FL, 2000.
- R. Tchoudakov, O. Breuer, M. Narkis, and A. Siegmann, Conductive polymer blends with low carbon black loading: polypropylene/polyamide, J. Polym. Eng. Sci. 36 (1996), pp. 1336–1346. doi:10.1002/pen.10528
- A.K. Pandaa and R. Singhb, Optimization of process parameters by taguchi method: catalytic degradation of polypropylene to liquid fuel, Int. J. Multidiscip. Curr. Res. (2013), pp. 50–54.
- Z.R. Lazic, Design of Experiments in Chemical Engineering: A Practical Guide, John Wiley & Sons, Weinheim, 2006.
- I. Ali, K. Jayaraman, and D. Bhattacharyya, Effects of resin and moisture content on the properties of medium density fibreboards made from kenaf bast fibres, Ind. Crop. Prod. 52 (2014), pp. 191–198. doi:10.1016/j.indcrop.2013.10.013
- B.M. Gopalsamy, B. Mondal, and S. Ghosh, Taguchi method and ANOVA: an approach for process parameters optimization of hard machining while machining hardened steel, J. Sci. Ind. Res. India. 68 (2009), pp. 686–695.
- O. Monticelli, S. Bocchini, A. Frache, E.S. Cozza, O. Cavalleri, and L. Prati, Simple method for the preparation of composites based on PA6 and partially exfoliated graphite, J. Nanometer. 25 (2012), pp. 1–5. doi:10.1155/2012/938962
- S.R. Ahmad, R.J. Young, and I.A. Kinloch, Raman spectra and mechanical properties of graphene/polypropylene nanocomposites, Int. J. Chem. Eng. Appl. 6 (2015), pp. 1–5. doi:10.7763/IJCEA.2015.V6.440
- D. Wang, X. Zhang, J.-W. Zha, J. Zhao, Z.-M. Dang, and G.-H. Hu, Dielectric properties of reduced graphene oxide/polypropylene composites with ultralow percolation threshold, Polymer 54 (2013), pp. 1916–1922. doi:10.1016/j.polymer.2013.02.012
- S. Devikala, P. Kamaraj, and M. Arthanareeswari, Conductivity and dielectric studies of PMMA composites, Chem. Sci. Trans. 2 (2013), pp. 129–134.
- K. Pielichowska, A. Szczygielska, and E. Spasówka, Preparation and characterization of polyoxymethylene-copolymer/hydroxyapatite nanocomposites for long-term bone implants, Polym. Adv. Technol. 23 (2012), pp. 1141–1150. doi:10.1002/pat.v23.8
- J.R. Potts, S.H. Lee, T.M. Alam, J. An, M.D. Stoller, R.D. Piner, and R.S. Ruoff, Thermomechanical properties of chemically modified graphene/poly(methyl methacrylate) composites made by in situ polymerization, Carbon 49 (2011), pp. 2615–2623. doi:10.1016/j.carbon.2011.02.023
- J. Brandrup, E.H. Immergut, and E.A. Grulke, Polymer Handbook, 4th ed., Wiley, New York, NY, 1999.
- J.A. Brydon, Plastics Materials, Butterworth-Heinemann, Oxford, 1999.
- K.W. Puts, O.C. Compton, M.J. Palmieri, S.T. Nguyen, and L.C. Brinson, High-nanofillers-content graphene oxide–polymer nanocomposites via vacuum-assisted self-assembly, Adv. Funct. Mater. 20 (2010), pp. 3322–3329. doi:10.1002/adfm.201000723

![Figure 1. Application areas of graphene-based materials [Citation7,Citation8].](/cms/asset/c9e9a421-e963-4ede-a1ed-59e0a17ab17a/tsnm_a_1237389_f0001_c.jpg)