?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Recent studies have highlighted the effects of various stimuli on the chemical reduction of graphene oxide (GO) through green reductant L-ascorbic acid (L-AA); however, the combination of near ultraviolet (NUV) light to increase the reduction rate has yet to be thoroughly explored. In this study, drop-casted GO films were subjected to chemical reduction through L-AA with various levels of exposure under 405 nm NUV radiation. The structure and uniformity of GO stackings that form the film were characterized through scanning electron microscopy (SEM) and wide-angle x-ray scattering (WAXS). Additionally, WAXS was used to track the removal of oxygen-containing functional groups along with Fourier-transform infrared (FT-IR) spectroscopy and x-ray photoelectron spectroscopy (XPS) as a function of L-AA and NUV light exposure times. XPS results demonstrated that the interaction between L-AA and NUV exposure has a significant effect on the reduction of films. Furthermore, the results that yielded the highest reduction (C-C bond concentration of 60.7%) were the longest L-AA and NUV light exposure times (48 hours and 3 hours, respectively). This report provides a study on the effects of NUV on the green reduction of GO films through L-AA with potential application in solar energy and chemical sensing applications.
Graphical abstract
Introduction
Graphene has been a highly regarded material since 2004 when coworkers Konstantin Novoselov and Andre Geim employed a simple mechanical exfoliation technique to obtain single-layered graphene [Citation1]. Graphene’s high modulus of elasticity [Citation2], thermal conductivity [Citation3], and electron mobility [Citation4] have enabled its use in various applications including high-end composite materials [Citation2,Citation5,Citation6], sensors [Citation7–9], electronics [Citation10,Citation11], and energy storage devices [Citation12–14]. Additionally, its optical transmittance [Citation15,Citation16] and high surface area [Citation17] make graphene an ideal material for energy collection devices [Citation18,Citation19]. Several methods have been developed over the years to produce graphene including mechanical exfoliation [Citation20–22], chemical vapor deposition [Citation23–25], and liquid phase exfoliation [Citation26–28]. Nevertheless, these production methods are associated with either high cost of production or low scalability which limit the use of graphene in commercial applications [Citation29]. Consequently, one of the more well-studied methods of recent years has been the reduction of graphene oxide (GO) due to its scalability [Citation22,Citation30,Citation31] and tunability [Citation32,Citation33]. GO is typically synthesized by oxidizing graphite using a strong acid mixture such as potassium chlorate (KClO3) with nitric acid (HNO3) as described by the Brodie and Staudenmaier methods [Citation34], or by using potassium permanganate (KMnO4) with sulfuric acid (H2SO4) in the more commonly used Hummer’s method [Citation35–37]. The produced multi-layered GO is then subjected to liquid exfoliation by sonication in water or organic solvents to produce single-layered GO [Citation38]. In order to remove oxygen functionalities and obtain a product with comparable properties to graphene, GO is reduced by various means [Citation39], although primarily through a chemical reduction process [Citation40,Citation41].
As an efficient chemical reductant, hydrazine has been the most widely used, but its toxicity and adverse effects on the environment [Citation42,Citation43] have prompted a search for green alternatives such as plant extracts [Citation44–46], microorganisms [Citation47–49], sugars [Citation50], proteins [Citation51,Citation52], and organic acids [Citation53]. While these alternatives are much safer for the environment, the quality of the reduced graphene oxide produced, as characterized by various chemical structural characterization methods, cannot yet reproduce the quality of reduced GO produced from using hydrazine [Citation54]. However, few alternatives including l-ascorbic acid [Citation55], glucose [Citation56], starch-based materials [Citation57], hydrogen-rich water [Citation58], ginseng [Citation59], natural cellulose [Citation60], backer’s yeast [Citation61], and gallic acid [Citation62], amongst others [Citation63–68] have shown reduction efficiencies comparable to hydrazine.
Among these, L-ascorbic acid (L-AA), an organic acid, has demonstrated its potential to be a green, low cost, and the best alternative to hydrazine due to its anti-oxidative properties and abundance. The reduction of GO through L-AA has been demonstrated to occur at room temperature [Citation69]; however, conditions such as alkaline pH, high L-AA to GO ratio, and applied heating can increase the rate of reduction from 48 hours, to nearly under 1 hour. In addition, stirring and sonication have also been shown to affect the rate of reduction as well as the quality of the reduced product [Citation70].
Previous studies have shown that L-AA is highly photosensitive in the ultraviolet spectrum [Citation71,Citation72], and as such, relevant parameters such as radiation type, wavelength, and power of light source could be used to directly affect the effectiveness of L-AA as a green reducing agent for graphene oxide. In this study, the effects of NUV light on the reductive behavior of green reductant L-AA in GO films are investigated. Our wide-angle x-ray scattering (WAXS) analysis after 48-hour exposure to L-AA, shows that GO films exhibit variable degrees of reduction depending on the length of exposure to L-AA, as it is revealed by the detection of two distinct peaks, corresponding to the c-lattice spacing of two distinct structural phases within the films. X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared (FT-IR) spectroscopy were also used to further analyze the degree of reduction and provide evidence that chemical methods under external stimuli can prove to be effective at obtaining metastable reduced graphene oxide films, in comparison to previous reports [Citation73]. Additionally, NUV-radiation was shown to assist in preventing the films from redispersing in the aqueous solution by making them hydrophobic in the surface. As a result, the films can be immersed in aqueous solutions for longer periods without the risk of resuspension.
Materials and methods
Materials
Graphene oxide in a 5 mg/mL suspension was purchased from Goographene (Virginia, United States). L-ascorbic acid (99% purity) was obtained from Sigma Aldrich (Darmstadt, Germany). Graphene oxide (GO) films were prepared by drop-casting 15 mL of GO solution onto a quartz glass substrate and dried overnight in air at room temperature. An aqueous solution composed of 5 g of L-ascorbic acid (L-AA) and 20 mL of deionized (DI) water, based on a previous report [Citation74], was carefully pipetted onto the GO films and left soaking for different time periods. GO films in L-AA solution were irradiated by a 405 nm wavelength near ultraviolet (NUV) light using a FormCure from Formlabs (Massachusetts, United States) for different lengths, 1 hour, 2 hours, and 3 hours. After the L-AA solution and NUV light exposure, films were washed and neutralized with DI water to remove the L-AA from the films. GO films were then left to dry overnight in air at room temperature.
Characterization
The thickness and surface morphology of GO films were characterized through scanning electron microscopy (SEM) on a thermionic ISM-IT500 from JEOL (Massachusetts, United States) under high vacuum, 10.0 kV accelerating voltage using a secondary electron detector. The efficiency of NUV exposure in the removal of oxygen-containing groups was analyzed through Fourier transform infrared (FT-IR) spectroscopy using a Cary 630 FTIR spectrometer from Agilent (California, United States). Wide-angle x-ray scattering (WAXS) measurements were carried out using a Xenocs Xeuss 2.0 HR SAXS/WAXS system (Sassenage, France) with a Cu source tuned to λ = 0.1542 nm. In WAXS, x-rays scattered as a function of the scattering angle 2θ with respect to the transmitted direct beam were collected on the detector. The 2-D data were azimuthally averaged and plotted as I(Q). Here, Q is given by Equationequation 1(1)
(1) ,
where θ is half the scattering angle, and λ is the wavelength of the source beam. I(Q) was scaled to units of differential scattering cross-section per unit volume (cm−1) using a glassy carbon intensity calibration standard [Citation75]. To ensure that samples produced isotropic scattering when hit by the x-ray beam, films were crushed into a fine powder and vacuum dried (Supplementary Section). Furthermore, the interlayer spacing, d, for the GO samples was calculated using Bragg’s law (Equationequation 2(2)
(2) )[Citation76],
X-ray photoelectron spectroscopy (XPS) was performed on an AXIS ULTRA X-Ray Photoelectron Spectrometer from Kratos (Manchester, United Kingdom). In order to acquire representative data for the entire film, samples were ground up into a powder and multiple readings were taken from different locations.
Results
Description of GO/rGO films
The drop-casting method produced uniform graphene oxide (GO) films ()); however, due to the hydrophilic properties of GO, special care was given to the samples when immersed in L-AA solution and when washed to avoid agitation that would lead to the films breaking apart from resuspension or redispersion. NUV-irradiated films and films with longer (over 4 hours) L-AA exposure demonstrated higher integrity in the aqueous solution than the controlled GO films and films with one, two, and 3 hours of exposure to L-AA solution, which is an indication of higher degree of reduction considering the hydrophobic nature of reduced graphene [Citation77,Citation78].
Scanning electron microscopy
The thickness of the GO films was estimated from the cross-section of the films ( (C,D)) at multiple locations through scanning electron microscopy (SEM). An average film thickness of 2.89 ± 0.14 μm was obtained. The cross-section of the GO films revealed a tightly packed, layered structure formed by graphene oxide laminates. Furthermore, small pockets can be observed in treated samples ()) likely due to partial intercalation of water and L-AA solution in the inner GO stackings without complete delamination.
Figure 2. Scanning electron micrographs of graphene oxide films treated with L-ascorbic acid and near ultraviolet light displaying folds on the surface (A and B). Cross-sectional micrographs of the film reveal waviness in a stacked structure
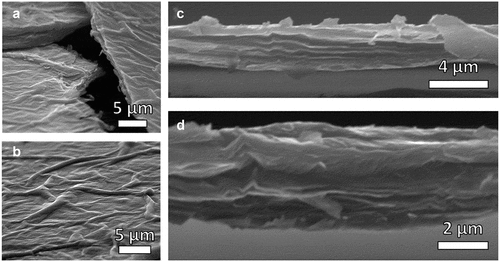
From the micrographs of the film’s surface ()), it can be noted that the films have wrinkles and folds throughout averaging a width of 1.73 μm and varying in length. These wrinkles are caused by drying and are prominent in graphene oxide films during the evaporation of water through drop-casting [Citation79–81].
Fourier transform infrared spectroscopy
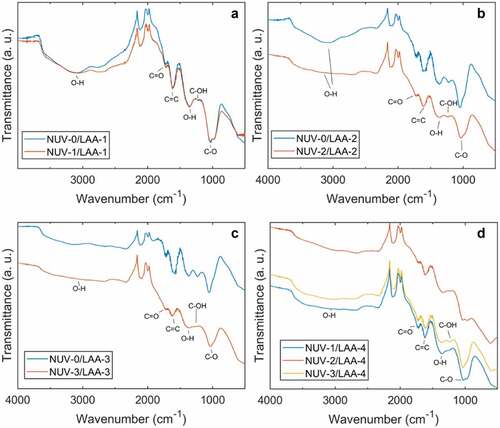
Graphene oxide peaks located at 1029 cm−1 (C-O stretching), 1262 cm−1 (C-OH bending), 1340 cm−1 (O-H bending), 1616 cm−1 (C = C stretching), 1728 cm−1 (C = O stretching), and 3116 cm−1 (O-H stretching) were observed (). Samples soaked in L-AA solution for 1 hour ()) did not show significant differences between NUV-irradiated and unirradiated GO. The presence of the narrow band of OH vibrations further suggests the presence of intercalated water in the films. Flattening of this band was observed to occur within 2 hours in irradiated samples ()) while the same feature was not observed in unirradiated samples until 3 hours of soak time in L-AA ()). After 3 hours of continuous NUV-radiation ()), samples show a clear flattening of the peak corresponding to the C-OH bonds bending, which is an indication reduction is taking place.
Wide-angle X-ray scattering
WAXS was used to quantify the changes in interlayer spacing caused by the removal of oxygen functionalities in the GO films. The peak shift within the first 3 hours of reduction was compared between NUV-irradiated and unirradiated films ()). A noticeable shift occurred within the first hour regardless of radiation conditions, decreasing the spacing of 8.07 Å by 0.91 Å and 0.76 Å from NUV-radiation with L-AA solution and L-AA solution alone, respectively. This large shift is caused by the repacking of the GO layers from the initial removal of oxygen-containing groups due to the exposure to L-AA. The peak at 2θ angle 26.5° (d = 3.36 Å) is observed in all samples and is due to residual graphite from the commercial GO solution [Citation82].
Figure 4. Wide-angle x-ray scattering (WAXS) spectra of pulverized graphene oxide films with increasing exposure to L-ascorbic acid alone (A) and NUV radiation (B). Additionally, WAXS spectra of GO films irradiated after 48 hours of exposure to L-AA solution compare (C) the two NUV radiation extremes (0 hours vs 3 hours) and (D) different NUV radiation times
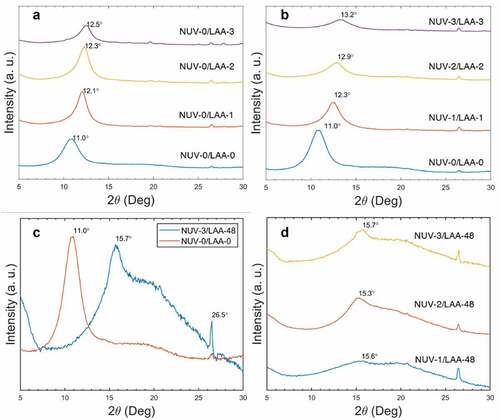
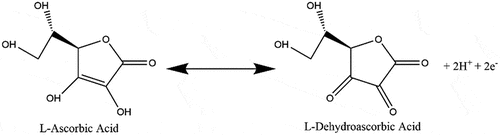
After the first hour, differences in peak location, and therefore interlayer spacing, between NUV-irradiated and unirradiated samples became more prominent. While samples immersed in L-AA solution indicated a decrease in spacing by 0.14 Å between the first and second hour and 0.10 Å between the second and third hour, irradiated samples saw a more notable decrease in spacing of 0.30 Å between the first and second hour and 0.10 Å between the second and third hour. This difference was attributed to NUV light assisting in further degrading the L-ascorbic acid ion, L-ascorbate, into dehydroascorbic acid at a faster rate (), an issue well studied in products containing high concentrations of vitamin C with beneficial effects in the reduction of GO [Citation69,Citation83]. It was also noted that the decrease in spacing does not occur at a linear rate as demonstrated by both conditions. A greater decrease in the rate is seen in NUV-irradiated samples potentially caused by the consumption of L-AA and decrease of oxygen-containing groups.
Figure 5. L-ascorbic acid degradation (sped up by NUV light) into L-dehydroascorbic acid yielding two H+ ions which act as antioxidants and react with hydroxyls and epoxies in the surface of graphene oxide
No significant reduction occurred past 24 hours in the segments of the GO film that were partially reduced. Films exposed to L-AA for longer periods, 24 hours to 48 hours, displayed relatively similar locations of peaks at 2θ angles near 15.6° (d = 5.68 Å) independent of radiation time as shown in ). Moreover, a broad, intense peak appeared past the 20.0° (d = 4.44 Å), characteristic of partially reduced graphene oxide and near full reduction [Citation84]. These peaks confirm the reduction of graphene oxide to various degrees within films, achieving results comparable to previous studies using hydrazine [Citation73] as the reductant. In the previous study of hydrazine-reduced GO films, a bimodal distribution of unreduced and reduced GO films was obtained.
In this study, all the starting material was involved in the reduction process. One fraction of the material gave rise to a broad diffraction peak (2θ = 15.6°-26.5°), indication of reduced graphene oxide and its corresponding distorted, non-crystalline stacking of graphene sheets; the other fraction of the material exhibited partial reduction with L-AA intercalation, but incomplete removal of resulting species from the interlayers ( (C,D)). The broadness is further evidence to suggest the uniformity of the reduction method.
X-ray photoelectron spectroscopy
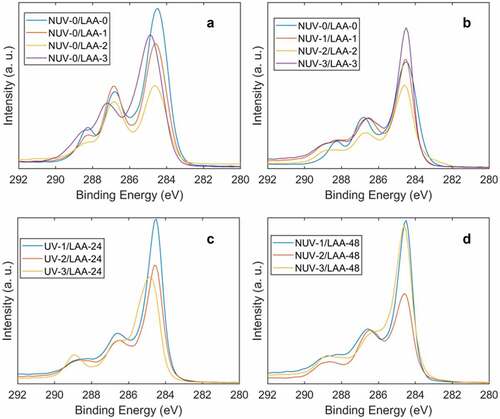
X-ray photoelectron spectroscopy (XPS) was used to track the decrease in concentration of oxidized C species (C-Ox) as a function of exposure time to NUV-radiation and L-ascorbic acid (L-AA). Initial GO films showed an O/C ratio of 0.29 which is much lower than GO synthesized through the modified Hummer Method (0.41) [Citation85]. The lower O/C ratio could be due to the presence of graphite contaminants in the commercial GO solution used. This is further supported by the WAXS results through the presence of the graphite peak at 2θ = 26°.5, present in all samples regardless of NUV radiation or L-AA exposure. The effects of continuous NUV-exposure were studied by comparing the C1s spectra () and C-C bond concentration ()) of irradiated films with non-irradiated films for the first 3 hours. In all three instances, irradiated films show a larger degree of reduction in comparison to the un-irradiated films as observed by the increase in intensity of the peak near a binding energy of 285 eV (C-C) and the decrease of the peak near a binding energy of 287 eV (O-C = O). Additionally, while irradiated samples displayed some reductive behavior having an increase in C-C bond concentrations, the opposite was observed in un-irradiated samples. A large variance in reduction of the film was noted in the data acquired for unirradiated samples increasing with every hour.
Discussion
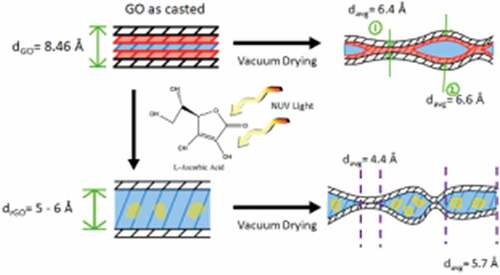
This variation in the graphene oxide reduction films could be largely due to intercalation of water and L-AA reduction byproducts in the GO film, a common problem with green reductants as illustrated in [Citation84]. When drop-casted, the graphene oxide tends to self-align and stack neatly on top of one another forming a film with roughly one water molecule hydrogen bonded between the oxygen functionalities of two GO layers, as evident by the presence of the OH band in the FT-IR results. Upon vacuum drying, the film begins to repack as moisture between the stacks exits [Citation86]. Due to the hydrophilic nature of GO, pockets can with water molecules remain, as demonstrated by the WAXS results (Supplemental Figure 1). When immersed in L-AA solution, reduction begins on the surface of the film and slowly makes its way through paths created from spaces and cavities in the loosely stacked structures [Citation86] entering tight areas of the inner layers of the film. Vacuum drying releases the moisture in the films but may leave adsorbed molecular water and L-AA residuals (glucuronic acid and oxalic acids) which may form hydrogen bonds with residual oxygen functionalities [Citation69] that could not be removed easily through washing.
Figure 8. Schematic of GO films demonstrating the effect that vacuum drying on the casted films before and after reduction based on WAXS and XPS results
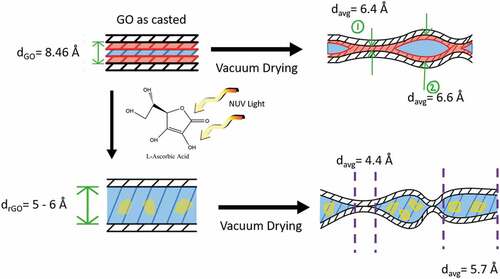
It is shown in the literature that graphene oxide paper/films can rapidly desorb around 80% of the adsorbed moisture using wrinkle-like tunnels such as those shown in ())[Citation81]; however, other factors affect the moisture desorption of the reduced graphene oxide film. As Boukhalov et al. have demonstrated through simulation, the interlayer distance between graphene sheets plays a crucial role in the water-permeation mechanism [Citation87]. The decrease in interlayer distance from the reduction of the graphene oxide can make the membrane 100 times less permeable to water and create blockage when the interlayer spacing drops to around 4 Å, as demonstrated experimentally by Nair et al. [Citation88]. While it is suggested that the formation of percolated capillaries from unoxidized or reduced regions assists the flow of water [Citation87], the probability of lenticular pores forming between impermeable passages which would essentially seal water in cannot be discarded. Due to the modest heat applied during the vacuum drying to limit the influence on reduction, it is unlikely that lenticular pores would be ruptured. In addition, intercalated water in the films can restrict the movement of other molecules, in this case, glucuronic acid and oxalic acid whose presence near the films’ surface can influence the XPS readings. Due to the impermeability to molecules other than water, chemical approaches are not suggested as appropriate methods to reduce graphene oxide stacked structures such as films, membranes, or papers.
Most samples that have been irradiated demonstrate significantly less variance in the degree of reduction. This could be due to the NUV light penetrating past the surface layers. Since graphene oxide has relatively lower absorbance at the visible NUV light range (400–405 nm) than UV light, this means that NUV light can reach deeper into the film and partially reduce the graphene oxide in the center layers of the film up to a certain depth [Citation89]. Go et al. [Citation90] have shown through UV-Vis that UV light is capable of reducing graphene oxide at a similar rate as L-AA alone; however, these experiments were done using 252 nm UV-radiation which can lead to some discrepancies since GO has a greater absorption at that particular wavelength.
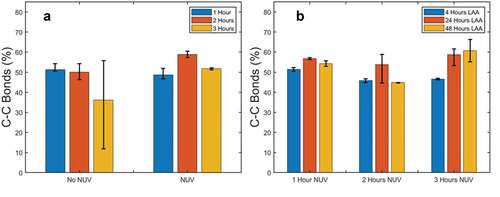
The concentrations of C-C bonds in the samples were measured after being exposed to L-AA for 4 hours, 24 hours, and 48 hours at the three NUV-radiation levels ()), and the statistical significance of these results was validated using Minitab. The normal probability plots provided in the supplemental () indicate that the results follow a normal distribution and are statistically acceptable for the design of experiment analysis. From the main effects plot (supplementary Figure 5), it was determined that all factors are significant with exposure to L-AA having the highest impact, followed by the interaction between L-AA and NUV exposure, and lastly NUV-radiation meaning that after the initial NUV-exposure, the reduction of the film became more dependent on the exposure to L-AA. While NUV light works in conjunction with L-AA to reduce the surface of the film, L-AA plays a more important role after the radiation in reducing the underlying layers, therefore, exposure to L-AA is necessary to reduce inner layers of the film and improve uniformity. The low impact of NUV exposure alone is likely caused by the reduction of the film’s surface. As the film was irradiated and reduced, graphene oxide began absorbing more light at 405 nm wavelength [Citation90] until reaching a point where the effects of NUV light were negligible. After this point, only the solution that has entered the inner layers of the film through gaps furthered the reduction.
The optimization graph obtained through the design of the experiment (supplementary Figure 7) suggests that the combination that will maximize the reduction is longer NUV-exposure time (3 hours) and longer exposure to L-AA (48 hours) producing samples with a C-C bond concentration of 60.70% and an O/C ratio of 0.23, which is comparable to, although higher than, GO suspensions reduced by green reductants ().
Table 1. Comparison of O/C ratio in most reduced samples reported (three-hour NUV-radiation and 48 hrs L-AA exposure) with values reported in the literature from other green reductants
Conclusion
The effect of NUV light in conjunction with L-ascorbic acid (L-AA) on the reduction of graphene oxide films was characterized using SEM, FT-IR, WAXS, and XPS. It was determined that NUV-radiation at 405 nm wavelength can promote the reduction of graphene oxide by not only accelerating the degradation of L-AA and subsequently the reduction of graphene oxide, but also increasing the depth of reduction from the surface layers to the layers in between the film. Samples exposed to 3 hours of NUV-radiation also showed that longer exposures of NUV-radiation can have a significant impact in the reduction of graphene oxide films within the first hours, meaning that longer exposure is recommended to obtain a more uniformly reduced film. Exposure time in L-AA was also proven to be a main factor in reducing the graphene oxide past the surface layer, with 48 hours of exposure showing concentrations of C-C bonds of up to 60.7% and the presence of an intense peak in the 2θ angle near 20.0°, comparable to solution processed graphene oxide reduced by other green methods. However, it is important to note that the lowered permeability of the reduced may entrap moisture and chemical residuals from the reductant. Still, alternately to using high-temperature thermal annealing which can result in blistering of the film due to the volatile release of vapors and air or other chemical methods with a large variance in reduction of the film, the use of L-AA synergistically with NUV-radiation can produce stable GO films with tailored reduction.
Supplemental Material
Download MS Word (1.8 MB)Acknowledgments
This work was supported by the US Department of Energy’s National Nuclear Administration under Grant No. DE-NA-0003865. Additionally, Jaime Regis would like to thank the National Science Foundation Bridge to the Doctorate Fellowship under Grant No. HRD-1810898 and the National Science Foundation Graduate Research Fellowship under Grant No. 1848741 for their support. The authors would also like to thank Angelica Benavidez for providing the X-Ray Photoelectron Spectroscopy data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Novoselov KS, Geim AK, Morozov SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. .
- Papageorgiou DG, Kinloch IA, Young RJ. Mechanical properties of graphene and graphene-based nanocomposites. Prog Mater Sci. 2017;90:75–127.
- Balandin AA, Ghosh S, Bao W, et al. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008;8(3):902–907. .
- Bolotin KI, Sikes KJ, Jiang Z, et al. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008;146(9–10):351–355. .
- Atif R, Shyha I, Inam F. Mechanical, thermal, and electrical properties of graphene-epoxy nanocomposites—A review. Polymers. 2016;8(8):281.
- Li Y, Feng Z, Huang L, et al. Additive manufacturing high performance graphene-based composites: a review. Compos Part Appl Sci Manuf. 2019;124:105483.
- Mehmood A, Mubarak NM, Khalid M, et al. Graphene based nanomaterials for strain sensor application—a review. J Environ Chem Eng. 2020;8:103743.
- Nag A, Mitra A, Mukhopadhyay SC. Graphene and its sensor-based applications: a review. 2018;270:177–194. Sens Actuators Phys.
- Wang Q, Arash B. A review on applications of carbon nanotubes and graphenes as nano-resonator sensors. Comput Mater Sci. 2014;82:350–360.
- Torrisi F, Carey T. Graphene, related two-dimensional crystals and hybrid systems for printed and wearable electronics. Nano Today. 2018;23:73–96.
- Tran TS, Dutta NK, Choudhury NR. Graphene inks for printed flexible electronics: graphene dispersions, ink formulations, printing techniques and applications. Adv Colloid Interface Sci. 2018;261:41–61.
- Lv W, Li Z, Deng Y, et al. Graphene-based materials for electrochemical energy storage devices: opportunities and challenges. Energy Storage Mater. 2016;2:107–138.
- Sun H, Mei L, Liang J, et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science. 2017;356(6338):599–604. .
- Hooch Antink W, Choi Y, Seong K, et al. Recent progress in porous graphene and reduced graphene oxide-based nanomaterials for electrochemical energy storage devices. Adv Mater Interfaces. 2018;5(5):1701212.
- Falkovsky LA. Optical properties of graphene. J Phys Conf Ser. 2008;129:012004.
- Zhu S-E, Yuan S, Janssen GCAM. Optical transmittance of multilayer graphene. EPL Europhys Lett. 2014;108(1):17007.
- Zhu Y, Murali S, Cai W, et al. Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater. 2010;22(35):3906–3924. .
- Altuntepe A, Seyhan A, Zan R. Graphene for Si-based solar cells. J Mol Struct. 2020;1200:127055.
- Chandrasekhar PS, Dubey A, Qiao Q. High efficiency perovskite solar cells using nitrogen-doped graphene/Zno nanorod composite as an electron transport layer. Sol Energy. 2020;197:78–83.
- Sinclair RC, Suter JL, Coveney PV. Micromechanical exfoliation of graphene on the atomistic scale. Phys Chem Chem Phys. 2019;21(10):5716–5722.
- Gao E, Lin S-Z, Qin Z, et al. Mechanical exfoliation of two-dimensional materials. J Mech Phys Solids. 2018;115:248–262.
- Yi M, Shen Z. A review on mechanical exfoliation for the scalable production of graphene. J Mater Chem A. 2015;3(22):11700–11715.
- Juang Z-Y, Wu C-Y, Lu A-Y, et al. Graphene synthesis by chemical vapor deposition and transfer by a roll-to-roll process. Carbon. 2010;48(11):3169–3174. .
- Li X, Colombo L, Ruoff RS. Synthesis of graphene films on copper foils by chemical vapor deposition. Adv Mater. 2016;28(29):6247–6252.
- Muñoz R, Gómez‐aleixandre C. Review of CVD synthesis of graphene. Chem Vap Depos. 2013;19(10–11–12):297–322.
- Amiri A, Naraghi M, Ahmadi G, et al. A review on liquid-phase exfoliation for scalable production of pure graphene, wrinkled, crumpled and functionalized graphene and challenges. FlatChem. 2018;8:40–71.
- Gu X, Zhao Y, Sun K, et al. Method of ultrasound-assisted liquid-phase exfoliation to prepare graphene. Ultrason Sonochem. 2019;58:104630.
- Hernandez Y, Nicolosi V, Lotya M, et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol. 2008;3(9):563–568. .
- Zhong YL, Tian Z, Simon GP, et al. Scalable production of graphene via wet chemistry: progress and challenges. Mater Today. 2015;18(2):73–78.
- Abdolhosseinzadeh S, Asgharzadeh H, Kim HS. Fast and fully-scalable synthesis of reduced graphene oxide. Sci Rep. 2015;5(1):1–7.
- Zhou X, Liu Z. A scalable, solution-phase processing route to graphene oxide and graphene ultralarge sheets. Chem Commun. 2010;46(15):2611–2613.
- Loh KP, Bao Q, Eda G, et al. Graphene oxide as a chemically tunable platform for optical applications. Nat Chem. 2010;2(12):1015–1024.
- Mei Q, Liu B, Han G, et al. Graphene oxide: from tunable structures to diverse luminescence behaviors. Adv Sci. 2019;6(14):1900855.
- Dreyer DR, Park S, Bielawski CW, et al. The chemistry of graphene oxide. Chem Soc Rev. 2010;39:228–240.
- Zaaba NI, Foo KL, Hashim U, et al. Synthesis of graphene oxide using modified Hummers method: solvent influence. Procedia Eng. 2017;184:469–477.
- Guerrero-Contreras J, Caballero-Briones F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater Chem Phys. 2015;153:209–220.
- Botas C, Álvarez P, Blanco P, et al. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon. 2013;65:156–164.
- Dideykin A, Aleksenskiy AE, Kirilenko D, et al. Monolayer graphene from graphite oxide. Diam Relat Mater. 2011;20(2):105–108. .
- Pei S, Cheng H-M. The reduction of graphene oxide. Carbon. 2012;50(9):3210–3228.
- Kiang Chua C, Pumera M. Chemical reduction of graphene oxide: a synthetic chemistry viewpoint. Chem Soc Rev. 2014;43(1):291–312.
- Guex LG, Sacchi B, Peuvot KF, et al. Experimental review: chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale. 2017;9(27):9562–9571. .
- Park S, An J, Potts JR, et al. Hydrazine-reduction of graphite- and graphene oxide. Carbon. 2011;49(9):3019–3023.
- Gao X, Jang J, Nagase S. Hydrazine and thermal reduction of graphene oxide: reaction mechanisms, product structures, and reaction design. J Phys Chem C. 2010;114(2):832–842.
- Khan M, Al-Marri AH, Khan M, et al. Green approach for the effective reduction of graphene oxide using Salvadora persica L. root (miswak) extract. Nanoscale Res Lett. 2015;10(1):281. .
- Lee G, Kim BS. Biological reduction of graphene oxide using plant leaf extracts, biotechnol. Prog. 2014;30:463–469.
- Maddinedi SB, Mandal BK, Vankayala R, et al. Bioinspired reduced graphene oxide nanosheets using terminalia chebula seeds extract. Spectrochim Acta A Mol Biomol Spectrosc. 2015;145:117–124.
- Gurunathan S, Han JW, Eppakayala V, et al. Microbial reduction of graphene oxide by Escherichia coli: a green chemistry approach. Colloids Surf B Biointerfaces. 2013;102:772–777.
- Wang G, Qian F, Saltikov CW, et al. Microbial reduction of graphene oxide by Shewanella. Nano Res. 2011;4:563–570.
- Akhavan O, Ghaderi E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon. 2012;50(5):1853–1860.
- Li B, Jin X, Lin J, et al. Green reduction of graphene oxide by sugarcane bagasse extract and its application for the removal of cadmium in aqueous solution. J Clean Prod. 2018;189:128–134.
- Guo C, Book-Newell B, Irudayaraj J. Protein-directed reduction of graphene oxide and intracellular imaging. Chem Commun. 2011;47(47):12658–12660.
- Sheng Z, Song L, Zheng J, et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials. 2013;34(21):5236–5243. .
- Kauppila J, Kunnas P, Damlin P, et al. Electrochemical reduction of graphene oxide films in aqueous and organic solutions. Electrochim Acta. 2013;89:84–89.
- De Silva KKH, Huang -H-H, Joshi RK, et al. Chemical reduction of graphene oxide using green reductants. Carbon. 2017;119:190–199.
- Fernández-Merino MJ, Guardia L, Paredes JI, et al. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J Phys Chem C. 2010;114(14):6426–6432. .
- Akhavan O, Ghaderi E, Aghayee S, et al. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J Mater Chem. 2012;22(27):13773–13781.
- Feng Y, Feng N, Du G. A green reduction of graphene oxide via starch-based materials. RSC Adv. 2013;3(44):21466–21474.
- Akhavan O, Azimirad R, Gholizadeh HT, et al. Hydrogen-rich water for green reduction of graphene oxide suspensions. Int J Hydrogen Energy. 2015;40(16):5553–5560.
- Akhavan O, Ghaderi E, Abouei E, et al. Accelerated differentiation of neural stem cells into neurons on ginseng-reduced graphene oxide sheets. Carbon. 2014;66:395–406.
- Peng H, Meng L, Niu L, et al. Simultaneous reduction and surface functionalization of graphene oxide by natural cellulose with the assistance of the ionic liquid. J Phys Chem C. 2012;116(30):16294–16299.
- Khanra P, Kuila T, Kim NH, et al. Simultaneous bio-functionalization and reduction of graphene oxide by baker’s yeast. Chem Eng J. 2012;183:526–533.
- Li J, Xiao G, Chen C, et al. Superior dispersions of reduced graphene oxide synthesized by using gallic acid as a reductant and stabilizer. J Mater Chem A. 2012;1(4):1481–1487.
- Chamoli P, Das MK, Kar KK. Temperature dependence green reduction of graphene oxide by urea. Adv Mater Lett. 2017;8(3):217–222.
- Akhavan O, Ghaderi E, Esfandiar A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and Inactivation by near-infrared irradiation. J Phys Chem B. 2011;115(19):6279–6288.
- Akhavan O, Ghaderi E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 Thin film for photoinactivation of bacteria in solar light irradiation. J Phys Chem C. 2009;113(47):20214–20220.
- Ghadim EE, Rashidi N, Kimiagar S, et al. Pulsed laser irradiation for environment friendly reduction of graphene oxide suspensions. Appl Surf Sci. 2014;301:183–188.
- Chae W, Kim M, Kim D, et al. Photo-Reduction of graphene oxide by using photographic flash-light. Sci Adv Mater. 2018;10(1):130–133.
- Zhou Y, Bao Q, Tang LAL, et al. Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem Mater. 2009;21(13):2950–2956.
- Zhang J, Yang H, Shen G, et al. Reduction of graphene oxide via l-ascorbic acid. Chem Commun. 2010;46(7):1112–1114.
- Abulizi A, Okitsu K, Zhu -J-J. Ultrasound assisted reduction of graphene oxide to graphene in l-ascorbic acid aqueous solutions: kinetics and effects of various factors on the rate of graphene formation. Ultrason Sonochem. 2014;21(3):1174–1181.
- Usaga J, Manns DC, Moraru CI, et al. Ascorbic acid and selected preservatives influence effectiveness of UV treatment of apple juice. LWT. 2017;75:9–16.
- Aguilar K, Garvín A, Lara-Sagahón AV, et al. Ascorbic acid degradation in aqueous solution during UV-Vis irradiation. Food Chem. 2019;297:124864.
- Hindawi, Synthesis and characterization of graphene thin films by chemical reduction of exfoliated and intercalated graphite oxide. Accessed on 04/17/2020. Available at https://www.hindawi.com/journals/jchem/2013/150536/.
- Dua V, Surwade SP, Ammu S, et al. All-organic vapor sensor using inkjet-printed reduced graphene oxide. Angew Chem Int Ed. 2010;49(12):2154–2157. .
- Zhang F, Ilavsky J, Long GG, et al. Glassy carbon as an absolute intensity calibration standard for small-angle scattering. Metall Mater Trans A. 2010;41(5):1151–1158.
- Moore DM, Reynolds RC. X-ray diffraction and the identification and analysis of clay minerals. X-Ray Diffr Identif Anal Clay Miner. 1989; pp. 332.
- Kanayama I, Miyaji H, Takita H, et al. Comparative study of bioactivity of collagen scaffolds coated with graphene oxide and reduced graphene oxide. Int J Nanomedicine. 2014;9:3363–3373.
- Wang K, Pang J, Li L, et al. Synthesis of hydrophobic carbon nanotubes/reduced graphene oxide composite films by flash light irradiation. Front Chem Sci Eng. 2018;12(3):376–382.
- Shen X, Lin X, Yousefi N, et al. Wrinkling in graphene sheets and graphene oxide papers. Carbon. 2014;66:84–92.
- Dikin DA, Stankovich S, Zimney EJ, et al. Preparation and characterization of graphene oxide paper. Nature. 2007;448(7152):457–460. .
- Lian B, Luca SD, You Y, et al. Extraordinary water adsorption characteristics of graphene oxide. Chem Sci. 2014;21(22):5106–5111. .
- Blanton TN, Majumdar D. X-ray diffraction characterization of polymer intercalated graphite oxide. Powder Diffr. 2012;27(2):104–107.
- Thomas JB, Yen JH, Sharpless KE. Characterization of NIST food-matrix standard reference materials for their vitamin C content. Anal Bioanal Chem. 2013;405(13):4539–4548.
- De Silva KKH, Huang -H-H, Yoshimura M. Progress of reduction of graphene oxide by ascorbic acid. Appl Surf Sci. 2018;447:338–346.
- Bo Z, Shuai X, Mao S, et al. Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci Rep. 2014;4(1):4684. .
- Chong JY, Wang B, Mattevi C, et al. Dynamic microstructure of graphene oxide membranes and the permeation flux. J Membr Sci. 2018;549:385–392.
- Boukhvalov DW, Katsnelson MI, Son Y-W. Origin of anomalous water permeation through graphene oxide membrane. Nano Lett. 2013;13(8):3930–3935.
- Nair RR, Wu HA, Jayaram PN, et al. Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science. 2012;335(6067):442–444.
- Korhonen H, Sinh LH, Luong ND, et al. Fabrication of graphene-based 3D structures by stereolithography. Phys Status Solidi A. 2016;213(4):982–985. .
- Go S-H, Kim H, Yu J, et al. Synergistic effect of UV and l-ascorbic acid on the reduction of graphene oxide: reduction kinetics and quantum chemical simulations. Solid State Sci. 2018;84:120–125.