ABSTRACT
Ionic conductive gels are promising soft conductors for stretchable and flexible electronics. Unfortunately, the basic requirements for an ideal ionic conductive gel, including environmental and chemical stability as well as low toxicity and low cost do not usually coincide in one material. Here we report an ionic conductive gel containing deep eutectic solvents (DES), which are specialized ionic liquids composed of propylene glycol (PG, hydrogen bonding donor) and various salts. In experiments, the DES-gel loses about 10% of the original weight in dry air (5– 9% relative humidity) for 7 days and its conductivity remains unaltered. Comparing to regular salt-containing hydrogels, it exhibits exceptional resistance to metal corrosion. We demonstrate the advantage of DES-gels as the ionic electrode in a flexible and stretchable electroluminescent device, especially in cold environment (−20°C). Through this study, we discover a new candidate for cheap and safe soft ionic conductors with excellent environmental and chemical stability.
Graphical abstract
1. Introduction
Due to its strong potentials in flexible electronics [Citation1,Citation2], soft robotics [Citation3–5] and biomedical engineering [Citation6,Citation7] etc., hydrogel-based ionic conductors have been extensively studied. Hydrogels are generally speaking unstable [Citation8]. For example, they tend to lose water when exposed in the air, especially in draught or hot environments. And at sub-zero temperatures, the conductivity is often deprived due to water freezing [Citation9]. Besides, they corrode metal electrodes rapidly caused by the indispensable high salt content in water.
To improve, researchers developed ionogels. Ionogels are polymer networks infused by ionic liquids [Citation10]. Unlike water, ionic liquids evaporate negligibly even at high temperature [Citation11]. And it shows better anti-corrosion property to metals comparing to regular salt-containing hydrogels [Citation12]. However, there are two basic attributes that prevent ionogels from being ideal, the high cost and high toxicity [Citation13–15]. Therefore, an ideal candidate for ionic conductors would be of good environmental and chemical stability, as well as low cost and low toxicity.
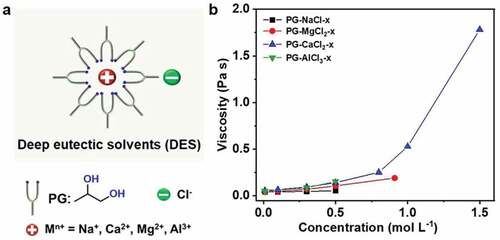
Deep eutectic solvents (DES) are co-solvents that have lower melting point than each individual componential solvent [Citation16–21]. There are many options for customizing particular DES, some of which generate basic merits of ionic liquids (such as conductivity, low volatility, etc.) [Citation22]. More importantly, the ionic-liquid-like DES are cost effective and low-toxic [Citation23], which make DES a charming substitute for traditional ionic liquids [Citation24]. Previously, DES are mainly utilized for solvothermal synthesis of nickel cobalt sulfides and electrodeposition of aluminum, and as versatile solvents for dissolving metal oxides etc [Citation19–21]. Despite significant achievements in DES, the case of using DES as the solvent for organogels and the topic of DES solvent influence in the organogels’ environmental and chemical stability in cold environments is less studied.
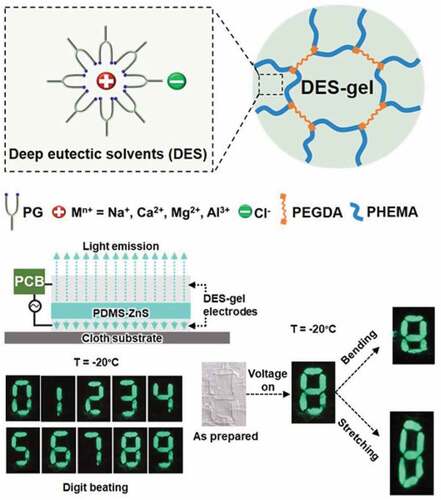
Here, we report a new type of organogel using a particular DES as solvent, which is composed by propylene glycol (PG) and various chloride salts. The DES-gel exhibit excellent environmental stability (anti-drying) and chemical stability (anti-corrosion). As a low temperature-tolerant ionic conductor, the DES-gel can be potentially used to fabricate stretchable electroluminescent devices. PG is utilized as the hydrogen bonding donor, because among the polyol family, PG is nontoxic comparing to ethylene glycol [Citation25], and less viscous comparing to glycerin. In fact, PG has long been utilized as a food additive and base oil for vapes. Its safety for ingestion has been certified by FDA in the US. As shown in , the hydrogen bonding donor PG breaks the static interaction between calcium and chlorine ions, forming a cluster based on the new hydrogen bonding structure [Citation26]. Together, the new cluster and chlorine ion form an ion pair with size asymmetry similar to traditional ionic liquids, which prevents the crystal formation, even when it is cooled down [Citation27]. Next, we use the DES as the solvent, 2-hydroxyethyl methacrylate (HEMA) as the monomer and poly(ethylene glycol)diacrylate (PEGDA) as the crosslinker to prepare DES-gels. The as-prepared DES-gels can maintain flexibility and stretchability at low temperatures and keep conductivity in the temperature range from −40°C to 80°C. Moreover, the DES-gels also possess high environmental and chemical stability. The DES-gels lose about 10% of the original weight in dry air (5– 9% relative humidity) for 7 days and conductivity remain unaltered. They also exhibit anti-corrosion behavior. We demonstrate the exceptional performance of DES-gels in a stretchable and flexible electroluminescent device.
2. Materials and methods
2.1. Materials
HEMA, PG and N, N’-methylenebisacrylamide (MBAA) are purchased from J&K (Beijing, China). PEGDA (average Mn = 575) was supplied by Sigma-Aldrich. Irgacure-2959 (I-2959), magnesium chloride (MgCl2), aluminum chloride (AlCl3) and α-ketoglutaric acid are purchased from Meryer. Sodium chloride (NaCl) is supplied by Macklin. Calcium chloride (CaCl2) and acrylamide (AAm) are purchased from Aladdin. Polydimethylsiloxane (Sylgard 184) is supplied by Dow corning. ZnS powder (electroluminescent material) and copper sheets (0.5 mm thickness, 20 mm diameter) are purchased from Taobao. All chemicals are used as received without further purification, and distilled water is used for the preparation of hydrogels.
2.2. Preparation of PG-MCln-x (DES)
Various salts (MCln = NaCl, MgCl2, CaCl2, and AlCl3) are dissolved in 100 mL PG under stirring at 60°C to obtain different salt concentrations of DES. Here, in PG-MCln-x, x represents the concentration of salt. For example, PG-CaCl2-0.5 means the concentration of CaCl2 in PG is 0.5 mol L−1. The feed ratios are summarized in Table S1.
2.3. Synthesis of [PG-MCln-x]-gels (DES-gels)
DES-gels are synthesized in the following process: monomer HEMA (2.603 g, 0.02 mol), crosslinker PEGDA (11.5 mg, 0.02 mmol) and initiator I-2959 (2.24 mg, 0.01 mmol) are added to 10 mL DES solution followed by vortex-mixing (Vortex 2-IKA) for several minutes. The resulting transparent solution is cast into acrylic molds (circular molds: 18 mm diameter, 3 mm thickness; square molds: 100 mm length, 25 mm width, 5 mm thickness) and subjected to 365 nm UV irradiation. After 90 min of UV irradiation, the gels named [PG-MCln-x]-gels (x also represents the concentration of salt) are obtained.
2.4. Synthesis of PAAm-MCln-y hydrogels
Firstly, NaCl (0.295 g, 5 mmol), MgCl2 (0.857 g, 9 mmol), CaCl2 (1.655 g, 15 mmol) and AlCl3 (0.798 g, 6 mmol) are respectively dissolved in 10 mL distilled water to obtain multiple MCln aqueous solution. Subsequently, monomer AAm (1.42 g, 0.02 mol), crosslinker MBAA (3.08 mg, 0.01 mmol) and initiator α-ketoglutaric acid (1.46 mg, 0.01 mmol) are added in various MCln aqueous solution and followed by 1 min vortex-mixing. The obtained hydrogel precursor is cast into circular acrylic molds (18 mm diameter, 3 mm thickness) and is exposed to 365 nm UV irradiation. Finally, the hydrogels named PAAm-MCln-y are acquired, y represents the concentration of salt, y = 0.5 (NaCl), 0.9 (MgCl2), 1.5 (CaCl2), 0.6 (AlCl3).
2.5. Preparation of PDMS-ZnS electroluminescent film
Sylgard 184 (base: crosslink agent = 20: 1 by weight), and ZnS powder (2 g) are mixed under stirring and is further subjected to an oven for 20 min to eliminate air bubbles. Then, the mixture is casted on a glass sheet fixed on a spin-coater (SU-8 Spin Coating Station, Reynolds Tech). The spinning coating process proceeded with the spinning rate of 500 rpm for 120 s. After that, the spinning coated glass sheet is stored in an oven to cure at 80°C for 3 h. An electroluminescent film named PDMS-ZnS with a thickness of 50 μm is finally obtained.
2.6. Characterization
The viscosity of DES is measured utilizing a rheometer (TA Instrument Discovery HR-2) with a 40 mm parallel plate. The shear rate varies from 1 to 100 s−1 at 20°C, and the gap is kept at 200 μm.
The conductivity of DES with the different concentration of salt is measured by a conductivity meter (SevenCompactTM S230 Conductivity Meter) at 25 ± 0.5°C.
Stress-strain curves of DES-gels are recorded using a universal test machine (Instron 5965, USA) at different temperatures, equipped with a 100 N load cell. The length, width and thickness of the specimens are 60, 25 and 5 mm, respectively. The gauge length is typically set to 9 mm and the stretching rate is 10 mm min−1. Each measurement is performed at least three times in the same condition.
Optical microscopy photographs are collected utilizing ZEISS Axioscope5.
2.7. Solvent-retention ability of the DES-gels and PAAm-MCln-y hydrogels
The DES-gels are respectively placed at 20°C under 30– 35% and 5 −9% relative humidity environments for 7 days (30– 35% relative humidity is a relatively stable condition in the laboratory environment, and 5– 9% relative humidity is obtained by utilizing a desiccator with ample desiccant). Change in the weight and conductivity of the specimens are recorded at predetermined time intervals. As the contrast, the PAAm-MCln-y hydrogels are also placed at 20°C for 24 h (30– 35% relative humidity), and the weight change of the samples is collected.
2.8. Chemical stability of the DES-gels and PAAm-MCln-y hydrogels
The DES-gels and PAAm-MCln-y hydrogels are placed on copper sheets. Then DES-gels are stored at room temperature. The hydrogel-copper junctions are sealed in Petri dish at room temperature. The corrosion behavior is directly observed and image-recorded.
Potentiostatic polarization measurements of DES-gels and PAAm-MCln-y hydrogels are carried out using an electrochemical workstation (CHI760E). A two-electrode system, with the counter electrode and the reference electrode connected as one electrode, the working electrode as another electrode is utilized to implement the potentiostatic polarization experiments. The two electrodes are respectively connected to both sides of the copper sheet (10 mV s−1 scan rate, −2 V – 2 V scanning voltage). The corrosion current densities and corrosion potentials are determined based on Tafel extrapolation.
2.9. Electrical behavior of the DES-gels
Electrical behavior of the DES-gels (18 mm diameter, 3 mm thickness) is performed on an electrochemical workstation (CHI760E). The resistance of the DES-gels is characterized by a two-electron system in a frequency range of 1–106 Hz. The conductivity of the DES-gels can be acquired by the formula σ = L/(RS), where σ (mS cm−1) represents the conductivity, L (m) represents the distance between the two electrodes, R (Ω) represents the resistance of the DES-gels, S represents the cross-sectional area of the DES-gels.
2.10 Preparation of a stretchable and flexible electroluminescent device and its working mechanism
In an electroluminescent device, a piece of PDMS-ZnS electroluminescent film is sandwiched between the top and bottom layer of DES-gel electrodes. The top electrodes are first connected to a printed circuit board (PCB), then the PCB outlet is connected to one polar of an alternating power source. The bottom electrode connects to the other polar of the power source. The electroluminescent layer glows in respond to the electric field generated by the power source. The PCB serves as a timer-controlled switch for the top electrodes, which are seven pieces of [PG-CaCl2-0.5]-gels arranged into a digit figure (denoted as a-g). When the power source is switched on, digits from 0 to 9 shows in sequence cyclically. Each number displays based on different combinations among the top electrodes. For example, when it is number ‘5,’ electrodes a, c, d, f and g are connected, while b and e are (Figure S1) disconnected.
3. Results and discussion
3.1. Preparation of DES
DES based on a variety of salts (NaCl, MgCl2, CaCl2, and AlCl3) and typical hydrogen bond donor PG are prepared ()[Citation23]. The as-prepared DES are transparent and colorless liquids (Figure S2). The saturated concentration of each salt in PG are in the order of CaCl2 > MgCl2 > AlCl3 > NaCl (Figure S3). Salts and PG with different ratios are utilized to fabricate a series of DES. shows that the viscosity of DES increases with the increase of the concentration of salt [Citation28]. Additionally, the conductivity of PG-CaCl2-x DES as a function of the concentration of CaCl2 is also measured. As illustrated in Figure S4, the conductivity of the PG-CaCl2-x increases first and then decreases with the increase of the concentration of CaCl2. The initial increase is due to the increased ion pairs, while the subsequent decline derives from the more rapidly growing viscosity (), the high viscosity results in the harder movement of the ions [Citation28,Citation29].
3.2. DES-gels synthesis and properties
We use DES, as a new solvent to prepare DES-gels. We synthesized DES-gels by free radical polymerization of HEMA in the DES (), and PEGDA and I-2959 are employed as crosslinker and initiator, respectively. After polymerization, various freestanding DES-gels are obtained. Similar to room temperature (20°C), the resultant DES-gels still show high optical transparency and flexibility even when stored at −60°C, indicating that the DES do not freeze in this condition (, c and S5).
Figure 2. (a) Preparation of DES-gels based on DES. Photos of [PG-CaCl2-0.5]-gel at (b) 20°C and (c) −60°C. The as-prepared gel possesses high optical transparency and flexibility over a wide temperature range
![Figure 2. (a) Preparation of DES-gels based on DES. Photos of [PG-CaCl2-0.5]-gel at (b) 20°C and (c) −60°C. The as-prepared gel possesses high optical transparency and flexibility over a wide temperature range](/cms/asset/f507db6d-e696-439b-a98e-d8c24f718ec6/tsnm_a_1972053_f0002_c.jpg)
The ion pairs in DES-gels make the DES-gels conductive. illustrates the conductivity of various DES-gels as a function of the concentration of salt. When the concentration is low, the conductivity increases with the concentration. However, when concentration is high, the conductivity decreases due to the more rapidly growing viscosity (). The high viscosity makes it harder to move the ions, thus the lower conductivity [Citation28,Citation29]. Note that this influence pattern on the conductivity is identical to that for pure DES, which indicates that the viscosity affects the conductivity in the same way whether there is a network confining the DES or not. The conductivity also depends on the temperature [Citation30]. As shown in , as raising the temperature, the conductivity of [PG-CaCl2-0.5]-gel rises significantly, this is due to the faster movement of the ions under higher temperature.
Figure 3. (a) Conductivity of various DES-gels at different concentration of MCln. In certain range of concentrations, the conductivity becomes much larger under a higher salt concentration. However, the conductivity shows a downward trend when the concentration of salt increases further. (b) Conductivity of [PG-CaCl2-0.5]-gel at different temperatures. Conductivity increases as the temperature increases
![Figure 3. (a) Conductivity of various DES-gels at different concentration of MCln. In certain range of concentrations, the conductivity becomes much larger under a higher salt concentration. However, the conductivity shows a downward trend when the concentration of salt increases further. (b) Conductivity of [PG-CaCl2-0.5]-gel at different temperatures. Conductivity increases as the temperature increases](/cms/asset/7b0955d9-6a97-4f0d-8693-f3974523f0d0/tsnm_a_1972053_f0003_c.jpg)
We investigate the mechanical properties of the DES-gels. As shown in , the tensile strength and Young’s modulus of the DES-gels increase with increasing the concentration of salt, the fracture strain decreases inversely. Specifically, when the concentration of NaCl increases from 0.01 to 0.5 mol L−1, the tensile tensile strength of [PG-NaCl-x]-gels increases from 6.40 ± 0.50 to 8.38 ± 0.48 kPa, the Young’s modulus aggrandizes from 6.07 ± 1.58 to 12.88 ± 0.58 kPa, and fracture strain decreases from 235 ± 30 to 124 ± 14% (Table S2). The mechanical properties of the other DES-gels are summarized in Table S3-S5. We suggest that the enhancements of the tensile strength and Young’s modulus and the reduction of fracture strain for DES-gels may relate to the growing viscosity when more salts are introduced ()[Citation31]. Additionally, previous researches demonstrated that the viscosity of DES increases with decreasing temperature [Citation32]. Thus, the mechanical properties of DES-gels may be also affected by temperature. are the tensile stress-strain curves of [PG-CaCl2-1.5]-gel at different temperatures. Because of the steeply rising viscosity of DES, the tensile strength and Young’s modulus of [PG-CaCl2-1.5]-gel increases drastically at the lower end of temperature. Cyclic tensile tests are also performed. In , the [PG-CaCl2-1.5]-gel shows a remarkable hysteresis under −50°C, possessing an enormous energy dissipation, which makes the [PG-CaCl2-1.5]-gel stronger and tougher at −50°C.
Figure 4. Mechanical properties of various DES-gels with the different concentrations of MCln. (a) [PG-NaCl-x]-gels. (b) [PG-MgCl2-x]-gels. (c) [PG-CaCl2-x]-gels. (d) [PG-AlCl3-x]-gels. The mechanical properties of DES-gels are influenced by the concentration of salt. (e) [PG-CaCl2-1.5]-gel under different temperature. The specimen illustrates higher tensile strength and Young’s modulus under the lower temperature. (f) Cyclic tensile test of the [PG-CaCl2-1.5]-gel under different temperatures. The sample shows an obvious hysteresis under −50°C, indicating a significant energy dissipation mechanism
![Figure 4. Mechanical properties of various DES-gels with the different concentrations of MCln. (a) [PG-NaCl-x]-gels. (b) [PG-MgCl2-x]-gels. (c) [PG-CaCl2-x]-gels. (d) [PG-AlCl3-x]-gels. The mechanical properties of DES-gels are influenced by the concentration of salt. (e) [PG-CaCl2-1.5]-gel under different temperature. The specimen illustrates higher tensile strength and Young’s modulus under the lower temperature. (f) Cyclic tensile test of the [PG-CaCl2-1.5]-gel under different temperatures. The sample shows an obvious hysteresis under −50°C, indicating a significant energy dissipation mechanism](/cms/asset/e2f4e4ad-3671-4eb9-9d77-89034c3f79bb/tsnm_a_1972053_f0004_c.jpg)
Dehydration is a challenge for traditional hydrogels [Citation33]. Here the solvent-retention ability of the DES-gels is estimated via a weighing method. As illustrated in , the DES-gels can maintain approximately 90% of the initial weight after storing for 7 days, and their size shrinkage are negligible in the experiment () (5– 9% relative humidity, temperature: 20– 25℃). The phenomenon stems from the DES. In a relatively higher humidity (30– 35% relative humidity), the DES-gels can also keep dimensional stability, but their weight increase slightly because PG is able to absorb water molecules from the air (Figure S6). As a contrast, the weight of PAAm-MCln-y hydrogels containing a large quantity of water drops 67– 86% from their initial weight as water evaporates in 24 h (). The size shrinkage of all PAAm-MCln-y hydrogels is visible (). Additionally, the conductivity of the DES-gels after storing for 7 days is characterized (). The conductivity of all specimens remains in the same level, which further confirms the high environmental stability of the DES-gel conductors. The above results also manifest that the DES-gels possess high environmental stability and would be suitable for a long-term operation. The high environmental stability derives from DES, which is similar to ionic liquids. What is more notable is that DES is low toxicity and low cost compared to ionic liquids.
Figure 5. Environmental stability of various DES-gels and PAAm-MCln-y hydrogels. (a) Plot of weight maintenance of the DES-gels under 5– 9% relative humidity versus time. The weight of DES-gels can maintain approximately 90% of their initial weights, showing high environmental stability even in an extremely dry condition. (b-e) Images of the DES-gels before and after storage for 7 days. The DES-gels show size stability. (f) Change of weight of the PAAm-MCln-y hydrogels for 24 h. The weight drops persistently fast as water evaporates. (g-j) Photos of the PAAm-MCln-y hydrogels before and after stored for 24 h. The sizes of all PAAm-MCln-y hydrogels are reduced remarkably due to water loss. Relative humidity: 30– 35%, temperature: 20– 25°C. (k) Change of conductivity of DES-gels under 5– 9% relative humidity versus time. Relatively stable conductivity further Proves the high environmental stability of the DES-gels
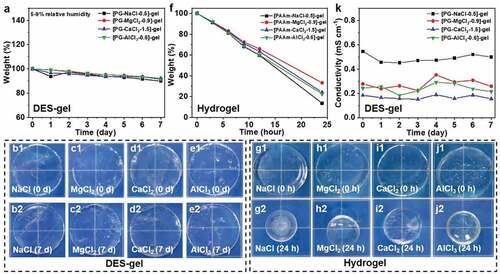
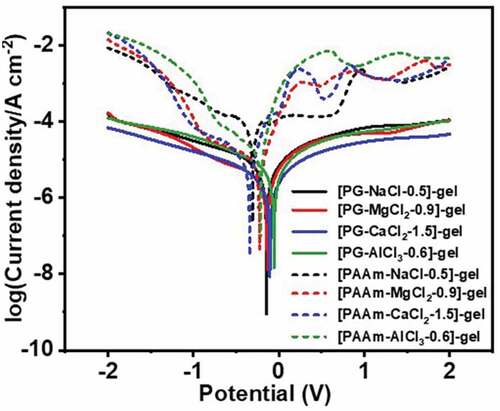
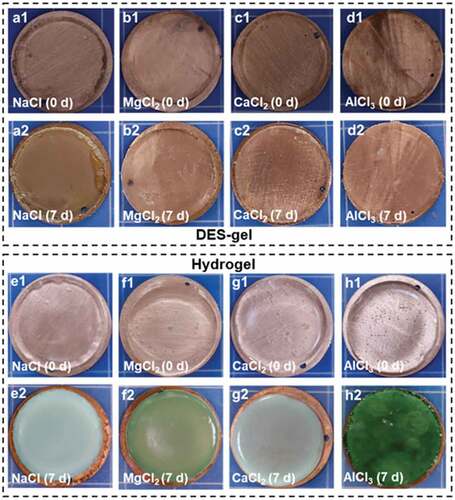
Except for facing the risk of evaporation in open atmosphere, hydrogel ionic conductors are also chemically corrosive to metal electrodes, which severely sabotage their practical application in any fields of electronics. Because DES consist non-water solvent, the as-prepared DES-gels can keep chemical stability, especially against electrochemical (redox) corrosion. To verify the anti-corrosive performance of DES gels, the DES-gels are put in contact with a copper sheet, and stored for 7 days. We find that the DES-gels not only remain the dimensional stability and optical transparency, but also are not chemically corrosive to copper sheets (). Contrary to the DES-gels, PAAm-MCln-y hydrogels have exerted severe corrosion toward copper sheets after storing in a confined space (for the purpose of retaining water) for 7 days (). Additionally, potentiostatic polarization experiments are conducted to further quantify the corrosion resistance behavior of DES-gels and PAAm-MCln-y hydrogels. presents the potentiostatic polarization curves, showing that the corrosion potential (Ecorr) and corrosion current density (icorr) of all the samples are fitted by Tafel extrapolation and the results are summarized in Table S6. As illustrated in and Table S6, all DES-gels exhibit more positive Ecorr and lower icorr than that of PAAm-MCln-y hydrogels. These results reveal that DES-gels possess better corrosion resistance. The above results indicate that the chemical stability of the DES-gels can ensure their application in gel-based soft electronic devices, especially at the gel-metal junctions.
Figure 6. The chemical stability of various DES-gels and PAAm-MCln-y hydrogels. (a-d) Photos of the DES-gels-copper junctions after storing for 7 days. DES-gels are not chemically corrosive to copper sheets, indicating good chemical stability. (e-f) Photographs of PAAm-MCln-y hydrogels-copper junctions after storing for 7 d in a confined space. The hydrogels severely corrode the copper sheets
3.3. Application in a stretchable and flexible electroluminescent device
Profiting from excellent flexibility, ionic conductivity, stretchability, cold environmental stability and chemical stability, the designed DES-gels can be applied in stretchable and flexible electroluminescent devices at ambient and low temperatures [Citation34]. [PG-CaCl2-0.5]-gel is chosen as a representative specimen to construct the electroluminescent devices because it possesses the highest conductivity (). In the following, we fabricate an electroluminescence-based digit displaying device. Ionic [PG-CaCl2-0.5]-gel conductors are placed on the top and bottom sides of a PDMS-ZnS electroluminescent layer (the optical microscope images are shown in Figure S7) [Citation34–36]. Figure S1 shows photographs of the electroluminescent device. The top electrodes consist of seven pieces of DES-gels arranged in a digital number ‘8,’ whereas the electroluminescent layer and bottom layer are plain films. In principle, the device emits visible light when the top-bottom electrodes are connected to an alternating power source (). The digit beating in sequence from ‘1’ to ‘0’ is manipulated by a PCB. Although the luminance depends on the temperature (), the device displays fair electroluminescence character at both 20°C and −20°C according to our experiments (Movie S1, S2). Note that the as-prepared electroluminescent device maintains luminescent character when its stretched and bended, thanks to the excellent stretchability and flexibility of DES-gels (, Movie S3-S6) and the PDMS-ZnS composite. In brief, the utilization of DES-gel-based soft electrodes in electroluminescent devices helps integrate advanced features such as good stretchability, flexibility solvent-retention, wide working temperature range into the devices, which may broaden the applications in wearable electronics for healthcare and outdoor activities.
Figure 8. [PG-CaCl2-0.5]-gel applied in stretchable and flexible electroluminescent devices at room and cold environment. (a) Diagram of the as-prepared electroluminescent device. (b) Luminance of the prepared electroluminescent device as a function of temperature, showing that luminance becomes brighter as the temperature increases. (c) Photos of the prepared electroluminescent device in darkroom at 20°C and −20°C. Images of the prepared electroluminescent device in darkroom at 20°C and −20°C with and without (d) stretching and (e) bending. The flexible and stretchable electroluminescent device works in both ambient and cold environments
![Figure 8. [PG-CaCl2-0.5]-gel applied in stretchable and flexible electroluminescent devices at room and cold environment. (a) Diagram of the as-prepared electroluminescent device. (b) Luminance of the prepared electroluminescent device as a function of temperature, showing that luminance becomes brighter as the temperature increases. (c) Photos of the prepared electroluminescent device in darkroom at 20°C and −20°C. Images of the prepared electroluminescent device in darkroom at 20°C and −20°C with and without (d) stretching and (e) bending. The flexible and stretchable electroluminescent device works in both ambient and cold environments](/cms/asset/c6608032-10c3-43ca-b105-cf1ee690ee70/tsnm_a_1972053_f0008_c.jpg)
4. Conclusions
In summary, we fabricated ionic conductive gels based on DES that merges high optical transparency, flexibility, stretchability and conductivity over a wide temperature range. DES also endows the designed ionic conductive gels with high environmental stability, chemical stability, low toxicity, and low cost. We demonstrate the application of DES-gels as the electrodes in a stretchable and flexible electroluminescent device at room and low temperatures. Strain sensors are widely used in flexible electronic devices [Citation37,Citation38]. DES-gels also possess the basic requirements to be utilized as a strain sensor, especially in cold environment (Figure S8). We consider DES-gel as an upgraded version of the traditional ionogels. That is, the DES-gels inherit most of the advantages of ionic liquids but generate much less safety concerns and more cost-effectiveness. In addition, the wide choices of hydrogen bond donors and salts/polymers guarantee diverse functions for specific requirements from users working in the vast fields of medicine and engineering.
Supplemental Material
Download Zip (92.6 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Rong Q, Lei W, Liu M. Conductive hydrogels as smart materials for flexible electronic devices. Chem A Eur J. 2018;24(64):16930–16943.
- Das S, Martin P, Vasilyev G, et al. Processable, ion-conducting hydrogel for flexible electronic devices with self-healing capability. Macromolecules. 2020;53(24):11130–11141.
- Lee Y, Song WJ, Sun JY. Hydrogel soft robotics. Mater Today Phys. 2020;15:100258.
- Lee HR, Kim CC, Sun JY. Stretchable ionics - A promising candidate for upcoming wearable devices. Adv Mater. 2018;30(42):1704403.
- Chen BH, Bai YY, Xiang F, et al. Stretchable and transparent hydrogels as soft conductors for dielectric elastomer actuators. J Polym Sci B Polym Phys. 2014;52(16):1055–1060.
- Pei XJ, Zhang H, Zhou Y, et al. Stretchable, self-healing and tissue-adhesive zwitterionic hydrogels as strain sensors for wireless monitoring of organ motions. Mater Horizons. 2020;7(7):1872–1882.
- Huang HL, Han L, Fu XB, et al. Multiple stimuli responsive and identifiable zwitterionic ionic conductive hydrogel for bionic electronic skin. Adv Electron Mater. 2020;6(7):2000239.
- Bai RB, Yang QS, Tang JD, et al. Fatigue fracture of hydrogels. Extreme Mech Lett. 2017;15:91–96.
- Morelle XP, Illeperuma WR, Tian K, et al. Highly stretchable and tough hydrogels below water freezing temperature. Adv Mater. 2018;30(35):1801541.
- Ding Y, Zhang JJ, Chang L, et al. Preparation of high-performance ionogels with excellent transparency, good mechanical strength, and high conductivity. Adv Mater. 2017;29(47):1704253.
- Yiming BB, Han Y, Han ZL, et al. A mechanically robust and versatile liquid-free ionic conductive elastomer. Adv Mater. 2021;33(11):2006111.
- Marino DD, Shalaby M, Kriescher S, et al. Corrosion of metal electrodes in deep eutectic solvents. Electrochem commun. 2018;90:101–105.
- Bubalo MC, Radosevic K, Redovnikovic IR, et al. Toxicity mechanisms of ionic liquids. Arh Hig Rada Toksikol. 2017;68(3):171–179.
- Pham TP, Cho CW, Yun YS. Environmental fate and toxicity of ionic liquids: a review. Water Res. 2010;44(2):352–372.
- Zhao DB, Liao YC, Zhang ZD. Toxicity of ionic liquids. CLEAN - Soil, Air, Water. 2007;35(1):42–48.
- Zhang H, Ferrer ML, Jiménez-Riobóo RJ, et al. Tools for extending the dilution range of the “Solvent-in-DES” regime. J Mol Liq. 2021;329:115573.
- Hansen BB, Spittle S, Chen B, et al. Deep eutectic solvents: a review of fundamentals and applications. Chem Rev. 2021;121(3):1232–1285.
- Tang BK, Row KH. Recent developments in deep eutectic solvents in chemical sciences. Monatshefte Für Chemie - Chemical Monthly. 2013;144(10):1427–1454.
- Jiang JY, Yan CY, Zhao XH, et al. A PEGylated deep eutectic solvent for controllable solvothermal synthesis of porous NiCo2S4 for efficient oxygen evolution reaction. Green Chem. 2017;19(13):3023–3031.
- Jiang JY, Bai XY, Zhao XH, et al. Poly-Quasi-Eutectic Solvents (pqess): versatile Solvent for Dissolving Metal Oxides. Green Chem. 2019;21(20):5571–5578.
- Jiang JY, Zhao WC, Xue ZM, et al. Pegylated quasi-ionic liquid electrolytes: fundamental physiochemical properties and electrodeposition of aluminum. ACS Sustain Chem Eng. 2016;4(10):5814–5819.
- Zhang QH, Vigier KDO, Royer S, et al. Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev. 2012;41(21):7108–7146.
- Santana-Mayor Á, Rodríguez-Ramos R, Herrera-Herrera AV, et al. Deep eutectic solvents. the new generation of green solvents in analytical chemistry. Trends Analyt Chem. 2021;134:116108.
- Wang HQ, Li JC, Yu X, et al. Cellulose nanocrystalline hydrogel based on a choline chloride deep eutectic solvent as wearable strain sensor for human motion. Carbohydr Polym. 2021;255(2):117443.
- LaKind JS, McKenna EA, Hubner RP, et al. A review of the comparative mammalian toxicity of ethylene glycol and propylene glycol. Crit Rev Toxicol. 1999;29(4):331–365.
- Farooq MQ, Abbasi NM, Anderson JL. Deep eutectic solvents in separations: methods of preparation, polarity, and applications in extractions and capillary electrochromatography. J Chromatogr A. 2020;1633:461613.
- Vekariya RL. A review of ionic liquids: applications towards catalytic organic transformations. J Mol Liq. 2017;227:44–60.
- Tsai CY, Soriano AN, Li M-H. Vapour pressures, densities, and viscosities of the aqueous solutions containing (Triethylene glycol or propylene glycol) and (LiCl or LiBr). J Chem Thermodyn. 2009;41(5):623–631.
- Abbott AP, Capper G, Davies DL, et al. Novel solvent properties of choline chloride/urea mixtures. Chem Comm. 2003;9(1):70–71.
- Sun TF, Teja AS. Density, viscosity, and thermal conductivity of aqueous ethylene, diethylene, and triethylene glycol mixtures between 290 K and 450 K. J Chem Eng Data. 2003;48(1):198–202.
- Baumberger T, Caroli C, Martina D. Solvent control of crack dynamics in a reversible hydrogel. Nat Mater. 2006;5(7):552–555.
- Karimi MB, Mohammadi F, Hooshyari K. Non-Humidified fuel cells using A Deep Eutectic Solvent (DES) as the electrolyte within A Polymer Electrolyte Membrane (PEM): the effect of water and counterions. Phys Chem Chem Phys. 2020;22(5):2917–2929.
- Yuk H, Zhang T, Parada GA, et al. Skin-Inspired hydrogel-elastomer hybrids with robust interfaces and functional microstructures. Nat Commun. 2016;7(1):12028.
- Yang CH, Chen BH, Zhou JX, et al. Electroluminescence of giant stretchability. Adv Mater. 2016;28(22):4480–4484.
- Larson C, Peele B, Li S, et al. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science. 2016;351(6277):1071–1074.
- Kim CC, Lee HH, Oh KH, et al. Highly stretchable, transparent ionic touch panel. Science. 2016;353(6300):682–687.
- Wang X, Liu XH, Schubert DW. Highly sensitive ultrathin flexible thermoplastic polyurethane/carbon black fibrous film strain sensor with adjustable scaffold networks. Nano-Micro Lett. 2021;13(64). DOI:10.1007/s40820-021-00592-9
- Sun HL, Liu XH, Liu CT, et al. The thermal management of wearable and stretchable electronics. Sci Bull. 2021;66(4):301–302.