ABSTRACT
Developing high-performance nanostructured materials is key to deliver the potential of hydrovoltaic technology into practical applications. As single-component materials have approached its limit in generating hydrovoltaic electricity, the development of multi-component hydrovoltaic materials has been necessary in continuously boosting the electricity output. Here, we report a hydrovoltaic material by integrating reduced graphene oxides and polypyrrole nanoparticles (rGO/PPy), where the rGO contributes improved conductivity and large specific surface area while PPy nanoparticles enable enhanced interaction with water. The device fabricated with this material generates a short-circuit current of 6 μA as well as a maximum power density of over 1 μW/cm3 from natural evaporation of water. And the substantial ion–PPy interaction enables robust voltage generation from evaporation of various salt solutions. Moreover, an outstanding scaling ability is demonstrated by connecting 10 devices in series that generate a sustainable voltage of up to ~2.5 V, sufficing to power many commercial devices, e.g. LED bulb and LCD screen
GRAPHICAL ABSTRACT
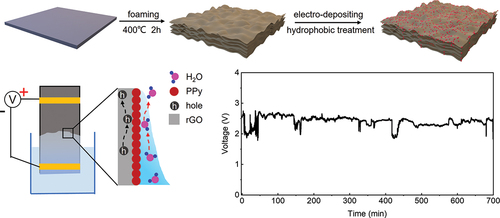
1. Introduction
Hydrovoltaic technology directly converts various forms of water energy [Citation1–3], including waves [Citation4], droplets [Citation5], moisture [Citation6–9], and evaporation [Citation10,Citation11] into useful electricity. Among them, water-evaporation induced electricity generation is particularly attractive by harvesting the low-grade heat from ambient environment through ubiquitous evaporation, referred to as evaporation potential. The evaporation potential stems from the interfacial interaction between capillary water and nanomaterials, and the output power holds potential for applications in self-powered devices [Citation12], distributed power supply [Citation13–15] and Internet of things (IoT) [Citation16,Citation17]. In the past decade, numerous nanomaterials have been investigated for harvesting the evaporation energy of water, and several mechanisms have been proposed for the evaporation-induced electricity, including streaming current [Citation1,Citation18], pseudo-streaming current [Citation19], waving potential [Citation4] and evaporation potential [Citation20]. Although a complete understanding of the evaporation-induced electricity is not achieved yet, years of experimental efforts have concluded several factors conducive to electricity generation, such as continuous water transport in nanochannels, strong interaction at the nanomaterial/water interface, large specific surface area and high carrier (ion) transport capability. However, a single-component material is often difficult to satisfy all these requirements [Citation21]. For example, Lao et al. have reported that conductive MXene nanosheets output a high ion current of 12 μA yet a relatively low voltage under solar irradiation [Citation22]. In contrast, nanomaterials that can substantially interact with capillary water have produced relatively high voltages but low currents at a level of 0.1 μA due to large inner resistances, as exemplified by carbon black [Citation10], metal-organic framework (MOF) [Citation23], metal oxides [Citation24,Citation25] and graphene oxides (GO) [Citation26,Citation27]. In this regard, developing multi-component hydrovoltaic materials by integrating compositionally and functionally distinct materials may represent a promising way to further boost the evaporation-induced electricity. Acting along this line, Shao et al. reported an interface charge recombination between Si nanowires generator and electrodes, generating remarkable electricity of ~30 μA/cm2 [Citation28]. Recently, Tan et al. realized a heterogenous material assembled from a hydrophilic LiCl-loaded cellulon paper and a hydrophobic carbon-black-loaded cellulon paper, with which the fabricated centimeter-sized device produced a high voltage of 0.78 V and a current of 7.5 μA from ambient humidity [Citation29]. These progresses greatly enriched the materials setting and choice in designing high-performance hydrovoltaic devices.
Polypyrrole (PPy) is a conductive polymer with excellent light-absorbing ability for water evaporation and desalination [Citation30,Citation31]. The gradient polymerized PPy nanowires have been utilized to fabricate moisture-driven hydrovoltaic devices [Citation32]. However, the device performance remains to be improved due to relatively low electricity and intermittent output. In this work, we report a new hybrid hydrovoltaic material by hydroplastically foaming the GO paper and then electro-depositing PPy nanoparticles on the reduced graphene oxide (rGO) aerogel. The device fabricated with such a centimeter-sized rGO/PPy material can produce a short-circuit current of ~6 μA and an open-circuit voltage of ~0.3 V, thanks to a synergy between a conductive network of rGO aerogel and a strong interaction of PPy nanoparticles with water. The output power can be further increased when using salt solutions due to additional interaction between ion and PPy nanoparticles. The generated electricity exhibits an excellent scaling behavior upon integration of multiple devices to sufficiently power commercial devices, such as LED bulb and LCD screen. These results suggest a new strategy to realize advanced hydrovoltaic materials for evaporation energy harvesting and pave the way for achieving an efficient generator for practical applications that can run across a range of aqueous solutions from deionized water to seawater.
2. Experiment section
2.1 Materials and reagents
Pyrrole, PVA (Mw ~ 67,000), NaCl, LiCl, KCl and CaCl2 were purchased from Aladdin Biochemical Technology Co., Ltd. N2H4 and ethanol were purchased from Nanjing Chemical Reagent Co., Ltd. Aqueous GO dispersions were purchased from Hangzhou Gaoxi Technology Co., Ltd. 1 H,1 H,2 H,2 H-Perfluorooctyltriethoxysilane (FOTS) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd.
2.2 Preparation of the rGO aerogels
The rGO aerogel was fabricated by hydroplastic foaming method based on the literature [Citation33]. First, aqueous GO suspension (10 mg/mL) was cast-dried on a PET substrate and peeled off to obtain freestanding GO films. Then, the obtained GO film was immersed into the N2H4/H2O solution (1 wt% N2H4) at 50°C for 30 minutes to get GO aerogel. After drying, the GO aerogel was further vacuum annealed at 400°C for 2 hours.
Fabrication of the rGO/PPy devices
mL pyrrole was uniformly mixed with 3 mL PVA aqueous solution (10 wt%), and labeled as solution A. The rGO aerogel (0.5 cm in width and 3 cm in length) was immersed into solution A for 10 minutes. Then, the as-prepared aerogel was employed as the anode and silver sheet as the cathode in 1.2 M HCl solution, with a bias voltage of 9 V applied for 30 min during the electro-depositing treatment process. The PPy nanoparticles were polymerized on the rGO surface. The obtained rGO/PPy aerogel was immersed in deionized water and ethanol for several hours to remove the residues. Finally, the obtained rGO/PPy was hydrophobized by FOTS vapor in a sealed vessel at 80°C for 6 h.
Characterizations and electric measurements
Field emission scanning electron microscopy (FESEM) imaging was performed by Zeiss-Sigma 300. X-ray photoelectron spectroscopy (XPS) measurements were performed by Thermo Scientific K-Alpha with Al Kα (1486.6 eV). The chemical structures of samples were investigated by Raman spectroscopy (Horiba JY Labram EVO) with a laser (532 nm). Zeta potential was measured by a Brookhaven NanoBrook zeta potential analyzer. Contact angle measurements were recorded by a ZHONGCHEN JC2000DS contact angle meter. Linear Sweep Voltammetry (LSV) measurements were processed by CHI760E electrochemical workstation. The environmental relative humidity and temperature were recorded with a TH22R-EX Temperature Humidity Meter. The time-dependent temperature change of samples was recorded by IR camera (FOTRIC 226s). The open-circuit voltage and short-circuit current of the hydrovoltaic devices were measured by a Keithley 6500 electrometer, and carbon tapes were used as electrodes to avoid electrochemical contribution at the electrodes.
3. Results and discussions
The fabrication process of the rGO/PPy hydrovoltaic device is shown in . First, GO aerogel was prepared by a hydroplastic foaming method [Citation33], which converted GO paper into aerogel bulk, and then the obtained aerogel was reduced following by a thermal reduction method in a tube furnace at 400°C in 2 h to remove partial oxygen-containing functional groups and form a conductive network. The reduced aerogel displays a rich wrinkle surface structure as shown in Fig. S1, which is suitable for nanoparticle absorption. Second, the rGO aerogel soaking with pyrrole/PVA solution was performed as anode electrode in the electro-depositing process, and a bias voltage was applied to the equipment for 30 min, with details illustrated in Fig. S2. PPy nanoparticles were thereby polymerized on the surface of rGO aerogel. Here, PVA chains form hydrogen bonds with rGO and PPy nanoparticles, which maintains a uniform distribution of the PPy nanoparticles within the rGO aerogel [Citation34]. Scanning electron microscopy (SEM) measurements on the surface morphology of the rGO/PPy show that PPy nanoparticles were uniformly attached onto the rGO surface with a size of ~10 nm (), and the cross-sectional SEM image showed a rich network (Fig. S3). Finally, the obtained rGO/PPy was hydrophobized by FOTS vapor to acquire appropriate capillary interaction, which will be discussed in details later. The schematic of measuring electricity output performance of the rGO/PPy hydrovoltaic device is illustrated in .
Figure 1. Schematic illustrations of (a) the fabrication process and (b) hydrovoltaic electricity generation of the rGO/PPy device. (c) SEM image of the rGO/PPy surface.
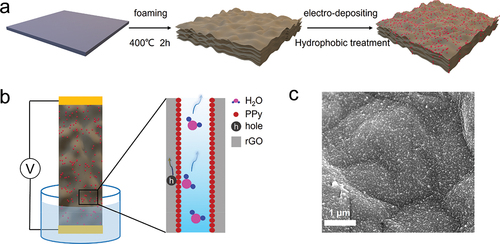
Brunauer – Emmett–Teller (BET) measurement shows that the rGO/PPy material has a large specific surface area of 34.2 m2/g with an average pore diameter of 6.4 nm ( and S4). The nano-pore distribution is mainly attributed to the stacking of graphene oxide sheets, which is consistent with previous results [Citation33]. A negative Zeta potential of −14.5 mV indicates that negative charges are distributed on the surface of the rGO/PPy material (). Besides, abundant pores and networks give the way for capillary water to pump up and substantially interact with PPy nanoparticles. The fast capillary ability of initial rGO/PPy material has also been verified by time-dependent water contact angle measurement (Fig. S5). When a 5 μL water droplet drops on the surface of initial 3D rGO/PPy without hydrophobic FOTS treatment, the surface shows a hydrophilic characteristic with a contact angle of 58°, and the droplet could be absorbed into a 3D network within 8 s, owing to the hydrophilicity of PVA and abundant capillary channels in rGO aerogel. However, an appropriate wettability is vital to the hydrovoltaic device since the electricity generation mainly depends on the evaporation interfaces between capillary water and nanomaterials [Citation1,Citation20]. After hydrophobic FOTS treatment, the contact angle increases to 120°, and a 5 μL water droplet is absorbed into 3D rGO/PPy within 30 min (). The influence of hydrophobic FOTS treatment on the capillary action for rGO/PPy device has also been investigated by infrared measurement (Fig. S6), where hydrophobically treated rGO/PPy maintained a stable evaporating interface, while the untreated one was totally filled with water in 3 min.
Figure 2. Property characterizations of the rGO/PPy materials. (a) N2 adsorption-desorption isotherm and (b) measured zeta potential of the rGO/PPy. (c) Raman spectra of the electro-deposited rGO/PPy under different bias voltage. (d) Time-dependent water contact angle on the hydrophobic treated rGO/PPy surface.
To optimize the electro-deposition process for PPy nanoparticles located on the rGO surface, the bias voltage is controlled to be 3, 6, 9, and 12 V, respectively. It has been dedicated that the open-circuit voltage (VOC) and short-circuit current (ISC) of the rGO/PPy device increase with the bias voltage of electro-deposition and reach a peak of ~0.3 V and ~6 μA, respectively, at a bias voltage of 9 V (Fig. S7). Furthermore, the corresponding Raman spectra have been studied to understand the influence of bias voltage for electro-deposition on the hydrovoltaic electricity generation, from the viewpoint of the chemical constituent of rGO/PPy material. Raman spectroscopy results exhibit two typical peaks of PPy centered at 842 and 1049 cm−1 [Citation32,Citation35,Citation36], respectively, indicating successful synthesis of PPy nanoparticles. There is also a peak intensity at 2888 cm−1, which corresponds to the C-H stretching vibrations of CH2 and CH3 functional groups of PVA chains [Citation37]. This peak intensity increases with the bias voltage, suggesting a higher PVA contribution and, thus, larger inner resistance of the rGO/PPy, consistent with ISC measurement in Fig. S7. The following rGO/PPy devices are electro-deposited under a bias voltage of 9 V unless specifically specified.
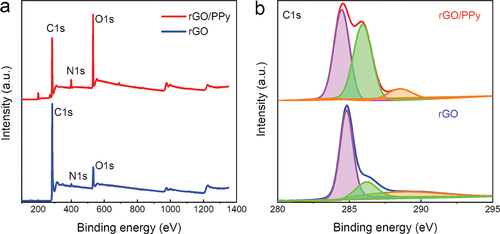
presents X-ray photoelectron spectroscopy (XPS) results for the rGO aerogel and rGO/PPy material, both displaying three dominant peaks, i.e. C 1s peak at 284 eV, N 1s peak at 399 eV and O 1s peak at 532 eV. The O 1s peak intensity of rGO/PPy is higher than that of rGO aerogel, suggesting more oxygen-containing functional groups due to the PVA, in line with above Raman analysis. The strong N 1s peak intensity of the rGO/PPy also confirmed the successful synthesis of PPy nanoparticles. In addition, the C 1s spectra can be resolved into C-C/C=C peak (284.8 eV), C-O/C-N peak (286 eV) and C=O/COOH peak (289 eV) [Citation38,Citation39], respectively (). The prominent C-O/C-N peak of rGO/PPy characterizes the contribution of oxygen-containing functional groups in the rGO/PPy material, which is crucial to capillary water supply [Citation40].
Figure 3. Component characterizations of the rGO/PPy materials. (a) XPS spectra and (b) high-resolution C 1s spectra of the rGO aerogel and rGO/PPy.
Having explored the chemical compositions of rGO/PPy, the output electricity performance is then carefully investigated. shows the recorded output voltage and current of the rGO/PPy device with deionized water at room temperature (24.0 ± 3°C) and relative humidity (RH) of 54 ± 10%, respectively. Such a device outputs a sustainable VOC of ~0.3 V and an initial ISC of ~6 μA. Similar results have also been obtained in the current-voltage (I-V) curve (). The individual roles of the rGO and PPy nanoparticles have also been confirmed by measuring the VOC and ISC curves of the GO aerogel, rGO aerogel and rGO/PPy device, respectively (Fig. S8). Compared to stable electricity generation during nature evaporation, VOC gradually decreases to zero when the device is initially placed in a sealed container (Fig. S9), which means continuous supply of capillary water and water evaporation are essential to the produced electricity. Benefiting from the conductive network and large specific surface area of the rGO aerogel, the evaporation-induced output current (~4 μA/cm2) of our rGO/PPy is at least one order of magnitude higher than that of most single-component hydrovoltaic materials under environment condition [Citation19,Citation23–25,Citation41–46], such as MOF (0.05 μA/cm2) [Citation23], natural mood (0.4 μA/cm2) [Citation45] and solid oxides (0.01–0.05 μA/cm2) [Citation24,Citation25,Citation46]. While it is less than the hydrogel-based (5.3 μA/cm2) [Citation47] and nanowire-array devices (10–30 μA/cm2) [Citation28,Citation48], these outstanding hydrovoltaic performances are attributed to the efficient water supply ability, abundant surface charges of nanomaterials and excellent carrier transport property.
Figure 4. Electricity output of the rGO/PPy device. (a) VOC, (b) ISC and (c) I–V curve of the rGO/PPy device under environment condition. (d) Dependence of the output voltage (blue curve) and current (red curve) of the device with different load resistances. The inset displays a circuit diagram.
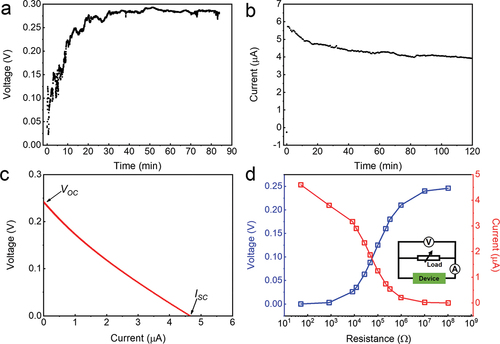
The output performance of the rGO/PPy device was further investigated by connecting different external load resistances (). When external load resistance in the circuit increases from 50 Ω to 100 MΩ, VOC gradually increases from 0.1 mV to 0.25 V, while ISC gradually decreases from 4.6 μA to 0. At an external load resistance of 47 kΩ, the device delivers a maximum power generation of 0.16 μW, corresponding to a power density of 1.1 μW/cm3 (Fig. S10). It is known that the optimal external load resistance equals to the inner resistance of rGO/PPy device, which is two orders of magnitude lower than that of previously reported carbon black sheets [Citation10] and metal oxides [Citation24]. The low inner resistance is attributed to the conductive rGO network in the device. Besides, the influences of evaporation rate, such as solar illumination, relative humidity and wind, on the output power of the device have also been explored. Figure S11 presented the VOC and ISC curves of the rGO/PPy device under 1 sun illumination, where VOC slightly decreased, while ISC increased more notably when the device is upon the sun illumination. This electrical variation might be attributed to solar-absorption capacity of PPy nanoparticles, in which the electrons in the antibonding orbitals of PPy nanoparticles absorb the solar energy and jump to higher energy orbitals [Citation31], thereby promoting the current output. Then the VOC measurement of the rGO/PPy device under the saturated moisture condition (100% RH) has also been shown in Fig. S12. As expected, the VOC significantly decreased when the device is at 100% RH from that under ambient condition since the evaporation process was completely suppressed. The recovery time of VOC to that under the ambient condition becomes longer when the device is repeatedly exposed to the saturated moisture, corresponding to the dynamic adsorption – desorption process of PPy nanoparticles with water. Also as expected, the produced voltage can also be enhanced to some extent by the wind (Fig. S13) for an increased evaporation rate.
To understand the effect of gradient water distribution on the evaporation-induced electricity, measurement with four electrodes (denoted as E1-E4) was conducted for the rGO/PPy device. As illustrated in , the bottom electrode E1 is immersed in water, while other electrodes are located above water surface. The distance between adjacent electrodes is 0.5 cm. When the rGO/PPy device contacts water, capillary water gradually infiltrates into rGO nanochannels induced by capillary force. Then, VOC between adjacent electrodes (i.e. V12, V23 and V34) as well as that between the top and bottom electrodes (V14) are measured for 100 s, respectively (). As the capillary front reaches the region between electrode E2 and E3, V12 is almost zero since that area is immersed with water without gradient distribution. Meanwhile, V34 is also negligible because there is no water distribution therein. In contrast, V23 is ~0.25 V, slightly lower than the total V14. Such distribution indicates that the evaporation-induced electricity is concentrated near the front edge of capillary water, consistent with the reported evaporation potential [Citation20]. At the molecular level, water molecules evaporate from the water–vapor interface, inducing gradient distribution of water in the rGO/PPy device. During the evaporating process, water molecules electrically interact with the PPy nanoparticles anchored on the rGO surface, then drawing hole carriers in the inner rGO to accumulate at water–vapor boundary to induce a potential difference across the gradient region. Note that the almost zero V12 largely rules out a significant contribution of the streaming potential to the generated voltage. And the electricity output measurements of the samples with different lengths have also showed the similar results (Fig. S14) [Citation49].
Figure 5. Factor analysis for the rGO/PPy device. (a) a schematic of a four-electrode rGO/PPy device and illustrated mechanism for electricity generation based on water evaporation. The distance between adjacent electrodes is 0.5 cm and the capillary front lies between E2 and E3. (b) Recorded voltages between two selected electrodes of the device, Δt = 100 seconds. (c,d) Generated voltages of the rGO/PPy device using (c) the NaCl solutions of different concentrations as well as (d) tap water and 1 M salt solutions.
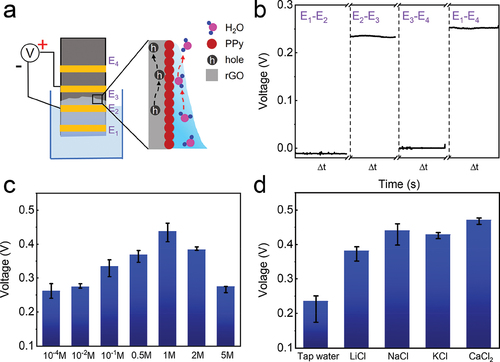
To further examine the effect of various ions on the power generation of rGO/PPy device, detail experiments using NaCl solutions of different concentrations were performed. shows that VOC increases from ~0.25 to ~0.4 V with increasing the solution concentration from 10−4 to 1 M. This is in contrast with carbon black sheets [Citation1] which exhibit an inversed trend of voltage with the salt concentration. The enhanced output voltage may be mainly attributed to ion adsorption on the surface of rGO/PPy device [Citation50]. When adsorbed with PPy nanoparticles, Na+ ions are prone to bind with electrons derived from the conductive network, further increasing the concentration of hole carriers in the inner rGO and thereby promoting generated output voltage [Citation51]. Besides, the interconnected porous network of rGO aerogel can partly avoid the salt accumulation [Citation52,Citation53]. However, VOC decreases with further increasing the concentration of NaCl solution, since the excessive salt concentration can suppress the water-evaporation due to salt crystallization at the water–vapor interface [Citation54]. Then, the sustainable VOC of the rGO/PPy device using 1 M NaCl solution has also been indicated. Though there are little salt crystals on the evaporation surface (Fig. S15), the produced voltage is only slightly decreased after measurement for 10 h. The sustainable electricity generation might be attributed to the excellent mechanical stability of the hydroplastic foamed GO aerogel. In addition, hydrovoltaic performances of the rGO/PPy devices with different salt solutions (1 M) and tap water were also studied, with the results shown in . The electricity generations from salt solutions strongly depend on the interactions of ions with PPy nanoparticles during the evaporation process. Besides, ions with larger ionic radius and higher ionic valence generally lead to stronger interaction with PPy nanoparticles, resulting in the different electric outputs. And the VOC of the rGO/PPy device using natural lake water (Donghua Lake, Nanjing) has also been measured to further verify the reusability of the device (Fig. S16). Such enhanced performance highlights the advantage of our rGO/PPy device for harvesting evaporation energy from a wide range of salt solutions.
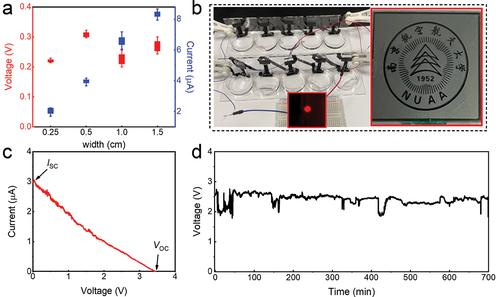
For practical applications, we further examined the scaling behavior of evaporation-induced electricity generation of our rGO/PPy device. First, the width effect of the rGO/PPy sample on the electricity generation was studied. As the width direction is perpendicular to the water capillary, increasing width of the sample is equivalent to parallel connection of devices. At a fixed length of 3 cm, ISC gradually increases from 2 to 8 μA as the width of rGO/PPy sample increases from 0.25 to 1.5 cm (), whereas VOC basically keeps a stable level around 0.25 V without notable decay. exhibits a generator made of ten rGO/PPy devices connected in series to harvest evaporation energy from seawater (0.6 M NaCl solution). The recorded I-V curve of such a generator shows that VOC and ISC reach up to 3.3 V and 3 μA, respectively, indicating an excellent scaling performance (). Moreover, the generator also exhibits a high long-term stability since VOC can still reach 2.5 V after 12-h operation in ambient condition (). The output power of the generator is sufficient to drive a commercial red LED blub and LCD screen with no need of an extra capacitor or rectifying circuit (, insets). The rGO/PPy generator array, if integrated on large scale with the maintained scaling capability, can provide enough electric energy to power a range daily electrical appliance, and even holds the potential of addressing the energy supply for living on remote islands, if the stability of electric generation could be further improved. Moreover, the sensitivity and rapid response of the rGO/PPy device to humidity and solar illumination can also be harnessed to develop sensors for solar and atmospheric data detection, respectively [Citation55].
Figure 6. Demonstration of the rGO/PPy device as a practical power source. (a) Generated voltages and currents as functions of the sample width of the rGO/PPy (with a fixed length of 3 cm). (b) Ten rGO/PPy devices connected in series to harvest electricity from 0.6 M NaCl solution (mimicking seawater). The output electricity can power a red LED (left inset) and a LCD screen (right inset). (c) I–V curve and (d) sustainable Voc of the generator with ten series-connected devices in ambient condition.
4. Conclusions
We have reported a multi-component hydrovoltaic material rGO/PPy, fabricated by hydroplastically foaming GO paper and then electro-depositing PPy nanoparticles on rGO aerogel. Owing to the conductive network of rGO aerogel and strong interaction of PPy nanoparticles with water, a rGO/PPy device with an area of ~1.5 cm2 outputs a VOC of ~0.3 V and a ISC of ~6 μA. Besides, the output electricity of this device can be further enhanced in salt solutions. And a four-electrode measurement has demonstrated that generated electricity is concentrated at water–vapor interface, consistent with the reported evaporation potential. Moreover, the rGO/PPy device exhibits an excellent scaling behavior, and a generator array can produce a sustainable VOC of ~2.5 V over 12 h, sufficing to drive a red LED bulb and LCD screen. These results suggest that integrating multi-component hydrovoltaic materials is an efficient strategy for enhancing electricity generation from water evaporation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Xue G, Xu Y, Ding T, et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nature Nanotechnol. 2017;12(4):317–321.
- Zhang Z, Li X, Yin J, et al. Emerging hydrovoltaic technology. Nature Nanotechnol. 2018;13(12):1109–1119.
- Wang X, Lin F, Wang X, et al. Hydrovoltaic technology: from mechanism to applications. Chem Soc Rev. 2022;51(12):4902–4927.
- Yin J, Zhang Z, Li X, et al. Waving potential in graphene. Nat Commun. 2014;5(1):3582.
- Xu W, Zheng H, Liu Y, et al. A droplet-based electricity generator with high instantaneous power density. Nature. 2020;578(7795):392–396.
- Xu T, Ding X, Huang Y, et al. An efficient polymer moist-electric generator. Energy & Environmental Science. 2019;12(3):972–978.
- Wang H, Sun Y, He T, et al. Bilayer of polyelectrolyte films for spontaneous power generation in air up to an integrated 1,000 V output. Nature Nanotechnol. 2021;16(7):811–819.
- Liu X, Gao H, Ward JE, et al. Power generation from ambient humidity using protein nanowires. Nature. 2020;578(7796):550–554.
- Guan P, Zhu R, Hu G, et al. Recent Development of Moisture-Enabled-Electric Nanogenerators, Small. 2022;18(46):2204603. DOI:10.1002/smll.202204603
- Ding T, Liu K, Li J, et al. All-printed porous carbon film for electricity generation from evaporation-driven water flow. Adv Funct Mater. 2017;27(22):1700551.
- Qin Y, Wang Y, Sun X, et al. Constant electricity generation in nanostructured silicon by evaporation-driven water flow. Angewandte Chemie. 2020;59(26):10619–10625.
- Zhao X, Xiong Z, Qiao Z, et al. Robust and flexible wearable generator driven by water evaporation for sustainable and portable self-power supply. Chem Eng J. 2022;434:134671.
- Yang S, Tao X, Chen W, et al. Ionic hydrogel for efficient and scalable moisture-electric generation. Adv Mater. 2022;34(21):2200693.
- Zhang Y, Guo S, Yu ZG, et al. An asymmetric hygroscopic structure for moisture-driven hygro-ionic electricity generation and storage. Adv Mater. 2022;34(21):2201228.
- Liu C, Wang S, Wang X, et al. Hydrovoltaic energy harvesting from moisture flow using an ionic polymer–hydrogel–carbon composite. Energy & Environmental Science. 2022;15(6):2489–2498.
- Guan H, Mao G, Zhong T, et al. A self-powered UV photodetector based on the hydrovoltaic and photoelectric coupling properties of ZnO nanowire arrays. J Alloys Compd. 2021;867:159073.
- Guan H, Zhong T, He H, et al. A self-powered wearable sweat-evaporation-biosensing analyzer for building sports big data. Nano Energy. 2019;59:754–761.
- Fang S, Chu W, Tan J, et al. The mechanism for solar irradiation enhanced evaporation and electricity generation. Nano Energy. 2022;101:107605.
- Yun TG, Bae J, Rothschild A, et al. Transpiration driven electrokinetic power generator. ACS Nano. 2019;13(11):12703–12709.
- Fang S, Li J, Xu Y, et al. Evaporating potential. Joule. 2022;6(3):690–701.
- Zheng C, Chu W, Fang S, et al. Materials for evaporation-driven hydrovoltaic technology. Interdisciplinary Materials. 2022;1(4):449–470.
- Lao J, Wu S, Gao J, et al. Electricity generation based on a photothermally driven Ti3C2Tx MXene nanofluidic water pump. Nano Energy. 2020;70:104481.
- Ma Q, He Q, Yin P, et al. Rational design of MOF-Based hybrid nanomaterials for directly harvesting electric energy from water evaporation. Adv Mater. 2020;32(37):2003720.
- Shao C, Ji B, Xu T, et al. Large-scale production of flexible, high-voltage hydroelectric films based on solid oxides. ACS Applied Materials & Interfaces. 2019;11(34):30927–30935.
- Li L, Feng S, Bai Y, et al. Enhancing hydrovoltaic power generation through heat conduction effects. Nat Commun. 2022;13(1):1043.
- Huang Y, Cheng H, Yang C, et al. Interface-mediated hygroelectric generator with an output voltage approaching 1.5 volts. Nat Commun. 2018;9(1):4166.
- Liang Y, Zhao F, Cheng Z, et al. Electric power generation via asymmetric moisturizing of graphene oxide for flexible, printable and portable electronics. Energy & Environmental Science. 2018;11(7):1730–1735.
- Shao B, Wu Y, Chen X, et al. Electron-selective passivation contacts for high-efficiency nanostructured silicon hydrovoltaic devices. Adv Mater Interfaces. 2021;8(18):2101213.
- Tan J, Fang S, Zhang Z, et al. Self-sustained electricity generator driven by the compatible integration of ambient moisture adsorption and evaporation. Nat Commun. 2022;13(1):3643.
- Zhao F, Zhou X, Shi Y, et al. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nature Nanotechnol. 2018;13(6):489–495.
- Qi D, Liu Y, Liu Y, et al. Polymeric membranes with selective solution-diffusion for intercepting volatile organic compounds during solar-driven water remediation. Adv Mater. 2020;32(50):2004401.
- Nie X, Ji B, Chen N, et al. Gradient doped polymer nanowire for moistelectric nanogenerator. Nano Energy. 2018;46:297–304.
- Pang K, Song X, Xu Z, et al. Hydroplastic foaming of graphene aerogels and artificially intelligent tactile sensors. Sci Adv. 2020;6(46) eabd4045. DOI:10.1126/sciadv.abd4045
- Liu J, Gui J, Zhou W, et al. Self-regulating and asymmetric evaporator for efficient solar water-electricity generation. Nano Energy. 2021;86:106112.
- Xue J, Hu C, Lv L, et al. Re-shaping graphene hydrogels for effectively enhancing actuation responses. Nanoscale. 2015;7(29):12372–12378.
- Jiang Y, Hu C, Cheng H, et al. Spontaneous, straightforward fabrication of partially reduced graphene oxide–polypyrrole composite films for versatile actuators. ACS Nano. 2016;10(4):4735–4741.
- Torrisi F, Popa D, Milana S, et al. Stable, surfactant-free graphene–styrene methylmethacrylate composite for ultrafast lasers. Adv Opt Mater. 2016;4(7):1088–1097.
- Zhao F, Cheng H, Zhang Z, et al. Direct power generation from a graphene oxide film under moisture. Adv Mater. 2015;27(29):4351–4357.
- Xie J, Wang Y, Chen S. Textile-based asymmetric hierarchical systems for constant hydrovoltaic electricity generation. Chem Eng J. 2022;431:133236.
- Zhang G, Duan Z, Qi X, et al. Harvesting environment energy from water-evaporation over free-standing graphene oxide sponges. Carbon. 2019;148:1–8.
- Sun J, Li P, Qu J, et al. Electricity generation from a Ni-Al layered double hydroxide-based flexible generator driven by natural water evaporation. Nano Energy. 2019;57:269–278.
- Das SS, Pedireddi VM, Bandopadhyay A, et al. Electrical power generation from wet textile mediated by spontaneous nanoscale evaporation. Nano Lett. 2019;19(10):7191–7200.
- Tian J, Zang Y, Sun J, et al. Surface charge density-dependent performance of Ni–Al layered double hydroxide-based flexible self-powered generators driven by natural water evaporation. Nano Energy. 2020;70:104502.
- Lee KH, Kang DJ, Eom W, et al. Holey graphene oxide membranes containing both nanopores and nanochannels for highly efficient harvesting of water evaporation energy. Chem Eng J. 2022;430:132759.
- Zhou X, Zhang W, Zhang C, et al. Harvesting electricity from water evaporation through microchannels of natural wood. ACS Applied Materials & Interfaces. 2020;12(9):11232–11239.
- Chi J, Liu C, Che L, et al. Harvesting water-evaporation-induced electricity based on liquid–solid triboelectric nanogenerator. Adv Sci. 2022;9(17):2201586.
- Li L, Hao M, Yang X, et al. Sustainable and flexible hydrovoltaic power generator for wearable sensing electronics. Nano Energy. 2020;72:104663.
- Ma X, Li Z, Deng Z, et al. Efficiently cogenerating drinkable water and electricity from seawater via flexible MOF nanorod arrays. ?J Mater Chem A. 2021;9(14):9048–9055.
- Zhao J, Wu X, Yu H, et al. Regenerable aerogel-based thermogalvanic cells for efficient low-grade heat harvesting from solar radiation and interfacial solar evaporation systems. EcoMat. 2023;5(3):e12302.
- Hou B, Kong D, Qian J, et al. Flexible and portable graphene on carbon cloth as a power generator for electricity generation. Carbon. 2018;140:488–493.
- Jin H, Yoon SG, Lee WH, et al. Identification of water-infiltration-induced electrical energy generation by ionovoltaic effect in porous CuO nanowire films. Energy & Environmental Science. 2020;13(10):3432–3438.
- He S, Chen C, Kuang Y, et al. Nature-inspired salt resistant bimodal porous solar evaporator for efficient and stable water desalination. Energy & Environmental Science. 2019;12(5):1558–1567.
- Wu M, Peng M, Liang Z, et al. Printed honeycomb-structured reduced graphene oxide film for efficient and continuous evaporation-driven electricity generation from salt solution. ACS Applied Materials & Interfaces. 2021;13(23):26989–26997.
- Sun Z, Han C, Gao S, et al. Achieving efficient power generation by designing bioinspired and multi-layered interfacial evaporator. Nat Commun. 2022;13(1):5077.
- Gui J, Li C, Cao Y, et al. Hybrid solar evaporation system for water and electricity co-generation: comprehensive utilization of solar and water energy. Nano Energy. 2023;107:108155.
