?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
For sustainable application of chloroprene rubber (CR), a new technology is developed by the vulcanization of CR using 2,2’-dithiodipyridine (DPD) as a cross-linking agent with the reprocessing performance owing to disulfide metathesis. When DPD was incorporated into CR vulcanization, the Menschutkin reaction between allyl chloride group and pyridine group occurred with a maximum exothermic peak at 184°C. The number of effective cross-linking bond at 0.5 phr DPD vulcanizate was higher than that at high DPD content. This vulcanizate showed high tensile strength (11.12 MPa) and elongation at break (1253 ± 120%) owing to the exchangeable disulfide bond in the system. Under the catalysis of triphenylphosphine, the metathesis of disulfide compound was improved obviously, which endowed CR/DPD vulcanizates with good recyclability performance. Disulfide cross-linkage maintains its stability at low temperature, thus ensuring the mechanical stability of CR/DPD vulcanizate under the ambient conditions. Vulcanization and reprocessing of CR/DPD vulcanizate can be conducted with common industrial rubber processing equipment. Such reprocessable chloroprene rubber could have potential application in CR industry, also serve to significantly improve environmental sustainability.
1. Introduction
Due to their unique elasticity, good mechanical properties as well as solvent resistance, rubbers are widely used in tires [Citation1], shock absorbers [Citation2], belts [Citation3], seals [Citation4], electromagnetic shielding [Citation5], flame retardant layer [Citation6], actuators [Citation7] and other functional materials [Citation8–10]. For engineering application, rubbers need to be vulcanized or cross-linked with the formation of 3D network structure. However, the covalent network prevents the reshaping or recycling of rubbery objects. As estimated, approximate 1000 million tires reach the end of their useful lives every year, of which 80% are reclaimed by grinding or pyrolysis process [Citation11], and the remainder is either burned or discarded in landfill or the ocean, causing a serious threat to ecological and environmental system [Citation12,Citation13].
With the deepening of the concept of sustainable development, rubber recycling has attracted widespread attention in the industry and has been continuously studied by researchers [Citation13,Citation14]. In this regard, it is of great significance to explore new strategies for manufacturing rubbers with high mechanical performance as well as the potential reprocess. Adaptable networks exhibiting reversible adaptive networks appear to be a possible way to achieve this goal. Vitrimers, recently introduced by Leibler and coworkers [Citation15], are fully processable materials, because their network contains chemical bonds that can undergo exchangeable reactions such as transesterification [Citation16,Citation17], olefin metathesis [Citation18,Citation19], disulfide exchange [Citation20–22], boronic ester exchange [Citation23–25], imine metathesis [Citation26,Citation27], and transcarbamoylation [Citation28]. For the industrialized rubbers, the dynamic chemical bond is convenient and economical to crosslink rubber to meet the requirements of recycling and reprocessing.
Chloroprene rubber (CR, Polychloroprene or Neoprene) is a traditional rubber, which was first successfully industrialized by DuPont in the 1920s [Citation29]. In industry, 2-chloroprene is used as the monomer to synthesize CR by free radical polymerization [Citation30]. C-Cl and C=C bonds in the polymer chain endow CR with some basic properties. For example, the strong polarity of C-Cl bond leads to high interchain force and excellent mechanical properties of CR. Meanwhile, chlorine atom contributes to the high limiting oxygen index (LOI) of CR due to the flame retardancy of halogens [Citation31,Citation32]. With its excellent mechanical properties and flame retardant properties, CR has been widely used in automobiles, aircrafts, protective layers of cables and wires, conveyor belts, V-belts, heat-resistant conveyor belts, chemical-resistant hoses, and other fields [Citation33].
The cross-linkages formed by CR vulcanization methods are mainly covalent bonds, which make it difficult to be recycled. Presently, the most common vulcanization method for the industrial application of CR is to use metal oxides (zinc oxide, magnesium oxide) for vulcanization [Citation34,Citation35], due to the advantages of low energy consumption, light environmental pollution and little harm to operators. Moreover, lead oxide and antimony trisulfide were also reported for its vulcanization; however, due to the environmental pollution of lead and antimony elements, these vulcanization methods are rarely used in the industrial application of CR [Citation33,Citation36]. Imidazole compounds were also reported in patents, but their vulcanization mechanism is not very clear. When the chlorine atom is directly connected to the C=C bond (C=C-Cl), the reactivity of allyl hydrogen group of CR is significantly different with that of the traditional rubber, so CR cannot be vulcanized with traditional sulfur [Citation37]. To enable CR with recyclability, Zhang [Citation38] prepared CR crosslinking agents containing dynamic bonds, and utilized the disulfide metathesis between disulfide and polysulfide in polymer chain under the catalysis of copper(II) methacrylate to realize the recycling of covalently bond crosslinked CR. Due to few S-S bonds in CR chain, the reprocessing process of CR vulcanizate is very cumbersome.
The reaction between pyridine and alkyl or allyl chloride group occurs easily and smoothly [Citation39–42]. Inspired by the reactions with these functional groups, it is envisioned that the reaction of allyl chloride group in CR molecular chain could also react with the pyridine group. In this study, 2,2’-dithiodipyridine (DPD) was first used as a vulcanizing agent for CR. The effects of DPD on the vulcanization reaction, mechanical properties, and recyclability of CR were studied in detail. The CR/DPD reaction is energy-saving and environmentally friendly. In line with the concept of green development of rubber industry, this vulcanization has potential application value.
2. Materials and methods
2.1 Materials
Chloroprene rubber CR3221 was supplied by Chongqing Changshou Chemical Co., Ltd, China. 2,2’-Dithiodipyridine (DPD) was supplied by Shanghai Yien Chemical Technology Co., Ltd, China. Zinc oxide and other chemical reagents were obtained from Aladdin.
2.2. Preparation of CRs
The X(S) K-160 double-roll mill was used for kneading at room temperature. Firstly, CR3221 rubber was put into the open mill and plasticized through the rolls for five times. Then, zinc oxide, DPD, triphenylphosphine and other additives were added and mixed evenly. Finally, the roll distance was adjusted to 2 mm to produce rubber sheets. The vulcanization and DSC analysis of rubber samples were performed after parking for 8 h.
2.3. Measurement and characterization
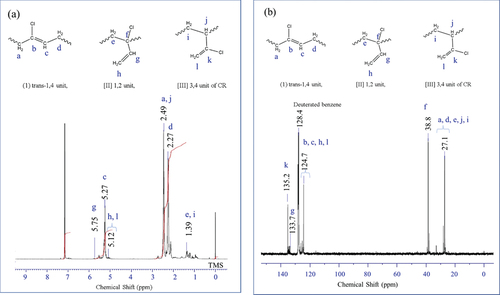
1H and 13C NMR spectra were recorded at 27°C using a JEOL JNM-ECA600 spectrometer (600 MHz). Chemical shift was referenced to tetramethylsilane (δ = 0), and the deuterated benzene was used as the solvent.
The vulcanization characteristics were tested according to ASTM D2084–2001 using a moving Die Rhometer GT-M2000-A (Gaotie Test Instruments Factory, China) at different temperatures. If not stated, the vulcanization temperature was 160°C.
The tensile properties of the rubber samples were tested according to GB/T 528–2009 using a GT-AI-7000-S high temperature stretching machine with a speed of 500 mm/min at room temperature. The average value of three individual samples was recorded for each sample.
Stress-relaxation experiments were carried out using Anton Paar MCR301 in a parallel-plate mode. Circular specimens with a diameter of 25 mm were punched out of the sample sheets. When the temperature reached the set ones, samples were initially compressed by a strain of 5% and the strain was maintained during the stress-relaxation experiments. Meanwhile, the stress and modulus were recorded as a function of time.
To evaluate the self-healing ability of rubber, the samples were completely cut in half and then manually recombined. After rehabilitation for a preset time period at 40°C, 60°C, 80°C, 100°C, and 120°C, respectively. The healed samples were again subjected to tensile tests. Healing efficiency (η) was calculated from the ratio of the tensile strength of the healed specimens to that of virgin specimens.
where is the tensile strength of virgin specimens,
is the tensile strength of the healed specimens.
For the recycling of CR-DPD, the specimens were cut into small particles (about 3 mm) by scissors. The particles were then pulverized at room temperature using a X(S)K-160 double-roll mill to produce sheets. Finally, the compression molding was carried out with a hydraulic press of 10 MPa at 170°C for 30 min. The mechanical properties of the recycled rubber were measured by tensile test to evaluate the recycling ability.
ATR-FTIR spectra were recorded on a TENSOR27 spectrometer (Bruker). Samples were characterized by signal averaging 32 scans at a resolution of 4 cm−1 in the wavenumber range of 500–4000 cm−1.
Differential scanning calorimetry (DSC) analysis was carried out to study the crosslinking behavior of samples using a TA equipment (SDTQ20). The samples were heated to 200°C at a rate of 10°C/min under N2 flow.
The thermal degradation behavior was studied using a thermal gravimetric analyzer (DTG-60 H, Shimadzu) at a heating rate of 10°C/min (from room temperature to 900°C) under nitrogen atmosphere. Each pulverized sample was measured in an open alumina crucible and weighed approximately 10 mg.
3. Results and discussion
For the convenience of discussion, the blend designations and compositions are shown in .
Table 1. Blend designations and compositions.
3.1 The crosslinking reaction between DPD and CR
The Menschutkin reaction (quaternization reaction) between pyridine and allyl chloride active group of geranyl chloride can occur smoothly at room temperature in chloroform with high yield [Citation42]. Therefore, it can be expected that the allyl chloride group of CR can also react with the pyridinyl group of DPD under mild conditions. To confirm the existence of allyl chloride group in CR molecular chain, NMR experiment was carried out. The 1H NMR and 13C NMR spectra of CR are shown in . The appearance of the signal at 5.75 ppm and 1.39 ppm in the 1H NMR spectrum and the signal at 135.2 ppm and 38.8 ppm in the 13C NMR spectrum confirmed that the CR polymer chain contains a small amount of allyl chloride groups, which was consistent with the results reported in the literatures [Citation43–45]. The primary goal is to investigate the Menschutkin reaction between the allyl chloride group of CR and the pyridine group of DPD. The CR/DPD vulcanizate cannot be completely dissolved in toluene at room temperature, indicating that the crosslinking reaction did occur during the vulcanization process. When the temperature of the mixture was raised to 90°C for 1 h, the CR/DPD gel gradually disappeared in the solution and it was difficult to observe the gel visually, which indicates that the disulfide bonds in solution occurred exchange reaction.
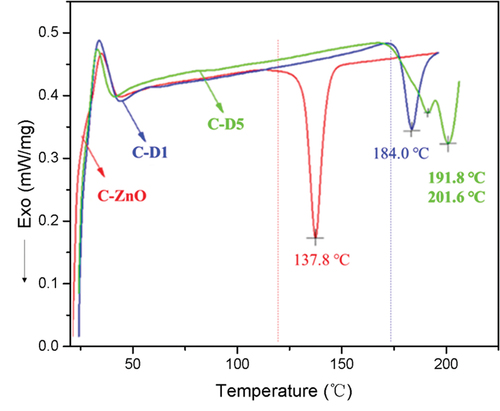
To demonstrate the feasibility of using DPD as a crosslinker for CR, the reaction between CR and DPD was verified by DSC tests with CR/ZnO as a control sample. As shown in , it can be found that the crosslinking reaction between CR and ZnO started at 125°C with an exothermic peak located at 137°C. The crosslinking reaction temperature is higher than the kneading temperature of CR compounds and much lower than the thermal decomposition temperature of the CR vulcanizate. Therefore, ZnO is widely used as the vulcanizing agent for CR in industrial application.
For the CR/DPD composites, the initial exothermic temperature of C-D1 reaction began at 173°C and the maximum exothermic peak located at 184°C (). When the DPD content increased to 12 phr (C-D5), the exothermic onset temperature remained unchanged, but two exothermic peaks appeared in the curve, corresponding to 191.8°C and 201.6°C, respectively. Excitingly, the crosslinking reaction temperature of CR/DPD was located between kneading temperature and vulcanization temperature. Therefore, the general rubber processing equipment is suitable for kneading and vulcanization of CR/DPD.
Here, ATR-FTIR was used to characterize the reaction of CR with different DPD proportions (vulcanized at 160°C for 30 minutes). As shown in , the characteristic absorption bands of DPD were located at 1570 and 1558 cm−1, which is consistent with the literature reported data [Citation46]. The characteristic absorption bands of CR () mainly presented at 2916 cm−1(stretching vibration of C-H), 1659 cm−1 (the stretching vibration of C=C), and 667 cm−1 (the stretching vibration of C-Cl) [Citation47]. Pure CR had almost no absorption at 1570, 1558 cm−1 in the FTIR spectra. Taking the characteristic absorption peaks of DPD at 1570, 1558 cm−1 as the reference peak, the reaction degree can be evaluated by the shift of these characteristic absorption bands.
Figure 3. ATR-FTIR spectra of different compounds. (a) CR, (b) DPD, (c) C-D1, (d) C-D2, (e) C-D3, (f) C-D4, (g) C-D5.
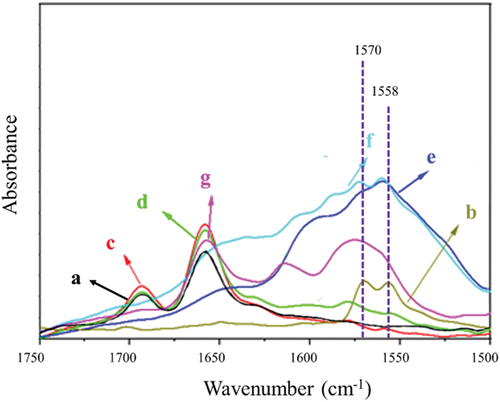
As shown in &d, the intensity of characteristic absorption peaks at 1570 and 1558 cm−1 of C-D1 and C-D2 samples became very weak, and shifted to a higher wavenumber at 1580 cm−1. This is due to the increase in stiffness of the pyridine ring arising from the formation of the quaternary ammonium salt between pyridine group and C=C-C-Cl bond of CR [Citation48–50], indicating the occurrence of Menschutkin reaction. When the DPD content was further increased in CR, the intensity of the characteristic peaks at 1570 and 1558 cm−1 gradually increased due to the excess of pyridine groups. Simultaneously, new absorption bands of the obtained the quaternary ammonium salt further shifted to higher wavenumbers, at 1597 cm−1 for C-D4 and 1615 cm−1 for C-D5, respectively. With the increase of DPD content, the excess of pyridine groups promoted the formation of quaternary ammonium salts, so the intensity of new absorption bands also increased accordingly. These results further confirmed that the crosslinking reaction did occur between CR and DPD.
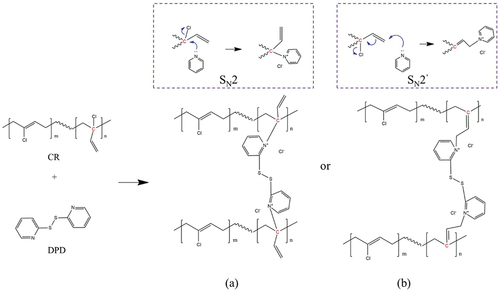
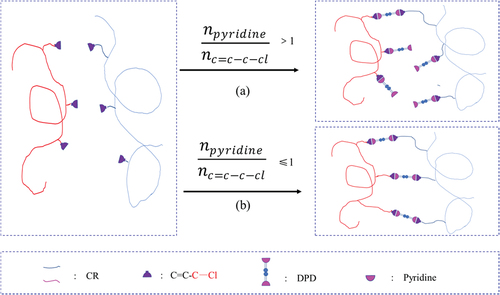
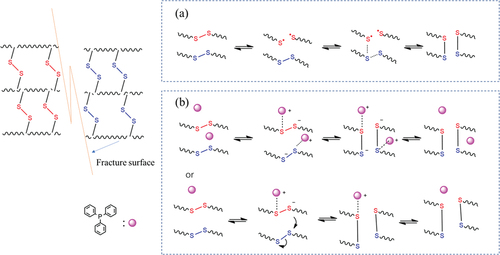
Due to the interaction between π and lone pair electron, the chlorine atom attached to the C=C bond inactivates the reactivity of the C=C bond. Correspondingly, the C=C bond also reduces the polarity of the C-Cl bond, resulting in the inability of CR to be vulcanized with sulfur. Benefiting from the structure of the presence of a small amount of allyl chloride in the CR polymer chain, C=C-C-Cl is more active than C=C-Cl due to the formation of a p-π conjugated system of allyl cation, which provides the active site for the CR vulcanization reaction. DSC and FTIR results confirmed the occurrence of vulcanization reaction between CR and DPD. As shown in , two possible types of mechanisms were proposed involving crosslinking reaction between CR and DPD. The two types of Menschutkin reactions exhibit the difference of the nucleophilic attack of pyridine group toward different carbocations of allyl chloride [Citation51]. The routes look reasonable, since it cannot be distinguished the detailed chemical structures of the cross-linkages of CR/DPD from FTIR spectra, we might expect faster attack at the terminated carbon of C=C bond. The reaction in the right-hand is preferred to the
in the left-hand box due to steric hindrance effect.
3.2. The vulcanization characteristics of CR/DPD
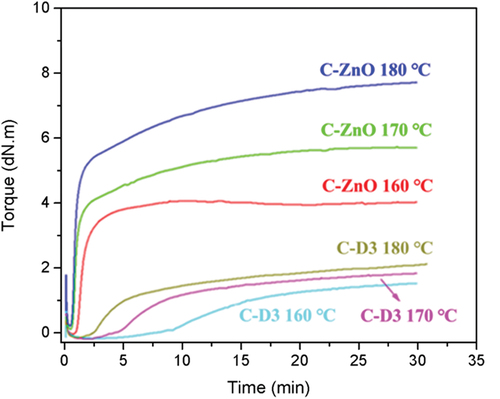
Vulcanization of rubber affects its industrial application. The vulcanization characteristics of CR/DPD were investigated with CR/ZnO as a control sample. Pure CR is unstable at high temperature and self-crosslinking reaction between polymer chains may occur [Citation52,Citation53]. Therefore, 1 phr antioxidant 1010 was added to eliminate the indiscernibility effect of self-crosslinking on vulcanization properties of samples. Taking C-D3 as an example, the vulcanization data of the C-D3 and C-ZnO at different temperatures are shown in and . Using ZnO as a vulcanizing agent, the vulcanization speed of CR was relatively high. As expected, the vulcanization rate of CR obviously increased with the increasing temperature. The scorch time (T10) at 160°C was 1.04 min, and it was shortened to 0.7 min at 180°C. Meanwhile, the vulcanization time (T90) was 2.93 min at 160°C, shortened to 1.95 min at 180°C. When DPD was used as a crosslinker for CR, the vulcanization reaction rate was slow, the vulcanization time was 16.8 min at 160°C, decreased to 6.33 min at 180°C.
For CR/ZnO, the maximum torque (MH) increased with increasing vulcanization temperature, because more chemical cross-linkages were formed at high temperature. For CR/DPD, the MH also increased with increasing vulcanization temperature, but not significantly, mainly due to the combined effect of increased crosslinking density and enhanced the exchange reaction of disulfide bonds. As shown in and , the torque of C-ZnO was higher than that of C-D3. C-ZnO and C-D3 form covalent crosslinking network and dynamic cross-linking network, respectively. Due to the reversible metathesis of disulfide bonds at high temperature, the MH of C-D3 samples was significantly lower than that of C-ZnO.
Table 2. Curing parameters and results of CR with DPD or ZnO.
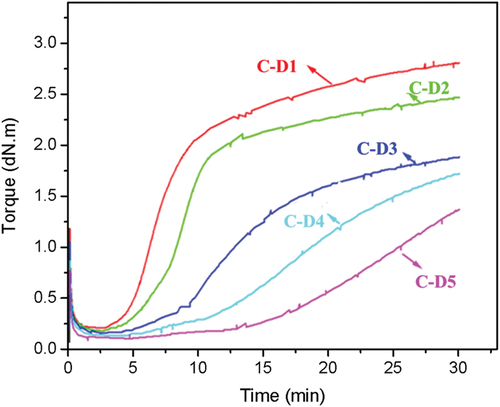
Furthermore, the effect of DPD content on the vulcanization properties of CR was investigated. As shown in , unexpectedly, the CR vulcanization rate (derived from the slope of the vulcanization curve) decreased with the increasing the content of DPD. It can be also found that the lower of the DPD content, the higher of the MH. The MH of C-D1 achieved the maximum value of 2.9 dN·m, which was 1.5 times that of the C-D3. These unexpected results of the vulcanization can be explained by the schematic diagram in . When the molar ratio of pyridine⁄(C=C-C-Cl) was higher than 1 (the number of pyridine group is greater than that of allyl chloride groups), only one pyridine group in most DPD molecules reacted with CR after vulcanization reaction, resulting in many pendant side chains (ineffective crosslinking bonds) located on the CR chain, therefore the number of effective crosslinking bonds becomes low. When the molar ratio of pyridine⁄(C=C-C-Cl) is less than 1, both pyridine groups of DPD could be involved in the Menschutkin reaction during vulcanization, thus forming more effective crosslinking bonds. Therefore, the vulcanization curves showed a higher crosslinking density and a faster vulcanization rate.
3.3. Tensile properties of CR/DPD
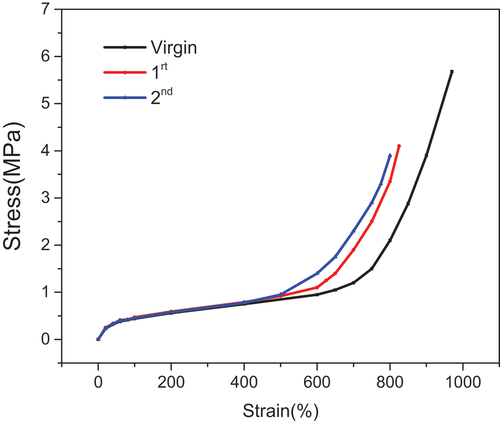
Before carrying out a comparative study on the static mechanical properties of DPD-crosslinked CR elastomers mentioned above, a covalently crosslinked CR sample was prepared as a benchmark by the crosslinking reaction between CR and ZnO (3phr). As shown in , it can be found that the tensile strength of the covalent bond crosslinked vulcanizate (C-ZnO) was higher than that of the dynamic bond crosslinked vulcanizate (C-D3), while the elongation at break of C-ZnO vulcanizate was lower than that of C-D3 vulcanizate. These results were consistent with the expected results of rubber crosslinking theory.
Figure 8. The stress and strain curves of C-Ds with dynamic crosslinking bond and C-ZnO with covalent crosslinking bond.
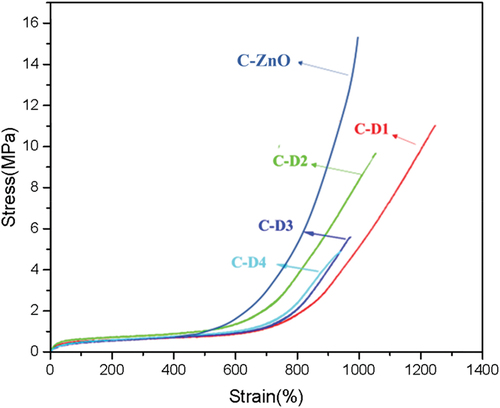
The effect of DPD content on the tensile properties of CR vulcanizate was investigated in detail. As shown in and , the tensile strength of CR vulcanizate decreased with the increasing DPD content. Surprisingly, the elongation at break of samples also decreased with increasing DPD content. Compared with CR vulcanizate with high DPD content, the C-D1 vulcanizate obtained the excellent mechanical properties, and its tensile strength and elongation at break were simultaneously improved. On the one hand, the crosslinking density of C-D1 vulcanizate was higher (illustrated in ), and during the stretching process, the continuous breaking and regeneration of the disulfide bond in the cross-linkage was favorable for the gradual orientation of the polymer chains along the stretching direction, effectively avoiding the stress concentration. Therefore, C-D1 vulcanizate has the highest tensile strength (11.12 ± 1.83 MPa) and elongation at break (1253 ± 120%), which was consistent with the results of the vulcanization curves (). Because most of the DPD participated in the formation of effective crosslinking bonds in the C-D1 vulcanizate. During the stretching process, the disulfide bond at the stress concentration position may preferentially break to generate sulfur radicals, which underwent a metathesis reaction with the other disulfide bond to form new disulfide bonds. In the system, the crosslinking network structure was constantly optimized while maintaining the crosslinking density of the network unchanged. When the DPD content was in excess, the sulfur radicals generated at the stress concentration position preferentially react with disulfide bond of ineffective cross-linkages. Thus, the density of the crosslinked network in the system cannot be maintained stable, and the structure of the crosslinked network cannot be well optimized. Therefore, the elongation at break of CR/DPD decreased with the increasing DPD content. The tensile modulus of covalently crosslinked CR-ZnO was basically consistent with that of dynamically crosslinked samples, and the tensile modulus of all samples at the initial stage was relatively low, indicating that the tensile modulus of low crosslinking degree CR samples is mainly related to the characteristics of CR itself.
Table 3. Mechanical properties of CR composites.
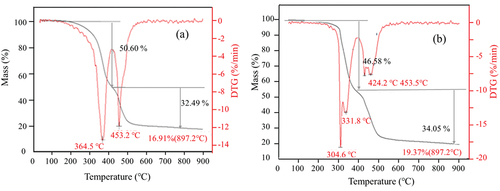
Thermogravimetric analysis revealed the two degradation stages of the composites in the temperature range of 100°C to 900°C (, ). The first-stage degradation peak temperatures of C-D3 and C-ZnO were 364.5°C and 304°C, it may correspond to the dehydrochlorination reaction of CR [Citation54,Citation55]. The weight loss of C-D3 and C-ZnO-3 in the first stage were 50.6% and 46.6%, respectively. shows that the peak temperature for the second stage degradation of C-D3 and C-ZnO occurred around 450°C, corresponding to the continuous degradation of CR via cracking and pyrolysis, resulting in the formation of low hydrocarbons with linear or cyclic structures. Over 30% weight loss of C-D3 and C-ZnO occurred at the second stage, indicating the completion of the degradation process. The remaining weight of C-D3 (16.9%) was lower than that of C-ZnO (19.4%). The possible reason is that DPD cross-linkages can be degraded and volatilized into the environment, while ZnO crosslinkages remain in the degraded product. Compared with the traditional ZnO crosslinked CR, the thermal stability of DPD crosslinked CR was not significantly reduced.
Table 4. Thermogravimetric analysis and char yield of unfilled and CR composites.
3.4. Self-healing and recyclability performances of CR/DPD vulcanizates
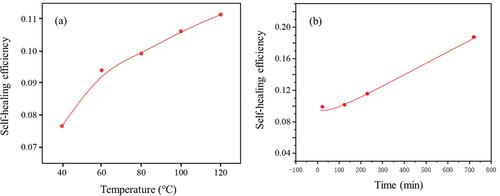
With a dynamic disulfide bond in each cross-linkage of CR system, the self-healing performance CR/DPD system was investigated. As shown in , it can be found that increasing the temperature and prolonging the healing time can promote the self-healing ability of C-D3, which was consistent with the phenomenon reported in the literature [Citation38]. However, the self-healing efficiency of C-D3 was poor, which may be due to the lack of reactive sites on the particle surface and the low density of effective cross-linkages in the C-D3 vulcanizate. The metathesis of disulfide bonds across the interface of C-D3 is difficult to occur even at high temperature (120°C), because the diffusion of the molecular segment movement at both sides of the interface is greatly restricted without external stimulation and the disulfide bond cannot exchange smoothly without catalyst at low temperature.
Figure 10. (a) effect of temperature on self-healing properties of C-D3 (healing time is 30 min). (b) effect of time on self-healing properties of C-D3 (healing temperature is 70°C).
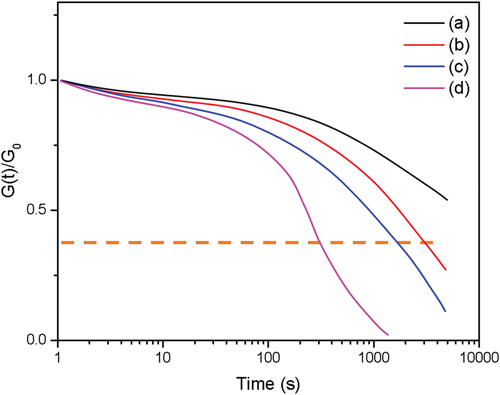
To further conform the dynamic properties of the CR/DPD samples with the reversible disulfide bonds, stress relaxation experiments were carried on at different temperatures (namely, 150, 160, 170°C). The normalized relaxation modulus as a function of time is shown in . The normalized relaxation modulus deceased rapidly with increasing temperature, but the characteristic relaxation time was longer. For example, the characteristic relaxation time of C-D3 was 26.7 min at 170°C, in agreement with the poor self-healing efficiency at lower temperature. The disulfide metathesis was enhanced by triphenylphosphine, as shown in ,the characteristic relaxation time of C-D6 was reduced to 5.1 min at 170°C.
Figure 11. Stress relaxation curves of CR/DPD samples. The dashed line indicates the characteristic relaxation time where the normalized modulus decreases to 1/e. (a) C-D3, 150°C, (b) C-D3, 160°C, (c) C-D3, 170°C, (d) C-D6, 170°C.
To obtain the excellent recyclability of CR-DPD through efficient exchange of disulfide cross-linkage, the high temperature and high pressure are essential factors. Triphenylphosphine (1 wt%) was also added to promote the metathesis of disulfide bonds. Therefore, the recyclability performance of C-D6 was investigated under a pressure of 10 MPa at 170°C. As shown in (route 1), the particles of C-D6 sample cannot be directed compress molded to produce a sheet at 170°C for 30 min, since the content of disulfide linkage in the system is low, the disulfide bond is stable below 170°C. As shown in (route 2), the particles of C-D6 sample were pulverized by double-roll mill at room temperature, and followed by a compress molding at 170°C for 30 min. C-D6 sample can be compressed and molded to produce a sheet via route 2. The smaller particles mean the larger specific surface area and the higher surface concentration of exposed disulfide linkages. Higher pressure also can reduce interstitial space of functional groups. The disulfide bonds of pulverized C-D6 sample could contact with triphenylphosphine to perform the reaction.
Figure 12. Pictures of reprocessing of C-D6 sample. (a) Particles of C-D6 sample. (b) Particles were compress molded under 10 MPa, at 170°C for 30 min. (c) Particles were pulverized to powders by a double-roll mill. (d) Powders were compress-molded under 10 MPa, at 170°C for 30 min.

As shown in , the tensile strength (4.1 MPa) and elongation at break (830%) of the first-recycled C-D6 sample were reduced compared with those of the virgin sample presumably due to the formation of a less uniform network structure and the fracture of CR chain during hot-pressing. The second-recycled sample obtained the almost similar mechanical properties with the once-recycled sample, confirming the possibility for repeated recycling of CR. Clearly, the recycled C-D6 can regain its mechanical properties to a great extent due to disulfide metathesis enabling crossover bonding among the polymer chains.
3.5. The mechanism for disulfide metathesis
The disulfide metathesis mechanism of CR/DPD is proposed, which is consistent with the mechanism reported in the literature [Citation56–58]. As shown in . the exchange reaction between disulfide compounds occurred via a radical-mediated mechanism without catalyst. In CR/DPD sample, the disulfide metathesis based on free radicals is insufficient due to low degree of crosslinking and low self-healing temperature, resulting in a poor self-healing performance. As shown in , under the catalysis of triphenylphosphine, the exchange reaction between disulfide compounds occurs via an ionic mechanism, the reaction involves a nucleophilic attack of triphenylphosphine on disulfide compounds, forming thiolate and the Ph3P+–S cation [Citation58]. Subsequently, based on two possible mechanisms, the disulfide exchange reaction is prone to occur, endowing C-D6 with good reprocessing performance.
4. Conclusions
Taking inspiration from the reaction of small molecule model compounds, a new technology was developed for the crosslinking of CR using DPD (2,2’−dithiodipyridine) as a crosslinking agent. When DPD was incorporated into CR fabricated following a vulcanization method commonly used in the rubber industry, the Menschutkin reaction between allyl chloride (C=C-C-Cl) group of CR and pyridine group of DPD occurred with a maximum exothermic peak temperature at 184°C. The number of effective crosslinking bond of C-D1 vulcanizate is higher than that of CR/DPD vulcanizates with high DPD content. When DPD is in excess, only one pyridine group of some DPDs is involved in the reaction with CR, resulting in many pendant side chains (ineffective crosslinking bonds). C-D1 vulcanizate obtained an excellent mechanical property, and its tensile strength and elongation at break were simultaneously improved. Compared with the traditional CR/ZnO vulcanizate, the thermal stability of DPD crosslinked CR is not significantly reduced. Disulfide bond in the cross-linkages maintains its stability at low temperatures, ensuring the mechanical stability of CR/DPD vulcanizate under ambient conditions. The metathesis of disulfide bond is improved obviously under the catalysis of triphenylphosphine, which endows CR/DPD vulcanizates with a good recyclability performance. These unique properties lead to the development of energy saving and environmentally friendly CR vulcanizates, which is essential for the green development of CR.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Saleh TA, Gupta VK. Processing methods, characteristics and adsorption behavior of tire derived carbons: a review. Adv Colloid Interface Sci. 2014;211:93–101. doi: 10.1016/j.cis.2014.06.006
- Abdelkareem M, Lin X, Ahmed A, et al. Vibration energy harvesting in automotive suspension system: a detailed review. Appl Energy. 2018;229:672–699. doi: 10.1016/j.apenergy.2018.08.030
- Shi TH, Wang MX. Zigzag hydrocarbon belts. CCS Chem. 2020;2:916–931. doi: 10.31635/ccschem.020.202000287
- Melanie B, Marie B, Joanna B, et al. Robotic seals as therapeutic tools in an Aged Care Facility: a qualitative study. J Aging Sci Res. 2016;2016:1–7. doi: 10.1155/2016/8569602
- Yang J, Liao X, Wang G, et al. Gradient structure design of lightweight and flexible silicone rubber nanocomposite foam for efficient electromagnetic interference shielding. Chem Eng J. 2020;390:124589. doi: 10.1016/j.cej.2020.124589
- Mensah B, Gil Kim H, Lee J-H, et al. Carbon nanotube-reinforced elastomeric nanocomposites: a review. Int J Smart Nano Mat. 2015;6(4):211–238. doi: 10.1080/19475411.2015.1121632
- Carrico JD, Tyler T, Leang KK. A comprehensive review of select smart polymeric and gel actuators for soft mechatronics and robotics applications: fundamentals, freeform fabrication, and motion control. Int J Smart Nano Mat. 2017;8(4):144–213. doi: 10.1080/19475411.2018.1438534
- T LY, J LW, X SF, et al. Processing, thermal conductivity and flame retardant properties of silicone rubber filled with different geometries of thermally conductive fillers: a comparative study. Compos Part B Eng. 2022;238:109907. doi: 10.1016/j.compositesb.2022.109907
- Chen HY, Chen ZY, Mao M, et al. Self‐Adhesive polydimethylsiloxane foam materials decorated with MXene/Cellulose nanofiber interconnected network for versatile functionalities. Adv Funct Mater. 2023;2304927. doi: 10.1002/adfm.202304927
- Xu H, Gong LX, Wang X, et al. Influence of processing conditions on dispersion, electrical and mechanical properties of graphene-filled-silicone rubber composites. Composites Part A: Applied Science And Manufacturingquery. 2016;91:53–64. doi: 10.1016/j.compositesa.2016.09.011
- Wemyss AM, Bowen C, Plesse C, et al. Dynamic crosslinked rubbers for a green future: a material perspective. Mater Sci Eng R Rep. 2020;141:100561. doi: 10.1016/j.mser.2020.100561
- Bao C, Jiang YJ, Zhang H, et al. Room-temperature self-healing and recyclable tough polymer composites using nitrogen-coordinated boroxines. Adv Funct Mater. 2018;28(23):1800560.1–.1800560.10. doi: 10.1002/adfm.201800560
- Dobrotă D, Gabriela D. An innovative method in the regeneration of waste rubber and the sustainable development. J Clean Prod. 2018;172:3591–3599. doi: 10.1016/j.jclepro.2017.03.022
- Myhre M, Saiwari S, Dierkes W, et al. Rubber recycling: chemistry, processing, and applications. Rubber Chem Technol. 2012;85(3):408–449. doi: 10.5254/rct.12.87973
- Aaontarnal D, Capelot M, Tournilhac F, et al. Silica-like malleable materials from permanent organic networks. Science. 2011;334(6058):965–968. doi: 10.1126/science.1212648
- Fu F, Huang M, Zhang W, et al. Thermally assisted self-healing behavior of anhydride modified polybenzoxazines based on transesterification. Sci Rep. 2018;8(1):10325. doi: 10.1038/s41598-018-27942-9
- Lu L, Jian P, Li G. Recyclable high performance epoxy based on transesterification reaction. J Mater Chem A. 2017;5(40):21505–21513. doi: 10.1039/C7TA06397K
- Lu Y-X, Guan Z. Olefin metathesis for effective polymer healing via dynamic exchange of Strong Carbon–Carbon Double bonds. J Am Chem Soc. 2012;134(34):14226–14231. doi: 10.1021/ja306287s
- Wang X, Liu K, Liu Y, et al. Dynamic ionic behavior of gel-free diolefin rubber-based carboxylate ionomers prepared via olefin metathesis. Soft Matter. 2022;18(13):2577–2584. doi: 10.1039/D2SM00098A
- Zhao D, Du Z, Liu S, et al. UV light curable self-healing superamphiphobic coatings by photopromoted disulfide exchange reaction. ACS Appl Polym Mater. 2019;1(11):2951–2960. doi: 10.1021/acsapm.9b00656
- Pepels M, Filot I, Klumperman B, et al. Self-healing systems based on disulfide–thiol exchange reactions. Polym Chem. 2013;4(18):4955–4965. doi: 10.1039/c3py00087g
- Xu Y, Chen D. A novel self-healing Polyurethane based on disulfide bonds. Macromol Chem Phys. 2016;217(10):1191–1196. doi: 10.1002/macp.201600011
- Zuo Y, Gou Z, Zhang C, et al. Polysiloxane-based Autonomic self-healing elastomers obtained through dynamic boronic ester bonds prepared by thiol-ene “click” chemistry. Macromol Rapid Commun. 2016;37(15):1300–1300. doi: 10.1002/marc.201600303
- Cash JJ, Kubo T, Dobbins DJ, et al. Maximizing the symbiosis of static and dynamic bonds in self-healing boronic ester networks. Polym Chem. 2018;9(15):2011–2020. doi: 10.1039/C8PY00123E
- Cho S, Hwang SY, Oh DX, et al. Recent progress in self-healing polymers and hydrogels based on reversible dynamic B–O bonds: boronic/boronate esters, borax, and benzoxaborole. J Mater Chem A. 2021;26:14630–14655. doi: 10.1039/D1TA02308J
- Chao A, Negulescu I, Zhang D. Dynamic covalent polymer networks based on degenerative imine bond exchange: tuning the malleability and self-healing properties by solvent. Macromolecules. 2016;49(17):6277–6284. doi: 10.1021/acs.macromol.6b01443
- Liu X, Liang L, Lu M, et al. Water-resistant bio-based vitrimers based on dynamic imine bonds: self-healability, remodelability and ecofriendly recyclability. Polymer. 2020;210:123030. doi: 10.1016/j.polymer.2020.123030
- Solouki Bonab V, Karimkhani V, Manas-Zloczower I. Ultra-fast microwave assisted self-healing of covalent adaptive polyurethane networks with Carbon nanotubes. Macromol Mater Eng. 2019;304(1):1800405. doi: 10.1002/mame.201800405
- Johnson PR. Polychloroprene Rubber. Rubber. Chem Technol. 1976;49(3):650–702. doi: 10.5254/1.3534978
- Carothers WH, Coffman DD. Homologs of chloroprene and their polymers (second paper on new synthetic rubbers). J Am Chem Soc. 1932;54(10):4071–4076. doi: 10.1021/ja01349a035
- Yuan W, Chen H, Chang R, et al. Synthesis and characterization of NaA zeolite particle as intumescent flame retardant in chloroprene rubber system. Particuology. 2011;9(3):248–252. doi: 10.1016/j.partic.2010.05.016
- Kobędza P, Smejda-Krzewicka A, Olejnik A, et al. Flame retardant and durable chloroprene rubber and styrene-butadiene rubber blends crosslinked with copper(I) oxide. Iran Polym J. 2021;30(2):149–165. doi: 10.1007/s13726-020-00881-x
- Wypych G, CR polychloroprene, in Handbook of Polymers, 2012, p. 71–74. doi:10.1016/C2011-0-04631-8
- Zxa B, Long Z, Swa B, et al. Graphene oxide-supported zinc oxide nanoparticles for chloroprene rubber with improved crosslinking network and mechanical properties. Compos Part A Appl Sci Manuf. 2019;124:105492–. doi: 10.1016/j.compositesa.2019.105492
- Kapgate BP, Das C, Das A, et al. Reinforced chloroprene rubber by in situ generated silica particles: evidence of bound rubber on the silica surface. J Appl Polym Sci. 2016;133(30):30. doi: 10.1002/app.43717
- Torrence MF. Vulcanization of neoprene with Antimony Trisulride. Ind Eng Chem. 1949;41(3):641–643. doi: 10.1021/ie50471a041
- Kong L, Zhu Y, Huang G, et al. Carbon nanodots as dual role of crosslinking and reinforcing chloroprene rubber. Compos Commun. 2020;22(3):100441. doi: 10.1016/j.coco.2020.100441
- Xiang HP, Rong MZ, Zhang MQS-H. Reshaping, and recycling of vulcanized chloroprene rubber: a case study of multitask cyclic utilization of cross-linked polymer. ACS Sustain Chem Eng. 2016;4(5):2715–2724. doi: 10.1021/acssuschemeng.6b00224
- Dan M, Qiao Y, Wang X, et al. DABCO-catalyzed knoevenagel condensation of aldehydes with ethyl cyanoacetate using hydroxy ionic liquid as a promoter. RSC Adv. 2018;8(53):30180–30185. doi: 10.1039/C8RA06506C
- Zhao S, He M, Guo Z, et al. [HyEtpy]cl–H2O: an efficient and versatile solvent system for the DABCO-catalyzed Morita–Baylis–Hillman reaction. RSC Adv. 2015;5(41):32839–32845. doi: 10.1039/C5RA02102B
- Musilek K, Komloova M, Zavadova V, et al. Preparation and in vitro screening of symmetrical bispyridinium cholinesterase inhibitors bearing different connecting linkage-initial study for myasthenia gravis implications. Bioorg Med Chem Lett. 2010;20(5):1763–1766. doi: 10.1016/j.bmcl.2010.01.034
- Reardon MB, Xu M, Tan Q, et al. Long-range reactivity modulations in geranyl chloride derivatives. J Org Chem. 2016;81(22):10964–10974. doi: 10.1021/acs.joc.6b01759
- Wei ZY, Yue DM, Mo XT, et al. Hydrogenation of neoprene latex with Diimide. Adv Mater Res. 2006;11-12:391–394. doi: 10.4028/www.scientific.net/AMR.11-12.391
- Makhiyanov N. Determination of the microstructure of polychloroprenes from 1H and 13C NMR data. Russian J Appl Chem. 2011;84(3):454–460. doi: 10.1134/S1070427211030219
- Saito T, Kawahara S, Ohtake YJRC, et al. Assignment of NMR signals for chloroprene rubber by two-dimensional NMR spectroscopy. Rubber Chem Tech. 2013;86(2):250–260. doi: 10.5254/rct.13.87981
- Talla A, Driessen B, Straathof NJW, et al. Metal-free photocatalytic aerobic oxidation of thiols to disulfides in batch and continuous-flow. Adv Synth Catal. 2015;357(10):2180–2186. doi: 10.1002/adsc.201401010
- Jiang J, Zhai J, Kong L, et al. Flame retardant chloroprene rubbers with high tensile strength and elongation at break via dual cross-linked networks. RSC Adv. 2022;12(42):27633–27640. doi: 10.1039/D2RA05389F
- Tang Z, Wu X, Guo B, et al. Preparation of butadiene–styrene–vinyl pyridine rubber–graphene oxide hybrids through co-coagulation process and in situ interface tailoring. J Mater Chem A. 2012;22(15):7492–7501. doi: 10.1039/c2jm00084a
- Lei Y, Tang Z, Zhu L, et al. Functional thiol ionic liquids as novel interfacial modifiers in SBR/HNTs composites. Polymer. 2011;52(5):1337–1344. doi: 10.1016/j.polymer.2011.01.024
- Salim NV, Hameed N, Guo Q. Competitive hydrogen bonding and self-assembly in poly(2-vinyl pyridine)-block-poly(methyl methacrylate)/poly(hydroxyether of bisphenol A) blends. J Polym Sci B Polym Phys. 2009;47(19):1894–1905. doi: 10.1002/polb.21792
- Streitwieser A, Jayasree EG, Hasanayn F, et al. A theoretical study of SN2′ reactions of Allylic Halides: role of Ion Pairs. J Org Chem. 2008;73(23):9426–9434. doi: 10.1021/jo8020743
- Zhang P, Huang G, Qu L, et al. Study on the self‐crosslinking behavior based on polychloroprene rubber and epoxidized natural rubber. J. 2012;125(2):1084–1090. doi: 10.1002/app.34508
- Antony P, De S, Duin M. Self-crosslinking rubber/rubber and rubber/Thermoplastic blends: a review. Rubber Chem Technol. 2001;74(3):376–408. doi: 10.5254/1.3547644
- Aracil I, Font R, Conesa JA, et al. TG–MS analysis of the thermo-oxidative decomposition of polychloroprene. J Anal Appl Pyrolysis. 2007;79(1):327–336. doi: 10.1016/j.jaap.2006.12.027
- Ren Y, Cao C, Hu H, et al. Transformation behavior and fate of chlorine in polychloroprene (PCP) during its pyrolysis. Fuel. 2022;317:123573. doi: 10.1016/j.fuel.2022.123573
- Lei ZQ, Xiang HP, Yuan YJ, et al. Room-temperature self-healable and Remoldable cross-linked polymer based on the dynamic exchange of disulfide bonds. Chem Mater. 2014;26(6):2038–2046. doi: 10.1021/cm4040616
- Caraballo R, Rahm M, Vongvilai P, et al. Phosphine-catalyzed disulfide metathesis. Chem Commun. 2008;48(48):6603–6605. doi: 10.1039/b815710c
- Nevejans S, Ballard N, Miranda JI, et al. The underlying mechanisms for self-healing of poly (disulfide) s. Phys Chem Chem Phys. 2016;18(39):27577–27583. doi: 10.1039/C6CP04028D