ABSTRACT
This review article comprehensively explores the electrochemical detection of organophosphate-based agents, including warfare agents, pesticides, and simulants. It provides an in-depth analysis of their molecular structures, emphasizing the inherent toxicity and environmental risks posed by these compounds. The review highlights the significant role of flexible sensors in facilitating the electrochemical detection of organophosphate-based agents, offering insights into their design, development, and application in detection methodologies. Additionally, the article critically evaluates the challenges encountered in this field, such as sensor sensitivity and sample complexity, and discusses potential solutions to address these challenges. Furthermore, it outlines the future scope and opportunities for advancement in electrochemical detection technologies, including the integration of novel materials and the exploration of innovative detection strategies. By synthesizing current research findings and identifying future research directions, this review contributes to the ongoing discourse on the detection and mitigation of organophosphate-based agents’ risks to human health and the environment.
1. Introduction
Chemical warfare agents (CWAs) have the potential to kill a person [Citation1]. Chemical compounds known as CWAs are synthetic and have the potential to be directly harmful to people, animals, and plants [Citation2]. CWAs, however, also include certain incendiary mixes and/or smoke, as well as combustible, toxic, irritating, and choking gasses [Citation3]. The hazardous substances included in CWAs may often cause rapid incapacitation, unexpected death, and long-term negative health impacts [Citation4]. CWAs were designed to be used as weapons in combat and were once accepted as legal weapons systems until an international treaty forbade their usage [Citation5]. During World War I, synthetic chemical CWAs were first used, first with chlorine gas and then with a range of other gases, including sulfur mustard [Citation6]. Nerve gasses were created and accumulated by many armed forces throughout the globe later in the 1930s as a consequence of research into insecticides. The Iraqis’ use of CWAs during the Iran-Iraq War in the 1980s caused thousands of deaths [Citation7], while the Aum Shinrikyo cult’s use of sarin gas in the Tokyo subway assault in 1995 left 12 people dead and 5,300 wounded [Citation8]. It is also believed that CWAs have been used in a number of instances related to the ongoing war in Syria, which has left victims with long-term health problems and multiple fatalities. The categories of CWAs include blood/asphyxiant, incapacitating/behavior-altering, choking, nerve, and blood-altering agents [Citation9]. Although there are many different kinds of CWAs, one of the deadliest is the nerve agent, which terrorists are likely to employ in an assault on people [Citation10]. Organophosphorus compounds, which are among the most poisonous chemicals, are often found in nerve agents [Citation11]. Although these compounds are often found in agricultural pesticides and insecticides, they have the potential to be abused as CWAs [Citation12]. The cholinesterase enzyme, which controls the acetylcholine required for nervous system activity (conduction), is impacted by organophosphorus chemicals (OPCs) [Citation13]. An organophosphorus chemical overdose usually causes muscular cramps, convulsions, miosis, and eventually death. Even at modest exposure levels, these effects may manifest [Citation14].
Among all the synthetic chemical derivatives, nerve agents are thought to be the most dangerous. Their mode of action, characterized by phosphorylation due to their organophosphonate structure (RO(O = P(R′)OR″), differentiates them from other categories of chemical warfare (CW) agents such as incapacitating, blood, blister, choking agents, etc. They are strong active agents for acetylcholinesterase (AChE). In particular, when compared to closely related species, the threat posed by mammalian toxicity is largely explained by this unique structure. Phosphorus (V) compounds with a terminal oxide and three singly bonded substituents (two alkoxyl and one alkyl group) make up nerve agents and insecticides (); these derivatives have sparked a broad range of investigations into their practical detection.
Figure 1. G-type and V-type agent names and structures. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).
![Figure 1. G-type and V-type agent names and structures. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).](/cms/asset/19af3667-09e9-4341-8bf1-e96c03bcf3cb/tsnm_a_2385350_f0001_b.gif)
This review article aims to briefly overview the electrochemical detection methods employed for identifying organophosphate-based agents, including warfare agents, pesticides, and simulants. These compounds pose significant risks to both human health and the environment due to their inherent toxicity. Particularly, organophosphate-based pesticides contribute to aquatic pollution and adversely affect human populations. Hence, the imperative need for their detection is underscored. Subsequent sections of this review will delve into the molecular structures and diverse types of organophosphate-based compounds. Emphasis is placed on elucidating the electrochemical detection techniques, with a particular focus on the role of flexible sensors in this domain. Noteworthy literature on flexible sensor development is examined, shedding light on the advancements made in this area. Furthermore, this review briefly addresses the challenges encountered and outlines the prospects in the field. By perusing this comprehensive review, readers will gain valuable insights into the electrochemical detection of organophosphate-based warfare agents.
2. Brief history and molecular structure
During human battlefield conflict, the practical use of certain chemical-based deterrents (such as smoke clouds, vapors containing arsenic, etc.) dates back thousands of years and is often a reflection of the technological and experimental expertise of the civilization and period. The understanding of synthetic chemistry has resulted in a clear advancement in the last century in the production of dispersible chemical media for use in human warfare. The substitution of mustard gas for phosgene (COCl2) and subsequently chlorine (Cl2) indicates a rise in complexity that is directly related to advances in science and chemistry. One may argue that such methods in contemporary conflict are derived from those used in trench warfare (Ypres, Belgium) during World War I. Chlorine-containing compounds were employed efficiently in Ypres, Belgium, and gas warfare resulted in around 91,000 fatalities (1.3 million casualties) altogether [Citation16]. An examination of the original chemical literature does not provide a precise history of the creation of nerve agents [Citation17]. This research was linked to covert wartime activities in the late 1930s and early 1940s that produced chemicals later identified as G-type (German-type) nerve agents and related substances (). In 1936, tabun was produced by G. Schrader and associates affiliated with I. G. Farbenindustrie (Leverkusen, Germany) [Citation18]. Then came the preparation of Sarin (1938) and Soman (1944). Tons upon tons of these chemicals were produced. In 1948, cyclosarin was created. Following the conclusion of World War II, these chemicals became well-recognized to the general public [Citation19]. In the early 1950s, at Porton Down, Great Britain, an even riskier extension of this clandestine work was carried out when VX (ethyl Sdiisopropylaminoethyl methyl phosphothioate), the first member of the V-class (venom or venomous-type), was developed. V agents were found by British scientists when creating new pesticides. The R-VX counterpart, a constitutional isomer containing diethylamino and isobutoxide units, was subsequently created by Russian scientists in 1963 (). These compounds were produced during the Cold War, which resulted in the current arsenals (shells, munitions) in a variety of amounts that are kept at military repository sites mostly in the former Soviet Union and the United States. News organizations have been keeping an eye on these stocks’ condition recently as decontamination operations have been intensified [Citation20]. Despite their close resemblance to [O=PV (OR)]-containing species (), the known nerve agents do vary significantly from one another. Furthermore, all have a p-CH3 group, with the exception of tabun [(EtO)O=P(−CN)(NMe2)] [Citation21]. Thus, the [O=P-Me] fragment is a common nerve agent moiety, with this one exception. Fluorophosphonates (PH → PF) are closely linked to sarin, soman, and cyclosarin [Citation22]. Within the short V-series, there are two compounds: VX [Citation23] and its isomer R-VX. Both include an extra sp3-hybridized group 15 center in addition to the pendant amino group. Compared to R-VX, Chinese VX has a butoxy group [Citation24]. More complex sensing procedures and breakdown chemistry result from the V-class. The element phosphorus (V) is necessary; at this point, PIII species and/or intermediaries are unknown, with the possible exception of hypothetical species in high-temperature surface chemistry.
2.1. Types of organophosphate based compounds
2.1.1. Phosphorus (V) parent compounds
By using simpler parent molecules as a guide, the complexity of compound nomenclature and agent replacement may be reduced. lists the names of the simple phosphorus (III) and (V) hydride, hydroxide, and oxide compounds. Acidity values expressed as pKa’s are given here. The phosphonate class gives names to all nerve agents. PH3 is the most basic analog of phosphorus. Formally, hydroxyl groups may be added to this homoleptic trivalent hydride to get phosphinous acid [PH2OH], phosphonous acid [PH(OH)2], and lastly phosphinic acid [P(OH)3]. Because of its strong p=O bond, phosphine oxide (O=PH3), the tautomeric form that predominates, is unstable in relation to phosphorus acid and its derivatives [Citation25]. (CF3)2POH is the sole known example of a stable phosphinous acid [Citation26]. Formally, phosphoryl oxygen [p=O] is absent from phosphonite species [Citation27]. One of the related compounds of pentavalent phosphorus is simple phosphine oxide (O=PH3). Though O=PPh3 and similar species’ hydrolysis chemistry might vary due to p-R bonds’ extreme stability against bond breakage, they may provide structural insights into the detection and detoxification of organophosphates (OPs). The formal addition of extra oxygen atoms results in the formation of phosphonic (phosphorus) acid [O=PH(OH)2] and phosphinic acid (O=PH2OH). It is noteworthy that these species have the ability to tautomerize between P(V) and P(III), or from H2P(O)OH to H=P(OH)2 (). Moreover, phosphonic acid, P(OH)3, is not as stable as its tetrahedral form [O=PH(OH)2]. Coordination with some metals may stabilize this form [Citation28]. There are further closely related simple species that include phosphorus. Numerous additional cyclic and chain phosphorus oxyacid derivatives are also known to exist [Citation29], such as orthophosphate [2-O3P-O-PO32-]. Analogs of arsenic could potentially be harmful [Citation30]. Because phosphorus sulfides are intermediates in the manufacture of nerve agents (NA), they are also significant in this context. The chemistry of p-F and other halide species is also important.
Figure 2. Names and structures of the basic mononuclear phosphorus hydrides, hydroxides, and oxides with three and five values. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).
![Figure 2. Names and structures of the basic mononuclear phosphorus hydrides, hydroxides, and oxides with three and five values. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).](/cms/asset/2a62205b-c9f4-4be6-b682-563b756f2df7/tsnm_a_2385350_f0002_c.jpg)
2.1.2. Pesticides
Man’s capacity to chemically eliminate pests may have existed before he was able to similarly eliminate or render human beings incapable of harming one another. As a result, organophosphates, or phosphorus triesters, were created during the same general post-World War II period (). Since nerve agents and insecticides have similar structural characteristics and formulations, their preparations are intertwined () [Citation31]. These species are less dangerous but have a similar mechanism of action like warfare agents and are located on the ‘toxicity continuum.’ Pesticides were organic chemicals that had been chlorinated until the 1930s. Phosphorus(V) compounds gradually replaced chlorine-containing pesticide chemicals, as was the case with analogous advancements in chemical warfare. Following World War II, there was a distinct phase of very rational development of organophosph(on)ate-based pesticides (insecticides) (1940s-1950s), which gave rise to many new closely related species. Pesticides are less instantly hazardous (to AChE) than nerve agents, according to established LD50 values in non-human model systems; yet, their increased usage and laxer supervision should raise more concerns. There are instances where pesticides have a terminal sulfide group [p=S]. This affects the physical characteristics and has implications for all applications (e.g. hydrogen bonding).
Figure 3. Various structures of OP-based pesticides. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).
![Figure 3. Various structures of OP-based pesticides. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).](/cms/asset/cd390112-738e-47e6-8e90-9c9975af1f68/tsnm_a_2385350_f0003_b.gif)
2.1.3. Simulants
Many relatively safe and easily accessible compounds are often employed in ‘nerve agent’ research because of the extreme toxicity of the nerve agent class. These surrogates, also known as mimics or surrogates, are present in the ‘continuum’ of reduced AChE toxicity caused by the drugs. Moreover, they are usually less costly. These are useful for evaluating a new chemical system for NA detection or cleaning capacity in less demanding laboratory environments. shows the architectures of the different simulants. Interestingly, some of them could not include phosphorus as well. A recognized pesticide may function as an NA simulant. It’s important to remember that simulators only ever provide a partial assessment of usefulness; they never behave precisely like ‘live’ agents. None, for example, provides ‘the unique intramolecular amino nitrogen effect in VX’ in terms of VX chemistry [Citation32]. The phosphorus chemistry is preserved in some compounds, but very few of them are very similar to the thiocholine moiety, which is by itself a crucial breakdown product in the process. The majority of them have one or more alkoxyl groups, and they are mostly P(V) centered [Citation33].
Figure 4. Names and structures of some pertinent mimics that are utilized in sensing as sarin, VX, and other mimics. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).
![Figure 4. Names and structures of some pertinent mimics that are utilized in sensing as sarin, VX, and other mimics. Reproduced with permission from ref [Citation15], copyright @ American Chemical Society (2011).](/cms/asset/19c3065b-86be-48bc-8c3f-5c31a1651cac/tsnm_a_2385350_f0004_b.gif)
2.2. Urgent necessity for detection
Usage of lethal chemical weapons by terrorists in entire or partially peaceful periods over the last 30 years has resulted in unease that persists to this day. Not only are these earlier occurrences well known but they are also connected to findings found in the main chemical literature. In 1988, during the Al-Anfal War, nerve (and mustard) agents were used on villages in Iraqi Kurdistan, resulting in thousands of fatalities [Citation34,Citation35]. And then there were other urban incidents in Japan that the Japanese Aum Shinrikyo cult was responsible for. These included (i) the sarin release in Matsumoto, Nagano in June 1994, which resulted in approximately 7 deaths and over 200 injuries; (ii) sporadic attempts at assassinations between 1994 and 1995 [Citation36,Citation37]; and (iii) the subway attack in Tokyo, Japan, in March 1995 [Citation38], which is considered the most widespread instance of ‘terrorist’ NA use (12 dead, approximately 5000 injured). During the Gulf War in 1991, more than 100,000 American soldiers may have been exposed to sarin and cyclosarin in Khamisiyah, Iraq [Citation39]. A sarin-filled artillery shell was used against coalition troops in May 2004 during the second Gulf War in Baghdad, Iraq, and this incident occurred more recently [Citation40,Citation41].
False positives for VX exposure, like the one that occurred in Washington, DC, in February 2006 [Citation42], have cast doubt on the accuracy of sensors and the public’s preparedness for agent-related assaults in the future in the United States. Exceptionally preventive measures have occasionally also resulted in controversy: in 1998, the United States launched a military strike against El Shifa Pharmaceuticals’ manufacturing facility in Khartoum, Sudan because the facility was suspected of producing organophosphonate nerve agents [Citation43]. This was prompted by fears of the covert manufacture of CWAs. As a result, effective sensing technology may also direct wise diplomacy.
3. Conventional methods for the determination of organophosphate nerve agents
Detecting and discriminating pesticides and organophosphates can be achieved through advanced techniques such as electrochemical sensors, chromatographic methods, spectroscopic methods, etc. Electrochemical sensors, using modified electrodes generate measurable signals upon interaction with target compounds. Chromatographic techniques like gas and liquid chromatography, often coupled with mass spectrometry, provide detailed molecular information. Spectroscopic methods identify functional groups and structures, while immunoassays use antibodies for specific detection. Molecularly imprinted polymers (MIPs), with tailored binding sites, offer selective binding and measurement of target molecules. Combining these techniques ensures high sensitivity and specificity in detecting and differentiating various pesticides and organophosphates. shows some important advantages and disadvantages of such conventional methods.
Table 1. Advantages and disadvantages of these conventional methods.
A few techniques, including gas chromatography (GC) [Citation44], liquid chromatography (LC) [Citation45], ion mobility spectrometry (IMS) [Citation46], and Fourier transform infrared spectroscopy (FTIR) [Citation47], have been employed throughout the last 50 years to identify organophosphorus compounds (OPCS).
3.1. Gas chromatography
Gas chromatography is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a substance or separating the different components of a mixture (the relative amounts of such components can also be determined). In preparative chromatography, GC can be used to prepare pure compounds from a mixture. With this pure compound, the GC procedure is often extended by employing different detectors in order to enhance its detection capability [Citation44].
3.2. Liquid chromatography
Liquid chromatography is a widely used analytical technique for separating, identifying, and purifying mixture components. It involves the interaction of a mobile phase (usually a liquid) with a stationary phase (a solid or liquid supported on a solid) to separate the components based on their properties such as polarity, size, charge, or affinity [Citation45].
3.3. Ion mobility spectrometry
Ion mobility spectrometry is a powerful analytical technique used in various fields, including environmental monitoring, industrial process control, drug development, and homeland security. It is based on the principle of separating and identifying ions in a gas phase by measuring their mobility under an electric field [Citation46].
3.4. Fourier transform infrared spectroscopy
FTIR is a powerful analytical technique used in various fields, including sensing applications. It offers high sensitivity and selectivity, fast analysis time, and the ability to detect trace amounts of substances. FTIR can be used in sensing applications such as detecting biomarkers in biological fluids, monitoring environmental pollutants, and detecting chemical warfare agents [Citation47].
3.5. Electrochemical method
In addition to the aforementioned techniques, electrochemical-based sensing systems have been extensively researched because of their promising technology for identifying and detecting organic pollutants [Citation48–54]. The ongoing development of electrochemical-based sensing systems may be attributed to features including selectivity, sensitivity, affordability, miniaturization, and a notable detection limit [Citation55]. In recent years, several innovations have been made to build this platform with increased selectivity and sensitivity, including unique surface modifications, enzyme immobilization, and sensor fabrication [Citation55,Citation56]. The sensors’ operation is based on the idea that the target and recognition chemical interact to produce an electrical signal, which is then used to quantify the amount of OPCs in the sample.
3.6. Electrochemical sensors
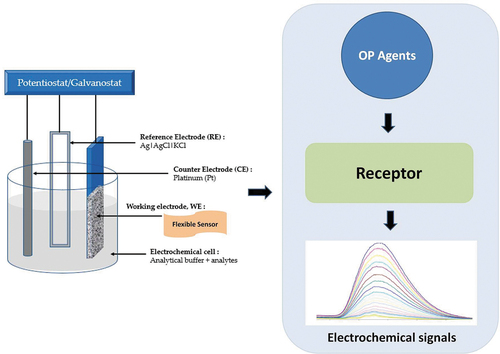
Electrochemical sensors are becoming more and more popular as a sensing platform for the identification of OPCs because of their high sensitivity, quick reaction time, and easy preparation. The contact of the target molecules with a sensing membrane, which is essentially a modified electrode material, produced a chemical signal, which was then transformed by a conversion element into a detectable electric signal. Based on the related association of the concentration of the standard analyte to be detected and the presented electrochemical signal, the content of the unknown analyte was computed. It may be categorized into four main categories: photoelectrochemical sensors, electrochemical luminescence sensors, current/potential sensors, and electrochemical impedance sensors. A working electrode, a reference electrode, and an auxiliary electrode made up the three-electrode system of the conventional electrochemical sensor, as seen in [Citation58–60]. In general, voltammetry, amperometry, and potentiometry are the three electrochemical methods utilized to detect OPPs. Notably, the most often used detection methodologies are voltammetric techniques such as square wave voltammetry (SWV), cyclic voltammetry (CV), and differential pulse voltammetry (DPV) [Citation61–63].
Figure 5. Diagrammatic representation of a traditional electrochemical sensor. Reproduced from [Citation57] under common creative license, MDPI (2020).
![Figure 5. Diagrammatic representation of a traditional electrochemical sensor. Reproduced from [Citation57] under common creative license, MDPI (2020).](/cms/asset/a852ec4f-60ce-44c3-90c1-86e5c3c6cc76/tsnm_a_2385350_f0005_c.jpg)
CV is utilized to measure alternating current by providing two potential differences in a cycle. DPV and SWV measure current generated based on analyte concentration through the application of a sequence of pulses with incrementally increasing voltage [Citation61]. CV, while being the simplest method, offers high sensitivity up to micromolar analyte concentrations [Citation62]. In contrast, DPV and SWV methods provide higher sensitivity, detecting analyte concentrations down to nanomolar levels [Citation63]. Furthermore, DPV can minimize the effect of charging current, which can interfere with faradaic current readings. Therefore, it can be concluded that DPV is the optimal choice for sensing applications, as it offers a low limit of detection due to its high sensitivity. To increase electrochemical activity and responsiveness in this process, the electrode material is essential [Citation64,Citation65].
An electrochemical sensor based on nanomaterials that has a high sensitivity and specificity is the best option for detecting OPCs. Thus, the electrochemical sensor research progress for OPC detection is presented in this study [Citation66]. In essence, OPCs are phosphoric acid esters that have varying ratios of sulfur, carbon, nitrogen, and oxygen. A phosphorus atom in the center forms a double bond with an oxygen or sulfur atom, and alkoxy/propyl/other substituents (X) and thioalkoxy/aryloxy (O-R, O-R’) are connected to the phosphorus atom. This structural pattern is shared by all OPCs. The toxicity of OPCs was caused by different X、R、R’ [Citation67].
3.6.1. Components of the sensor
As per the International Union of Pure and Applied Chemistry (IUPAC), A chemical sensor is a device designed to convert chemical information, ranging from the concentration of a specific component within a sample to a comprehensive analysis of its composition, into a useful and analytically relevant signal [Citation68]. This signal can take various forms depending on the sensor’s design and detection principle, such as electrical, optical, or mechanical signals. The primary objective of a chemical sensor is to provide accurate and reliable information about the presence and concentration of target analytes in a sample. By detecting and quantifying specific chemical substances, chemical sensors facilitate applications in fields such as sensing, environmental monitoring, industrial process control, medical diagnostics, etc [Citation69]. Their ability to transform chemical data into actionable insights enables the monitoring of processes, identification of contaminants, and assurance of product quality and safety. A receptor and a transducer are the two fundamental functional components of a typical chemical sensor [Citation70] (). The receptor converts the analyte’s chemical information into an energy form that the transducer can measure [Citation72]. Chemical sensors’ receptor components might be derived from chemical, biological, or physical theories [Citation73].
Figure 6. Diagrammatic depiction of common sensor parts. Reproduced from [Citation71] under common creative license, MDPI (2023).
![Figure 6. Diagrammatic depiction of common sensor parts. Reproduced from [Citation71] under common creative license, MDPI (2023).](/cms/asset/ba98a937-6935-4573-a149-ab29aaf7c125/tsnm_a_2385350_f0006_c.jpg)
Enzymes and MIPs interact with organophosphate compounds (OPCs) in distinct ways, and these interactions are translated into measurable signals by the transducer. Enzymes such as acetylcholinesterase (AChE) bind to OPCs, inhibiting their activity and preventing the breakdown of acetylcholine, which leads to an accumulation of acetylcholine [Citation74]. This inhibition is detected by the transducer as a change in current or potential, with the magnitude of the change proportional to the concentration of OPCs. On the other hand, MIPs are synthetic polymers with specific binding sites tailored to the target OPC molecules. When OPCs bind to these sites, the physical or chemical properties of the MIP layer change [Citation75]. In both cases, the transducer converts the chemical interactions into electrical signals, enabling the detection and quantification of OPCs. For sensors to be used effectively, several requirements must be met. When a sensor is used as an analytical tool, a few important aspects must be satisfied, namely, the sensor must be selective, sensitive, and stable. Selectivity is the most important feature of a sensor. The molecular recognition between the analyte and the sensing molecule represents a base for analytical sensor development. This recognition usually involves non-covalent interactions [Citation75]. The selectivity of electrochemical sensors toward organophosphate compounds is crucial for accurate detection. Recent advancements in electrochemical sensors have demonstrated significant improvements in selectivity and sensitivity [Citation13,Citation76]. MIPs have been used to enhance the selectivity of non-enzymatic sensors, while nanomaterials and composites have also improved the selectivity by providing enhanced electroactive surface areas and catalytic sites [Citation75]. Synergistic effects between carbonaceous nanomaterials and metals, metal oxide alloys, and other alternative approaches have also been explored to enhance the selectivity of non-enzymatic sensors [Citation77].
3.7. Flexible sensors and their manufacturing approaches
Developing economically feasible methodologies for the integration of sensors into portable and flexible devices represents a swift and efficient approach to pesticide detection in real samples [Citation78–80]. Among the prevalent techniques, lithographic methods stand out for their scalability in sensor manufacturing [Citation81]. Notably, lithography offers high spatial resolution, facilitating dense sensor arrays and miniaturization [Citation82]. However, achieving an optimal balance between sensor cost and performance parameters, such as resolution and throughput, is essential [Citation83,Citation84]. For instance, Narakathu et al. [Citation85] have demonstrated the development of a photolithographic gold-interdigitated sensor for detecting organophosphorus compounds. In disposable electrochemical sensors, the selection of substrate and electrode fabrication methods is pivotal, spanning different scales of implementation [Citation58,Citation65,Citation78,Citation79]. Employing a soft substrate and conductive electrode materials is paramount for realizing flexible sensors, wherein this selection significantly influences device performance and flexibility [Citation86]. The modification process of electrodes can be accomplished utilizing a screen-printing technique (SPT), known for its cost-effectiveness and suitability for large-scale production () [Citation88,Citation90,Citation91]. Utilizing this method, along with incorporating various nanomaterials [Citation92,Citation93], has propelled the development of flexible electronics. Thin coatings in the nanometer range offer increased catalytic activity due to their high surface-to-volume ratio. Achieving sensor homogeneity relies on the preparation of ink and its penetration into the substrate.
Figure 7. (a) electrochemical sensor produced by photolithography and manufacturing process schematic [Citation85,Citation87]. Reproduced with permission from, ref [Citation85], copyright @ Elsevier (2011) and ref [Citation87], copyright @ Wiley (2008). (b) SPE production steps; reproduced from [Citation88] under common creative license, MDPI (2020); (c) an electrochemical sensor made entirely of roll-to-roll graphene nanoplatelets and ZrO2; reproduced with permission from ref [Citation89], copyright @ American Chemical Society (2021).
![Figure 7. (a) electrochemical sensor produced by photolithography and manufacturing process schematic [Citation85,Citation87]. Reproduced with permission from, ref [Citation85], copyright @ Elsevier (2011) and ref [Citation87], copyright @ Wiley (2008). (b) SPE production steps; reproduced from [Citation88] under common creative license, MDPI (2020); (c) an electrochemical sensor made entirely of roll-to-roll graphene nanoplatelets and ZrO2; reproduced with permission from ref [Citation89], copyright @ American Chemical Society (2021).](/cms/asset/91d10f23-4861-44d0-9ad0-995b2c8b2bb7/tsnm_a_2385350_f0007_c.jpg)
The representative evaluation index of flexible electrochemical sensors includes key performance metrics such as limit of detection (LOD), selectivity, linearity, response time, stability, reproducibility, and flexibility. LOD indicates the lowest detectable concentration, while selectivity measures the sensor’s ability to distinguish the target analyte from other substances. Linearity is the range over which the sensor’s response is proportional to the analyte concentration. Response time is the duration to reach a stable reading, stability reflects consistent performance over time, reproducibility ensures consistent results across trials, and flexibility measures performance under mechanical stress. These indices are crucial for evaluating the applicability and reliability of these sensors in environmental monitoring, medical diagnostics, and wearable technology. The shape of the electrode significantly influences the output current values. Flexible sensors are designed to be wearable and stretchable, which requires the electrodes to be flexible and maintain their performance under deformation.
The shape of the electrode can impact the sensitivity and response time of the sensor [Citation94]. For example, three-dimensional electrode geometries like trough-shaped microelectrodes can enhance sensitivity by focusing the diffusion flux and increasing the impedance change upon analyte binding [Citation95]. Additionally, using nanomaterials and two-dimensional (2D) materials can improve the performance of flexible sensors by increasing the rate of electron transmission and enhancing the electrochemical signal [Citation96]. The shape of the electrode can also influence the detection sensitivity, with smaller radii generating more sensitive impedance responses to cell density changes [Citation96]. By considering these specifications, we can select the electrode shape for the electrochemical sensors to enhance their sensitivity and response time.
Previous research has delved into the utilization of three-dimensional (3D) printed electrodes composed of nanocarbon and polylactic acid for the detection of four organophosphorus compounds (OPs): methyl parathion, fenitrothion, paraoxon, and parathion [Citation97]. However, these methodologies are still in the preliminary stages, particularly concerning in-situ monitoring techniques, with limited reported attempts at developing portable electrochemical sensors. An emerging fabrication method known as roll-to-roll processing has gained traction for its ability to construct various functional structures on substrate materials at an industrial scale and low cost [Citation98]. While there are a few reports on roll-to-roll manufacturing for pesticide detection and other applications, only one study has specifically investigated the sensing of OPCs () [Citation89,Citation99]. Stanciu et al. devised a roll-to-roll enzyme-less electrochemical sensor incorporating graphene nanoplatelets and ZrO2 nanoparticles for detecting nitroaromatic OP pesticides [Citation89].
4. Applications in real-time monitoring
In sensor technology, real-time monitoring emerges as a crucial facet, necessitating sensors to operate effectively in real-world scenarios amidst varied environmental challenges [Citation100]. While sensors often perform optimally under controlled laboratory conditions, their true efficacy is determined by their ability to function efficiently in practical environmental contexts. Flexible sensors exhibit exceptional resilience and adaptability, rendering them indispensable for operation in harsh environments [Citation101]. Their durability withstands mechanical stress, vibrations, and impacts, while chemical resistance ensures longevity in environments laden with corrosive substances. Waterproofing capabilities enable reliable performance in wet or humid conditions, and tolerance to extreme temperatures facilitates operation in fluctuating thermal environments. Their inherent flexibility allows seamless integration onto irregular surfaces, including curved or moving structures, while low power consumption ensures prolonged operation in remote or hazardous locations [Citation64,Citation65]. Moreover, their capacity for remote monitoring facilitates real-time data collection without physical presence, enhancing safety and efficiency. Flexible sensors play a pivotal role in the real-time monitoring of organophosphates, enabling comfortable integration into wearable devices for continuous monitoring of the environment [Citation78,Citation79].
Graphene, Carbon nanotubes (CNTs) and their composites with various metals, metal oxides, polymers, etc. have gained huge attention in recent years, owing to their good conductivity, high sensitivity, and good electron transfer [Citation102]. These materials have also been used for the electrochemical detection of OPs. Size and shape of the particles during synthesis along with the composition of the constituents greatly influences the sensitivity and selectivity of the electrochemical sensor. Moreover, the choice of the substrate for electrode fabrication is also very crucial. The conductivity, stability, and surface area of the substrate plays important role in the performance of the electrode and sensor. In this section, we will discuss some recent research works related to real-time monitoring of OPs via wearable electrochemical sensors.
Zhao et al. [Citation103] developed a smart plant-wearable biosensor for in-situ analysis of organophosphorus pesticides on crop surfaces. They utilized laser-induced graphene (LIG) technology to fabricate a flexible three-electrode system capable of conforming to crop leaves and fruits. Incorporating organophosphorus hydrolase (OPH) modifications enabled selective capture and recognition of methyl parathion. shows few images of the biosensors utilized for real-time monitoring of methyl parathion. The obtained data was transmitted to a smartphone device, facilitating real-time analysis of agricultural products for pesticides. Further enhancement of device performance was achieved by modifying three-dimensional (3D) porous LIG with gold nanoparticles (AuNPs). The limit of detection (LOD) for methyl parathion was determined to be 0.01 μM, which is in agreement with the allowed limit of methyl parathion directed by the European Commission.
Figure 8. Images of the biosensor along with real-time monitoring of methyl parathion. Reproduced with permission from ref [Citation103], copyright @ Elsevier (2020).
![Figure 8. Images of the biosensor along with real-time monitoring of methyl parathion. Reproduced with permission from ref [Citation103], copyright @ Elsevier (2020).](/cms/asset/9e97c3c3-ef05-47cd-9ca2-f3d0900b3f2e/tsnm_a_2385350_f0008_c.jpg)
Raymundo-Pereira et al. [Citation104] reported selective and sensitive detection of carbendazim (carbamate), diuron (phenylamide), paraquat (bipyridinium) and fenitrothion (organophosphate). A set of three glove-embedded sensors printed on three fingers of a rubber glove () were used for the electrochemical detection of these pesticides. The sensors comprise a screen-printed carbon electrode along with two additional electrodes based on carbon spherical shells (CSS) or Printex carbon nanoballs (PCNB). The LOD of 4.7 × 10−8 and 9.2 × 10−7 mol L−1 was achieved for carbendazim and diuron, respectively, using the DPV technique. Conversely, LODs of 2.4 × 10−8 and 6.4 × 10−7 mol L−1 was obtained for paraquat and fenitrothion (in PBS) via square wave voltammetry (SWV). The sensors exhibited good selectivity for these pesticides in real samples of orange juice, apple, and cabbage. This significantly highlights the compatibility and efficiency of the developed sensor. These gloves have very high potential for on-site analysis and detection owing to their good efficiency, high sensitivity, and selectivity toward the selected pesticides.
Figure 9. Image of the wearable glove sensor and real time monitoring of carbendazim, diuron, paraquat, and fenitrothion in cabbage, apple, and orange juice. Reproduced with permission from ref [Citation104], copyright @ Elsevier (2021).
![Figure 9. Image of the wearable glove sensor and real time monitoring of carbendazim, diuron, paraquat, and fenitrothion in cabbage, apple, and orange juice. Reproduced with permission from ref [Citation104], copyright @ Elsevier (2021).](/cms/asset/c9cdea10-9809-4678-97a4-d3f0a0287cf9/tsnm_a_2385350_f0009_c.jpg)
Mishra et al. [Citation105] developed a flexible tattoo and textile-based electrochemical biosensor for vapor-phase detection of organophosphorus (OP) nerve agents. The electrodes with OPH enzyme were integrated into a flexible electronic interface for the detection of OP vapor. The data obtained was easily transferred to a mobile device via wireless transmission. The sensor displayed good sensitivity of 10.7 µA cm3 mg−1 (R2 0.983), LOD of 12 mg/L, and good reproducibility. The utilization of wireless tattoo and biosensor systems presents a promising setup for individuals to alert against exposure to OP nerve agents. This technology holds considerable potential for deployment across various decentralized security applications. In another work, Mishra et al. [Citation106] detected G-type nerve agent simulants on a human body using a sensor in the shape of a tattoo. The biosensor effectively detected di-isopropyl fluorophosphate (DFP), a fluorine-containing organophosphate, in both liquid and vapor phases. The electrode is fabricated by coating polyaniline (PANI) on a flexible tattoo paper and connected to an electronic interface. The biosensor displayed good sensitivity and selectivity toward DFP even in the presence of mechanical stress and strain. Thus, the work reported a robust wearable and flexible biosensor for real-time monitoring of DFP nerve agents. shows the application of tattoo-type sensors on human body parts to diagnose the health status via electrochemical technique.
Figure 10. Images of the tattoo-type sensor and its electrochemical performance. Reproduced with permission from ref [Citation105], copyright @ Elsevier (2018).
![Figure 10. Images of the tattoo-type sensor and its electrochemical performance. Reproduced with permission from ref [Citation105], copyright @ Elsevier (2018).](/cms/asset/ed7ba0d0-8226-40c5-a843-554723554d74/tsnm_a_2385350_f0010_c.jpg)
Thangarasu et al. [Citation107] reported the synthesis of MnO2/PANI/rGO nanocomposite via one-pot chemical polymerization for the effective detection of methyl parathion (MP). The sensor displayed a LOD of 7.4 nM with excellent selectivity and reproducibility. The efficient sensing capabilities are attributed to the mechanical properties of (rGO) and the excellent conductivity of PANI. Real-time monitoring was conducted for apple, water, and milk, demonstrating the sensor’s exceptional detection of methyl parathion (MP) and highlighting its potential application in the fruit and beverage industry.
Porto et al. [Citation108] for the first time, developed a fast and highly sensitive electrochemical method for the detection of diazinon (DZN), malathion (MLT), and chlorpyrifos (CLPF). In this study, a modified pyrolytic graphite electrode (PGE) was employed for the detection of these OPs. The modification in PGE was done using a nanocomposite of functionalized carbon nanotubes and silver nanoparticles. The analysis of OPs was done by placing the sample (at pH 6) on the surface of the modified electrode connected with the BIA-MPA system. The sensor displayed LOD of 0.35, 0.89, and 0.53 μmol L−1 and LOQ of 1.18, 2.98, and 1.78 μmol L−1 for DZN, MLT, and CLPF, respectively. Moreover, the sensor displayed high sensitivity of 0.068, 0.030 and 0.043 mA L μmol−1 for DZN, MLT, and CLPF, respectively. The real-sample analysis was done for orange juice, tap water, and apple fruit. The analytical performance of the developed sensor offers a compelling alternative approach for the determination of OPs, demonstrating significant potential for rapid and sensitive application in contaminated samples containing these pesticides.
Cioffi et al. [Citation109] developed cost-effective and portable electrochemical sensor based on common office paper for the detection of paraxon-ethyl (an OP) in soil and vegetables. Using office paper helps in reducing plastic waste produced by plastic strips mostly used for fabricating electrochemical sensors. Enzyme butyrylcholinesterase, Prussian blue, and carbon black were mixed together and drop casted over the screen-printed paper strips and developed into a portable sensing system. (a) represents the procedure used for the development of the sensor and (b) represents the performance of the device. A low LOD of 1.3 ng/mL was observed for OPs present in the soul. Moreover, the fruits and vegetables were also analyzed for OPs using the developed sensors. Recovery percentages ranging from 90% to 110% have been attained across various matrices, indicating the suitability of the developed sensor for field measurements. Goud et al. [Citation110] developed a printed and flexible textile-based sensor for the detection of G-type nerve agents such as sarin or soman. These nerve agents contain fluorine which is released upon their biocatalytic hydrolysis and are easily detected by fluoride-selective enzymes. The sensor was used to detect diisopropyl fluorophosphate (DFP), a common simulant of F-containing G-type nerve agents using anhydrolylase (OPAA) and organophosphorus hydrolase (OPH) enzyme modified printed electrodes. These enzymes help in the biocatalytic hydrolysis of DFP, releasing fluoride. The resulting OPAA and OPH biosensors offer highly sensitive DFP detection with LOQ of 200 μM and 50 μM. Using stress-resistant inks, serpentine structures, and stretchable textiles, the fluoride sensor shows strong mechanical resilience under significant strains. Moreover, a flexible, wearable, wireless, portable, and user-friendly electronic module was developed for alerting the wearer instantaneously about potential chemical threats. (c) represents the fabricated textile electrode along with its performance and developed portable electronic device. The list of some electrochemical sensors for the detection of OPCs is given in .
Figure 11. Office paper-based electrochemical sensor with (a) printing method, and (b) device performance towards paraoxon-ethyl [Citation109]. Reproduced with permission from ref [Citation109], copyright © 2021, American Chemical Society. (c) Textile-based sensor for the detection of DFP. Reproduced with permission from ref [Citation110], copyright © 2021 Elsevier B.V.
![Figure 11. Office paper-based electrochemical sensor with (a) printing method, and (b) device performance towards paraoxon-ethyl [Citation109]. Reproduced with permission from ref [Citation109], copyright © 2021, American Chemical Society. (c) Textile-based sensor for the detection of DFP. Reproduced with permission from ref [Citation110], copyright © 2021 Elsevier B.V.](/cms/asset/8104008d-77b7-4e8a-8548-cf8d6ceab432/tsnm_a_2385350_f0011_c.jpg)
Table 2. Electrochemical sensors for organophosphate detection.
The integration of contemporary electrochemical techniques with advancements in microelectronics and miniaturization enables the development of robust analytical devices for the efficient detection of pollutants. Advanced real-time on-site monitoring technologies effectively mitigate the temporal limitations inherent in traditional laboratory analyses. The electrochemical sensors help facilitate the acquisition of requisite analytical data with enhanced expediency, simplicity, and cost-effectiveness in contrast to conventional laboratory-based instrumentation. As a result of these advancements, substantial emphasis is now placed on conducting on-site and real-time electrochemical measurements, signifying a significant shift in prioritization within the field. Despite advancements, electrochemical devices remain constrained in their applicability and are unable to fulfill all requirements for environmental or industrial monitoring. While electrochemical sensors hold promise as analytical tools for wearable devices, several issues must be resolved to achieve an optimal design. The performance of the devices in robust environment conditions derails with time. The presence of interferents, pH values of the sample, etc. also affects the performance of the device. The sensor must be free from the external interferents and proper calibration and stability studies need to be performed before using sensor for real-time monitoring [Citation138]. Moreover, various available platforms such as rings, glasses, gloves, cloths, human skin, etc. for wearable sensors must be compatible with the device design and should not alter the performance of device [Citation139–141]. Despite significant progress and applications, wearable sensors has seen uneven advances in research and development [Citation142,Citation143]. The acceptance of wearable sensor technology hinges on creating products that seamlessly integrate into users’ lifestyles. Improving sensitivity, selectivity, long-term stability, reliability, and multiplexing capabilities are key challenges that need to be addressed. The multidisciplinary synergy of chemistry, biology, electrical engineering, and software engineering will play a central role in creating innovative portable, wireless wearable microdevices with excellent performance. Increased commercialization, in addition to regulatory endorsement, is anticipated to facilitate the transition of the electrochemical sensors into widespread adoption across environmental and industrial domains.
5. Challenges and future perspectives
While prior research has laid the groundwork for the development of rapid, highly sensitive, reliable, and easily deployable electrochemical sensors for the detection of organophosphorus compounds (OPCs), certain areas necessitate further exploration to realize rapid monitoring systems suitable for integration into flexible electronics. A primary challenge in employing nanostructured materials for these electrochemical sensors is the occurrence of surface-fouling at electrode surfaces due to nonspecific binding interactions with interferents present in wastewater, leading to a restricted sensor lifespan. One effective strategy to impart antifouling properties to electrode surfaces involves the use of materials inherently resistant to biofouling, such as metallic nanostructures [Citation144]. For instance, incorporating gold nanoparticles (AuNPs) on the electrode surface has been shown to enhance the sensor’s resistance to protein adsorption and biofouling [Citation145]. The unique properties of AuNPs, including their high surface area and ability to resist nonspecific adsorption, make them an attractive choice for antifouling coatings. Another promising approach is the utilization of zwitterionic polymers, which have been extensively studied for their excellent antifouling properties [Citation146]. Polymers such as poly(carboxybetaine) and poly(sulfobetaine) can form a hydrated layer on the electrode surface that effectively repels biomolecules and prevents fouling [Citation77]. Incorporating zwitterionic polymers into the sensor design has been demonstrated to improve long-term stability and performance in complex biological matrices. Additionally, developing disposable sensor strips presents a viable solution to mitigate surface fouling [Citation147]. By employing a fresh, pre-fabricated sensor strip for each measurement, the sensor can be protected from the accumulation of fouling agents, thereby extending its operational lifespan. This approach is particularly useful for field-based applications where frequent sensor replacement is more feasible. Furthermore, integrating antifouling strategies with other sensor design elements, such as MIPs or carbon nanomaterials, can further enhance the selectivity and sensitivity of sensors toward organophosphate compounds (OPCs) [Citation144,Citation145]. The combination of these approaches can lead to the development of robust, long-lasting electrochemical sensors capable of reliable OPC detection in complex environmental and biological matrices.
The successful determination of organophosphorous compounds (OPCs) in wastewater samples poses a significant challenge due to the considerable interference from small ions and molecules, impeding the selective detection of OPCs. While some studies have explored the binding mechanisms of target OPCs, further scientific investigations are warranted to gain deeper insights into these interactions at the atomic level. Additionally, the integration of portable sensor technologies faces challenges related to stability and repeatability in large-scale manufacturing processes. Future endeavors should prioritize feasibility studies aimed at industrializing both types of electrochemical sensors. Current methods predominantly rely on a limited range of nanomaterials, with only a few molecules explored for OPC sensor fabrication. The detection of organophosphorus compounds (OPCs) has long been a significant challenge. However, the emergence of nanomaterials has opened new possibilities for developing highly sensitive and selective sensors. Researchers have explored using various carbon-based nanomaterials, such as carbon nanotubes (CNTs) and graphene, as well as metal and metal oxide nanostructures, for OPC sensing applications [Citation148,Citation149]. These nanomaterials offer several advantages, including improved electrical conductivity, large surface area, and enhanced sensitivity and selectivity [Citation149]. Integrating carbon nanomaterials with metal oxides in nanocomposite structures has demonstrated superior sensing performance for OPC detection [Citation150].
Array technology emerges as a promising solution to mitigate signal alterations caused by multiple pesticides in sample mixtures; however, its implementation remains at an early stage of development. Therefore, further research efforts are essential to advance enzyme-free sensing devices for the accurate determination of OPC pesticides, given their significant implications for human health. The core attributes of emerging electrochemical sensors designed for the detection of organophosphorous agents encompass portability, flexibility, and wearability. Nanomaterials have emerged as pivotal contributors to the integration of flexible electrochemical sensors, facilitating real-time monitoring capabilities. By leveraging novel hybrid materials, enhanced sensitivity, selectivity, and reliability can be attained. The future trajectory of flexible sensor development necessitates meticulous selection of nanomaterials, considering factors such as abundance, reduced toxicity, biodegradability, and cost-effectiveness, thereby minimizing risks across diverse domains including health, food safety, military applications, forensics, and ecology. While current sensor applications predominantly target health-related domains, there exists a pressing need for their expansion into broader fields. Nonetheless, the proliferation of disposable sensors, including electrochemical variants, has underscored concerns regarding environmental pollution. Addressing the issue of electronic waste (E-waste) emerges as a critical imperative to safeguard environmental integrity. Disposable sensor fabrication predominantly relies on conventional materials such as micro- and nanoelectromechanical systems, synthetic polymers, cellulose-based materials, or hybrid compositions.
The development of sensors for OPC detection increasingly focuses on biodegradable and recyclable materials. Researchers are utilizing natural and renewable polymers such as polylactic acid (PLA), polyhydroxyalkanoates (PHA), chitosan, and cellulose as substrates, supporting matrices, and packaging materials [Citation151]. These biodegradable polymers offer the advantage of reduced environmental impact, as they degrade naturally without leaving persistent waste. Additionally, recyclable materials like graphene oxide (GO) and carbon nanotubes (CNTs) have been investigated for their high conductivity and potential for reuse [Citation151]. Despite barriers such as production costs, regulatory hurdles, and consumer acceptance, the growing demand for sustainable, eco-friendly products and innovative applications presents significant opportunities for incorporating biodegradable and recyclable materials in OPC sensor development. Incorporating properties such as recyclability, biodegradability, or composability into these materials offers a promising avenue for enhancing environmental sustainability. It is pertinent to note that not all materials need to be inherently biodegradable; however, the adoption of greener, eco-friendly materials and low-waste fabrication approaches holds significant potential for mitigating the adverse environmental repercussions associated with disposable electrochemical sensors. The commercialization and scalability of electrochemical sensor development represent burgeoning domains within contemporary environmental technology, driven by heightened awareness of global environmental and health ramifications.
For instance, the Innovation Quarter in North Carolina has intentionally established focus areas such as health, personalized care, and precision medicine, and has leveraged its partnerships with institutions like the Wake Forest Health System to become a leader in these sectors [Citation152]. By identifying anchor institutions and their strengths, innovation districts can attract complementary firms and encourage cross-sector relationships that drive research and development forward [Citation153]. Furthermore, industry-academia collaborations have been shown to enhance innovation and growth for companies. A Harvard Business Review study found that companies with higher levels of collaboration with universities had higher innovation output, higher quality patents, and more successful new ventures [Citation154]. These partnerships allow companies to leverage the theoretical expertise and innovative capabilities of academic institutions to address complex challenges and push technological boundaries [Citation155]. Such platforms are indispensable for addressing next-generation human health challenges and fostering improved quality of life.
6. Conclusion
In recent years, the development of electrochemical sensors for the detection of organophosphorus (OP) based warfare agents has garnered significant attention among researchers as a viable alternative to conventional detection methods. Electrochemical sensors offer distinct advantages, including high sensitivity, rapid response times, cost-effectiveness, and straightforward instrumentation. Moreover, their compatibility with miniaturization and portability makes them particularly promising for field applications. Organophosphate-based warfare agents present unique challenges due to their toxic nature and potential threat levels, requiring sensors to detect extremely low concentrations with high accuracy. Second, integrating these sensors into wearable or portable devices for real-time monitoring in field environments presents challenges in terms of power consumption, data transmission, and device durability. Addressing the specificity of detection to differentiate between organophosphate compounds and other environmental factors or interferents is essential for reliable detection. While non-enzymatic sensors do have poor selectivity, researchers have been working to address this issue through various strategies. The use of MIPs, synergistic effects, metal oxide alloys, and electrode design can all contribute to improving the selectivity of non-enzymatic sensors, making them more suitable for detecting specific targets in complex solutions. The major challenges in flexible nanosensors encompass ensuring mechanical flexibility without compromising performance or durability, maintaining consistent sensing capabilities under varying environmental conditions, and integrating seamlessly into existing technologies. Addressing these challenges requires advancements in material science, fabrication techniques, and sensor design. Future scope lies in developing novel nanomaterials tailored for flexibility and enhanced sensing properties, as well as advancing scalable fabrication methods like additive manufacturing and roll-to-roll processing. Integration with emerging technologies such as the Internet of Things (IoT) and wearable electronics offers new opportunities for real-time monitoring in healthcare, environmental monitoring, agriculture, and military applications. The development of portable and wearable devices equipped with flexible nanosensors could revolutionize battlefield surveillance and protection strategies, providing timely detection and response to organophosphate-based threats. Hence, addressing the challenges and advancing the future scope of flexible nanosensors for organophosphate-based warfare agents require interdisciplinary collaboration, technological innovation, and strategic partnerships between academia, industry, and government agencies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Urabe T, Takahashi K, Kitagawa M, et al. Development of portable mass spectrometer with electron cyclotron resonance ion source for detection of chemical warfare agents in air. Spectrochim Acta A Mol Biomol Spectrosc. 2014;120:437–444. doi: 10.1016/j.saa.2013.10.041
- Geoghegan J, Tong JL. Chemical warfare agents. Continuing education in Anaesthesia. Crit Care & Pain. 2006;6(6):230–234. doi: 10.1093/bjaceaccp/mkl052
- Chan JTS, Yeung RSD, Tang SYH. An overview of chemical warfare agents. HKJEM. 2002;9(4):201–205. doi: 10.1177/102490790200900404
- Sferopoulos R. A review of chemical warfare agent (CWA) detector technologies and commercial-off-the-shelf items. 2009. https://apps.dtic.mil/sti/citations/ADA502856.
- Pacsial-Ong EJ, Aguilar ZP. Chemical warfare agent detection: a review of current trends and future perspective. Front Biosci. 2013;5(2):516–543. doi: 10.2741/S387
- Borak J, Sidell FR. Agents of chemical warfare: sulfur mustard. Ann Emerg Med. 1992;21(3):303–308. doi: 10.1016/S0196-0644(05)80892-3
- Gravett MR, Hopkins FB, Main MJ, et al. Detection of the organophosphorus nerve agent VX and its hydrolysis products in white mustard plants grown in contaminated soil. Anal Methods. 2013;5(1):50–53. doi: 10.1039/C2AY25883H
- Eiceman GA, Stone JA. Peer reviewed: ion mobility spectrometers in national defense. Anal Chem. 2004;76(21):390 A–397 A. doi: 10.1021/ac041665c
- Diauudin FN, Rashid JIA, Knight VF, et al. A review of current advances in the detection of organophosphorus chemical warfare agents based biosensor approaches. Sens And Bio-Sens Res. 2019;26:100305. doi: 10.1016/j.sbsr.2019.100305
- Tomchenko AA, Harmer GP, Marquis BT. Detection of chemical warfare agents using nanostructured metal oxide sensors. Sens Actuators B Chem. 2005;108(1–2):41–55. doi: 10.1016/j.snb.2004.11.059
- Wang J, Chatrathi MP, Mulchandani A, et al. Capillary electrophoresis microchips for separation and detection of organophosphate nerve agents. Anal Chem. 2001;73(8):1804–1808. doi: 10.1021/ac001424e
- Joshi AK, Tang J, Haddon R, et al. A disposable biosensor for organophosphorus nerve agents based on carbon nanotubes modified thick film strip electrode. Electroanalysis: An Int J Devoted To Fundamental And Practical Aspects Of Electroanalysis. 2005;17(1):54–58. doi: 10.1002/elan.200403118
- Liu G, Lin Y. Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents. Anal Chem. 2005;77(18):5894–5901. doi: 10.1021/ac050791t
- Sporty JL, Lemire SW, Jakubowski EM, et al. Immunomagnetic separation and quantification of butyrylcholinesterase nerve agent adducts in human serum. Anal Chem. 2010;82(15):6593–6600. doi: 10.1021/ac101024z
- Kim K, Tsay OG, Atwood DA, et al. Destruction and detection of chemical warfare agents. Chem Rev. 2011;111(9):5345–5403. doi: 10.1021/cr100193y
- Szinicz L. History of chemical and biological warfare agents. Toxicology. 2005;214(3):167–181. doi: 10.1016/j.tox.2005.06.011
- Newmark CJ. The birth of nerve agent warfare: lessons from Syed Abbas Foroutan. Neurology. 2004;62(9):1590–1596. doi: 10.1212/01.WNL.0000124519.85516.50
- Marrs TC. Organophosphates: history, chemistry, pharmacology. Organophosphates And Health. 2001:1–36. doi: 10.1142/9781848161443_0001
- Lukey BJ, Romano Jr JA, Salem H, editors. Chemical warfare agents: chemistry, pharmacology, toxicology, and therapeutics. United States: CRC Press; 2007.
- Ackerman G, Bale J, Moran K. Assessing terrorist motivations for attacking Critical “Chemical”. Livermore (CA) (United States): Lawrence Livermore National Lab (LLNL). 2004. doi: 10.2172/897993. Infrastructure (No. UCRL-SR-208717).
- Degenhardt CE, Van den Berg GR, Jong D, et al. Enantiospecific complexation gas chromatography of nerve agents. Isolation and properties of the enantiomers of ethyl N, N-dimethylphosphoramidocyanidate (tabun). JACS. 1986;108(26):8290–8291. doi: 10.1021/ja00286a043
- Krejcova G, Kuca K, Sevelova L. Cyclosarin-an organophosphate nerve agent. Def Sci J. 2005;55(2):105. doi: 10.14429/dsj.55.1974
- Balmer B. A secret formula, a rogue patent and public knowledge about nerve gas: secrecy as a spatial–epistemic tool. Soc Stud Sci. 2006;36(5):691–722. doi: 10.1177/0306312706063786
- Aurbek N, Thiermann H, Szinicz L, et al. Analysis of inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds with human and pig acetylcholinesterase. Toxicology. 2006;224(1–2):91–99. doi: 10.1016/j.tox.2006.04.030
- Kenttamaa KH, Cooks RG. Tautomer characterization by energy resolved mass spectrometry. Dimethyl phosphite and dimethyl phosphonate ions. JACS. 1985;107(7):1881–1886. doi: 10.1021/ja00293a013
- Griffiths JE, Burg AB. The phosphinous acid (CF3) 2POH and the diphosphoxane (CF3) 2POP (CF3) 21. JACS. 1960;82(6):1507–1508. doi: 10.1021/ja01491a062
- Cummings DA, McMaster J, Rieger AL, et al. EPR spectroscopic and theoretical study of chromium (I) carbonyl phosphine and phosphonite complexes. Organometallics. 1997;16(20):4362–4368. doi: 10.1021/om970252h
- Sokolov MN, Virovets AV, Dybtsev DN, et al. Phosphorous acid and arsenious acid as ligands. Inorg Chem. 2001;40(19):4816–4817. doi: 10.1021/ic015520p
- Greenwood NN, Earnshaw A. Chemistry of the elements. Oxford, (UK): Pergamon Press; 1984.
- Griffiths TR, Volkovich VA, Robert Carper W. The structures of the active intermediates in catalyst-enhanced molten Salt oxidation and a new method for the complete destruction of chemical warfare arsenicals. Struct Chem. 2010;21(2):291–297. doi: 10.1007/s11224-009-9530-0
- Eto M. Organophosphorus pesticides. United States: CRC Press; 2017.
- Yang YC. Chemical detoxification of nerve agent VX. Acc Chem Res. 1999;32(2):109–115. doi: 10.1021/ar970154s
- Mandal D, Mondal B, Das AK. Isomerization and decomposition of a model nerve agent: a computational analysis of the reaction energetics and kinetics of dimethyl ethylphosphonate. J Phys Chem A. 2010;114(39):10717–10725. doi: 10.1021/jp106270d
- Black RM, Clarke RJ, Read RW, et al. Application of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry to the analysis of chemical warfare samples, found to contain residues of the nerve agent sarin, sulphur mustard and their degradation products. J Chromatogr A. 1994;662(2):301–321. doi: 10.1016/0021-9673(94)80518-0
- Okumura T, Ariyoshi K, Hitomi T. Lessons learned from nerve agent attacks in Iran and Japan: Is it really necessary to stockpile oximes? Toxin Rev. 2009;28(4):255–259. doi: 10.3109/15569540903338040
- Wilhelm CM, Snider TH, Babin MC. A comprehensive evaluation of the efficacy of leading oxime therapies in guinea pigs exposed to organophosphorus chemical warfare agents or pesticides. Toxicol Appl Pharmacol. 2014;281(3):254–265. doi: 10.1016/j.taap.2014.10.009
- Tsuchihashi H, Katagi M, Nishikawa M, et al. Identification of metabolites of nerve agent VX in serum collected from a victim. J Anal Toxicol. 1998;22(5):383–388. doi: 10.1093/jat/22.5.383
- Ember L. Chem. Eng News. 1995;73(Mar 27):6. doi: 10.1021/cen-v073n010.p006
- Chao LL, Rothlind JC, Cardenas VC, et al. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf war on brain function and brain structure in US veterans. Neurotoxicology. 2010;31(5):493–501. doi: 10.1016/j.neuro.2010.05.006
- Ember LR, Destroying VC, News E. DESTROYING VX. Chem & Eng News Archiv. 2004;82(15):28–30. doi: 10.1021/cen-v082n015.p028
- Ember L, Chem. Eng. News. 2004;82(24):15. doi: 10.1021/cen-v082n024.p015
- Matsushita K, Sekiguchi H, Seto Y. Performance of portable surface acoustic wave sensor array detector for chemical agents. Bunseki Kagaku. 2005;54(1):83–88. doi: 10.2116/bunsekikagaku.54.83
- Ember L. Soil sample key to U.S. missile strike in Sudan. Chem Eng News. 1998;76(35):6. doi: 10.1021/cen-v076n035.p006a
- Driskell WJ, Shih M, Needham LL, et al. Quantitation of organophosphorus nerve agent metabolites in human urine using isotope dilution gas chromatography-tandem mass spectrometry. J Anal Toxicol. 2002;26(1):6–10. doi: 10.1093/jat/26.1.6
- Mawhinney DB, Hamelin EI, Fraser R, et al. The determination of organophosphonate nerve agent metabolites in human urine by hydrophilic interaction liquid chromatography tandem mass spectrometry. J Chromatogr B. 2007;852(1–2):235–243. doi: 10.1016/j.jchromb.2007.01.023
- Steiner WE, Harden CS, Hong F, et al. Detection of aqueous phase chemical warfare agent degradation products by negative mode ion mobility time-of-flight mass spectrometry [IM (tof) MS]. J Am Soc Mass Spectrom. 2006;17(2):241–245. doi: 10.1016/j.jasms.2005.11.004
- Gäb J, Melzer M, Kehe K, et al. Quantification of hydrolysis of toxic organophosphates and organophosphonates by diisopropyl fluorophosphatase from Loligo vulgaris by in situ Fourier transform infrared spectroscopy. Anal Biochem. 2009;385(2):187–193. doi: 10.1016/j.ab.2008.11.012
- Gomes NO, de Campos AM, Calegaro ML, et al. Core-Shell Nanocables Decorated with carbon spherical shells and silver nanoparticles for sensing ethinylestradiol hormone in water sources and pills. ACS Appl Mater & Interface. 2024;16(8):10897–10907. doi: 10.1021/acsami.3c16249
- Martoni LV, Gomes NO, Prado TM, et al. Carbon spherical shells in a flexible photoelectrochemical sensor to determine hydroquinone in tap water. J Environ Chem Eng. 2022;10(3):107556. doi: 10.1016/j.jece.2022.107556
- Raymundo-Pereira PA, Gomes NO, Machado SA, et al. Wearable glove-embedded sensors for therapeutic drug monitoring in sweat for personalized medicine. Chem Eng J. 2022;435:135047. doi: 10.1016/j.cej.2022.135047
- Sá ACD, Barbosa SC, Raymundo-Pereira PA, et al. Flexible carbon electrodes for electrochemical detection of bisphenol-a, hydroquinone and catechol in water samples. Chemosensors. 2020;8(4):103. doi: 10.3390/chemosensors8040103
- Gomes NO, Mendonça CD, Machado SA, et al. Flexible and integrated dual carbon sensor for multiplexed detection of nonylphenol and paroxetine in tap water samples. Microchim Acta. 2021;188(10):1–10. doi: 10.1007/s00604-021-05024-4
- Baccarin M, Ciciliati MA, Oliveira ON Jr, et al. Pen sensor made with silver nanoparticles decorating graphite-polyurethane electrodes to detect bisphenol-A in tap and river water samples. Mater Sci Eng: C. 2020;114:110989. doi: 10.1016/j.msec.2020.110989
- Raymundo-Pereira PA, Gomes NO, Machado SA, et al. Simultaneous, ultrasensitive detection of hydroquinone, paracetamol and estradiol for quality control of tap water with a simple electrochemical method. J Electroanal Chem. 2019;848:113319. doi: 10.1016/j.jelechem.2019.113319
- Uniyal S, Sharma RK. Technological advancement in electrochemical biosensor based detection of organophosphate pesticide chlorpyrifos in the environment: a review of status and prospects. Biosens And Bioelectron. 2018;116:37–50. doi: 10.1016/j.bios.2018.05.039
- Moon K, Lee SH, Kim YH. Evaluation of reference genes for quantitative real-time PCR to investigate seasonal and labor-specific expression profiles of the honey bee abdomen. J Asia Pac Entomol. 2018;21(4):1350–1358. doi: 10.1016/j.aspen.2018.10.014
- Bohari NA, Siddiquee S, Saallah S. Optimization and analytical behavior of electrochemical sensors based on the modification of indium tin oxide (ITO) using PANI/MWCNTs/AuNPs for mercury detection. Sensors. 2020;20(22):6502. doi: 10.3390/s20226502
- Gomes NO, Teixeira SC, Calegaro ML, et al. Flexible and sustainable printed sensor strips for on-site, fast decentralized self-testing of urinary biomarkers integrated with a portable wireless analyzer. Chem Eng J. 2023;472:144775. doi: 10.1016/j.cej.2023.144775
- Gomes NO, Paschoalin RT, Bilatto S, et al. Flexible, bifunctional sensing platform made with biodegradable mats for detecting glucose in urine. ACS Sustain Chem & Eng. 2023;11(6):2209–2218. doi: 10.1021/acssuschemeng.2c05438
- Gomes NO, Raymundo‐Pereira PA. On‐Site therapeutic drug monitoring of paracetamol analgesic in Non‐Invasively collected saliva for personalized medicine. Small. 2023;19(12):2206753. doi: 10.1002/smll.202206753
- Amali RKA, Lim HN, Ibrahim I, et al. Significance of nanomaterials in electrochemical sensors for nitrate detection: a review. Tren Environ Anal Chem. 2021;31:00135. doi: 10.1016/j.teac.2021.e00135
- Anu Prathap MU, Kaur B, Srivastava R. Electrochemical sensor platforms based on nanostructured metal oxides, and zeolite‐based materials. Chem Rec. 2019;19(5):883–907. doi: 10.1002/tcr.201800068
- Bo X, Zhou M, Guo L. Electrochemical sensors and biosensors based on less aggregated graphene. Biosens Bioelectron. 2017;89:167–186. doi: 10.1016/j.bios.2016.05.002
- Raymundo-Pereira PA, Gomes NO, Shimizu FM, et al. Selective and sensitive multiplexed detection of pesticides in food samples using wearable, flexible glove-embedded non-enzymatic sensors. Chem Eng J. 2021;408:127279. doi: 10.1016/j.cej.2020.127279
- Teixeira SC, Gomes NO, de Oliveira TV, et al. Review and perspectives of sustainable, biodegradable, eco-friendly, and flexible electronic devices and (bio) sensors. Biosens And Bioelectron. 2023;X:100371. doi: 10.1016/j.biosx.2023.100371
- Liu T, Chu Z, Jin W. Electrochemical mercury biosensors based on advanced nanomaterials. J Mater Chem B. 2019;7(23):3620–3632. doi: 10.1039/C9TB00418A
- Pundir CS, Malik A. Preety bio-sensing of organophosphorus pesticides: a review. Biosens Bioelectron. 2019;140:111348. doi: 10.1016/j.bios.2019.111348
- Hulanicki A, Glab S, Ingman FOLKE. Chemical sensors: definitions and classification. Pure Appl Chem. 1991;63(9):1247–1250. doi: 10.1351/pac199163091247
- Volpatti LR, Yetisen KA. Commercialization of microfluidic devices. Trends Biotechnol. 2014;32(7):347–350. doi: 10.1016/j.tibtech.2014.04.010
- Raymundo‐Pereira PA, Baccarin M, Oliveira ON Jr, et al. Thin films and composites based on graphene for electrochemical detection of biologically relevant molecules. Electroanalysis. 2018;30(9):1888–1896. doi: 10.1002/elan.201800283
- Lazarević-Pašti T. Carbon materials for organophosphate pesticide sensing. Chemosens. 2023;11(2):93. doi: 10.3390/chemosensors11020093
- Aquino A, Paschoalin VMF, Tessaro LLG, et al. Updating the use of nano-biosensors as promising devices for the diagnosis of coronavirus family members: a systematic review. J Pharm Biomed Anal. 2022;211:114608. doi: 10.1016/j.jpba.2022.114608
- Raymundo-Pereira PA, Silva TA, Caetano FR, et al. Polyphenol oxidase-based electrochemical biosensors: a review. Anal Chim Acta. 2020;1139:198–221. doi: 10.1016/j.aca.2020.07.055
- Zhou N, Li C, Mo R, et al. A graphene/enzyme-based electrochemical sensor for sensitive detection of organophosphorus pesticides. Surf Rev Lett. 2016;23(2):1550103. doi: 10.1142/S0218625X15501036
- Jiménez D, Díaz-Díaz G, Blanco-López MC, et al. Chapter 1—molecularly imprinted electrochemical sensors: past, present, and future. Amsterdam, The Netherlands: Elsevier; 2012. p. 1–34. doi: 10.1016/B978-0-444-56331-6.00001-3
- Hossain MI, Hasnat MA. Recent advancements in non-enzymatic electrochemical sensor development for the detection of organophosphorus pesticides in food and environment. Heliyon. 2023;9(9):e19299. doi: 10.1016/j.heliyon.2023.e19299
- Pathiraja G, Bonner CD, Obare SO. Recent advances of enzyme-free electrochemical sensors for flexible electronics in the detection of organophosphorus compounds: a review. Sensors. 2023;23(3):1226. doi: 10.3390/s23031226
- Paschoalin RT, Gomes NO, Almeida GF, et al. Wearable sensors made with solution-blow spinning poly (lactic acid) for non-enzymatic pesticide detection in agriculture and food safety. Biosens And Bioelectron. 2022;199:113875. doi: 10.1016/j.bios.2021.113875
- Teixeira SC, Gomes NO, Calegaro ML, et al. Sustainable plant-wearable sensors for on-site, rapid decentralized detection of pesticides toward precision agriculture and food safety. Biomater Adv. 2023;155:213676. doi: 10.1016/j.bioadv.2023.213676
- Raymundo‐Pereira PA, Gomes NO, Carvalho JH, et al. Simultaneous detection of quercetin and carbendazim in wine samples using disposable electrochemical sensors. ChemElectrochem. 2020;7(14):3074–3081. doi: 10.1002/celc.202000788
- Raymundo-Pereira PA, Shimizu FM, Coelho D, et al. A nanostructured bifunctional platform for sensing of glucose biomarker in artificial saliva: synergy in hybrid Pt/Au surfaces. Biosens And Bioelectron. 2016;86:369–376. doi: 10.1016/j.bios.2016.06.053
- Fruncillo S, Su X, Liu H. Lithographic processes for the scalable fabrication of micro-and nanostructures for biochips and biosensors. ACS Sens. 2021;6(6):2002–2024. doi: 10.1021/acssensors.0c02704
- Martoni LV, Gomes NO, Oliveira ON Jr, et al. Low-cost photoelectrochemical sensor sensitized with carbon spherical shells and cobalt (II) phthalocyanine for fast acetaminophen determination. Microchem J. 2024;197:109780. doi: 10.1016/j.microc.2023.109780
- Bondancia TJ, Soares AC, Popolin-Neto M, et al. Low-cost bacterial nanocellulose-based interdigitated biosensor to detect the p53 cancer biomarker. Biomater Adv. 2022;134:112676. doi: 10.1016/j.msec.2022.112676
- Narakathu BB, Guo W, Obare SO, et al. Novel approach for detection of toxic organophosphorus compounds. Sens Actuators B Chem. 2011;158(1):69–74. doi: 10.1016/j.snb.2011.05.040
- Jeerapan I, Poorahong S. Flexible and stretchable electrochemical sensing systems: materials, energy sources, and integrations. J Electrochem Soc. 2020;167(3):037573. doi: 10.1149/1945-7111/ab7117
- Park TK, Shuler ML. Integration of cell culture and microfabrication technology. Biotechnol Prog. 2003;19(2):243–253. doi: 10.1021/bp020143k
- Pérez-Fernández B, Costa-García A, Muñiz ADLE. Electrochemical (bio) sensors for pesticides detection using screen-printed electrodes. Biosensors (Basel). 2020;10(4):32. doi: 10.3390/bios10040032
- Ulloa AM, Glassmaker N, Oduncu MR. Roll-to-roll manufactured sensors for nitroaromatic organophosphorus pesticides detection. ACS Appl Mater Interfaces. 2021;13(30):35961–35971. doi: 10.1021/acsami.1c08700
- Stefano JS, Orzari LO, Silva-Neto HA. Different approaches for fabrication of low-cost electrochemical sensors. Curr Opin Electrochem. 2022;32:100893. doi: 10.1016/j.coelec.2021.100893
- Liang G, He Z, Zhen J, et al. Development of the screen-printed electrodes: a mini review on the application for pesticide detection. Environ Technol Innov. 2022;28:102922. doi: 10.1016/j.eti.2022.102922
- Khairy M, Ayoub HA, Banks CE. Non-enzymatic electrochemical platform for parathion pesticide sensing based on nanometer-sized nickel oxide modified screen-printed electrodes. Food Chem. 2018;255:104–111. doi: 10.1016/j.foodchem.2018.02.004
- Trojanowicz M. Impact of nanotechnology on design of advanced screen-printed electrodes for different analytical applications. TrAC Trends In Analytical Chem. 2016;84:22–47. doi: 10.1016/j.trac.2016.03.027
- Zhao Y, Jin KQ, Li JD, et al. Flexible and stretchable electrochemical sensors for biological monitoring. Adv Mater. 2023;2305917. doi: 10.1002/adma.202305917
- Raza T, Qu L, Khokhar WA, et al. Progress of wearable and flexible electrochemical biosensors with the aid of conductive nanomaterials. Front Bioeng Biotechnol. 2021;9:761020. doi: 10.3389/fbioe.2021.761020
- Mathew M, Radhakrishnan S, Vaidyanathan A, et al. Flexible and wearable electrochemical biosensors based on two-dimensional materials: recent developments. Anal Bioanal Chem. 2021;413(3):727–762. doi: 10.1007/s00216-020-03002-y
- Jyoti RE, Alduhaish O, Alduhaish O, et al. 3D‐printed electrochemical sensor for organophosphate nerve agents. Electroanalysis. 2023;35(1):202200047. doi: 10.1002/elan.202200047
- Ibáñez-Redín G, Cagnani GR, Gomes NO, et al. Wearable potentiometric biosensor for analysis of urea in sweat. Biosens And Bioelectron. 2023;223:114994. doi: 10.1016/j.bios.2022.114994
- Liedert C, Rannaste L, Kokkonen A. Roll-to-roll manufacturing of integrated immunodetection sensors. ACS Sens. 2020;5(7):2010–2017. doi: 10.1021/acssensors.0c00404
- Yuan F, Xia Y, Lu Q. Recent advances in inorganic functional nanomaterials based flexible electrochemical sensors. Talanta. 2022;244:123419. doi: 10.1016/j.talanta.2022.123419
- Almuslem AS, Shaikh SF, Hussain MM. Flexible and stretchable electronics for harsh‐environmental applications. Adv Mater Technol. 2019;4(9):1900145. doi: 10.1002/admt.201900145
- Zhou C, Feng J, Tian Y, et al. Non-enzymatic electrochemical sensors based on nanomaterials for detection of organophosphorus pesticide residues. Environ Sci: Adv. 2023;2(7):933–956. doi: 10.1039/D3VA00045A
- Zhao F, He J, Li X. Smart plant-wearable biosensor for in-situ pesticide analysis. Biosens Bioelectron. 2020;170:112636. doi: 10.1016/j.bios.2020.112636
- Raymundo-Pereira PA, Gomes NO, Shimizu FM, et al. Selective and sensitive multiplexed detection of pesticides in food samples using wearable, flexible glove-embedded non-enzymatic sensors. J Chem Eng. 2021;408:127279. doi: 10.1016/j.cej.2020.127279
- Mishra RK, Martín A, Nakagawa T, et al. Detection of vapor-phase organophosphate threats using wearable conformable integrated epidermal and textile wireless biosensor systems. Biosens Bioelectron. 2018;101:227–234. doi: 10.1016/j.bios.2017.10.044
- Mishra RK, Barfidokht A, Karajic A, et al. Wearable potentiometric tattoo biosensor for on-body detection of G-type nerve agents simulants. Sens Actuators B Chem. 2018;273:966–972. doi: 10.1016/j.snb.2018.07.001
- Thangarasu R, Victor VD, Alagumuthu M. MnO2/PANI/rGO-A modified carbon electrode based electrochemical sensor to detect organophosphate pesticide in real food samples. Anal Bioanal Electrochem. 2019;11(4):427–447.
- Porto LS, Ferreira LF, Dos Santos WTP, et al. Determination of organophosphorus compounds in water and food samples using a non-enzymatic electrochemical sensor based on silver nanoparticles and carbon nanotubes nanocomposite coupled with batch injection analysis. Talanta. 2022;246:123477. doi: 10.1016/j.talanta.2022.123477
- Cioffi A, Mancini M, Gioia V, et al. Office paper-based electrochemical strips for organophosphorus pesticide monitoring in agricultural soil. Environ Sciamp; Technol. 2021;55(13):8859–8865. doi: 10.1021/acs.est.1c01931
- Goud KY, Sandhu SS, Teymourian H, et al. Textile-based wearable solid-contact flexible fluoride sensor: toward biodetection of G-type nerve agents. Biosens And Bioelectron. 2021;182:113172. doi: 10.1016/j.bios.2021.113172
- Ma H, Wang L, Liu Z. Ionic liquid–graphene hybrid nanosheets-based electrochemical sensor for sensitive detection of methyl parathion. J Environ Anal Chem. 2016;96(2):161–172. doi: 10.1080/03067319.2015.1114111
- Wei P, Gan T, Wu K. N-methyl-2-pyrrolidone exfoliated graphene as highly sensitive analytical platform for carbendazim. Sens. Actuators B: Chem. 2018;274:551–559. doi: 10.1016/j.snb.2018.07.174
- Masibi KK, Fayemi OE, Adekunle AS. Electrochemical detection of endosulfan using an AONP-PANI-SWCNT modified glassy carbon electrode. Mater. 2021;14(4):723. doi: 10.3390/ma14040723
- Zhang J, Hu H, Wang P, et al. A stable biosensor for organophosphorus pesticide detection based on chitosan modified graphene. Biotechnol Appl Biochem. 2022;69(2):567–575. doi: 10.1002/bab.2133
- Itkes MP, de Oliveira G G, Silva TA, et al. Voltammetric sensing of fenitrothion in natural water and orange juice samples using a single-walled carbon nanohorns and zein modified sensor. JEAC. 2019;840:21–26. doi: 10.1016/j.jelechem.2019.03.055
- Okumura LL, Saczk AA, Oliveira MFD, et al. Electrochemical feasibility study of methyl parathion determination on graphite-modified basal plane pyrolytic graphite electrode. J Braz Chem Soc. 2011;22:652–659. doi: 10.1590/S0103-50532011000400007
- Pedrosa VA, Miwa D, Machado SA, et al. On the utilization of boron doped diamond electrode as a sensor for parathion and as an anode for electrochemical combustion of parathion. Electroanalysis: Electroanalysis (NYNY). 2006;18(16):1590–1597. doi: 10.1002/elan.200603561
- An Y, Dong S, He Z, et al. Heteroatom-doped Co-MOF derivative enhancing immobilization and activity of two enzymes for small-molecules electrochemical determination. Microchem J. 2022;172:106942. doi: 10.1016/j.microc.2021.106942
- Maji B, Achary LSK, Barik B, et al. MnCo2O4 decorated (2D/2D) rGO/g-C3N4-based non-enzymatic sensor for highly selective and sensitive detection of Chlorpyrifos in water and food samples. JEAC. 2022;909:116115. doi: 10.1016/j.jelechem.2022.116115
- Mersal GA, El-Sheshtawy HS, Amin MA, et al. Simultaneous hydrolysis and detection of organophosphate by benzimidazole containing ligand-based zinc (II) complexes. Cryst. 2021;11(6):714. doi: 10.3390/cryst11060714
- Devi RK, Ganesan M, Chen TW, et al. Tailored architecture of molybdenum carbide/iron oxide micro flowers with graphitic carbon nitride: an electrochemical platform for nano-level detection of organophosphate pesticide in food samples. Food Chem. 2022;397:133791. doi: 10.1016/j.foodchem.2022.133791
- Saranya S, Deepa PN. Perforated oxidized graphitic carbon nitride with nickel spikes as efficient material for electrochemical sensing of chlorpyrifos in water samples. Surf Interface. 2022;32:102170. doi: 10.1016/j.surfin.2022.102170
- Khoshsafar H, Karimian N, Nguyen TA, et al. Enzymeless voltammetric sensor for simultaneous determination of parathion and paraoxon based on Nd-based metal-organic framework. Chemosphere. 2022;292:133440. doi: 10.1016/j.chemosphere.2021.133440
- Rajaji U, Chinnapaiyan S, Chen TW, et al. Rational construction of novel strontium hexaferrite decorated graphitic carbon nitrides for highly sensitive detection of neurotoxic organophosphate pesticide in fruits. Electrochim Acta. 2021;371:137756. doi: 10.1016/j.electacta.2021.137756
- Motaharian A, Motaharian F, Abnous K, et al. Molecularly imprinted polymer nanoparticles-based electrochemical sensor for determination of diazinon pesticide in well water and apple fruit samples. Anal Bioanal Chem. 2016;408(24):6769–6779. doi: 10.1007/s00216-016-9802-7
- Radi AE, Oreba R, Elshafey R. Molecularly imprinted electrochemical sensor for the detection of organophosphorus pesticide profenofos. Electroanalysis. 2021;33(8):1945–1951. doi: 10.1002/elan.202100175
- Khadem M, Faridbod F, Norouzi P, et al. Modification of carbon paste electrode based on molecularly imprinted polymer for electrochemical determination of diazinon in biological and environmental samples. Electroanalysis. 2017;29(3):708–715. doi: 10.1002/elan.201600293
- Jangid K, Sahu RP, Pandey R, et al. Multiwalled carbon nanotubes coated with nitrogen–sulfur Co-doped activated carbon for detecting fenitrothion. ACS Appl Nano Mater. 2021;4(5):4781–4789. doi: 10.1021/acsanm.1c00376
- Raymundo-Pereira PA, Gomes NO, Shimizu FM. Selective and sensitive multiplexed detection of pesticides in food samples using wearable, flexible glove-embedded non-enzymatic sensors. Chem Eng J. 2021;408:127279. doi: 10.1016/j.cej.2020.127279
- Khalifa ME, Abdallah AB. Molecular imprinted polymer based sensor for recognition and determination of profenofos organophosphorous insecticide. Biosens Bioelectron. 2019;2:100027. doi: 10.1016/j.biosx.2019.100027
- Deosarkar T, Chandrasekaran N, Mukherjee A. An ultra-sensitive and selective AChE based colorimetric detection of malathion using silver nanoparticle-graphene oxide (ag-GO) nanocomposite. Anal Chim Acta. 2021;1142:73–83. doi: 10.1016/j.aca.2020.10.057
- Loganathan C, Gowthaman NS, John SA. Chain-like 2-amino-4-thiazoleacetic acid tethered AuNPs as colorimetric and spectrophotometric probe for organophosphate pesticide in water and fruit samples. Microchem J. 2021;168:106495. doi: 10.1016/j.microc.2021.106495
- Li W, Rong Y, Wang J, et al. MnO2 switch-bridged DNA walker for ultrasensitive sensing of cholinesterase activity and organophosphorus pesticides. Biosens Bioelectron. 2020;169:112605. doi: 10.1016/j.bios.2020.112605
- Liu X, Wu Z, Yang X, et al. Photothermal and fluorescent dual-mode assay based on the formation of polydopamine nanoparticles for accurate determination of organophosphate pesticides. Microchim Acta. 2020;187(12):652. doi: 10.1007/s00604-020-04629-5
- Ashrafi Tafreshi F, Fatahi Z, Ghasemi SF, et al. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS One. 2020;15(3):0230646. doi: 10.1371/journal.pone.0230646
- Zhang C, Jiang Z, Jin M, et al. Fluorescence immunoassay for multiplex detection of organophosphate pesticides in agro-products based on signal amplification of gold nanoparticles and oligonucleotides. Food Chem. 2020;326:126813. doi: 10.1016/j.foodchem.2020.126813
- Wang J, Zhang J, Wang J, et al. Fluorescent peptide probes for organophosphorus pesticides detection. J Hazard Mater. 2020;389:122074. doi: 10.1016/j.jhazmat.2020.122074
- Wang B, Wilhelm A, Wilhelm A, et al. Wearable chemical sensors. In: Parlak O, Salleo A Turner A, editors. Wearable bioelectronics. Elsevier; 2020, Chapter 2. doi: 10.1021/acsnano.0c01804
- Tai L-C, Gao W, Chao M, et al. Adv. Mater. 2018;30(23):1707442. doi: 10.1002/adma.201707442
- Manjakkal L, Dang W, Yogeswaran N, et al. Textile-based potentiometric electrochemical pH sensor for wearable applications. Biosensors (Basel). 2019;9(1):14. doi: 10.3390/bios9010014
- Keum DH, Kim S-K, Koo J, et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci Adv. 2020;6(17):eaba3252. doi: 10.1126/sciadv.aba3252
- Sempionatto JR, Nakagawa T, Pavinatto A, et al. Lab on a chip. Lab Chip. 2017;17(10):1834–1842. doi: 10.1039/C7LC00192D
- Steinberg MD, Kassal P, Kereković I, et al. A wireless potentiostat for mobile chemical sensing and biosensing. Talanta. 2015;143:178–183. doi: 10.1016/j.talanta.2015.05.028
- Lin PH, Li BR. Antifouling strategies in advanced electrochemical sensors and biosensors. Analyst. 2020;145(4):1110–1120. doi: 10.1039/C9AN02017A
- Song G, Han H, Ma Z. Anti-fouling strategies of electrochemical sensors for tumor markers. Sensors. 2023;23(11):5202. doi: 10.3390/s23115202
- Hossain MI, Hasnat MA. Recent advancements in non-enzymatic electrochemical sensor development for the detection of organophosphorus pesticides in food and environment. Heliyon. 2023;9(9):e19299. doi: 10.1016/j.heliyon.2023.e19299
- Zhou L, Li X, Zhu B, et al. An overview of antifouling strategies for electrochemical analysis. Electroanalysis. 2022;34(6):966–975. doi: 10.1002/elan.202100406
- Itkes MP, de Oliveira GG, Silva TA, et al. Voltammetric sensing of fenitrothion in natural water and orange juice samples using a single-walled carbon nanohorns and zein modified sensor. J Electroanal Chem. 2019;840:21–26. doi: 10.1016/j.jelechem.2019.03.055
- Ma H, Wang L, Liu Z, et al. Ionic liquid–graphene hybrid nanosheets-based electrochemical sensor for sensitive detection of methyl parathion. Int J Environ Anal Chem. 2016;96(2):161–172. doi: 10.1080/03067319.2015.1114111
- Devi RK, Ganesan M, Chen TW, et al. Tailored architecture of molybdenum carbide/iron oxide micro flowers with graphitic carbon nitride: an electrochemical platform for nano-level detection of organophosphate pesticide in food samples. Food Chem. 2022;397:133791. doi: 10.1016/j.foodchem.2022.133791
- Piro B, Tran HV, Thu VT. Sensors made of natural renewable materials: efficiency, recyclability or biodegradability—the green electronics. Sensors. 2020;20(20):5898. doi: 10.3390/s20205898
- Lin ST, Klabunde KJ. Thermally activated magnesium oxide surface chemistry. Adsorption and decomposition of phosphorus compounds. Langmuir. 1985;1(5):600–605. doi: 10.1021/la00065a015
- Lucietto AM, Peters DL, Taleyarkhan MR, et al. Academic and industry collaboration: a literature review; ASEE Virtual Annual Conference; 2021 June 27-30; California. 2021.
- Amini L, Chen CH, Cox D, et al. Experiences and insights for collaborative industry-academic research in artificial intelligence. AI Mag. 2020;41(1):70–81. doi: 10.1609/aimag.v41i1.5201
- Tseng FC, Huang MH, Chen DZ. Factors of university–industry collaboration affecting university innovation performance. The J Technol Transf. 2020;45(2):560–577. doi: 10.1007/s10961-018-9656-6