Abstract
This study investigates the contextual effect of neighbourhood socio-economic status (SES) on the risk of preterm birth (PTB) using multilevel models. Birth data retrieved from 2000 Georgia Vital Records were geocoded and joined to their respective census tracts. The census tract level Index of Deprivation (IoD) was calculated using nine 2000 Census variables based on a previously proposed ‘standard’ index. Two-level random intercept regression models were developed using 117,329 live and singleton births at the individual level and 1618 census tracts at the neighbourhood level. After adjustment for individual-level factors, IoD generated an odds ratio of 1.006 (95% CI 1.00–1.01), showing a modest but significant effect on PTB. Intra-class correlation (ICC) was 0.83% after adjusting for individual-level factors and the census tract level IoD. A wide IOR-80% interval (0.74–1.36) suggests large unexplained residual in between census tract variation remained. The median odds ratio (MOR) value of 1.17 revealed that the unmodelled neighbourhood effect was stronger than the fixed effect of census tract-level predicting variable, IoD, but weaker than the effects of several individual-level predictor variables, including race, tobacco use, prenatal care, foetal death history and marital status. Overall, better census tract-level SES would have a modest protective effect for PTB risk. The full strength of multilevel models should be exploited further to help our understanding of PTB aetiology.
1. Introduction
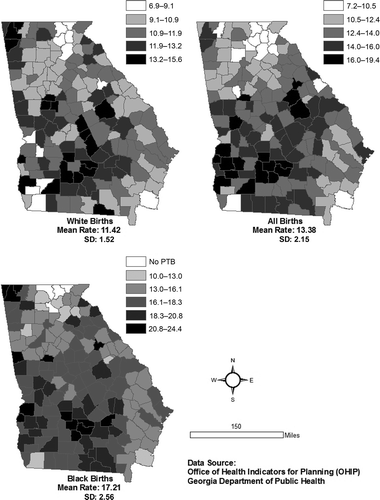
A preterm birth (PTB)Footnote1 is a birth occurring before the 37th week of pregnancy. In the United States, the PTB rate was 12.8% in 2006, more than 20% higher than in 1990. Although the US PTB rate has since been decreasing slowly and continuously, 11.7% of all the live births (or nearly 500,000) were PTB in 2011. In the State of Georgia, the PTB rates were 11.9%, 14.2% and 13.2% in 1995, 2006 and 2011, respectively, reflecting a similar pattern of rate change to the national trend in the past two decades. There have also been distinctive spatial and racial disparities in the raw PTB rates at the county level in Georgia (). Furthermore, both the US and Georgia PTB rates in 2011 were much higher than the Healthy People 2020 target rate of 9.6% (March of Dimes Citation2012).
PTB is a significant public health issue. PTB was responsible for more than one-third of all infant deaths in 2008, more than any other single cause (Callaghan et al. Citation2006; US Centers for Disease Control and Prevention Citation2013). PTB is also a primary contributor of infant morbidity and children’s developmental disabilities (Berkowitz and Papiernik Citation1993). In addition, PTB causes significant financial burdens to the impacted families and the society, and the US health system pays an estimated $26 billion each year for PTB-related health problems (Institute of Medicine Citation1985; US Centers for Disease Control and Prevention Citation2013). Previous studies suggested that a history of PTB is the strongest predictor of current PTB trends (Ensher and Clark Citation1994; Eibner and Sturm Citation2006; Goldenberg and Rouse Citation1998; Goldenberg et al. Citation2008), and widely reported individual-level PTB risk factors include mother’s socio-economic status (SES, e.g. income), demographic (e.g. race) and behavioural (e.g. smoking during pregnancy) characteristics and exposure to pollution (Ritz et al. Citation2000; Kyrklund-Blomberg, Granath, and Cnattingius Citation2005; Behrman and Rosenzweig Citation2004; Collins et al. Citation2007; Kramer and Hogue Citation2009; Darrow et al. Citation2009; Miranda, Messer, and Kroeger Citation2011).
More recent studies have investigated the impact of neighbourhood-level SES on adverse birth outcomes, including PTB and low birth weight. For instance, Herrick (Citation1996) reported the association between residence in low-income areas and PTB (prior to 33 weeks’ gestation) among urban black mothers in North Carolina; Roberts (Citation1997), in a Chicago case study, reported a significant positive relationship between low birth weight and community economic hardship and housing costs. Kaufman et al. (Citation2003) found a significant association between reduced PTB risk and census tract-level wealth. DeFranco et al. (Citation2008) analysed birth data (1989–1997) in Missouri with the conclusion that high county-level poverty was significantly associated with higher PTB risk. The interaction between neighbourhood and individual-level effects was also examined. For example, in a Baltimore study, O’Campo et al. (Citation1997) found that prenatal care had less protective effect on low birth weight risk for women living in high-risk regions than in low-risk regions.
Numerous studies have confirmed the neighbourhood effects (or contextual effects) on health outcomes, although in many cases the effects tend to be modest and smaller than compositional effects. Pickett and Pearl (Citation2001) reviewed 25 published studies with heterogeneous study designs and neighbourhood measures and, after adjusting for individual-level SES, they found statistically significant but modest association between at least one measure of social environment and a health outcome in all but two studies. Because efforts to predict PTB, as well as efforts to prevent it, have had only limited success at the best (Shiono and Klebanoff Citation1993), prevention remains the key to reducing PTB risk (Behrman and Butler Citation2007; Bahl et al. Citation2012). As such, a better understanding of health risks associated with the social structure and neighbourhood ecology could help the design of neighbourhood-level intervention strategies, identify concise geographical regions for intervention, facilitate allocation of resources for local intervention, monitor disparities among various population subgroups and track progress towards Healthy People 2020 goals at the local level depending on the magnitude of the variation among areas.
Multilevel models have been the most common type of analytical methods for assessing the effects of neighbourhood residential environments on health outcomes (Duncan, Jones, and Moon Citation1998; Merlo Citation2003). Multilevel perspectives recognize the hierarchical nesting of people within places and bring the two divergent epidemiological paradigms, individual risk factor epidemiology and an ecological approach, into one analytical framework. Multilevel models produce not only association measures (in the format of regression coefficients, odds ratios, etc.), but also partition the variance between individual and contextual levels and compute measures of cluster for understanding the distribution of health outcomes in the general population (Kearns and Moon Citation2002).
Our literature review also revealed three gaps in the multilevel model applications on adverse birth outcomes. First, the neighbourhood-level SES tended to be modelled using individual census variables. Between the different studies, a range of variables used for modelling included household income, poverty level, crime rate and education, to name a few (O’Campo et al. Citation1997; Roberts Citation1997; English et al. Citation2003). These individual measures can capture only one of many aspects of the neighbourhood SES, and the considerable variation in the SES measure also makes it very difficult to compare the results among different studies.
Second, several statistical measures including median odds ratio (MOR) and interval odds ratio (IOR-80%) have been developed to help the interpretation of the neighbourhood effect of multilevel logistic models (Merlo et al. Citation2006). While these measures have been used and have proven to be effective in many health studies (Sanagou et al. Citation2012; Larsen and Merlo Citation2005), the measures have yet to be applied in adverse birth outcomes studies to our best knowledge.
Third, according to 2013 Premature Birth Report Card (March of Dimes Citation2012), the lowest grades were found in Southeast US. Although many agree that results gleaned from multilevel studies can help quantify the magnitude of the geographical variation in PTB (Behrman and Butler Citation2007; Bahl et al. Citation2012), cases studies focusing on these poorly performed states including Georgia remained very limited. The good news is that two recent Georgia studies confirmed the neighbourhood SES on PTB risk. Ren (Citation2013) reported the association between residential instability and an increased risk of PTB using singleton birth data in Georgia between 1998 and 2002. Additionally, Messina (Citation2012) modelled singleton birth data in the city of Atlanta from 1998 to 2006 and found a statistically significant association between PTB risk and violent crime at both the census block group and census tract levels. However, more multilevel applications are still expected to provide more insights on the geographical variations of PTB risk to facilitate the design of preventive programme targeting specific geographical areas.
In this study, we attempted to bridge these gaps by demonstrating a substantive application and interpretation of multilevel models using year 2000 birth data in Georgia. We adapted and used a standardized census tract-level SES index in our models as an effort to allow a comprehensive representation of the neighbourhood SES and to facilitate the systematic comparison of across different studies (Eibner and Sturm Citation2006; Rajaratnam, Burke, and O’Campo Citation2006). We calculated MOR and IOR-80% and used these two measures to compliment the interpretation of the neighbourhood effect on PTB risk. The main objectives of this study were to (1) describe the extent of the variation in PTB risk among census tracts in Georgia; (2) determine the extent of the variation in PTB that can be explained by differences in mothers’ characteristics who resided in these census tracts and (3) determine whether living in a census tract with a lower SES increased the likelihood of having a PTB over and above individual characteristics.
2. Data and methods
2.1. Study population
The birth data were obtained from the electronic birth certificate data (BCD) from Georgia Vital Records Office (Georgia Department of Public Health Citation2010). A total of 132,286 births occurred in the State of Georgia in the year 2000. This study included only live and singleton births with the complete data of the nine individual-level predictor variables (n = 117,329). The mother’s self-reported residential addresses were geocoded as the locations of the births, and these addresses were subsequently linked to the census tracts.
2.2. Individual- and neighbourhood-level variables
The model outcome is a binary variable, 1 for PTB (births before completing 37 weeks of pregnancy) and 0 for full-term births (births on or after 37 weeks of pregnancy). Nine individual-level predicting variables were included in the models: race (black, white or others), sex (male or female), age (mother’s age in years), marital status (married or unmarried), education (mother’s total year of education), mother’s previous number of foetal death, mother received late or no prenatal care (yes or no), smoking during pregnancy (yes or no) and alcohol use during pregnancy (yes or no). These variables were considered as better-established risk factors according to two authoritative studies on premature births (Institute of Medicine Citation1985; Behrman and Butler Citation2007).
One of the research objectives of this study was to use a standardized neighbourhood-level SES in multilevel models so results from different studies can be compared in a similarly systematic and replicable manner. In a previous study, Messer and her colleagues identified 20 census variables after a comprehensive literature review of socio-economic and demographic domains associated with health outcomes. Using principal components analysis (PCA) and factor analysis (FA), investigators selected the eight most significant variables that included the percent of males in management and professional occupations, percent of crowded housing, percent of households in poverty, percent of female headed households with dependents, percent of households on public assistance and households earning <$30,000 per year, percent less than a high school education and the percent unemployed (Messer et al. Citation2006).
The first principal component resulting from PCA analysis using the eight variables was then defined as the deprivation index. Subsequently, the index was found to be associated with the unadjusted prevalence of PTB and low birth weight for white non-Hispanic and black non-Hispanic women in the eight study areas across the states of Maryland, Michigan, North Carolina and Pennsylvania. Investigators concluded that using a deprivation index would benefit research of neighbourhood effects on adverse birth outcomes.
In this study, we created a neighbourhood SES index based on the deprivation index. Our index contains nine instead of eight census variables, including poverty, female household head, household income <$25,000, occupation in management sectors, unemployment, percent population receiving public assistance, average household size, vehicle ownership and population receiving less than high school education. We had one more census variable, vehicle ownership, in our index because we believe that mothers’ mobility is an important aspect of the overall neighbourhood SES that should be included. In addition, we used household income <$25,000 instead of <$30,000 in our index.
The census data were obtained from US Census Bureau’s decennial census website (US Census Bureau Citation2013) and they were standardized, oriented to the same direction (higher values, lower SES) and combined into one variable, Index of Deprivation (IoD), using the following equations:
2.3. Selection of neighbourhood
Census tracts were chosen as the contextual unit of analysis to approximate neighbourhoods. According to the US Census Bureau, census tracts are small, relatively stable statistical sub-county units with fairly homogenous SES and living conditions, containing on average 4000 residents (US Census Bureau Citation2010). Despite the potential pitfalls of using census tract, it remains to be a convenient choice (particularly for data collection) of the unit of analysis in health studies and has overall been considered as a satisfactory approximation of a person’s immediate residential environment (O’Campo et al. Citation1997; Rauh, Andrews, and Garfinkel Citation2001; Kaufman et al. Citation2003; Collins, Rankin, and David Citation2011).
2.4. Statistical analysis
The multilevel logistic regression (MLR) models reported in this paper were all two-level models in which individuals (mothers, level 1) were nested within neighbourhoods (census tracts, level 2). The full multilevel model is described conceptually below and the formal statistical notations and explanations can be found elsewhere (Demidenko Citation2004; Gelman and Hill Citation2007):
Birth outcome (yes or no) = baby’s sex + mother’s age + mother’s race/ethnicity + mother’s marital status + mother’s education + mother’s foetal death history + mother’s alcohol use during pregnancy + mother’s tobacco use during pregnancy + census tract-level SES index (IoD) + random effects (at the census tract level).
Maximum likelihood with the Laplace approximation was used to estimate the random part (level 1 and 2 variances) and fixed part (regression coefficients) of the models. All the multilevel logistic models were developed and fitted using R v. 2.15 (R Core Team Citation2013).
The intraclass correlation coefficient (ICC) is the percentage of the neighbourhood-level variance in the total (individual and neighbourhood) variance. ICC can also be considered as a variance partition coefficient. A high ICC indicates more individual difference is from the difference of the neighbourhoods (Snijders and Bosker Citation2012). The total variance in the outcome variable is the sum of individual- and neighbourhood-level variances. In MLR, the unobserved individual variable follows a logistic distribution with an individual-level variance of 3.29. Therefore, ICC can be calculated as
However, variances at the two levels are on difference scale, the individual level is on the probability scale and the neighbourhood level is on the logistic scale. Therefore, ICCs calculated from MLR models may not accurately represent the partitioning of variance and they may also have interpretational and generalizability issues (Goldstein, Browne, and Rasbash Citation2002). To provide a clearer interpretation of neighbourhood-level variance, we calculated MOR in addition to ICCs. MOR is the median of a set of odds ratios that are obtained by comparing two mothers with identical individual-level characteristics from two randomly chosen, different neighbourhoods (i.e. with different neighbourhood random effect). MOR is thus the median odds between a mother in the neighbourhood with higher PTB propensity and another mother in the neighbourhood with lower PTB propensity. The value of MOR is always equal to or larger than 1. If MOR is 1, there is no between-neighbourhood variation. The larger the MOR than 1, there are more between-neighbourhood variation that is not explained by the modelled predicting variables (Merlo et al. Citation2006). MOR is shown in Equation (4) as a function of , the variance of neighbourhood effect:
In MLR models, usual interpretation of odds of individual-level predicting variables applies to compare individuals located within the same neighbourhood. For example, a marital status effect may be interpreted as the odd ratio of having a PTB between a married and an unmarried mother who live in the same neighbourhood and with the same individual predicting variables except for marital status.
For neighbourhood-level predicting variables, the odds ratio of PTB should be interpreted as comparing two neighbourhoods with one-unit difference in the value of the predicting variable but having the identical random effect. For example, the odds ratio of having a PTB between two mothers living in two census tracts with one-unit difference in IoD value and with the identical random effect (R Core Team Citation2013).
To accommodate the need of comparing individuals from neighbourhoods with different random effects, we calculated the IOR-80%, which incorporates both the fixed neighbourhood-level predicting variable effect and the unexplained between-neighbourhood heterogeneity in an interval (Merlo et al. Citation2006; Sanagou et al. Citation2012). Considering all possible pairs of mothers with identical individual-level predicting variables from different neighbourhoods with a one-unit difference in the value of the neighbourhood-level predicting variable (e.g. IoD), we calculate the odds ratio for each pair of mothers first. We then examine the distribution of these odds ratios. Therefore, IOR-80% is defined as an interval at the median of the distribution that contains 80% of the odds ratio values. The lower and upper bounds of the IOR-80% can be approximated using the following equation:
According to Equation (5), small between-neighbourhood variation (τ2) will lead to a narrow IOR-80%, whereas a large between-neighbourhood variation (τ2) will lead to a wide IOR-80%. IOR-80% is a combined measure of unexplained between-neighbourhood variation and the effect of the modelled neighbourhood-level predicting variable, so IOR-80% will contain 1 if the value of τ2 is large compared with the effect of the neighbourhood-level predicting variable.
3. Results
We fitted three MLR models. M0 was a null model with no predicting variables, M1 included the individual-level predicting variables and M2 added census tract-level predicting variable, IoD, to M1. No generally accepted criterion exists to evaluate the adequacy of multilevel models for small area estimation. We calculated the proportional change in variance (PCV) and followed the recommendation that a multilevel model should explain at least 40% (i.e. PCV > 40%) between area-level variance for the outcome measure of interest to justify model adequacy (Merlo et al. Citation2005). Compared with the null model, M0, the full model (M2) explained 59.8% census-level variances associated with PTB. For the purpose of comparison, we also fitted an ordinary logistical regression model that included all the individual-level predicting variables and census tract-level IoD. The Akaike information criterion (AIC) value of M2 was 74,837, smaller than the ordinary model, 74,862 (Akaike Citation1974).
3.1. Descriptive statistics
A total of 117,329 live and singleton births from Georgia’s 1613 census tracts (no births were reported in five census tracts in 2000) were included in our analyses. provides summary statistics of individual- and census tract-level variables. At the individual level, 50.9% of the babies were male and the percentages of mothers self-identified as white, black and other race/ethnicity were 63.3% (n = 74,274), 33.5% (n = 39,322) and 3.2% (n = 3733), respectively. In addition, the crude PTB rates for all, white and black mothers were 10.2%, 8.7% and 13.1%, respectively. We decided to limit our analyses to white and black mothers because these two population groups accounted for nearly 97% of the total births in 2000 and showed the most pronounced disparity in raw PTB rates. Sixty-three percent of the mothers were reported as married, mothers’ average age was 26.5 years and average educational level was 12.8 years. Approximately 12.6% of the mothers reported no or late initiation of prenatal care. In addition, 0.7% and 8.2% of the mothers admitted the consumption of alcohol and tobacco products during pregnancy, respectively. Significant variations in census tract-level SES were also observed from the nine selected variables. For example, poverty rate ranged from 0 and 75.7% with the mean of 15.9%.
Table 1. Individual- and neighbourhood-level descriptors based on Georgia 2000 Vital Records and census data and used in the logistic regression analysis.
3.2. Results of MLR models
contains results from the ordinary linear regression model and the three MLR models M0 (the null model), M1 (including only individual-level predicting variables) and M2 (the full model, including both the individual- and the census tract-level IOD). M2 estimated that the odds ratio of the census tract-level IoD was 1.0064. If comparing two mothers with identical risk factors residing in two census tracts with one-unit difference in IoD and if the two census tracts are otherwise identical with regards to PTB risk, then the odds of PTB was increased 1.0064-fold for the mother residing in the census tract with the higher IOD.
Table 2. Results of ordinary and multilevel logistic regression models.
As discussed earlier in the paper, it is neither very intuitive nor very useful to interpret directly the odds of the neighbourhood effect. M2 estimated that the IOR-80% for IoD was 0.74 to 1.36, which can provide further insight about the neighbourhood effect on PTB risk. The data interval suggests that when comparing two randomly chosen mothers with identical risk factors, one from a census tract with one unit higher IoD than the census tract the other was from, and those census tracts possibly differing in other ways regarding PTB risk, the odds ratio for the comparison will, with 80% probability, lie between 0.74 and 1.36. The wide IOR-80% reflects considerable uncertainty in the impact of the census tract-level IoD on mothers’ PTB risk due to substantial residual variation in PTB risk between census tracts. This residual was not accounted for by either census tract-level IoD or individual-level characteristics included in the MRL model.
The odds ratios IoD in the ordinary logistic regression and M2 were very close and the effects were statistically significant in both models (P < 0.001). However, the 95% CIs for IoD in M2 were slightly wider than in the ordinary logistic regression, reflecting that the MLR model accounted for a small portion of between census tract heterogeneity.
M2 estimated that the proportion of the variance in PTB risk between census tracts was less than 1% (ICC = 0.083). The low ICC value for PTB suggests much greater heterogeneity within census tracts than between census tracts. MOR provides information on unexplained heterogeneity between census tracts. From M2, MOR was 1.17, which can be interpreted as, if a mother moved from one census tract to the other with a higher PTB propensity, the median increase of the odds of having PTB was 1.17. This number also indicated that the effect of unexplained neighbourhood variation on PTB was weaker than the effects of several individual-level predictor variables, including race, tobacco use, prenatal care, foetal death history and marital status, but stronger than the fixed effect of census tract-level predicting variable, IoD.
4. Discussions
The results from our MLR model indicate that the association between census tract-level SES and PTB was weak but statistically significant and that the size of the variance among census tracts was modest. However, a small neighbourhood variance does not necessarily mean that the contextual factors are unimportant to PTB. Statistically, the result shows that the effect of unexplained heterogeneity between census tracts was stronger than the fixed effect of census tract-level SES. Substantively, uneven geographic distribution of raw premature birth rates has been consistently observed in the United States in the past two decades, and physical and socio-economic environment also varies significantly across different geographic regions across the country. In addition, from a modelling perspective, the geographic boundaries (i.e. census tracts) used in out models to define the actual neighbourhood might not correspond well with the boundaries shaping the relevant environment for higher PTB risk.
Little evidence could be found from this study to support policies that would allocate additional resources to improving SES in specific neighbourhoods to reduce PTB risk. However, the result should not prevent us from seeking innovative approaches in improving the overall neighbourhood SES. From a multilevel perspective, neighbourhood SES is viewed as an upstream effect in the causal pathway influencing individual health outcome. Neighbourhood-level changes may affect downstream individual characteristics, which in turn influence individual health outcomes including PTB. This chain of events may be critical in understanding and enhancing the probability of success of community-based intervention programmes. For example, an educational programme at the neighbourhood level may reduce mothers’ smoking behaviour, which in turn may reduce the PTB risk. In addition, the cost savings of preventing even a modest number of PTB could be substantial.
We investigated in this study the neighbourhood SES as a possible mediator of PTB risk. Other possible pathways by which mothers who reside in low SES areas may be more likely to have PTB include accessibility of health-care services, exposure to environmental toxicants and cofounding effects of individual characteristics. These pathways need to be examined to deepen our understanding on the neighbourhood effect on adverse birth outcomes.
The results of this study should be considered in light of its limitations. First, the reliability and quality of vital records data are likely to vary and cannot be independently verified. In particular, the data did not provide information about the lengths of time the mothers had lived in their respective addresses. This information was correlated to the exposure time of the specific neighbourhood-level SES. This problem could be addressed by using incorporating survey data with birth certificate data (O’Campo Citation2003) or by designing a cohort study so that the relationship between residential area and time-at-risk of study subjects would be more accurately estimated (Caughy, O’Campo, and Muntaner Citation2003). Nevertheless, the best available data were utilized in our analyses when this study was conducted. In addition, validation studies of vital records have reported that while the quality of sensitivity of accurate recording for risk factors and outcomes was instable (Reichman and Hade Citation2001), the specificity of those same data fields in most cases were more than acceptable (Reichman and Schwartz-Soicher Citation2007).
Second, although commonly used, census tracts should still be treated as a crude proxy of ‘neighbourhood’. Census tracts are artificial administrative boundaries designed for census data gathering and reporting. Given this limitation, it is important to note that it is reasonable to assume that the populations who share these same artificial administrative boundaries are more likely than not to share the same cultural practices in terms of customs, values and perceptions. In other words, individuals who practice the same cultural norms are more likely to cluster together in residential areas. Given this assumption, more salient definition of ‘neighbourhood’ from some recent research should be tested in the future to better address the boundary issue as well as the Multiple Area Unit Problem (Riva et al. Citation2008; Haynes et al. Citation2007; Mu and Wang Citation2008; Openshaw et al. Citation1987).
Third, we applied a modified version of a ‘standardized’ neighbourhood deprivation index based on the work of Messer et al. (Citation2006). While this index allowed more convenient comparison of our results with those conducted in other regions, especially in the United States, the reader should be aware that this index is imperfect. The usefulness of the original index was tested only in the 19 cities and 5 counties in 4 states in the United States. In addition, we chose to use an equally weighted and linearly combined index to summarize the overall neighbourhood SES. More sophisticated weighting approaches (e.g. Z-score, experts weightings) and index construction techniques (e.g. PCA, FA) can be tested in the future to improve the model performance (Eibner and Sturm Citation2006; Messer et al. Citation2006; Mu, Wang, and McLafferty. 2010; Lalloué et al. Citation2013).
Last but not the least, the research design of this study is vulnerable to the so-called uncertain geographic context problem (UGCoP). Coined by Kwan (Citation2012, p. 959), UGCoP refers to ‘…the spatial uncertainty in the actual areas that exert the contextual influences under study and the temporal uncertainty in the timing and duration in which individuals experienced these contextual influences…’ (959). Because the contextual variable (in our case, IoD) is sensitive to how contextual units are delineated, it is problematic to rely solely on the model fit to evaluate the model results.
To mitigate UGCoP and its potential confounding effect with the famous modifiable areal unit problem (Openshaw Citation1984), Kwan suggests conceptualizing geographic context dynamically in health research. Methodologically, several recent approaches have pointed out some promising directions in addressing UGCoP. One is to conduct sensitivity analysis to assess how contextual variables and study results respond to changes in delineations of contextual units (Shi Citation2010). The second is to use basic census units or other arbitrary spatial units (e.g. zip code areas) to construct larger geographic areas (or regionalization). Featured by large and more stable base population, coherent areal socio-economic characteristics and spatial closeness, these larger geographic areas will be more robust unit of analysis in health studies (Wang, Guo, and McLafferty Citation2012). The third is to conduct space–time analysis simultaneously to allow a more nuanced understanding of the dynamic and complex health outcomes (McLafferty et al. Citation2012).
5. Conclusions
In this study, we constructed a simple two-level random intercepts logistic regression model to investigate the impact of neighbourhood SES on PTB using vital records data in Georgia, USA. We provided measures to allow more interpretable information about the modelled and unmodelled neighbourhood effects on PTB. We used a ‘standardized’ neighbourhood SES index in the model to make it easier to compare our results with others. Compared with the ordinary regression model, MLR is capable of between neighbourhood heterogeneity.
Our MLR model estimated that the proportion of the variance in the probability of having PTB between census tracts was less than 1% (ICC = 0.84), after adjusting the individual factors and census tract-level IoD. The fixed census tract-level effect, IoD, was 1.006, suggesting a weak but significant association between low neighbourhood SES and elevated PTB risk. In other words, higher census tract-level SES was a small protective factor of PTB risk. The MLR model also estimated that if a mother moved to another census tract with a higher probability of having PTB, the median increase in their odds of having PTB would be 1.17-fold (MOR = 1.17), a modest effect compared with several individual-level effects including race, tobacco use, prenatal care, foetal death history and marital status patient-level risk factor effects but higher than the census tract-level fixed effect, IoD. This result suggests that unexplained heterogeneity between census tracts was of greater relevance than the census tract-level IoD for understanding the individual PTB risk. The wide IOR-80% interval (0.74–1.36) suggested large unexplained residual in between census tract variation. Census tract-level IoD alone could not clearly distinguish low- from high-propensity PTB areas.
Health outcomes are intrinsically nested in hierarchical structure (Moellering and Tobler Citation2010; Jones Citation1991), the ‘ecological fallacy’ (Robinson Citation1950), transferring observations at an aggregate-level to an individual-level outcome and the ‘atomistic fallacy’ (Macintyre, Ellaway, and Cummins Citation2002), ignoring the socio-economic context that may alter an individual-level outcome, remain to be the two key challenges in finding causal inference of diseases. More sophisticated modelling strategies (e.g. random slope, interaction between predicting variables at different levels) are available for future exploration to untangle the complicated and often confounded nature of ecological and atomistic fallacies and to seek more clues of the neighbourhood effect on adverse birth outcomes (Gelman and Hill Citation2007). The results from these models can not only further our understanding of the compositional and contextual effects on PTB risk, but also provide useful information for the formulation of place- and population-specific prevention and interventions programmes to improve adverse birth outcomes including PTB.
Acknowledgements
We would like to thank Scott Markely for providing research assistance for the manuscript. We thank Dr Hui Lin for his excellent work in handling the blind review process and making editorial decisions for this article. We appreciate the constructive comments from the two anonymous reviewers which have helped improve the article considerably. The analyses and opinions in this article are entirely the responsibility of the authors.
Notes
1. Abbreviations: PTB, preterm birth; MLR, multilevel logistic regression; SES, socio-economic status; MOR, median odds ratio; IOR-80%, 80% interval odds ratio; IoD, Index of Deprivation; ICC, intra-class correlation coefficient; AIC, Akaike information criterion; PVC, proportional change in variance.
References
- Akaike, H. 1974. “A New Look at the Statistical Model Identification.” IEEE Transactions on Automatic Control 19 (6): 716–723. doi:10.1109/TAC.1974.1100705.
- Bahl, R., J. Martines, N. Bhandari, Z. Biloglav, K. Edmond, S. Iyengar, M. Kramer, et al. 2012. “Setting Research Priorities to Reduce Global Mortality from Preterm Birth and Low Birth Weight by 2015.” Journal of Global Health 2 (1): 10403–10403. doi:10.7189/jogh.01.010403.
- Behrman, J. R., and M. R. Rosenzweig. 2004. “The Returns to Increasing Body Weight.” Review of Economics and Statistics 86 (2): 586–601.
- Behrman, R. E., and A. S. Butler. 2007. “Preterm Birth [Electronic Resource]: Causes, Consequences, and Prevention/Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Board on Health Sciences Policy.” Institute of Medicine of the National Academies, edited by R. E. Behrman and A. S. Butler. Washington, DC: National Academies Press. c2007. Bibliographies.
- Berkowitz, G. S., and E. Papiernik. 1993. “Epidemiology of Preterm Birth.” Epidemiologic Reviews 15 (2): 414–443.
- Callaghan, W. M., M. F. MacDorman, S. A. Rasmussen, C. Qin, and E. M. Lackritz. 2006. “The Contribution of Preterm Birth to Infant Mortality Rates in the United States.” Pediatrics 118 (4): 1566–1573. doi:10.1542/peds.2006-0860.
- Caughy, M. O., P. J. O’Campo, and C. Muntaner. 2003. “When Being Alone Might be Better: Neighborhood Poverty, Social Capital, and Child Mental Health.” Social Science & Medicine 57 (2): 227–237. doi:10.1016/S0277-9536(02)00342-8.
- Collins, J., K. Rankin, and R. David. 2011. “Low Birth Weight Across Generations: The Effect of Economic Environment.” Maternal & Child Health Journal 15 (4): 438–445. doi:10.1007/s10995-010-0603-x.
- Collins, J. W., R. J. David, D. M. Simon, and N. G. Prachand. 2007. “Preterm Birth among African American and White Women with a Lifelong Residence in High-Income Chicago Neighborhoods: An Exploratory Study.” Ethnicity & Disease 17 (1): 113–117.
- Darrow, L. A., M. J. Strickland, M. Klein, L. A. Waller, W. D. Flanders, A. Correa, M. Marcus, and P. E. Tolbert. 2009. “Seasonality of Birth and Implications for Temporal Studies of Preterm Birth.” Epidemiology (Cambridge, Mass) 20 (5): 699–706. doi:10.1097/EDE.0b013e3181a66e96.
- DeFranco, E. A., M. Lian, L. A. Muglia, and M. Schootman. 2008. “Area-Level Poverty and Preterm Birth Risk: A Population-Based Multilevel Analysis.” BMC Public Health 8: 316–324. doi:10.1186/1471-2458-8-316.
- Demidenko, E. Z. 2004. Mixed Models: Theory and Applications/Eugene Demidenko, Wiley Series in Probability and Statistics. Hoboken, NJ: Wiley Interscience. c2004. Bibliographies.
- Duncan, C., K. Jones, and G. Moon. 1998. “Context, Composition and Heterogeneity: Using Multilevel Models in Health Research.” Social Science & Medicine 46 (1): 97–117. doi:10.1016/S0277-9536(97)00148-2.
- Eibner, C., and R. Sturm. 2006. “US-Based Indices of Area-Level Deprivation: Results from Healthcare for Communities.” Social Science & Medicine 62 (2): 348–359. doi:10.1016/j.socscimed.2005.06.017.
- English, P. B., M. Kharrazi, S. Davies, R. Scalf, L. Waller, and R. Neutra. 2003. “Changes in the Spatial Pattern of Low Birth Weight in a Southern California County: The Role of Individual and Neighborhood Level Factors.” Social Science & Medicine (1982) 56 (10): 2073–2088. doi:10.1016/S0277-9536(02)00202-2.
- Ensher, G. L., and D. A. Clark. 1994. Newborns at Risk: Medical Care and Psychoeducational Intervention. 2nd ed. Gaithersburg, MD: Aspen Publishers.
- Georgia Department of Public Health. 2010. “Vital Records.” Accessed December 19, 2013. http://dph.georgia.gov/VitalRecords
- Gelman, A., and J. Hill. 2007. “Data Analysis Using Regression and Multilevel/Hierarchical Models/Andrew Gelman, Jennifer Hill.” In Analytical Methods for Social Research, edited by R. M. Alvarez, N. L. Beck, L. L. Wu, and S. L. Morgan. Cambridge: Cambridge University Press.
- Goldenberg, R. L., J. F. Culhane, J. D. Iams, and R. Romero. 2008. “Epidemiology and Causes of Preterm Birth.” The Lancet 371 (9606): 75–84. doi:10.1016/S0140-6736(08)60074-4.
- Goldenberg, R. L., and D. J. Rouse. 1998. “Prevention of Premature Birth.” The New England Journal of Medicine 339 (5): 313–320. doi:10.1056/NEJM199807303390506.
- Goldstein, H., W. Browne, and J. Rasbash. 2002. “Partitioning Variation in Multilevel Models.” Understanding Statistics 1 (4): 223–231. doi:10.1207/S15328031US0104_02.
- Haynes, R., K. Daras, R. Reading, and A. Jones. 2007. “Modifiable Neighbourhood Units, Zone Design and Residents’ Perceptions.” Health & Place 13 (4): 812–825. doi:10.1016/j.healthplace.2007.01.002.
- Herrick, H. 1996. “The Association of Poverty and Residence in Predominantly Black Neighborhoods with the Occurrence of Preterm Births among Black Women: A Case-Control Study of Three North Carolina Metropolitan Areas.” Vol. 99, Special Report by the State Center for Health and Environmental Statistics. Raleigh: North Carolina Department of Environment, Health, and Natural Resources.
- Institute of Medicine 1985. Preventing Low Birthweight [Electronic Resource]/Committee to Study the Prevention of Low Birthweight, Division of Health Promotion and Disease Prevention, Institute of Medicine. 1985. Standards/specifications. Washington, DC: National Academy Press.
- Jones, K. 1991. “Specifying and Estimating Multi-Level Models for Geographical Research.” Transactions of the Institute of British Geographers 16 (2): 148. doi:10.2307/622610.
- Kaufman, J. S., N. Dole, D. A. Savitz, and A. H. Herring. 2003. “Modeling Community-Level Effects on Preterm Birth.” Annals of Epidemiology 13 (5): 377–384. doi:10.1016/S1047-2797(02)00480-5.
- Kearns, R., and G. Moon. 2002. “From Medical to Health Geography: Novelty, Place and Theory after a Decade of Change.” Progress in Human Geography 26 (5): 605–625. doi:10.1191/0309132502ph389oa.
- Kramer, M. R., and C. R. Hogue. 2009. “What Causes Racial Disparities in Very Preterm Birth? A Biosocial Perspective.” Epidemiologic Reviews 31: 84–98.
- Kwan, M.-P. 2012. “The Uncertain Geographic Context Problem.” Annals of the Association of American Geographers 102 (5): 958–968. doi:10.1080/00045608.2012.687349.
- Kyrklund-Blomberg, N. B., F. Granath, and S. Cnattingius. 2005. “Maternal Smoking and Causes of Very Preterm Birth.” Acta Obstetricia Et Gynecologica Scandinavica 84 (6): 572–577. doi:10.1080/j.0001-6349.2005.00848.x.
- Lalloué, B., J.-M. Monnez, C. Padilla, W. Kihal, N. Le Meur, D. Zmirou-Navier, and S. Deguen. 2013. “A Statistical Procedure to Create a Neighborhood Socioeconomic Index for Health Inequalities Analysis.” International Journal for Equity in Health 12 (1): 21–31. doi:10.1186/1475-9276-12-21.
- Larsen, K., and J. Merlo. 2005. “Appropriate Assessment of Neighborhood Effects on Individual Health: Integrating Random and Fixed Effects in Multilevel Logistic Regression.” American Journal of Epidemiology 161 (1): 81–88. doi:10.1093/aje/kwi017.
- Macintyre, S., A. Ellaway, and S. Cummins. 2002. “Place Effects on Health: How can we Conceptualise, Operationalise and Measure Them?” Social Science & Medicine (1982) 55 (1): 125–139. doi:10.1016/S0277-9536(01)00214-3.
- March of Dimes. 2012. “2012 Premature Birth Report Card.” Accessed December 19, 2013. http://www.marchofdimes.com/peristats/pdflib/998/us.pdf
- McLafferty, S., M. Widener, R. Chakrabarti, and S. Grady. 2012. “Ethnic Density and Maternal and Infant Health Inequalities: Bangladeshi Immigrant Women in New York City in the 1990s.” Annals of the Association of American Geographers 102 (5): 893–903. doi:10.1080/00045608.2012.674901.
- Merlo, J. 2003. “Multilevel Analytical Approaches in Social Epidemiology: Measures of Health Variation Compared with Traditional Measures of Association.” Journal of Epidemiology and Community Health 57 (8): 550–552. doi:10.1136/jech.57.8.550.
- Merlo, J., M. Yang, B. Chaix, J. Lynch, and L. Råstam. 2005. “A Brief Conceptual Tutorial on Multilevel Analysis in Social Epidemiology: Investigating Contextual Phenomena in Different Groups of People.” Journal of Epidemiology & Community Health 59 (9): 729–736. doi:10.1136/jech.2004.023929.
- Merlo, J., B. Chaix, H. Ohlsson, A. Beekman, K. Johnell, P. Hjerpe, L. Ṙstam, and K. Larsen. 2006. “A Brief Conceptual Tutorial of Multilevel Analysis in Social Epidemiology: Using Measures of Clustering in Multilevel Logistic Regression to Investigate Contextual Phenomena.” Journal of Epidemiology and Community Health 60: 290–297. doi:10.1136/jech.2004.029454.
- Messer, L. C., B. A. Laraia, J. S. Kaufman, J. Eyster, C. Holzman, J. Culhane, I. Elo, J. G. Burke, and P. O’Campo. 2006. “The Development of a Standardized Neighborhood Deprivation Index.” Journal of Urban Health: Bulletin of the New York Academy of Medicine 83 (6): 1041–1062. doi:10.1007/s11524-006-9094-x.
- Messina, L. C. 2012. “Investigating the Association Between Small-Area Violent Crime and Preterm Birth in Atlanta, GA: 1998–2006.” Master diss., Emory University, Atlanta, GA.
- Miranda, M. L., L. C. Messer, and G. L. Kroeger. 2011. “Associations between the Quality of the Residential Built Environment and Pregnancy Outcomes among Women in North Carolina.” Environmental Health Perspectives 120 (3): 471–477. doi:10.1289/ehp.1103578.
- Moellering, H., and W. Tobler. 2010. “Geographical Variances.” Geographical Analysis 10.1111/j.1538-4632.1972.tb00455.x.
- Mu, L., and F. H. Wang. 2008. “A Scale-Space Clustering Method: Mitigating the Effect of Scale in the Analysis of Zone-Based Data.” Annals of the Association of American Geographers 98 (1): 85–101. doi:10.1080/00045600701734224.
- Mu, L., F. Wang, and S. McLafferty. 2010. “Analyzing Spatial Patterns of Late-Stage Breast Cancer in Chicago Region: A Modified Scale-Space Clustering Approach.” Geospatial Analysis & Modelling of Urban Structure & Dynamics 99. doi:10.1007/978-90-481-8572-6_18.
- O’Campo, P. 2003. “Invited Commentary: Advancing Theory and Methods for Multilevel Models of Residential Neighborhoods and Health.” American Journal of Epidemiology 157 (1): 9–13. doi:10.1093/aje/kwf171.
- O’Campo, P., X. Xue, M.-C. Wang, and M. Caughy. 1997. “Neighborhood Risk Factors for Low Birthweight in Baltimore: A Multilevel Analysis.” American Journal of Public Health 87 (7): 1113–1118. doi:10.2105/AJPH.87.7.1113.
- Openshaw, S. 1984. The Modifiable Areal Unit Problem. Norwich: Geo Books.
- Openshaw, S., M. Charlton, C. Wymer, and A. Craft. 1987. “A Mark 1 Geographical Analysis Machine for the Automated Analysis of Point Data Sets.” International Journal of Geographical Information Systems 1 (4): 335–358. doi:10.1080/02693798708927821.
- Pickett, K. E., and M. Pearl. 2001. “Multilevel Analyses of Neighbourhood Socioeconomic Context and Health Outcomes: A Critical Review.” Journal of Epidemiology & Community Health 55 (2): 111–122. doi:10.1136/jech.55.2.111.
- R Core Team. 2013. “R: A Language and Environment for Statistical Computing.” Accessed March 10, 2014. http://www.r-project.org/
- Rajaratnam, J. K., J. G. Burke, and P. O’Campo. 2006. “Maternal and Child Health and Neighborhood Context: The Selection and Construction of Area-Level Variables.” Health & Place 12 (4): 547–556. doi:10.1016/j.healthplace.2005.08.008.
- Rauh, V. A., H. F. Andrews, and R. S. Garfinkel. 2001. “The Contribution of Maternal Age to Racial Disparities in Birthweight: A Multilevel Perspective.” American Journal of Public Health 91 (11): 1815–1824. doi:10.2105/AJPH.91.11.1815.
- Reichman, N. E., and E. M. Hade. 2001. “Validation of Birth Certificate Data a Study of Women in New Jersey’s HealthStart Program.” Annals of Epidemiology 11 (3): 186–193. doi:10.1016/S1047-2797(00)00209-X.
- Reichman, N. E., and O. Schwartz-Soicher. 2007. “Accuracy of Birth Certificate Data by Risk Factors and Outcomes: Analysis of Data from New Jersey.” American Journal Of Obstetrics And Gynecology 197 (1): 32.e1–e8. doi:10.1016/j.ajog.2007.02.026.
- Ren, X. 2013. “Investigating the association of preterm birth and residential stability in Georgia.” Master diss., Emory University.
- Ritz, B., F. Yu, G. Chapa, and S. Fruin. 2000. “Effect of Air Pollution on Preterm Birth among Children Born in Southern California between 1989 and 1993.” Epidemiology 11: 502–511. doi:10.1097/00001648-200009000-00004.
- Riva, M., P. Apparicio, L. Gauvin, and J.-M. Brodeur. 2008. “Establishing the Soundness of Administrative Spatial Units for Operationalising the Active Living Potential of Residential Environments: An Exemplar for Designing Optimal Zones.” International Journal of Health Geographics 7: 43–13. doi:10.1186/1476-072X-7-43.
- Roberts, E. M. 1997. “Neighborhood Social Environments and the Distribution of Low Birthweight in Chicago.” American Journal of Public Health 87: 597–603. doi:10.2105/AJPH.87.4.597.
- Robinson, W. S. 1950. “Ecological Correlations and the Behavior of Individuals.” American Sociological Review 15: 351–357. doi:10.2307/2087176.
- Sanagou, M., R. Wolfe, A. Forbes, and C. M. Reid. 2012. “Hospital-Level Associations with 30-Day Patient Mortality after Cardiac Surgery: A Tutorial on the Application and Interpretation of Marginal and Multilevel Logistic Regression.” BMC Medical Research Methodology 12: 28–28. doi:10.1186/1471-2288-12-28.
- Shi, W. 2010. Principles of Modeling Uncertainties in Spatial Data and Analyses. Boca Raton, FL: CRC Press/Taylor & Francis. http://www.crcnetbase.com/isbn/978-1-4200-5928-1
- Shiono, P. H., and M. A. Klebanoff. 1993. “A Review of Risk Scoring for Preterm Birth.” Clinics in Perinatology 20 (1): 107–125.
- Snijders, T. A. B., and R. J. Bosker. 2012. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. 2nd ed. Los Angeles, CA: SAGE. Bibliographies.
- US Census Bureau. 2010. “Geographic Terms and Concepts.” Accessed December 19. http://www.census.gov/geo/reference/gtc/gtc_ct.html
- US Census Bureau. 2013. “American Factfinder.” Accessed December 19. http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml
- US Centers for Disease Control and Prevention. 2013. “Preterm Birth.” Accessed December 19. http://www.cdc.gov/reproductivehealth/MaternalInfantHealth/PretermBirth.htm
- Wang, F. H., D. S. Guo, and S. McLafferty. 2012. “Constructing Geographic Areas for Cancer Data Analysis: A Case Study on Late-Stage Breast Cancer Risk in Illinois.” Applied Geography 35 (1–2): 1–11. doi:10.1016/j.apgeog.2012.04.005.