ABSTRACT
In the past several decades, epidemic modelling for communicable diseases has experienced transitions from treating the entire study area as a whole to addressing spatial variation within the area, and from targeting the entire population to incorporating characteristics of categorized subpopulations and finally going down to the individual level. These transitions have been first driven by the recognition that generalizations of space and population in conventional epidemic modelling may have hampered the effectiveness of the modelling; they then have been supported by increasingly available data that allow depiction of detailed spatiotemporal characteristics of an epidemic, as well as those characteristics of the environment in both human and natural aspects; and finally they have been facilitated by developments in geographic information science, data science, computer science, and computing technologies. Based on a review of a variety of recently developed communicable disease models, we explicitly put forward the notions of spatialization and individualization in this area, and point out that the integration of the two is the future of communicable disease modelling. We also point out that in this area models based on the object conceptualization are good at modelling spatiotemporal process, whereas models based on the field conceptualization are good at representing spatialized information. We propose a procedural framework of epidemic modelling that implements the integration of individualization and spatialization, integration of object-based process and field-based representation, and integration of modelling that retrospectively traces infection relationships based on historical patient data and modelling that prospectively predicts such relationships of future epidemics.
Introduction
Human communicable diseases, transmitted among people through direct contact or media (e.g. vectors), are serious threats to public health and cause great public concerns (World Health Organization Citation2019). Modelling the spatiotemporal dynamics of epidemics is important to preventing and intervening epidemics of communicable diseases. Conventional epidemic models are non-spatial and population-oriented, i.e. they treat the entire study area as a whole and characterize the epidemic by the population-level measurements. In the past two decades, spatialization and individualization became trends in epidemic modelling. Here, spatialization means characterizing and representing spatial variation in modelling, rather than using a single value to represent the overall situation of the entire area (Yang, Shoff, and Noah Citation2013; Souris Citation2019). For communicable disease modelling, spatialization is implemented through mapping the disease, i.e. depicting local characteristics of the transmission process or epidemic state. The information obtained through spatialization supports location-targeted and thereby possibly leads to more efficient and effective disease preventions and interventions, and is also helpful in understanding and interpreting patterns in disease spread and in detecting disease-environment associations. Likewise, individualization means atomizing the population under modelling, aiming to achieve treating each individual as a unique subject in the modelling (Parmet Citation2009; Stroeken Citation2017). An individual has her/his own properties, such as health state, age, gender, occupation, address, and behaviour. The disease dispersion is caused by interactions among individuals and is represented by the change of health state of the individual.
The trends of spatialization and individualization have been first driven by the recognition that generalizations in conventional epidemic modelling may have hampered the effectiveness of the model. Particularly, more than often a study area, e.g. a city, may feature considerable spatial variation in both human and natural aspects, leading to highly different local characteristics in the disease transmission. A non-spatial model treating the entire area as a whole ignores those local characteristics and may result in an overall model that cannot well fit any particular region in the study area and thus is less informative in prevention/intervention planning and policymaking. Similarly, a population-oriented model is inherently limited in incorporating individuals’ characteristics in terms of demographics, socioeconomics, and behaviours, but those characteristics may play a significant role in the person-to-person transmission and in turn the general spatiotemporal pattern of the epidemic.
Spatialization and individualization in epidemic modelling have meanwhile been supported by the increasing availability of detailed data of patients, population, pathogen, media, and environment. As communicable diseases have emerged or re-emerged under contemporary global change, globalization, and urbanization, and as they imposed serious and sometimes acute economical and societal burdens at the local, regional, national, and even global scales, exemplified by the recent COVID-19 pandemic, governments at different levels and other institutions have started to respond to the challenge. A major effort in such responses is the construction of data infrastructure, which aims to support informed decision-making in preventions and interventions. Government-sponsored data collection mechanisms and systems have been established in many countries and continue expanding and improving (World Health Organization Citation2006; Citation2016). Resources allocated to hospital/clinic-based reporting schemes and field epidemiological investigations have been rapidly growing (Fatema et al. Citation2016). New portable monitoring devices and ‘big’ data about people’s movements make the data collection easier (Gonzalez, Hidalgo, and Barabasi Citation2008; Fiosina, Fiosins, and Müller Citation2013; Blondel, Decuyper, and Krings Citation2015). As an outcome, detailed and comprehensive data about a communicable disease epidemic in an area become increasingly common, supplying the accuracy and precision of information that were not available before but are required by the spatialization and individualization in epidemic modelling.
Driven by the recognition of importance, and supported by the data with unprecedented quality, new concepts, theories, and methodologies have been constantly developed to achieve spatialization and individualization in epidemic modelling (Bian Citation2013). These concepts, theories, and methodologies intend to take advantage of the quality of new data and the development in geographic information science, data science, computer science, and computing technologies. A highlight in this kind of methodological development is the shift towards non-parametrical local approaches and empirical simulations/inductive procedures that are based on intensive computation, from traditional parametrical global approaches and analytical/deductive procedures that frequently rely on mathematical/statistical assumptions.
Bian (Citation2013) provides an excellent review of recent development in spatiotemporal modelling of communicable diseases, which we will briefly summarize in next section as a basis for our discussion in the rest of this paper. In the discussion, we point out that the integration of spatialization and individualization is the future of communicable disease modelling. We propose a procedural framework that implements the integration based on individual-level data. This framework puts the people-oriented model based on the object conceptualization and the location-oriented model based on the field conceptualization into order, aiming to achieve a procedure from retrospectively tracing infection relationships based on historical patient data to prospectively predicting spatiotemporal patterns of future epidemics.
A brief review of recent development in communicable diseases modelling
Traditional epidemic modelling usually focuses on the temporal dimension and uses global measurements to represent the general situation of the entire study area, without considering the spatial variation within the area. The generic SIR (Susceptible-Infectious-Recovered) model divides the population in an area into three subpopulations, namely the susceptible (S), the infectious (I), and the recovered (R) (Kermack and McKendrick Citation1927). Which subpopulation an individual belongs to changes as her/his health status changes, and the model is eventually for deriving number of people in each subpopulation at different time points. The model is typically represented as a set of differential equations, with parameters to be estimated with historical epidemic data or a priori knowledge. Based on the size of each subpopulation and the estimated parameter values, further measurement like the cases-reproduction ratio, Rt, defined as the average number of people infected by a new case emerging during period t, can be calculated to represent the state of the epidemic, e.g. Rt > 1 indicates that the epidemic is in its growing state (Dietz Citation1993). Based on the generic SIR model, more complex models have been developed by incorporating more factors to make the situation more realistic or suit properties of specific diseases. However, all these traditional models share the assumptions that the spatial variation in the study area has nothing to do with the transmission (spatial homogeneity) and all individuals in a subpopulation are the same (population homogeneity).
In the past several decades, spatialization and individualization became trends in epidemic modelling. Motivated by practical demands, many efforts have been made in those two aspects. Some scholars in epidemiology have listed spatialization as one of the five major challenges in epidemic modelling (Riley et al. Citation2015). Bian (Citation2013) provided a comprehensive review to this change. Bian (Citation2013) classified the models that deviate from the traditional non-spatial and population-oriented framework into six categories, including the population-based wave model (Cliff, Haggett, and Ord Citation1986; Rhodes and Anderson Citation1997; Li, Li, and Hethcote Citation2009; Nie and Li Citation2020), the subpopulation model (Szymanski and Caraco Citation1994; Johst, Brandl, and Eber Citation2002; Keeling and Rohani Citation2011; Zhu et al. Citation2020), the individual-based cellular automata model (Holmes, Tilman, and Kareiva Citation1997; Sirakoulis, Karafyllidis, and Thanailakis Citation2000; Li and Guo Citation2012), the mobile subpopulation model (Smallmanâ Raynor and Cliff Citation2001), the individual-based spatial implicit model (Ghani, Swinton, and Garnett Citation1997a; Grimm Citation1999; Koopman and Lynch Citation1999; Riley Citation2007; Emch et al. Citation2012), and the individual-based mobile model (Bian Citation2004; Eubank et al. Citation2004; Bian and Liebner Citation2007; Mao and Bian Citation2011; Bian et al. Citation2012; Janko and Emch Citation2016). Bian (Citation2013) attributed the former three to the field conceptualization in GIS, i.e. their modelling unit or primary concern is the location, and the people and their health states are considered as properties of the location. Apparently, the finer the spatial resolution, the more expressive the model is for representing spatial variation. Also, the finer the spatial resolution, the smaller size of population within a defined location, until a location contains only one individual. Interactions (transmissions) may occur among people within a defined location and between people at different locations, and different models have different ways to implement these intra- and inter-location processes. Theoretically, such models can adopt either vector or raster data models. However, increasingly detailed segmentation of the study area naturally leads to the adoption of the raster data model. The outputs of these models are typically raster layers with cells holding the information about the health state of the population within them at a given time point. While such a representation precisely depicts the spatial variation of the epidemic in the study area, an inherent difficulty of this kind of model lies in its awkwardness trying to model dynamics resulting from human mobility based on immobile locations.
The latter three models reviewed by Bian (Citation2013), including the mobile subpopulation model, the individual-based spatial implicit model, and the individual-based mobile model, at the conceptual level belong to the object model in GIS. They explicitly target people and treat the location as a property of people. The modelling unit in these models is the subpopulation, which is conceptualized as an object. A subpopulation is typically defined by a particular health state or other attributes. When the modelling unit is people rather than a location (a piece of geographic area), the unit becomes movable and this is why Bian (Citation2013) used mobility to label the three models based on the object conceptualization. The spatial nature of a subpopulation is represented and characterized by its location and mobility. Furthermore, the subpopulations are not fixed but can be merged or further divided due to the continuous changes of their health states through interactions. The reorganization and decomposition of the subpopulations represent the transmission process of the epidemic. As the division of population incorporates more information and gets more specific, the spatial information about the subpopulation tends to get more detailed, and this process will finally go down to the individual level, which is indeed the situation in the last two individual-based models in this category (the individual-based spatial implicit model and the individual-based mobile model). Particularly, the individual-based mobile model (Bian et al. Citation2012), which employs the agent-based modelling (ABM) approach, creates simulated individuals in a study area, each with certain attributes and behaviours, assigned to the individual according to the characteristics of the demographic and socioeconomic categories the individual belongs to. The model then let all simulated individuals live their daily lives, moving around following their assumed routine travel trajectories, mainly about how much time they spend at home, workplace, shopping places, and others. An individual’s infection state may change during her/his daily activities through contacts with others. The model can prospectively estimate numbers of people in the S, I, and R compartments at a given time point.
Based on the Bian (Citation2013) review, we summarize features of models based on the field conceptualization and models based on the object conceptualizations in epidemic modelling in .
Table 1. A summary of features of two kinds of models in spatiotemporal modelling of communicable diseases.
Indeed, during those efforts to spatialized and individualized models, challenges have been encountered and have been tackled. Our understanding is that those challenges are mainly due to the fact that the traditional epidemic models, typically based on SIR, are inherently incompatible with spatialization and individualization, as they are designed to target the population in an area. Many ‘spatialized’ epidemic models simply apply the model to different areas in a separate way and then combine the results into a collage, which does not involve the essence of spatial modelling such as scale, spatial autocorrelation, and spatial dependence. There are works that employ the concepts and methodologies of network analysis, intending to model interactions among different areas during an epidemic. We consider this latter approach promising, and we would like to push it even further to have the network to be about individual-human subjects rather than geographic areas. Not only this individual-human network approach pushes the spatialization to the extreme (as nothing can be more spatialized than individual’s locations in an epidemic modelling), but it also addresses another big challenging issue in traditional epidemic modelling: a population-oriented model is very limited in taking advantage of the rich information in the individual-level data that are increasingly available today.
It is worth to reemphasize that here, spatialization and individualization are not just about specific techniques in the modelling process, or necessary procedures of data pre-processing before modelling. That is to say, they are not just at the technical level; rather, they are more at the conceptual level regarding the nature and core concepts of the modelling. Moreover, the spatialization is also not just for spatializing the individual information, although this is a natural requirement and demand when the information goes down to the individual level. At the conceptual level, spatialization is for characterizing the spatial variation of an epidemic, through measurement like local Rt.
Integration of spatialization and individualization in epidemic modelling
As discussed earlier, there have been works that apply modelling to different parts of the study area separately, and some even model the interaction among those different parts through network analysis, based on aggregate patient and population data. On the other hand, there are works that analyse individual-level attribute information (e.g. age, sex, race, career, and socioeconomic status) without considering locations. Also, there are techniques to extract individual-level information from aggregate data, through the process of disaggregation. For example, we have developed a Monte Carlo process to extract individual activity trajectories from aggregate population movement data. As the spatialization and individualization keep evolving, inevitably they are going to merge, and the modelling becomes a process to couple or even integrate the two so as to be coherent and optimal. This is not only because practically such an integration is most ideal for accurately and precisely modelling epidemics but is also because methodologically the two trends will inherently move towards each other and thereby such an integration is inevitable if the trends continue. On the spatialization side, dividing the study area into small pieces is also a process dividing the population in the area into subpopulations. A higher degree of spatialization usually means a higher spatial resolution of the information representation, i.e. smaller resulting area divisions, which also means smaller subpopulation in the area division, until the subpopulation becomes an individual. On the individualization side, it is eventually a process of obtaining and utilizing increasingly accurate and precise information about the people under concern, including these people’s spatial information, until the information reaches its most accurate and precise level when it is about each individual.
The review in the previous section leads to the understanding that, in terms of achieving an integration of spatialization and individualization in epidemic modelling, the models based on the field conceptualization and the models based on the object conceptualization have their respective advantages and disadvantages, but neither is generally optimal.
On the one hand, spatialization is about the location, which is what conceptualized by the field model, and naturally leads to the adoption of raster data model at the technical level. The raster model uses small and regular cells to represent the local situation (property of a small piece of geographic area), not only in a precise way when the cell size is small but also making situations at different locations more comparable, thanks to the regularity of the cell. The modelling process, e.g. solving differential equations, can be applied to the data of a raster cell, and can also be implemented through defining and creating interactions among different cells. However, both ways would encounter serious difficulties. First, as the spatial resolution increases (i.e. the cell gets smaller), the people within a cell become fewer and therefore harder to support meaningful epidemic modelling; second, as the cell is immobile, by nature it is hard for this data model represent a process that is basically a result of people’s interactions through movements. For example, the individual-based cellular automata models can only accommodate interactions between adjacent cells, obviously not appropriate for representing transmissions caused by people’s long-distance travels, but such transmissions are common in contemporary epidemics; and third, in a model based on the field conceptualization, the people within a cell are only determined by the spatial definition of the cell, without considering the attributes of the people that are highly relevant to the epidemic process, and even if the researcher has an intention to attach those attributes to the cell, such data at the cell level would be hardly available or difficult to generate.
On the other hand, the epidemic models in the object model category, while by nature are good at modelling a spatiotemporal process resulted from people’s movements and interactions, are limited in the aspect of spatialized representation. If the model is at the population level, the spatial representation is usually based on subjectively defined polygons, almost always suffering a low resolution and the modifiable areal unit problem (MAUP). If the model is at the individual level, the immediate output from the modelling is usually points, representing individuals with different health states at different locations. The point representation can serve the purpose of visualization, but it cannot be directly used for quantitatively characterizing and evaluating overall spatial variation of the epidemic and specific situation at each and every location.
If we recognize that models can be used for modelling process and/or for representing information, then according to above discussion, the models based on the field conceptualization are effective for spatialized representation of the epidemic state, but would encounter a dead end for spatialized modelling of the epidemic process. On the other hand, the models based on the object conceptualization, especially those directly modelling individual behaviours, enable the maximum flexibility and thus possibility to realistically model the transmission process. Moreover, the increasingly available detailed data about individuals have been and will continue to be supporting and encouraging such individual-level modelling. However, the immediate output of this kind of modelling, either represented by polygons or points, is not as effective as the raster representation, in terms of quantitatively evaluating spatial variation.
Therefore, a natural speculation is that an optimal approach to achieving the integration of spatialization and individualization is incorporating both types of models. This idea has been implemented by the recently proposed Epidemic Forest model (Li et al. Citation2019). The Epidemic Forest model treats each patient as an object, and intends to build the infection relationship between individual patients. It organizes such relationships into the tree structure. A node on a tree represents a patient, and the edge between two nodes represents an immediate infection relationship between the two patients. The infection relationship can be constructed deterministically or stochastically by evaluating the contact strength between patients. The contact strength can be evaluated based on the patients’ individual information, such as age, career, residential location, and moving trajectories during a specific period. Each tree represents an epidemic process started by a primary case, e.g. an imported dengue fever case in an area where dengue fever is not endemic. Multiple trees form a forest, which represents the entire epidemic. Once an Epidemic Forest is constructed, a process that is essentially the kernel density estimation (KDE) can be used to calculate the case reproduction ratio (Rt) for each and every location in the study area, using the information that can be extracted directly from the constructed trees (Li et al. Citation2019). The output of this calculation is a series of raster layers, each showing the spatial variation of Rt in the study area at a given time point. In this way, not only can the Epidemic Forest model take full advantage of the detailed individual-level disease data for modelling the transmission process, but it can also represent and present high-resolution information about spatiotemporal characteristics of the transmission as the result of the modelling. Basically, the modelling process of the Epidemic Forest model is mostly object-based and works on points, whereas the representation of the modelling results is on the field, which is implemented by the raster data model.
Analytical framework for individual datasets
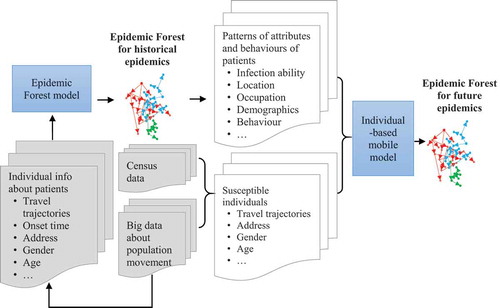
The Epidemic Forest model currently uses data of historical epidemics to retrospectively trace the infection relationship between known patients. Its aim is to derive, from data of previous epidemics, spatiotemporal patterns of the transmission process and relationships between the disease and the environment, so as to provide empirical support for predicting future epidemics. However, by itself, its retrospective modelling cannot directly make prediction. Another individualized model, the individual-based mobile model (Bian et al. Citation2012), which is reviewed earlier in this paper, works on each and every individual in the population, and intends to predict the occurrence of transmission between individuals. However, so far the prediction from this model is solely based on the assumed daily routines of the subpopulation the individual belongs to, not explicitly supported by empirical patient information.
Apparently, the retrospective and prospective approaches can be integrated into a more robust process. Here, we propose an analytical procedure that integrates the two, which eventually also integrates spatialization and individualization, as well as object-based process and field-based representation. As illustrated by , for a particular communicable disease in a study area, the procedure starts with the Epidemic Forest as a retrospective model to trace and spatialize the transmission relationship between individual patients, and then feed the output of the Epidemic Forest modelling to the individual-based mobile model to support its prediction of spatiotemporal dynamics of future epidemics. Specifically, we first construct epidemic trees of the historical epidemics of a disease in the study area, taking full advantage of the historical information about individual patients, including onset time, residential location, daily movement trajectories for a certain period, and demographic and occupational attributes. From the constructed epidemic forest, we can derive patterns of the epidemic of this disease, especially about individuals’ attributes and behaviours. We will use such information to support specification of the parameter values for the individual-based mobile model. For example, using the information from the retrospective modelling, we can specify that individuals with certain occupations are more susceptible than others; infections are more likely to occur in certain areas during certain periods of a day; and a parent case would usually infect a certain number of child cases at a certain stage of the epidemic. We then run the individual-based mobile model to predict about individuals with different health states (e.g. susceptible, infectious, and recovered) at different time points. Finally, we can use the KDE approach calculate the spatialized (rasterized) case reproduction ratio (Rt) directly from the infections relationships constructed by the individual-based mobile model between predicted patients.
Concluding remarks
Individualization and spatialization have been the trends in epidemic modelling, and the integration of the two is the future of such modelling. Models based on the object conceptualization are good at modelling the process that incorporates the integration of the two, whereas models based on the field conceptualization are good at representing the output of the process. Currently available process models that handle individual-level data can be recognized as retrospective and predictive. We propose a nonparametric analytical procedure that integrates all these notions, starting from retrospectively modelling individual-level transmissions, to using the information derived from the retrospective modelling to support individual-level predictive modelling based on the ABM approach, and to finally calculating local Rt to produce a quantitative, high-resolution, and continuous representation of a spatialized and individualized modelling of the communicable disease.
The access to individual-level data is indeed a big challenge to the non-parametric approach we are promoting. The challenge is not that the data do not exist – they do exist – but how to access them, as evidenced in current COVID-19 pandemic. From our own experience, we see that this spatialized and individualized non-parametric approach has shown great potential in battling the COVID-19 pandemic, especially in city-level control and intervention action planning and policymaking. Thus, the struggle with and uncertainty in the issue of accessing data demand immediate attention from lawmakers, government agencies, and researchers. Hopefully, with the development of regulations, data infrastructures, and technologies (e.g. the techniques of geomasking and disaggregation), we will find a reasonable way to get around or mitigate this problem. All in all, when we state that ‘spatialization’ and ‘individualization’ are the future of epidemic modelling, the point we are trying to make is threefold: 1) we want to explicitly and clearly define these two concepts and notions within the context of epidemic modelling and predict that they will flourish in this area (but we are not implying that they did not exist in this area); 2) we particularly point out that the integration of spatialization and individualization is the future of epidemic modelling; in other words, we consider that the two will evolve in this area in a coupling rather than separate way, as we see that this coupling is natural and inevitable; 3) we promote a non-parametric approach that is alternative to the traditional parametric approach to accommodate and facilitate the integration of spatialization and individualization in epidemic modelling to overcome the limitation of the traditional approach. We consider that this main point we intend to make is a novel contribution of this position paper to the area of epidemic modelling.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bian, L. 2004. “A Conceptual Framework for an Individual-based Spatially Explicit Epidemiological Model.” Environment and Planning. B, Planning & Design 31 (3): 381–395. doi:10.1068/b2833.
- Bian, L. 2013. “Spatial Approaches to Modeling Dispersion of Communicable Diseases: A Review.” Transactions in GIS 17 (1): 1–17. doi:10.1111/j.1467-9671.2012.01329.x.
- Bian, L., Y. Huang, L. Mao, E. Lim, G. Lee, Y. Yang, M. Cohen, and D. Wilson. 2012. “Modeling Individual Vulnerability to Communicable Diseases: A Framework and Design.” Annals of the Association of American Geographers 102 (5): 1016–1025. doi:10.1080/00045608.2012.674844.
- Bian, L., and D. Liebner. 2007. “A Network Model for Dispersion of Communicable Diseases.” Transactions in GIS 11 (2): 155–173. doi:10.1111/j.1467-9671.2007.01039.x.
- Blondel, V. D., A. Decuyper, and G. Krings. 2015. “A Survey of Results on Mobile Phone Datasets Analysis.” EPJ Data Science 4 (1): 10. doi:10.1140/epjds/s13688-015-0046-0.
- Cliff, A. D., P. Haggett, and J. K. Ord. 1986. Spatial Aspects of Influenza Epidemics. Abingdon: Routledge.
- Dietz, K. 1993. “The Estimation of the Basic Reproduction Number for Infectious Diseases.” Statistical Methods in Medical Research 2 (1): 23–41. doi:10.1177/096228029300200103.
- Emch, M., E. D. Root, S. Giebultowicz, M. Ali, C. Perez-Heydrich, and M. Yunus. 2012. “Integration of Spatial and Social Network Analysis in Disease Transmission Studies.” Annals of the Association of American Geographers 102 (5): 1004–1015. doi:10.1080/00045608.2012.671129.
- Eubank, S., H. Guclu, V. S. Anil Kumar, M. V. Marathe, A. Srinivasan, Z. Toroczkai, and N. Wang. 2004. “Modelling Disease Outbreaks in Realistic Urban Social Networks.” Nature 429 (6988): 180. doi:10.1038/nature02541.
- Fatema, K., N. A. Zwar, A. H. Milton, L. Ali, and B. Rahman. 2016. “Prevalence of Risk Factors for Cardiovascular Diseases in Bangladesh: A Systematic Review and Meta-analysis.” PloS One 11 (8): e160180. doi:10.1371/journal.pone.0160180.
- Fiosina, J., M. Fiosins, and J. P. Müller. 2013. “Big Data Processing and Mining for Next Generation Intelligent Transportation Systems.” Jurnal Teknologi 63 (3): 21–38. doi:10.11113/jt.v63.1949.
- Ghani, A. C., J. Swinton, and G. P. Garnett. 1997. “The Role of Sexual Partnership Networks in the Epidemiology of Gonorrhea.” Sexually Transmitted Diseases 24 (1): 45–56. doi:10.1097/00007435-199701000-00009.
- Gonzalez, M. C., C. A. Hidalgo, and A. Barabasi. 2008. “Understanding Individual Human Mobility Patterns.” Nature 453 (7196): 779. doi:10.1038/nature06958.
- Grimm, V. 1999. “Ten Years of Individual-based Modelling in Ecology: What Have We Learned and What Could We Learn in the Future?” Ecological Modelling 115 (2–3): 129–148. doi:10.1016/S0304-3800(98)00188-4.
- Holmes, E. E.. 1997. “Basic Epidemiological Concepts in a Spatial Context.” In Spatial Ecology: The Role of Space in Population Dynamics and Interspecific Interactions (MPB-30), edited by T. DAVID and KAREIVA PETER, 111-36. PRINCETON, NEW JERSEY: Princeton University Press, 1997. Accessed May 18, 2020. doi:10.2307/j.ctv36zpzm.11.
- Janko, M., and M. Emch. 2016. “The Geography of Malaria Control in the Democratic Republic of Congo.” In Population Health Intervention Research, 109–122. Abingdon: Routledge.
- Johst, K., R. Brandl, and S. Eber. 2002. “Metapopulation Persistence in Dynamic Landscapes: The Role of Dispersal Distance.” OIKOS 98 (2): 263–270. doi:10.1034/j.1600-0706.2002.980208.x.
- Keeling, M. J., and P. Rohani. 2011. Modeling Infectious Diseases in Humans and Animals. Princeton, New Jersey: Princeton University Press.
- Kermack, W. O., and A. G. McKendrick. 1927. “A Contribution to the Mathematical Theory of Epidemics.” Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences 115 (772): 700–721.
- Koopman, J. S., and J. W. Lynch. 1999. “Individual Causal Models and Population System Models in Epidemiology.” American Journal of Public Health 89 (8): 1170–1174. doi:10.2105/AJPH.89.8.1170.
- Li, M., X. Shi, X. Li, W. Ma, J. He, and T. Liu. 2019. “Epidemic Forest: A Spatiotemporal Model for Communicable Diseases.” Annals of the American Association of Geographers 109 (3): 812–836. doi:10.1080/24694452.2018.1511413.
- Li, T., Y. Li, and W. H. Hethcote. 2009. “Periodic Traveling Waves in SIRS Endemic Models.” Mathematical and Computer Modelling 49 (1–2): 393–401. doi:10.1016/j.mcm.2008.07.033.
- Li, W. D., and X. H. Guo. 2012. “Using Cellular Automata to Study the Effect of Competition for Epidemic Diseases.” Procedia Environmental Sciences 13: 1010–1018. doi:10.1016/j.proenv.2012.01.094.
- Mao, L., and L. Bian. 2011. “Agent-based Simulation for a Dual-diffusion Process of Influenza and Human Preventive Behavior.” International Journal of Geographical Information Science 25 (9): 1371–1388. doi:10.1080/13658816.2011.556121.
- Nie, S., and W. Li. 2020. “Using Lattice SIS Epidemiological Model with Clustered Treatment to Investigate Epidemic Control.” Biosystems 191: 104119. doi:10.1016/j.biosystems.2020.104119.
- Parmet, W. E. 2009. “Dangerous Perspectives: The Perils of Individualizing Public Health Problems.” The Journal of Legal Medicine 30 (1): 83–108. doi:10.1080/01947640802694593.
- Rhodes, C. J., and R. M. Anderson. 1997. “Epidemic Thresholds and Vaccination in a Lattice Model of Disease Spread.” Theoretical Population Biology 52 (2): 101–118. doi:10.1006/tpbi.1997.1323.
- Riley, S. 2007. “Large-scale Spatial-transmission Models of Infectious Disease.” Science 316 (5829): 1298–1301. doi:10.1126/science.1134695.
- Riley, S., K. Eames, V. Isham, D. Mollison, and P. Trapman. 2015. “Five Challenges for Spatial Epidemic Models”. Epidemics 10: 68–71. doi:10.1016/j.epidem.2014.07.001.
- Sirakoulis, G. C., I. Karafyllidis, and A. Thanailakis. 2000. “A Cellular Automaton Model for the Effects of Population Movement and Vaccination on Epidemic Propagation.” Ecological Modelling 133 (3): 209–223. doi:10.1016/S0304-3800(00)00294-5.
- Smallmanâ Raynor, M., and A. D. Cliff. 2001. “Epidemic Diffusion Processes in a System of US Military Camps: Transfer Diffusion and the Spread of Typhoid Fever in the Spanish-American War, 1898.” Annals of the Association of American Geographers 91 (1): 71–91. doi:10.1111/0004-5608.00234.
- Souris, M. 2019. Epidemiology and Geography: Principles, Methods and Tools of Spatial Analysis. Hoboken, NJ: John Wiley & Sons.
- Stroeken, K. 2017. “The Individualization of Illness: Bewitchment and the Mental in Postcolonial Tanzania.„ In African Medical Pluralism, edited by Olsen William C. and Sargent Carolyn, 151-69. Bloomington, Indianapolis: Indiana University Press, 2017. Accessed May 18, 2020. www.jstor.org/stable/j.ctt1zxz1b8.10
- Szymanski, B., and T. Caraco. 1994. “Spatial Analysis of Vector-borne Disease: A Four-species Model.” Evolutionary Ecology 8 (3): 299–314. doi:10.1007/BF01238280.
- World Health Organization. 2006. “World Health Organization. Weekly Epidemiological Record (WER).” [online]. Accessed 26 December 2019. https://www.who.int/wer/en/
- World Health Organization. 2016. “World Health Organization. Global Health Observatory Data Repository.” [online]. Accessed 26 December 2019. http://apps.who.int/gho/data/?theme=main
- World Health Organization. 2019. “World Health Organization. Communicable Diseases.” [online]. Accessed 26 December 2019. https://www.afro.who.int/health-topics/communicable-diseases
- Yang, T., C. Shoff, and A. J. Noah. 2013. “Spatializing Health Research: What We Know and Where We are Heading.” Geospatial Health 7 (2): 161. doi:10.4081/gh.2013.77.
- Zhu, X., A. Zhang, S. Xu, P. Jia, X. Tan, J. Tian, T. Wei, Z. Quan, and J. Yu. 2020. Spatially Explicit Modeling of 2019-nCoV Epidemic Trend Based on Mobile Phone Data in Mainland China. medRxiv.