 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study investigated childhood cancer disparities in the state of Texas based on data from 1995 to 2014 from the perspective of race/ethnicity, geographic location, and social factors. The enhanced 2-step floating catchment area (E2SFCA) method was used to measure relative spatial access to Children’s Oncology Group (COG) hospitals. This study also examined the effect of spatial access to specialized COG services along with other variables in explaining the variations of late-stage diagnosis of childhood cancer. Multilevel logistic regression was used to analyse how individual- and contextual-level factors affect the occurrence of childhood cancer disparities (i.e. late-stage diagnosis). The study revealed that Hispanic children were more likely to be diagnosed at a late-stage, after adjusting for age, race/ethnicity, socioeconomic status (SES), socio-culture, education, spatial access to COG hospitals, percent African American, and health insurance coverage. The study also identified that the childhood cancer stage at diagnosis is associated with spatial access to COG services as well as levels of urbanization. Moreover, findings indicate that contextual-level factors, such as SES, socio-cultural factors, education level, and percent health insurance coverage partially explained some of the childhood cancer disparities. Results from this study will contribute to developing more effective and targeted childhood cancer intervention programs in socially underprivileged areas, focusing on population with lower socioeconomic status and lower education levels, limited English-speaking households, areas with a higher percentage of Hispanics and African Americans, locations with a low level of spatial access to COG services.
Introduction
In the United States, cancer is the second leading cause of death and is a major public health concern. In 2021, it was estimated that 1,898,160 new cases will be diagnosed, which is equivalent to around 5,200 new cases each day (Siegel et al. Citation2021). Although cancer is not the most common disease in children, it accounts for considerable mortality, shortened lifespan, and decreased quality of life in children. The survival rate and quality of life have improved for children with cancer in the last few decades, in part because approximately 70% of paediatric cancer patients successfully enrol in clinical trials (Bleyer et al. Citation1997; Lund et al. Citation2009). However, racial/ethnic and income-related disparities in both diagnosis and access to treatment remain.
The principal goal in oncology is the early diagnosis of cancer since it permits an opportunity for timely treatment (Dang-Tan and Franco Citation2007). The short latency period is an important characteristic of childhood cancers compared with adult cancers, and most often these cancers grow rapidly (Dang-Tan and Franco Citation2007), (Whitworth, Symanski, and Coker (Citation1995-2004). Early stage childhood cancer diagnosis can positively affect prognosis(Abdelkhalek et al. Citation2014) and survival, which decreases the chance of morbidity (Araz and Guler Citation2015). Three main factors can be attributed to a delay in diagnosis of childhood cancer, including patient and/or parent-related factors (Abdelkhalek et al. Citation2014; Dang-Tan, Trottier, and Mery et al. Citation2008; Haimi Citation2004; Thulesius, Pola, and Hakansson Citation2000), healthcare facilities (Dang-Tan, Trottier, and Mery et al. Citation2008; Haimi Citation2004; Brown et al. Citation2009; Dang-Tan, Trottier, and Mery et al. Citation2010) and factor related to the diseases. (Dang-Tan and Franco Citation2007)
Individual-level variables include race/ethnicity, age at diagnosis, and stage at diagnosis. Contextual-level factors are defined by available resources and priorities (Stewart and Wild Citation2014). In a healthcare system, resource means specialized care by qualified personnel, technology that supports the management of health-related information, diagnostic services and drugs, therapeutic equipment, and facilities which include access to transportation and health care services and financial resources (Stewart and Wild Citation2014). In addition, spatial access to Children’s Oncology Group (COG) hospitals could play an important role in the outcome of childhood cancer patients. This contradicts results for adult cancer outcomes based on the spatial access factor. Two previous studies did not identify a significant association between spatial access to healthcare services and cervical cancer stage at diagnosis (Brewer et al. Citation2012; Zhan and Lin Citation2014). Some other similar studies suggested that spatial access to healthcare services was not a consistent predictor of racial/ethnic disparities in cervical cancer(Lin and Zhan Citation2014) and breast cancer(Tian et al. Citation2012) mortality. However, other studies revealed statistically significant disparities of colorectal cancer late-stage diagnosis and their association with spatial accessibility to PCPs (Wan et al. Citation2013).
Extensive research has been conducted for racial/ethnic and SES disparities in cancer (Lund et al. Citation2009; Lemstra, Neudorf, and Opondo Citation2006; Byers Citation2010; Krieger Citation2005; Lin, Schootman, and Zhan Citation2015; Murphy, Tseng, and Shah Citation2010; Read, Emerson, and Context Citation2005; Ward, Jemal, and Cokkinides et al. Citation2004). Racial disparities for African-Americans and Hispanic women were prominent in breast cancer mortality compared to non-Hispanics White women based on a rate difference measurement approach (Tian et al. Citation2010). An Egyptian study found a significant association between SES and delayed childhood cancer diagnosis(Abdelkhalek et al. Citation2014), whereas a study in northern Israel did not find a correlation between SES and delay in diagnosis (Haimi Citation2004).
However, there are remaining knowledge gaps about childhood cancer disparities in Texas. First, no study has examined the impact of both individual- and contextual-level factors on racial/ethnic and SES disparities in late-stage diagnosis of childhood cancer in Texas. Second, there has been no reported study that investigated how spatial access to specialized Children’s Oncology Group (COG) hospitals would affect childhood cancer disparities using geographic data at a fine scale such as census tract level data.
Population health and health disparities have been discussed in terms of three primary levels of determinants: distal, intermediate, and proximal (Gehlert and Colditz Citation2011). This study incorporated the childhood cancer stage at diagnosis (early-stage and late-stage) as distal determinants. Intermediate determinants are described as social interactions and the physical context of a neighbourhood or community (Gehlert and Colditz Citation2011). In a built-up environment, spatial accessibility to healthcare residential segregation based on race/ethnicity and socioeconomic status (SES), and their opportunities for social interactions fall into the bigger picture of intermediate determinants. In the United States, the world’s childhood cancer experts at COG member institutions cared for more than 90% of the children and adolescents diagnosed with cancer (Children’s Oncology Group: The World’s childhood cancer experts Citation2021). Spatial accessibility to these specialized cancer institutions significantly affects childhood cancer stage at diagnosis.
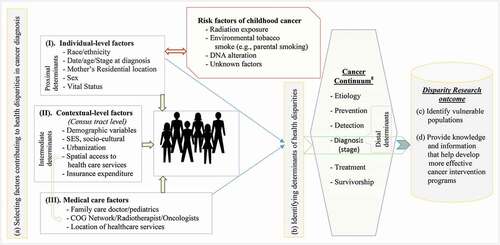
Based on the definitions described above, this study proposes a conceptual model for population-based health disparity research for cancer patients from multiple perspectives () Morshed, Haskard-Zolnierek, and Zhan Citation2020). In the conceptual model, distal effects represent the experience at the community level resulting from contextual variables, variables associated with census tracts are intermediate determinants, and individual-level factors of the cancer patients are treated as proximal determinants. The analysis procedure based on this conceptual framework contains four major steps. First, researchers selected factors contributing to childhood cancer health disparities. Second, we identify determinants of childhood cancer health disparities attributed to late-stage diagnosis. Third, identify vulnerable population groups based on racial/ethnic background. In the fourth and final steps, researchers provides important knowledge and information to develop intervention programmes in targeted areas aimed at eliminating health disparities from the perspective of cancer control continuum(Dang-Tan, Trottier, and Mery et al. Citation2008; Cancer Control Continuum Citation2021) in children.
Figure 1. Conceptual framework of the current childhood cancer late-stage diagnosis disparities research
There are a number of ways to measure racial/ethnic disparities (Chu, Miller, and Springfield Citation2007; Keppel, Pamuk, and Lynch et al. Citation2005). The most conventional way to quantify the excess cancer burden of racial/ethnic groups is to compare it with a reference group. The same principle applies analyses aim to uncover racial/ethnic disparities of childhood cancer incidence, late-stage diagnosis, and mortality. As stated above, the purpose of this study was to examine disparities in childhood cancer late-stage diagnosis in Texas from the perspective of race/ethnicity, geographic location, and various social factors using data from 2005 to 201433. This study investigated the role of individual-level and contextual-level variables in these disparities. Spatial access to COG hospitals was used as a factor reflecting accessibility to healthcare services in this study.
This study provides additional demonstration as to how GIS-based methodology and spatial analysis techniques can be utilized in health disparity studies. Studying underlying factors in childhood cancer disparities from a geographic perspective will help in identifying geographic areas of interest, where elimination of cancer disparity is required. Findings from this study will contribute to the development of more effective childhood cancer intervention programmes.
Materials and methods
Study population
This study used statewide childhood cancer data from the Texas Cancer Registry (TCR) in the Texas Department of State Health Services (TDSHS). TCR is one of the largest cancer registries in the United States and is Gold Certified by the North American Association of Central Cancer Registries (NAACCR). There were 7,700 childhood cancer incidences in the state of Texas from 2005 to 2014. The dataset was limited to cases of individuals between 0 and 19 years of age (DSHS (Department of State Health Services) Citation2005–2014). The study did not require consent to participate because the project did not involve direct recruitment of or contact with human participants. The Institutional Review Boards (IRBs) of the Texas Department of State Health Services and Texas State University approved the study protocol and use of the data.
Study variables
This study included three mutually exclusive racial groups: Non-Hispanic White, Hispanic and African American because of the small proportion (3.69%) of reported cases for Native Americans, Asians, and other racial groups. Race/ethnicity, age at diagnosis, stage at diagnosis, and tumour grade were listed as individual-level variables. The Surveillance Epidemiology End Result (SEER) program from the National Cancer Institute (NCI) has categorized childhood cancer cases at diagnosis into localized, regional, and distant stages. This categorization approach was used in the current study. The number of cases categorized as in situ and localized, regional, distant, and not applicable or unknown stage was 2,157 (29%), 835 (11%), 3,177 (43%), and 1,232 (17%), respectively. Cases classified as ‘not applicable’ and ‘unknown stage’ were excluded from the study. Based on clinical and pathological information, in situ and the localized stage were categorized as early-stage, whereas the regional and distant stages were characterized as late-stage.
The study used the American Community Survey (ACS) 5-year estimates (2006–2010) for census tracts in the state of Texas. The ACS is a continuing national survey that generates period estimates of demographic, housing, and socioeconomic characteristics of the U.S. population. There are three primary uses of ACS estimates, including (a) understanding the demographic characteristics of an area for local planning purposes, (b) comparing across areas, and (c) measuring change over time. It is recommended to use 5-year estimates for small geographic areas (populations of less than 20,000) because of the strong statistical reliability of the data for small population subgroups. (US Census Bureau Citation2008)
This study also used contextual-level variables, which include census demographics, socio-environmental characteristics (i.e. percent Hispanic and percent African American), spatial accessibility to COG hospitals, and percent health insurance coverage at the census tract level. The study used 11 census tract level demographic variables available in the ACS 2010 dataset: percent below poverty level, percent unemployed, median household income, median home value, percent without a high-school degree, percent without a college degree, percent limited English-speaking household, female household with children under 18 years of age, average family size, percent foreign-born under 18 years of age, and percent household without a car. Socio-environmental variables such as children by age (0–19 years) and race/ethnicity, percent African Americans, and percent Hispanics were extracted from the Census 2010 summary file 1 (SF1). These variables have been used in cancer disparity research in the past, and they fall under the broad umbrella of socio-environmental, socio-cultural, and socio-economic domains.
The level of urbanization at the census tract level was determined using 10 primary RUCA (Rural–Urban Commuting Area) codes based on the 2006–2010 ACS and the 2010 decennial census tracts. The study delineated four major areas at the census tract level to reflect the level of urbanization. The four levels are metropolitan, micropolitan, small town, and rural.
Methodology
Relative spatial accessibility to COG medical services was calculated using an enhanced 2-step floating catchment area (E2SFCA) method (Luo and Qi Citation2009) which is an updated version of the basic gravity-based spatial accessibility model (Hansen Citation1959; Joseph and Bantock Citation1982). The E2SFCA method first calculates the spatial accessibility index (SPAI) for each census tract, and the level of relative spatial accessibility of the entire region is then computed using a ratio of SPAI in each census tract to the average SPAI. The measurement of supply-to-demand ratio, Rj in census tracts of j is determined using Expression (1) below.
Where denotes the health care capacities at location j,
represents the population size of any census tract k,
refers to the subzones of the catchment in terms of time intervals, and
is the impedance weight for
based on the Gaussian function (
).(Luo and Qi Citation2009). The SPAI of each area unit i (
is calculated using Expression (2).
Where denotes the supply-to-demand ratio for any health care service location
inside the catchment and
represents the travel cost between
and l. The study used travel time as the network travel impedance/cost with a focal catchment area (FCA) dimension of 300 minutes. The COG health care service locations represent the service supply point locations and the total population under the age of 19, which represents the service demand in census tracts.
Factor analysis was performed to analyse the covariation among the observed variables and to reduce the number of dimensions of census demographic variables. We employed the function factanal () with a varimax rotation that uses the ‘maximum likelihood’ function as opposed to ‘principal component’ to derive the factors. (Factor Analysis and Analysis Citation2014) When the maximum likelihood function is used, there is a (conservative) significance test for the null hypothesis that the extracted factors are sufficient. The higher eigenvalue by a factor is helpful in explaining the variance in the variables. If the eigenvalue is greater than 1 by a factor; then, it is useful/important based on the Kaiser rule. Kaiser Citation1960)
Multilevel logistic regression was used to analyse how individual- and contextual-level factors predict the occurrence of childhood cancer by race/ethnicity and social domains. A mixed-effects logistic regression in the R 3.5.140 package ‘glmer’ (Generalized Linear Mixed-Effects Models) was employed to model binary outcome variables. (Agresti Citation2002; Bruin Citation2006). The relationship between several predictor variables (,
,
, … .
) to a dichotomous dependent variable (
) can be described using a mathematical model called logistic regression. Here, Y is typically coded as 1 (early-stage) or 0 (late-stage) for its two possible categories (Kleinbaum et al. Citation2008; Tian, Wilson, and Zhan Citation2011). Hierarchical or clustered data at different levels (i.e. patient-level and census tract-level) were analysed using a multilevel regression model, accounting for the variability associated with each level of the hierarchy. This model shown below can be applied to data with a binary outcome variable(Dai, Li, and Rocke Citation2006)
Where Yij is a binary outcome variable, i is a patient-level indicator, j is a census tract-level indicator, i is the probability of the late-stage diagnosis for patient i in census-tract j, and
is a patient-level random error for patients i in census-tract. The logit function assumes that each census tract has its own intercept
measuring census tract-level effects. The intercept
is a linear combination of the grand mean
and a deviation
from that mean. As a result, the hierarchical model has both fixed effects (α, β) and random effects
. There are two essential benefits of using the multilevel logistic analysis model. First, this function is easy to use and very flexible. Second, it provides a more meaningful interpretation for health research when the data are from different levels. Because the traditional multivariate logistic regression only uses variables from the same level, it fails to account for correlations among individuals within the same neighbourhood, and it does not adequately explain the geographic variations in the study area because of random effect (Austin and Merlo Citation2017).
Results
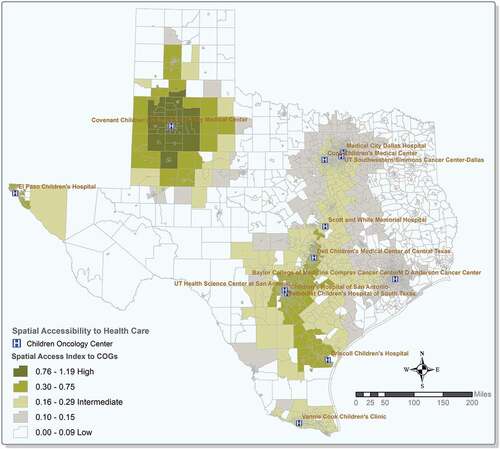
shows the geographic variations of spatial access to COG hospitals in Texas. COG hospitals are located in urban areas where most of the population live. Based on the accessibility results, urban areas with a higher population density exhibit better spatial access to COG services compared to their rural counterparts. The Upper East, Southeast, the tip of the high plains, parts of northwest and west Texas, and the Upper Rio Grande have lower levels of spatial access to COG hospitals. Areas along interstate highway I-35 from the Gulf of Mexico to the Dallas metropolitan area have high levels of spatial access due to the population distribution and proximity to the COG hospitals in these areas.
Figure 2. Spatial access to Children’s Oncology Group (COG) services in Texas.(Note: Values close to 1 or higher represents high level of spatial accessibility)
Factor analysis extracted four factors (). Depending on the factor analysis results and authors’ knowledge and experience, factors were labelled as socioeconomic status (SES) (Factor 1), socio-cultural (Factor 2), education level (Factor 3), and percent African Americans (Factor 4), respectively. Although in the literature education level is used to reflect SES, in this study, both percent of cases without a high-school diploma and percent without a college degree had independent and high factor loadings in the results of the factor analysis. Percent African Americans also had a significantly higher sum of squared (SS) loading and was considered an independent factor. Results in show that all four factors had high eigenvalues (>1.00) and high relative cumulative variance. The standard alpha and Guttman’s λ6 (G6) were 0.84 and 0.89, respectively, meaning the variables were highly correlated with each other. Although Cronbach’s α is a popular measure, it overestimates the first-factor saturation and underestimates the reliability of a test. (Revelle Citation2018)
Table 1. Factor loadings and the percentage of cumulative variance explained by each factor
A Chi-square test of independence of the factors was performed to see if there was a significant difference between early-stage and late-stage diagnosed groups in terms of selected factors (). There was a statistically significant difference between early- and late-stage diagnosis cases based on individual-level characteristics, including race/ethnicity and contextual-level characteristics including SES, sociocultural factors, spatial accessibility to COG hospitals, and percent health insurance coverage. Results in also shows that a large proportion of cases (91.05%) were diagnosed at younger ages (0–14) compared to older ages (15–19 years of age). Findings from this study revealed that the children in socioeconomically advantaged group was more likely to be diagnosed at an early stage. Although there is no significant difference in childhood cancer stage at diagnosis in different levels of urbanization, a large number of cases (87.19%) with late-stage diagnosis lived in metropolitan areas. The percentage of late-stage diagnosis cases increased gradually with the decrease in the percentage of people with health insurance coverage in census tracts.
Table 2. Summary information of data used in the analysis
illustrates statistically significant racial/ethnic disparities in childhood cancer late-stage diagnosis. Compared with non-Hispanic Whites, Hispanics had an elevated risk of diagnosis at a late-stage (Odds Ratio [OR] = 1.25; 95% CI, (1.09–1.43) after adjusting for both individual and contextual variables. African Americans had a lower risk of late-stage diagnosis compared with Whites (OR = 0.92; 95% CI = 0.75–1.13) after adjusting for both individual and contextual covariates. Possible reasons for this result may be due to the limited numbers of cases for African Americans and the fact that 40% of these cases were diagnosed at an early stage. Furthermore, results in suggest that children living in areas of a low SES status is 11% more likely to experience a late-stage diagnosis (OR = 1.11; 95% CI = 0.92–1.33) compared with those living in socioeconomically advantaged areas.
Table 3. Odds ratio showing association between childhood cancer late-stage diagnosis and race/ethnicity and other social factors
Socio-cultural factors showed elevated risk for late-stage diagnosis when they were fitted alone in the logistic regression (model I), especially for lower socio-cultural factors (OR = 1.25; 95% CI = 1.07–1.47), and the risk decreased slightly after adjusting for other factors. Children with a lower parental education level had a significantly higher risk (OR = 1.23; 95% CI = 1.02–1.48) for late-stage diagnosis in all three models (with or without other variables). The percent of African Americans did not have a significant effect on stage at diagnosis.
Compared with census tracts with the highest level of spatial access to COG hospitals, census tracts with lower spatial access to COG hospitals were more likely to have a higher rate of late-stage diagnosis, after adjusting for covariates (OR = 1.26; 95% CI = 1.07–1.48). The study revealed that micropolitan areas had a higher risk of late-stage diagnosis after adjusting for covariates (OR = 1.18; 95% CI = 0.95–1.45) when compared with large metropolitan areas. It was apparent that rural areas had a slightly higher risk of late-stage diagnosis compared to small towns after adjusting for other variables (OR = 1.08; 95% CI = 0.68–1.70). Percent health insurance coverage showed a significant effect on stage at diagnosis, particularly census tracts in the lowest percentile were more likely to have a higher rate of late-stage diagnosis (OR = 1.26; 95% CI = 0.97–1.63) ().
Discussion
One of the goals of the Children’s Oncology Group (COG) is the inclusion of proportional racial/ethnic groups diagnosed with cancer in clinical trials. The COG encompasses more than 200 paediatric cancer programmes in the United States (Lund et al. Citation2009; Children’s Oncology Group: The World’s childhood cancer experts Citation2021) with only 15 COG sites in Texas, mostly clustered in urban areas (Children’s Oncology Group: The World’s childhood cancer experts Citation2021). Texas is the second-largest state in the United States and features a population distributed in a wide variety of rural and urban environments. The spatial access result in this study indicates that most of the rural areas in Texas are highly inaccessible to COG services (), which decreases the quality and span of life for children with cancer. However, results of the logistic regression showed that micropolitan areas are more likely to have a higher risk of late-stage diagnosis compared with large metropolitan areas after adjusting for covariates. In addition, small towns and rural areas also displayed certain risks for late-stage diagnosis. The findings of this study suggest that poor geographical accessibility to those COG service institutions may pose a significant risk of childhood cancer late-stage diagnosis. This is the first study to examine the role of spatial accessibility to COG in childhood cancer, focusing on stage at diagnosis.
The current study reported a significant disparity in childhood cancer late-stage diagnosis for Hispanics compared to Whites after adjusting for age, race/ethnicity, SES, and contextual factors. Hispanic children were more likely to experience an advanced stage of cancer at diagnosis, which corroborates the findings of two other studies of childhood melanoma in Texas(Hamilton, Nguyen, and Chang et al. Citation2016) and New Mexico(Rajput, Faizi, and Nir et al. (Citation2014-2009). In addition, several other studies have found significant disparities in late-stage diagnosis for Hispanics and African Americans (Tian et al. Citation2012; Wan et al. Citation2013; Lin, Schootman, and Zhan Citation2015; Ward, Jemal, and Cokkinides et al. Citation2004). After adjusting for both individual and contextual covariates, African Americans showed a lower risk of late-stage diagnosis compared to Whites. One important reason for this result might be that there were insufficient number of cases used in this study.
Differences in SES status is known to affect cancer outcomes. In this study, SES (Factor 1) was constructed based on multiple indicators such as percent people living in poverty, percent unemployed, median household income, and percent household without a car. SES has not been frequently examined in studies involving childhood cancer patients. In several studies, SES was not found to be significantly associated with delayed diagnosis(Haimi Citation2004; Dang-Tan, Trottier, and Mery et al. Citation2010; Martin et al. Citation2007) and it showed no indication of association with increased risk of childhood cancer (Marquant, Goujon, and Faure et al. Citation2016). The current study revealed that higher rate of childhood cancer late-stage diagnosis is associated with higher levels of social deprivation. In addition, results from this study suggested that there were significant contextual SES disparities in childhood cancer late-stage diagnosis, which is consistent with the results reported in previous disparity studies (Araz and Guler Citation2015; Tian et al. Citation2012; Wan et al. Citation2013; Fajardo-Gutiérrez et al. Citation2002).
Children are completely dependent on their family and parents for their medical attention and well-being. Parental education levels were found to be an important predictor for childhood cancer late-stage diagnosis. Census tracts with lower parental education levels showed higher late-stage diagnosis risk, as education level is closely associated with socioeconomic status. Education level can be used as a proxy for SES status because it remains fairly stable through adulthood, whereas the change in health status is less affected by occupational status and income (Krieger, Williams, and Moss Citation1997). A retrospective study of Egyptian children found that the education level of parents was a statistically significant predictor of total delay in diagnosis (Abdelkhalek et al. Citation2014). Moreover, two other studies in Mexico concluded that lower parental education level was an important factor for children with cancer to be diagnosed at advanced stages. (Fajardo-Gutiérrez et al. Citation2002; Ramírez-Ortiz et al. Citation2014)
This study revealed significant differences in selected characteristics of childhood cancer stage at diagnosis based on socio-cultural factors. The number of cases increased steadily for both early- and late-stage diagnosis in terms of socio-culturally disadvantaged groups, which is similar to past research on cervical cancer(Zhan and Lin Citation2014) and colorectal cancer(Henry, Sherman, and Lm Citation2009) disparities in Texas and New Jersey, respectively. It is worth noting that the percentage of limited English-speaking households, average family size, and percent Hispanics loaded heavily on sociocultural factor (Factor 2), which is not surprising based on the sociodemographic characteristics of Texas. The socio-cultural factor may limit patients’ ability to navigate the medical system, affect the level of health literacy, and hinder communications with health care professionals (Henry, Sherman, and Lm Citation2009).
This study reported a relatively low proportion of late-stage cases with increased health insurance enrolment. Lack of sufficient health insurance coverage can pose a major obstacle to adequate treatment and preventive health care services; more importantly, it can adversely affect the incidence and mortality throughout the cancer control continuum, which extends from aetiology, prevention, detection, diagnosis, treatment, and survival to palliative care (Ward, Halpern, and Schrag et al. Citation2008).
This study has several limitations to be noted when interpreting the results. First, we had to exclude 1,232 (16.61% of total) cases from the logistic regression analysis due to ‘not applicable/unknown stage’ at diagnosis in the records of the data. In addition, tumour grade was excluded from the analysis because grade descriptions were unknown (not stated, or not applicable) for more than 50% of the cases in the data. Second, the centroid of each census tract was used in the spatial access analysis, meaning we assumed that everyone lived at the location represented by the centroid of the respective census tract in question. This assumption affects the accuracy of the spatial access analysis, particularly for large census tracts. However, in the real world, the distribution of shape and size of the census tracts, and the number of people in each tract varies in space and time. Finally, the study also assumed that each COG centre would provide equal services in terms of medical professionals and logistics, which is unrealistic and calls for further refinement in the future research.
Conclusion and future research
Childhood cancer stage at diagnosis is a critically important determinant of overall health outcomes of children experiencing cancer, because early diagnosis allows timely treatment, and can have positive effects on prognosis and survival rate. Among other factors affecting childhood cancer stage at diagnosis, this current study highlights the importance of spatial accessibility to Children’s Oncology Group (COG) hospitals in reducing late-stage paediatric cancer disease burden. The study identified statistically significant differences in early- and late-stage diagnosis for childhood cancer patients in terms of spatial accessibility to COG services. Findings from this study suggests that poor spatial accessibility to COG hospitals increased the risk of late-stage diagnosis after adjusting for other covariates, which corroborates with the results from other studies(Onega et al. Citation2008; Wang and Late-Stage Breast Citation2008; Wan et al. Citation2012.) Based on the different levels of urbanization, this study also reported an increased risk of childhood cancer late-stage diagnosis for micropolitan areas compared to small towns and rural areas. This effect is another geographic factor that warrants additional investigation in the future research.
This study also revealed a statistically significant difference between early- and late-stage diagnosis groups based on race/ethnicity, socio-economic status, and socio-cultural factors. Hispanic children showed a higher risk of late-stage diagnosis compared to non-Hispanic Whites, after adjusting for both individual- and contextual-level factors. It was also reported that there is a significant association between childhood cancer late-stage diagnosis and lower socio-economic status, disadvantaged socio-cultural factors, lower parental education level, and living in areas with lower percentage of health insurance coverage.
This study represents the first attempt in the United States to analyze spatial access to COG healthcare services at a fine spatial resolution at the census tract level. Results from this study about the factors associated with childhood cancer stage at diagnosis would be useful for the development of more effective childhood cancer intervention programmes. Findings from this study suggest that more resources should be allocated in the targeted socioeconomically disadvantaged areas, focusing on those with a high percentage of linguistically isolated households, households without a car and in areas with a higher percentage of Hispanic and African American populations. Public awareness, especially parental health literacy of this deadly disease may save thousands of lives. Future work should explore how racial/ethnic disparities change over geographic space and at a finer geographic scale. It is also recommended that additional studies are needed to investigate the effect of race/ethnicity on the overall survival of paediatric cancer patients, while mediated through spatial access and socioeconomic status mediators.
Disclosure of financial support
The study did not receive any financial support from any individual or entity.
Institutional review board approval
The study protocol and the use of the cancer registry data were approved by the Institutional Review Boards (IRB) of the Texas Department of State Health Services and Texas State University.
Acknowledgements
This article is based on Niaz Morshed’s dissertation completed at Texas State University under F. Benjamin Zhan’s supervision. The authors wish to thank the Texas Department of State Health Services (DSHS) and the Texas Cancer Registry (TCR) for providing the data used in the research. The contents are solely the responsibility of the authors and does not necessarily represent the official views of the Texas DSHS and the TCR. We would like to thank Dr. Russell Weaver (Associate Professor, Geography, Texas State University) for his input on the analysis. The authors also wish to thank Dr. Kelly Haskard-Zolnierek (Associate professor, Psychology, Texas State University) and Dr. Mark A. Deka (Postdoctoral Fellow at Centres for Disease Control and Prevention) for their feedback on writing–review and editing section. The authors do not endorse the purchase of any commercial products or services mentioned in the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during and/or analysed during the current study are not publicly available due to the risk involving a breach of confidentiality, allowing for identification of individual cases.
References
- Abdelkhalek, E. R., L. M. Sherief, N. M. Kamal, and R. M. Soliman. 2014. “Factors Associated with Delayed Cancer Diagnosis in Egyptian Children.” Clin Med Insights 8: 39–44. doi:https://doi.org/10.4137/CMPed.S16413.
- Agresti, A. 2002. “Categorical Data Analysis.„ 2nd Edition, John Wiley & Sons, Inc., Hoboken, New Jersey
- Araz, N. C., and E. Guler. 2015. “Delays in Diagnosis of Childhood Cancer in Southeastern Turkey and the Associated Factors.” Pediatric Hematology and Oncology 32 (2): 153–163. doi:https://doi.org/10.3109/08880018.2013.874511.
- Austin, P. C., and J. Merlo. 2017. “Intermediate and Advanced Topics in Multilevel Logistic Regression Analysis.” Statistics in Medicine 36 (20): 3257–3277. doi:https://doi.org/10.1002/sim.7336.
- Bleyer, W. A., H. A. Tejeda, S. B. Murphy, O. W. Brawley, M. A. Smith, and R. S. Ungerleider. 1997. “Equal Participation of Minority Patients in U.S. National Pediatric Cancer Clinical Trials.” Journal of Pediatric Hematology/oncology 19 (5): 423–427. doi:https://doi.org/10.1097/00043426-199709000-00003.
- Brewer, N., N. Pearce, P. Day, and B. Borman. 2012. “Travel Time and Distance to Health Care Only Partially Account for the Ethnic Inequalities in Cervical Cancer Stage at Diagnosis and Mortality in New Zealand.” Australian and New Zealand Journal of Public Health 36 (4): 335–342. doi:https://doi.org/10.1111/j.1753-6405.2012.00843.x.
- Brown, B. J., B. O. James, S. O. Ajayi, O. A. Ogun, and R. E. Oladokun. 2009. “Factors Influencing Time to Diagnosis of Childhood Cancer in Ibadan, Nigeria.” African Health Sciences 9 (4): 247–253. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3074401&tool=pmcentrez&rendertype=abstract
- Bruin, J. 2006. “Newtest: Command to Compute New Test.” In UCLA: Statistical Consulting Group.https://stats.idre.ucla.edu/stata/ado/analysis/ . Accessed 15 September 2021.
- Byers, T. 2010. “Two Decades of Declining Cancer Mortality: Progress with Disparity.” Annual Review of Public Health 31 (1): 121–132. doi:https://doi.org/10.1146/annurev.publhealth.121208.131047.
- Cancer Control Continuum. “National Institute of Health (NIH): Division of Cancer Control & Population Sciences. National Cancer Institute”. Bethesda, MD. https://cancercontrol.cancer.gov/od/continuum.html Published Accessed 30 April 2021. Accessed April 30, 2021
- Children’s Oncology Group: The World’s childhood cancer experts. https://childrensoncologygroup.org/locations/ Published 2021. “Accessed” January 1, 2021.
- Chu, K. C., B. A. Miller, and S. A. Springfield. 2007. “Measures of Racial/ethnic Health Disparities in Cancer Mortality Rates and the Influence of Socioeconomic Status.” Journal of the National Medical Association 99 (10): 1092–1100, 1102–1104. http://www.ncbi.nlm.nih.gov/pubmed/17987912%5Cnhttp
- Dai, J., Z. Li, and D. Rocke. 2006. Hierarchical Logistic Regression Modeling with SAS GLIMMIX. … Proc West Users SAS, 1–9. http://www.lexjansen.com/wuss/2006/analytics/ANL-Dai.pdf
- Dang-Tan, T., and E. L. Franco. 2007. “Diagnosis Delays in Childhood Cancer: A Review.” Cancer 110 (4): 703–713. doi:https://doi.org/10.1002/cncr.22849.
- Dang-Tan, T., H. Trottier, L. S. Mery, H. I. Morrison, R. D. Barr, M. L. Greenberg, and E. L. Franco. 2008. “Delays in Diagnosis and Treatment among Children and Adolescents with Cancer in Canada.” Pediatric Blood & Cancer 51 (4): 468–474. doi:https://doi.org/10.1002/pbc.21600.
- Dang-Tan, T., H. Trottier, L. S. Mery, H. I. Morrison, R. D. Barr, M. L. Greenberg, and E. L. Franco. 2010. “Determinants of Delays in Treatment Initiation in Children and Adolescents Diagnosed with Leukemia or Lymphoma in Canada.” International Journal of Cancer.126 (8): 1936–1943. doi:https://doi.org/10.1002/ijc.24906.
- DSHS (Department of State Health Services). “Texas Cancer Registry Statewide Incidence File”, 2005–2014. http://www.dshs.state.tx.us/tcr/researchers.shtm#datasets Published 2017. Accessed 15 January 2020
- Factor Analysis, T. E., and C. Analysis. 2014. http://langcog.stanford.edu/expts/MLL/Coursework/Psych. 253/powerpoints and handouts/Factor Analysis/ho3-factoranal (1).pdf
- Fajardo-Gutiérrez, A., A. M. Sandoval-Mex, J. M. Mejía-Aranguré, M. E. Rendón-Macías, and M. D. C. Martínez-García. 2002. “Clinical and Social Factors that Affect the Time to Diagnosis of Mexican Children with Cancer.” Medical and Pediatric Oncology 39 (1): 25–31. doi:https://doi.org/10.1002/mpo.10100.
- Gehlert, S., and G. A. Colditz. 2011. “Cancer Disparities: Unmet Challenges in the Elimination of Disparities.” Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 20 (9): 1809–1814. doi:https://doi.org/10.1158/1055-9965.EPI-11-0628.
- Haimi, M. 2004. “Delay in Diagnosis of Children with Cancer: A Retrospective Study of 315 Children.” Pediatr Hematol 21 (1): 37–48. doi:https://doi.org/10.1080/08880010490263579.
- Hamilton, E. C., H. T. Nguyen, Y. C. Chang, J. M. Eberth, J. Cormier, L. S. Elting, and M. T. Austin. 2016. “Health Disparities Influence Childhood Melanoma Stage at Diagnosis and Outcome.” The Journal of Pediatrics 175 (2016): 182–187. doi:https://doi.org/10.1016/j.jpeds.2016.04.068.
- Hansen, W. G. 1959. “How Accessibility Shapes Land Use.” J Am Plan Assoc 25 (2): 73–76. doi:https://doi.org/10.1080/01944365908978307.
- Henry, K., R. Sherman, and R. Lm. 2009. “Colorectal Cancer Stage at Diagnosis and Area Socioeconomic Characteristics in New Jersey.” Health & Place 15 (2): 505–513. doi:https://doi.org/10.1016/j.healthplace.2008.09.003.
- Joseph, A. E., and P. R. Bantock. 1982. “Measuring Potential Physical Accessibility to General Practitioners in Rural Areas: A Method and Case Study.” Social Science & Medicine 16 (1): 85–90. doi:https://doi.org/10.1016/0277-9536(82)90428-2.
- Kaiser, H. F. 1960. “The Application of Electronic Computers to Factor Analysis.” Educational and Psychological Measurement 20 (1): 141–151. doi:https://doi.org/10.1177/001316446002000116.
- Keppel, K., E. Pamuk, J. Lynch, O. Carter-Pokras, I. Kim, V. Mays, J. Pearcy, V. Schoenbach, and J. S. Weissman. 2005. “Methodological Issues in Measuring Health Disparities.” Vital Heal Stat 2 (141): 1–16. https://pubmed.ncbi.nlm.nih.gov/16032956/ .
- Kleinbaum, D. G., L. L. Kupper, A. Nizam, and K. E. Muller. 2008. Applied Regression Analysis and Other Multivariable Methods. Fourth Edit. Duxbury Press, An International Thomson Publishing Company,Pacific Grove, USA.
- Krieger, N. 2005. “Defining and Investigating Social Disparities in Cancer: Critical Issues.” Cancer Causes & Control 16 (1): 5–14. doi:https://doi.org/10.1007/s10552-004-1251-5.
- Krieger, N., D. R. Williams, and N. E. Moss. 1997. “Measuring Social Class in US Public Health Research.” Annual Review of Public Health 18 (1): 341–378. doi:https://doi.org/10.1146/annurev.publhealth.18.1.341.
- Lemstra, M., C. Neudorf, and J. Opondo. 2006. “Health Disparity by Neighbourhood Income.” Can J Public Heal 97 (6): 435–439. November – December 2006. doi:https://doi.org/10.1007/BF03405223.
- Lin, Y., and F. B. Zhan. 2014. “Geographic Variations of Racial/ethnic Disparities in Cervical Cancer Mortality in Texas.” Southern Medical Journal. doi:https://doi.org/10.1097/SMJ.0000000000000100.
- Lin, Y., M. Schootman, and F. B. Zhan. 2015. “Racial/ethnic, Area Socioeconomic, and Geographic Disparities of Cervical Cancer Survival in Texas.” Applied Geography 56: 21–28. doi:https://doi.org/10.1016/j.apgeog.2014.10.004.
- Lund, M. J., M. T. Eliason, A. E. Haight, K. C. Ward, J. L. Young, and R. D. Pentz. 2009. “Racial/ethnic Diversity in Children’s Oncology Clinical Trials: Ten Years Later.” Cancer 115 (16): 3808–3816. doi:https://doi.org/10.1002/cncr.24437.
- Luo, W., and Y. Qi. 2009. “An Enhanced Two-step Floating Catchment Area (E2SFCA) Method for Measuring Spatial Accessibility to Primary Care Physicians.” Heal Place 15 (4): 1100–1107. doi:https://doi.org/10.1016/j.healthplace.2009.06.002.
- Marquant, F., S. Goujon, L. Faure, S. Guissou, L. Orsi, D. Hemon, B. Lacour, and J. Clavel. 2016. “Risk of Childhood Cancer and Socio-economic Disparities: Results of the French Nationwide Study Geocap 2002-2010.” Paediatric and Perinatal Epidemiology 30 (6): 612–622. doi:https://doi.org/10.1111/ppe.12313.
- Martin, S., C. Ulrich, M. Munsell, S. Taylor, G. Lange, and A. Bleyer. 2007. “Delays in Cancer Diagnosis in Underinsured Young Adults and Older Adolescents.” Oncologist 12 (7): 816–824. doi:https://doi.org/10.1634/theoncologist.12-7-816.
- Morshed, N., K. Haskard-Zolnierek, and F. B. Zhan. 2020. “Geographic Variations of Racial/Ethnic Disparities in Late-Stage Diagnosis of Childhood Cancer in Texas.” Southern Medical Journal 113 (5): 224–231. doi:https://doi.org/10.14423/smj.0000000000001097.
- Murphy, M. M., J. F. Tseng, and S. A. Shah. 2010. “Disparities in Cancer Care: An Operative Perspective.” Surgery 147 (5): 733–737. doi:https://doi.org/10.1016/j.surg.2009.10.050.
- Onega, T., E. J. Duell, X. Shi, D. Wang, E. Demidenko, and D. Goodman. 2008. “Geographic Access to Cancer Care in the U.S.” Cancer 112 (4): 909–918. doi:https://doi.org/10.1002/cncr.23229.
- Rajput, A., S. A. Faizi, I. Nir, K. T. Morris, B. Fahy, J. Russell, and C. Wiggins. 2014. “Pediatric Melanoma in New Mexico American Indians, Hispanics, and non-Hispanic Whites, 1981–2009.” American Journal of Surgery207 (3):412–416. 10.1016/j.amjsurg.2013.10.015
- Ramírez-Ortiz, M. A., M. V. Ponce-Castañeda, M. L. Cabrera-Muñoz, A. Medina-Sansón, X. Liu, and M. A. Orjuela. 2014. “Diagnostic Delay and Sociodemographic Predictors of Stage at Diagnosis and Mortality in Unilateral and Bilateral Retinoblastoma.” Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 23 (5): 784–792. doi:https://doi.org/10.1158/1055-9965.EPI-13-1069.
- Read, J. G., M. O. Emerson, and R. Context. 2005. “Black Immigration and the U .S. Black/White Health Disparity.” Social Forces; a Scientific Medium of Social Study and Interpretation 84 (1): 181–199. doi:https://doi.org/10.1353/sof.2005.0120.
- Revelle, W. “Find Two Estimates of Reliability : Cronbach’s Alpha and Guttman’s 6. R Doc Alpha {psych}”. 2018. http://personality-project.org/r/html/alpha.html
- Siegel, R. L., K. D. Miller, H. E. Fuchs, and A. Jemal. 2021. “Cancer Statistics.” CA: A Cancer Journal for Clinicians 71 (1): 7–33. 2021. doihttps://doi.org/10.3322/caac.21654.
- Stewart, B. W., and C. P. Wild World Cancer Report 2014. “International Agency for Research on Cancer”. World Health Organization (WHO) Press, Geneva, Switzerland. Geneva, Switzerland; 2014. http://www.thehealthwell.info/node/725845
- Thulesius, H., J. Pola, and A. Hakansson. 2000. “Diagnostic Delay in Pediatric Malignancies: A Population-based Study.” Acta Oncol (Madr) 39 (7): 873–876. doi:https://doi.org/10.1080/028418600750063640.
- Tian, N., J. G. Wilson, and F. B. Zhan. 2011. “Spatial Association of Racial/ethnic Disparities between Late-stage Diagnosis and Mortality for Female Breast Cancer: Where to Intervene?” International Journal of Health Geographics 10 (1): 24. doi:https://doi.org/10.1186/1476-072X-10-24.
- Tian, N., P. Goovaerts, F. B. Zhan, and J. G. Wilson. 2010. “Identification of Racial Disparities in Breast Cancer Mortality: Does Scale Matter?” International Journal of Health Geographics 9 (1): 35. doi:https://doi.org/10.1186/1476-072X-9-35.
- Tian, N., P. Goovaerts, F. B. Zhan, T. E. Chow, and J. G. Wilson. 2012. “Identifying Risk Factors for Disparities in Breast Cancer Mortality among African-American and Hispanic Women.” Women’s Heal Issues 22 (3): e267–e276. doi:https://doi.org/10.1016/j.whi.2011.11.007.
- US Census Bureau. 2008. A Compass for Understanding and Using American Community Survey Data: What General Data Users Need to Know. Washington DC: U.S Government Printing office. https://www.census.gov/content/dam/Census/library/publications/2008/acs/ACSGeneralHandbook.pdf
- Wan, N., F. B. Zhan, B. Zou, and E. Chow. 2012. “A Relative Spatial Access Assessment Approach for Analyzing Potential Spatial Access to Colorectal Cancer Services in Texas.” Applied Geography 32 (2): 291–299. doi:https://doi.org/10.1016/j.apgeog.2011.05.001.
- Wan, N., F. B. Zhan, B. Zou, and J. G. Wilson. 2013. “Spatial Access to Health Care Services and Disparities in Colorectal Cancer Stage at Diagnosis in Texas.” The Professional Geographer : The Journal of the Association of American Geographers 65 (3): 527–541. doi:https://doi.org/10.1080/00330124.2012.700502.
- Wang, F., S. McLafferty, V. Escamilla, and L. Luo. 2008. “Late-Stage Breast Cancer Diagnosis and Health Care Access in Illinois.” The Professional Geographer60 (1): 54–69. https://doi.org/10.1080/00330120701724087
- Ward, E., A. Jemal, V. Cokkinides, G. K. Singh, C. Cardinez, A. Ghafoor, and M. Thun. 2004. “Cancer Disparities by Race/ethnicity and Socioeconomic Status.” CA: A Cancer Journal for Clinicians 54 (2): 78–93. 10.3322/canjclin.54.2.78
- Ward, E., M. Halpern, N. Schrag, V. Cokkinides, C. DeSantis, P. Bandi, R. Seigel, A. Stewart, and A. Jemal. 2008. “Association of Insurance with Cancer Care Utilization and Outcomes.” CA: A Cancer Journal for Clinicians 58 (1): 9–31. doi:https://doi.org/10.3322/CA.2007.0011.
- Whitworth, K. W., E. Symanski, and A. L. Coker. 1995-2004. “Childhood Lymphohematopoietic Cancer Incidence and Hazardous Air Pollutants in Southeast Texas.” Environmental Health Perspectives 116 (11): 1576–1580. 2008. doihttps://doi.org/10.1289/ehp.11593.
- Zhan, F. B., and Y. Lin. 2014. “Racial/Ethnic, Socioeconomic, and Geographic Disparities ofCervical Cancer Advanced-Stage Diagnosis in Texas.” Women’s Health Issues 24 (5): 519–527. doi:https://doi.org/10.1016/j.whi.2014.06.009.
