Abstract
A synthesis of field data carried out in the Mediterranean Sea are presented, aimed at contributing to the knowledge of three prokaryotic-mediated processes and their implications on the Carbon cycle. The distribution of exoenzymatic activities, secondary production and respiration rates was studied together with the prokaryotic abundances. Particular attention was paid to the meso- and bathypelagic layers which play an important role in the Mediterranean carbon cycle. The study is noteworthy because of its large spatial scale spanning the entire Mediterranean Sea over 4 years. In addition, two Atlantic stations in front of the Gibraltar Strait were investigated. The longitudinal distribution of prokaryotic activities and abundance along the MED showed different trends along the depth-layers. In particular, higher exoenzymatic rates were detected in the Eastern basin compared to the Western one; carbon respiration rate showed patterns variable with the sampling periods in the epipelagic and bathypelagic layers, while a consistent Westwards decreasing trend at the mesopelagic layers occurred. Specific enzyme activities per cell showed high values in the deepest layers for leucine aminopeptidase. Comparison with Carbon respiration rate data collected before the 2000s showed changing patterns of microbial heterotrophic processes in the Mediterranean Sea.
1. Introduction
The biogeochemical cycle of carbon in the sea contributes to the equilibrium of the biosphere, by means of the solubility, carbonate and biological pumps Citation1,Citation2 that assume great importance in mitigating the effects of the increasing atmospheric CO2 by its sequestration in the marine depths. The major actors in the biological pump, by means of their anabolic and catabolic activities, are the dark ocean microbial communities Citation3.
The deep-sea microbes change the chemistry and productivity of the oceanic water column by performing complex transformations on both organic and inorganic molecules. In spite of the several studies concerning the biogeochemical fluxes of elements and the role of microbial assemblage within them Citation4–6, the mechanisms by which these reactions proceed, their in situ transformation rates, their quantitative significance to element cycling are still poorly understood Citation3,Citation7.
For a long time, the twilight and dark ocean were considered to be sites of negligible biological activity compared to the photic layers. On the contrary, they represent key sites for remineralization of organic matter and long-term carbon storage and burial in the biosphere. They contain the largest pool of microbes in aquatic systems, harbouring nearly 75 and 50% of the prokaryotic biomass and production, respectively, of the global ocean Citation3.
Most of the organic carbon, produced by photosynthesis in the epipelagic zone, is transported into the twilight and dark ocean, respired there by prokaryotes, and returned back to the atmosphere as CO2 within months to years Citation3.
Despite emerging evidence pointing to the deep ocean as a site of active biogeochemistry, until now few studies have recognised the importance of meso- and bathypelagic trophic processes Citation8–12. Recent attempts to derive estimates of carbon cycling in the global ocean conclude that about one-third of biological CO2 production in the ocean occurs in the deep pelagic layers Citation13,Citation14, where relatively intense microbial activity take place driven by prokaryotes (both Bacteria and Archaea) in a complex community featuring virus, protist, zooplankton, and nekton interactions Citation15–18. In the Mediterranean Sea this biological complexity is compounded even more by dynamic hydrographic and atmospheric processes Citation19–24. In simplified terms, three main water layers can be distinguished: a surface layer, an intermediate layer, and a deep layer that sinks to the bottom. A separate bottom layer is absent Citation25. Fragmentary studies on microbial processes in the Mediterranean Sea have been carried out within the framework of different multidisciplinary cruises and projects (i.e. MEDGOOS, FIRB, CIESM-SUB, DYFAMED, VECTOR). Moreover, the Mediterranean basin has recently attracted scientific attention because it is a sensitive ecosystem to climate changes Citation26–28.
Deep water formation, exchange rates, and the processes of heat and water exchange make the Mediterranean an excellent model for studying the global climatic change. In fact, it represents a natural ‘laboratory’ for mesoscale studies on oceanic processes due to its small size and the brief residence time and higher turnover rates of its deep waters. These deep waters in the western Mediterranean have been warming and becoming more saline since the 1950s. This tendency has accelerated after the climate event called Eastern Mediterranean Transient (EMT) Citation29–31. In addition, there is evidence of changing biogeochemical dynamics in the Eastern MED Citation32,Citation33, evolving microbial assemblages, increasing deep-respiration in the Levantine and Ionian Seas, and reduced remineralization in the South Adriatic Sea. All these changes seem to be caused by enhanced deep-water renewal and organic matter injection following the EMT Citation9. Furthermore, superimposed on these changes related to the EMT there are newly discovered associations of heterotrophic processes other than respiration, such as bacterial production and exoenzymatic activities that appear associated with the main Mediterranean water masses Citation11.
After 2000, the propagation of EMT from eastern to western Mediterranean basin occurred again Citation29,Citation34. The Eastern Mediterranean basin appears to be a nutrient source for the Western one while renewing the old resident western deep water Citation35. This results in changing chemical signatures and DOC pool distribution in the Western Mediterranean basin Citation36,Citation37.
Adding to this knowledge base, this work presents a study of three key-processes controlled by the marine microbial assemblage. These are the carbon respiration rate, the activities of two exoenzymes involved in Carbon cycle (Leucine aminopeptidase and beta glucosidase), and the rate of heterotrophic prokaryotic production. Moreover, the prokaryotic (Archaea and Bacteria) standing stock in several provinces of the MED is presented for 2004, 2005 and 2007. A major contribution of this study lies in the large spatial scale covered by the investigations. As the microbial variables are recognised to be strictly related to both physical and chemical parameters, three main questions are addressed in this paper: (1) the distribution of three prokaryotic heterotrophic activities in the Mediterranean Sea, (2) the contribution of the microbial assemblage to carbon flux in the meso- and bathypelagic layers of different Mediterranean provinces, and (3) the identification of unique biogeochemical properties of the Mediterranean deep-water.
2. Materials and methods
The data here derive from five multidisciplinary field studies carried out in the Mediterranean Sea in 2004, 2005, and 2007 on the R/Vs Urania of the Italian National Research Council (CNR) and Universitatis of the National Interuniversity Consortium For Marine Sciences (CoNISMa). The surveys had similar scientific objectives, research approaches, sampling strategies and methods. As a matter of fact, in the different sampling sites and cruises, the same depths were collected (2, 10, 25, 50, 75, 100, 200, 500, 750, 1000, 1500, 2000, 2500, 3000, 3500 and 3700 m) according to the bathymetry. In the names of cruises and projects, sampling periods, studied provinces, depths, parameters, sampling size, and bibliographic references are reported Citation22,Citation38–41. A CTD probe 911plus SeaBird was used to record conductivity, temperature, pressure and oxygen content at all the stations. Salinity values were also checked in comparison with the measurements on discrete samples made with an induction salinometer AutoSal Guildline Model 8004B. The water samples were collected at different depths from surface to bottom, using a rosette sampler equipped with 10-L acid rinsed Niskin bottles. The samples were either immediately processed for specific measurements aboard the R/Vs or stored for subsequent analyses in laboratory.
Table 1. Names of cruises or projects, sampling periods and provinces, depths, parameters and sampling size together with references.
Simultaneous measurements of prokaryotic abundances and metabolism were carried out. In particular, the data set covered the distribution of the following parameters: exoenzymatic hydrolysis (EEA) by leucine aminopeptidase (LAP) and β-glucosidase (β-Glu) activities by using fluorogenic substrates Citation42,Citation43, heterotrophic prokaryotic production (HPP) by [3H]-leucine uptake Citation44,Citation45, potential respiration rates measured via ETS assay, converted into Carbon Respiration (CR) which represents the Carbon Dioxide Production Rates Citation10,Citation46, as well as prokaryotic abundances (PA) by image analysis Citation47,Citation48.
2.1. Study areas
The study area was divided into 6 provinces: Atlantic (ATL), Alboran-Almerian (ALB), Algero-Provençal (APR), Southern Tyrrhenian Sea (TYR), Ionian (ION), Eastern Mediterranean (EM) sub-basins (). The ATL province lies near to the west of the Strait of Gibraltair which connects to the Alboran Sea, the first Mediterranean province (ALB). ALB is a transition zone between ATL and the Mediterranean (MED) Sea. Here, surface currents flows eastward, bringing water from the ATL into the MED. Deeper subsurface currents flow westward, carring saltier Mediterranean Water into Atlantic Ocean. There is often a gyre as a result of this exchange of water. The APR is located east of ALB Basin and west of Sardinia and Corsica, extending from the Algerian littoral to the French littoral. The TYR is about 760 km long and from 97 to 483 km wide, between the Ligurian Sea, the Italian peninsula, Sicily, Sardinia, and Corsica. The ION represents a crossing point between the Western and the Levantine MED Sea; it is a site where the major water masses of the Eastern MED are transformed as they spread from their sites of formation Citation25 and it is directly influenced by Adriatic outflow Citation49. Finally, the EM basin, in particular the Levantine Sea, is the most important site of the Intermediate Water (LIW) formation, which spreads throughout the entire MED. Moreover, from the Aegean Sea the climatic event called EMT is formed.
Figure 1. Map of the Mediterranean Sea with locations of the studied provinces: Atlantic (ATL), Alboran (ALB), Algero-Provencal (APR), Tyrrhenian (TYR), Ionian (ION) and Eastern Basin (EB) provinces.
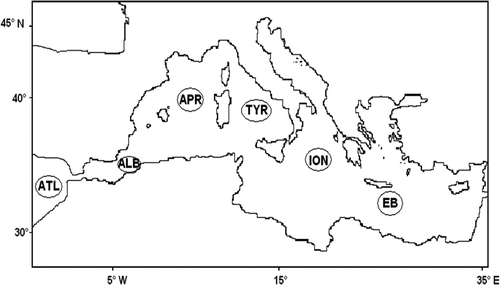
During MEDGOOS cruise 2, 3, 5 and 4 stations in the ATL, ALB, APR and TYR provinces, respectively, were collected; during MEDBIO cruise 7 stations in ION; during CIESM and FIRB-MIUR cruises 14 and 4 stations, respectively, in TYR; during TRANSMED cruise a single water column was sampled for each province with the exception of ION and EB, where 3 and 2 stations for provinces, respectively, were considered.
2.2. Data processing
Data were integrated with depth according to the trapezoidal method and normalized to the depth: from 2 to 200 m for the epipelagic layer; from 200 to 1000 m for the mesopelagic layer; and from 1000 to the bottom depth for the bathypelagic layer. Normalized values are reported throughout the paper.
Specific cell activity was determined by dividing the activities by cell numbers in each water sample and then averaging them.
CR power functions were obtained by fitting the data with the following power function:
Ri = yZ X , where y is the CR in µg C m−3 h−1, Z is the depth below the surface in metres and x is the exponent of depth Citation2. The depth-integrated rate (∫R dz in mg C m−2 d−1) in the water column was calculated within the depth interval between Z 1 and Z 2 using the following formula Citation9:
From each parameter, significant differences between two or more groups were analyzed applying the non-parametric multivariate analysis of variance NPMANOVA test (known also as PERMANOVA) Citation50. The test was performed using Bray–Curtis distance measure on untransformed data; P values were calculated from 4999 random permutations. Analysis were done using PAST Statistics V 1.97 software (Ø. Hammer, University of Oslo).
3. Results
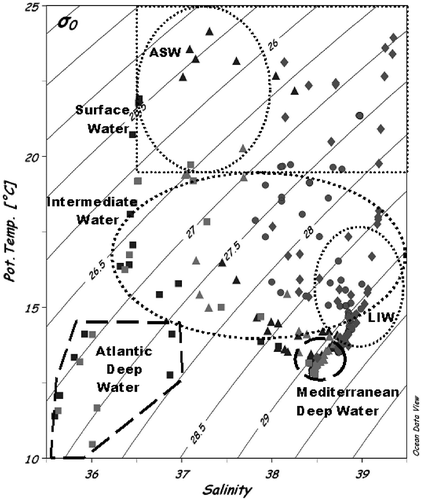
The hydrological signatures of the water samples such as the cumulative potential temperature (PT) versus salinity (S) are shown in . It allowed us to hydrographically identify the most important water masses and their thermohaline modifications across the studied provinces. On the left of the figure, a layer of relatively low salinity waters of Atlantic origin are recognizable–the Atlantic Surface Water (ASW) characterized by temperature and salinity around to 13.04 and 36.32°C–followed by their thermohaline modifications of the surface waters (Modified Atlantic Water, MAW) across the ALB, APR and TYR provinces. On the right, the warmer and saltier Ionian and Eastern surface waters can be distinguished. An underlying layer composed of LIW (on the right of the figure) and their thermohaline modifications across the basin between 250 and 700 m depths, were clearly recognized. Finally, the Mediterranean Deep Water (MDW) filled the water column from 700 m down to the bottom with cold temperatures ranging between 13.08 and 14.24°C and the Atlantic deep water (T ≤ 13°C).
Figure 2. The cumulative potential temperature (PT) versus salinity (S) diagram reassuming the most important hydrographic characteristics of the water column during the sampling cruises and the studied provinces (![]()
reports the PA, CR, b-GLU, LAP and HPP mean values, standard deviations, sample numbers obtained in the Western and Eastern MED basins and in the ATL Stations, within the epi-, meso- and bathypelagic layers.
Table 2. Mean values and standard deviations of the PA, CR, b-GLU, LAP and HPP in the Western and Eastern MED basins and in the Atlantic Stations, in the epi-, meso- and bathypelagic layers.
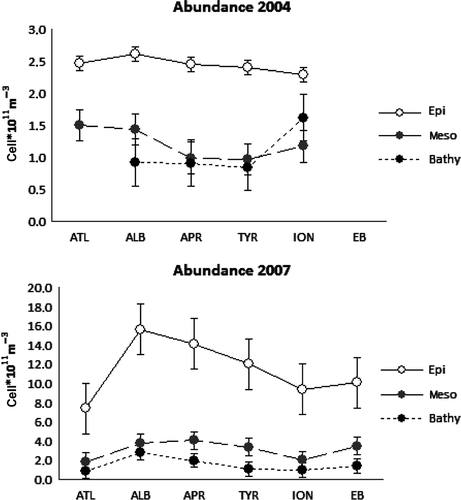
reports the depth-integrated and normalized values of PA, LAP, b-GLU, HPP and CR obtained at the different provinces in the epi-, meso- and bathypelagic layers. The values of the parameters changed between the three sampling periods. In 2004, PA showed low depth-normalized values in all three layers and low variability along the whole MED basin and ATL province. Higher values in the epi- than in the meso- and bathypelagic layers were always observed (ANOVA: epi- vs. mesopelagic, P = 0.00087 and epi- vs. bathypelagic P = 0.00118). In fact, in the meso- and bathypelagic layers, PA represented about a half and a quarter of the values observed in the epipelagic one, respectively. Significant differences were found between the whole MED and the ATL stations in the mesopelagic layers (ANOVA: P = 0.029). In 2005, depth-normalized PA values () showed higher values than those in the TYR during 2004 in the epi- and meso-pelagic layers (ANOVA: P < 0.01) but not in the bathypelagic. Again a decreasing trend along the water column from surface toward the bottom was observed. Finally, in 2007, the depth-normalized PA values were higher than in 2004 and 2005, especially in the epipelagic layer where the cell counts were one order of magnitude higher. In this upper layer, a significant decreasing west-to-east trend was observed (ANOVA: P = 0.0001) and the same appeared in the meso- and bathypelagic layers, also if to a lesser extent. Higher values in MED basin than in ATL stations were observed (ANOVA: P = 0.013 in the epipelagic; PERMANOVA: P = 0.002 in the mesopelagic).
Table 3. Prokaryotic Abundance (PA), Leucine-aminopeptidase (LAP), beta-Glucosidase (b-GLU), Heterotrophic Prokaryotic Production (HPP) and Carbon Respiration (CR) depth-integrated and normalized values in the epi-, meso- and bathypelagic zones of the different MED provinces.
From 2004 to 2007 PA significantly increased by about 7 and 2 times in the epi- and mesopelagic layers, respectively (ANOVA: epi2004 vs. epi2007, P < 0.0001; meso2004 vs. meso2007, P < 0.0001). In the bathypelagic layer, the differences were significant with PERMANOVA (P < 0.0001). Finally, also in ATL province higher values in 2007 than 2004 were observed in the epipelagic layer (ANOVA: P = 0.0018) but lower in the mesopelagic one. In , the distribution of the depth-normalized PA values within the three layers and along the MED provinces in 2004 and 2007 are reported.
Figure 3. Distribution of the depth-normalized PA values in the different provinces along the MED in the epi- meso- and bathypelagic layers in 2004 and 2007. The values are expressed as cells × 1011 m−3.
Regarding the EEA activities, during November 2004 only the ION province was studied. Depth-normalized LAP values were low (), prevailing at the epi- and bathypelagic layers, where it was about 150% of the mesopelagic value. b-GLU activity was 3 times higher in the epipelagic layer than in deeper ones.
During 2005, depth-normalized LAP values increased progressively over depth along the water column. b-GLU values showed the highest activity at the mesopelagic layer.
Comparing the b-GLU and the LAP data from 2004 (ION) and 2005 (TYR) to the 2007 data, a reduction of enzyme levels was observed for both the enzyme activities. However, no statistically significant differences were found for b-GLU values. In 2007, LAP values at epi- and mesopelagic layers were significantly lower than in 2004 in the ION province (ANOVA: P < 0.05; P < 0.01, respectively) and lower than in 2005 in the TYR one (ANOVA: P < 0.05).
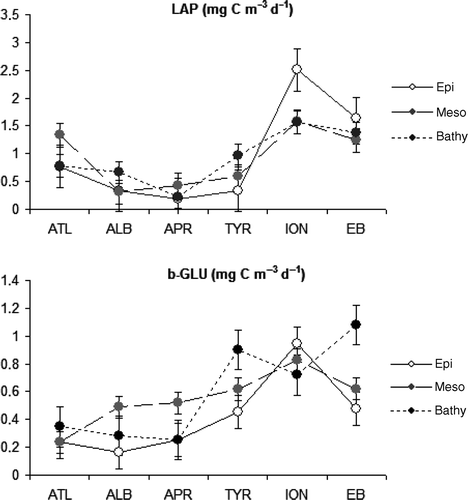
In 2007, in the ATL province, LAP activity in the mesopelagic layer exceeded that observed in the epi- and bathypelagic ones; this behaviour was also observed in the APR basin (). LAP was higher in the bathypelagic layer compared to the other ones only at the ALB and TYR provinces. b-GLU activity showed lower absolute values than LAP, except in the APR and TYR where it exceeded LAP activity in the surface layer. b-GLU generally exhibits greater importance in the meso- and bathypelagic areas (). Both activities increased from Western to Eastern provinces at all depths ().
Figure 4. EEA distribution along the MED in 2007: depth-normalized LAP and b-GLU values (expressed as mg C m−3 d−1) in the different provinces at the epi- meso- and bathypelagic layers.
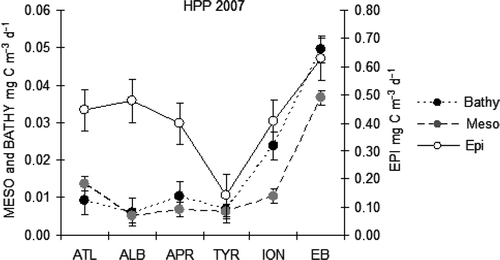
In 2004 in the ION province, the depth-normalized HPP rate was 8 times higher in the epi- than in the meso- and bathypelagic layers, reaching 0.244 mg C m−3 d−1. In 2005, HPP showed the highest values in the epipelagic layer (0.323 mg C m−3 d−1) and the lowest in the mesopelagic layer; values in the bathypelagic layer fell in between. In 2007, high values were always observed in the epipelagic (), where they were over 0.397 mg C m−3 d−1, with the only exception of the TYR province (). The maximum value occurred in the EB with rates exceeding 0.627 mg C m−3 d−1. In the meso- and bathypelagic layers, low values were found although several high HPP rates were frequently detected in the deeper layers. At the sub-basin scale, higher HPP activities in the mesopelagic layer were observed in the Eastern MED as compared to the Western one.
Figure 5. Distribution along the MED of the HPP depth-normalized values (mg C m−3 d−1) in the different provinces at the epi- meso- and bathypelagic layers in 2007.
In 2004, CR showed higher depth-normalized values in MED basin with respect to the ATL station, with significant differences at epipelagic layers (PERMANOVA: P < 0.0001). In the MED basin, at the epipelagic layer an eastward increasing trend was observed reaching the maximum value in the TYR. At the mesopelagic layer, low CR values were observed, reflecting a trend similar to the upper layer. In the bathypelagic layer, the CR rates decreased again, with the only exception in the ALB, where the highest data was detected. The CR values were not statistically different between the Western and Eastern MED in the epi- and mesopelagic layers, whilst significant decreases were observed in the bathypelagic layers (PERMANOVA P < 0.0001).
In 2005, depth-normalized values of CR were lower than in 2004, with the values decreasing by about a factor of 6 and 2 in the epi- and bathypelagic-layers, respectively, but only the decrease in the epipelagic layer was significant (ANOVA: P < 0.01).
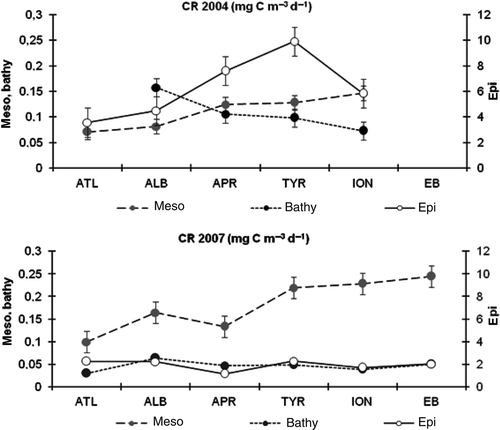
In 2007, CR rates showed significantly lower depth-normalized values than in 2004 () in both the epi- and bathypelagic layers (PERMANOVA: both P < 0.0001). On the contrary, in the mesopelagic layer the CR values significantly increased (ANOVA: P = 0.00035). Moreover significant differences were found between Western MED basin and ATL province (ANOVA P < 0.0001). In , the variability of CR along a West-East transect in 2004 and 2007 is reported. In 2004, positive W-E trends in both the epi- and mesopelagic layers were observed, whilst a negative W-E tendency in the bathypelagic layer was found. In fact, the highest respiratory rates were observed in the ALB province and the lowest in the ION. In 2007, lower CR values were detected in the epi- and bathypelagic layers where no evident longitudinal variations were observed, while in the mesopelagic layer, the eastwards increasing distribution of CR values was confirmed.
Figure 6. Distribution of the depth-normalized CR values in the different provinces along the MED in the epi- meso- and bathypelagic layers in 2004 and 2007. The values are expressed as mg C m−3 d−1.
In , the power functions of the CR distribution in the water column, the coefficients of the power functions, and the depth-integrated CR values for 2004 and 2007 in the six provinces are shown. In 2004, APR and ION depth-integrated rates were comparable and higher than those in the TYR. In 2007 the rates in the ALT, APR and ION provinces were again similar to each other but lower than those in the ALB, TYR and EB provinces. Steep slopes of the CR distribution within the deep-water were computed at all the MED provinces. Decreasing rates were recorded in the water column below 200 m, so that the exponents (x) of the CR power functions were negative. Only the ATL exponent (x) in November 2004 was positive. In a different way, in 2004, the exponents (x) of the ALB function were the lowest followed by the exponents (x) of the ION, indicating a slower decrease of the respiration along the entire water column. It is important to remember that at low x, the exponents correspond to steep slopes. In 2007, the power functions changed for all the provinces, with the exception of the slope calculated for the ION equation (). reports the ‘shallow’ CR (depth integrated from 200 to 1200 m), the ‘deep’ CR (depth integrated from 1500 to 2500 m) as mg C m−2 d−1 as well as the deep/shallow ratios (as percentages) estimated in the studied areas. These ratios have been calculated using the CR integrated data derived from each equation reported in . Such a ratio was suggested to quantify the relative importance of deep vs. shallow layer respiration as a percentage of the deepest C respiration (1500–2500 m) with respect to the remaining upper aphotic layers (200–1200) Citation51. The deep C respiration was stronger in November 2004 than in June 2007. In particular, in 2004 the APR, TYR, ION rates, calculated at the same depth range, were 1.76, 1.18 and 1.92 times higher than in 2007, respectively.
Table 4. CR power functions and CR depth-integrated values in 2004 and 2007 in the studied provinces.
Table 5. Shallow respiratory rates (depth integrated from 200 to 1200 m), deep respiratory rates (depth integrated from 1500 to 2500 m) and deep/shallow ratios (as percentages) estimated in the studied Mediterranean provinces.
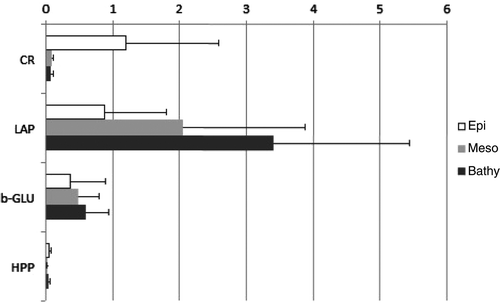
Assuming that the activities we measured were mainly due to the prokaryotic fraction and that all the cells have similar activity levels, cell specific activity was calculated to compare surface-water with deep sea cell activity (from the whole data set). In , CR, LAP, b-GLU and HPP cell specific activities are presented. CR rates per cell decreased with increasing depth, whilst LAP and b-GLU cell specific rates generally showed an opposite behavior and increased with depth. For HPP, the trend of cell specific activity was: epi > bathy > meso-pelagic layers.
4. Discussion
4.1. Uncertainties in the estimation of the prokaryotic activities
This study contributes to the knowledge of three microbial heterotrophic key-processes that rule the cycle of the carbon in the sea. To our knowledge, there are few synoptic reports available on these activities across the whole MED basin particularly in its dark water column. We know that some uncertainties exist with the adopted analytical procedures as already discussed Citation12,Citation52. The determination of respiration from ETS relies on some empirically determined factors to convert the ETS Vmax into actual rates of O2 consumption and CO2 production Citation53. However, the ETS data interpretation ‘faces problems, but no more so than those of other commonly used rate process techniques (14C and 3H-tymidine) Citation54 or extrapolations of remotely sensed ocean colour data to sea surface (Chl and primary production estimates)’ Citation55. Currently, the ETS assay has gained acceptance due to the ubiquity of the ETS and its role as an universal tracer of life and metabolism. Furthermore the ETS assay offers sensitivity and resolution levels as well as data acquisition rates that are not attainable with incubation-based methods. It is a sensitive biochemical assay especially suited for estimating the community respiration of organisms in the meso- and bathypelagic waters Citation12,Citation14,Citation56–59. Moreover, it facilitates the collection of large temporal and spatial data sets rendering it suitable for oceanographic surveys.
With regard to all enzyme assays, it is likely that pressure affects the expression of microbial enzymes; consequently the true HPP and EEA in situ activities could be higher than that estimated in atmospheric pressure. A threefold to fivefold decrease in the bacterial uptake rates of glucose and aminoacids, when deep water samples were analyzed at sea surface pressure, was found Citation60, while in other study Citation61 a 50% underestimation of EEA values in decompressed water samples was found. The effect of in situ pressure on prokaryotic production and enzyme activities is still a matter of debate; nevertheless they are potential rates and they indicate the capability of the prokaryotic community to decompose organic polymers and incorporate DOC into the microbial food chain.
Despite the uncertainties concerning the microbial activity levels measured here, it is noteworthy that the information reported in this study covering a wide spatial scale is not frequently available in the pertinent literature, excepting for a few other studies Citation7,Citation62,Citation63.
4.2. Experimental observations in 2004, 2005 and 2007
The synthesis of the observations from 2004, 2005 and 2007 showed high variability in general, both with time and space of the microbial processes in the water column. With time, the prokaryotic abundances increased while the bacterial activities decreased from 2004 (ION) or 2005 (TYR) to 2007. The time-changes of b-GLU at TYR in the bathypelagic layer were the only exception. In the context of the global warming, previous investigations recognized that seasonal variability could increase remineralization in warm periods. Consequently strong heterotrophy was predicted Citation10. In our case, the microbial parameters were not impacted by temperature changes, although prokaryotic activities did decrease from November–October 2004 to June 2007.
The PA, EEA and CR in the water column showed linear decreasing trends with depth in all provinces with the exceptions of LAP at ION (2004) and TYR (2005) and CR at ALB in 2004, when an increase between meso- and bathypelagic layers occurred. Altogether these findings confirmed the negative relationship between respiration and depth. Respiration decreases by about 53% over the mesopelagic layer have been observed Citation13 but, at the same time, enhanced respiratory activity at 1000–2000 m depth often occurs and seems to be a consistent feature in various seas and oceans. In our study case, in 2004, CR declined by 57% from the epi- to meso-pelagic, but in 2005 and 2007 by only 15 and 10%, respectively. The declines from epi- to bathypelagic were 58, 20, and 42% in 2004, 2005 and 2007, respectively.
With regards to the cell specific activities, increases of exoenzymatic and respiratory activities with depth but not for HPP were reported in the meso- and bathypelagic layers of the sub-tropical Atlantic Citation12. Decreasing cell-specific HPP values with depth, as observed in this study, were also noted in a synthesis on microbial activity in the dark ocean Citation3. This behavior means that DOC uptake per cell is reduced, especially in the mesopelagic layer where the lowest single-cell HPP was observed, thus suggesting a low cell metabolism in these dark waters. The meaning of the EEA per cell increases was probably related to availability of labile polymeric OM (both dissolved and particulate), so the cells produced more enzyme to degrade OM, however as HPP per cell decreased, the monomers released cannot be taken up by prokaryotes for new biomass production, causing an uncoupling of degradation and production processes. This phenomenon was reported for bacteria living in aggregates in euphotic zones Citation44 but never in the aphotic zones. Biomass production decreases with the increasing expression of extracellular enzymes was observed only in the sub tropical Atlantic Ocean Citation12.
4.3. Longitudinal patterns of cell abundances and activities
More interesting findings were the occurrences of the longitudinal patterns of the prokaryotic activities and cell abundance along the MED basin and their variability among the years. In 2004, PA were homogeneously distributed throughout the whole MED basin in the three layers. The cell abundances were in agreement with a previous study carried out in 2004 Citation18 and showed a similar increase at meso- and bathypelagic layers in the Eastern stations. Instead, in two MED transects done during 1999, similar abundance but different longitudinal distribution were observed in the epipelagic layer Citation63. In 2007, the situation changed with increasing prokaryotic abundances and statistically different distribution in each layers compared to 2004. Significant differences were observed between Western and Eastern basin at all layers.
Enzyme levels showed a high occurrence of LAP activity during summer 2005 in the TYR province, confirming the availability of greater amounts of labile, proteinaceous matter prone to microbial decomposition. Conversely, high levels of b-GLU activity measured during autumn 2004 in the ION province suggested with the presence of high concentrations of refractory compounds, especially at the epipelagic layers. In 2007, LAP distribution was in the same order of magnitude as that found in a previous study Citation63. The prevalence of LAP at the bathypelagic layer observed at the ALB and TYR basins indicated an active microbial community metabolizing proteinaceous substrates still present even in the deepest waters. High cell-specific activities in the meso- and bathypelagic layers are a common feature of the deep-sea prokaryotes Citation7,Citation12,Citation61. Thus, they might express more enzymes than epipelagic cells to cleave the same amount of monomers, due probably to the more recalcitrant nature of organic matter at depth Citation12. Moreover, our hypothesis is that the renewal of the deep water in the MED probably introduces new un-degraded organic matter Citation32.
HPP rates were in agreement with those previously reported in the MED Sea Citation63, moreover along a longitudinal transect the Western distribution was similar as well as in the Easternmost regions an increase occurred.
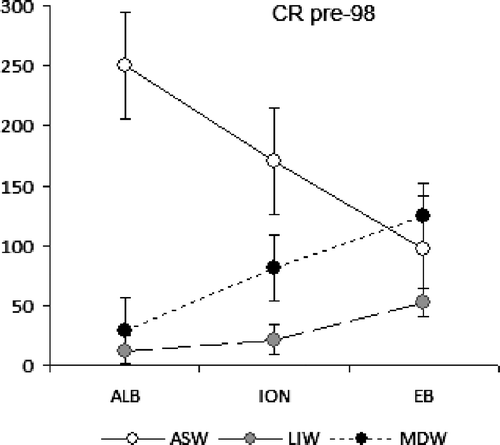
The distribution of the respiratory rates showed trends in relation with isobaths. In 2004, a general W-E increasing pattern was observed in the epipelagic layer in the western MED basin and, more clearly, in the mesopelagic layers; an opposite decreasing trend in the bathypelagic layer also occurred. In 2007, lower values were detected in the epi- and bathypelagic layers compared to 2004, while the mesopelagic layer showed the same Eastward increasing slope. For comparison, the data of CR referred by several authors before 1998 in the MED were reported and assigned within the different main MED water masses Citation64 (). The analysis of the time evolution of CR along MED transect, by comparison with and , has shown the occurrence of variable scenarios in the C respiration. In fact, before 1998 the CR values appeared associated to the circulatory patterns of the main water masses such as Atlantic Surface Water (ASW), LIW and Mediterranean Deep Water (MDW) roughly and partially superimposed on the epi-, meso- and bathypelagic layers, respectively, and with the preformed organic load injected from the new dense water formation sites (both deep and intermediate layers) (see Citation64). In fact, in the epipelagic layer the CR values decreased Eastward, in mesopelagic layer the values decreased Westward and in bathypelagic layer the values decreased from the deep water formation sites toward the Western and Eastern deep MED basins Citation25. Moreover, in the MDW of the EB basin, the enhanced oxidative processes depended on the newly entrapped organic matter in the younger outflowing Aegean water and served as a first signal of the EMT event. After 1998, the EMT has spread into the rest of the MED.
Figure 8. Distribution of CR mean values (mg C m−3 d−1) in the ALB, ION and EB provinces within the Atlantic Surface Water (ASW, 0–200 m), Levantine Intermediate Water (LIW, 200–600 m) and Mediterranean Deep Water (MDW, 600 m–bottom depth) before 1998 (modified from Citation64).
In 2004 throughout the MED, the CR levels fell consistently, in comparison to earlier studies and the organic matter transported by the EMT spread along the western part of the MED basin. In the surface layer, the enhanced respiration rates in the Eastern provinces evidenced that the close relationship between CR and the ASW circulatory pathway was missing. In the mesopelagic layers the scenario observed before 2000 occurred again, although to a lower extent. In the bathypelagic the EMT, spreading towards the Western basin, probably sustained the increase of CR values in the Western provinces while, in the Eastern provinces, the pre-2000 situation tended to be restored. In 2007, at the epi- and bathypelagic layers the CR values decreased about 5 and 3 times, respectively, with respect to 2004. In the mesopelagic layer the CR values kept the same trend already observed in 2004 and the pre-1998 years. Briefly, considering the westward diffusion of the EMT within the whole MED, implying, among other things, the spreading of waters mass with peculiar physical and chemical characteristics (more saltier and rich-in-organic-carbon waters), a speculative synoptic picture could indicate the tight coupling between microbial respiration and the hydrological and circulatory patterns in the upper and deep layers. Nevertheless, the LIW contribution to the mesopelagic water renewal continues to be the prevailing engine of the MED circulatory patterns and the deep biological activity, even though at different levels, the CR distribution suggests that the mesopelagic layer was less affected by climatic changes.
4.4. Evidence of the unique biogeochemical peculiarities of the Mediterranean Sea
ATL prokaryotic activities and abundances were different from those of the MED provinces. These findings need to be confirmed by more data, nevertheless, the data we have support the hypothesis that the deep-metabolism of the MED is different from other seas. However, comparing the ‘deep’-integrated CR and the ‘shallow’-integrated CR throughout the MED, the deep aphotic zone metabolism contributes more than 76% of the shallow C respiration. This argues for the occurrence of important mechanisms other than sinking and export production. For example, it is known that the lateral advection of new-formed water masses (both intermediate and deep) from convective regions as well as the lateral injection in the winter of organic matter from the canyons and shelves of the northern MED enhanced C respiration in deep layers Citation65.
Hence, the export of biogenic carbon from the surface layers to deeper waters–normally driven by the vertically oriented biological pump–is enhanced by a considerable amount of ‘preformed’ organic products conveyed and respired within laterally moving water masses Citation56.
Previous studies showed that the ratio of oceanic ‘deep’-integrated CR to the ‘shallow’-integrated CR ratios were always below 21%. The only exception was the ratio of 44% calculated in 2007 in the ATL province, in this study. This occurrence was probably strongly connected with the outflowing of dense LIW waters from the MED to the Atlantic and, presumably, the pool of respired carbon is transported along the isopycnal surfaces in this outflowing LIW Citation66. Hence, notwithstanding climate events that temporarily changed the MED hydrological patterns, the uniqueness of the MED circulation is still associated with its intermediate waters and their important physical and biological contributions to the Atlantic waters.
5. Conclusions
In conclusion, our field studies have confirmed a great variability in the different steps in the C cycle, in contrast to the old view of a steady-state system of MED. The occurrence of diverse patterns in microbial metabolism has emphasized the MED's sensitivity to climate changes. They suggest that the export of biogenic carbon depends not only on the biological pump but also on lateral advection of organic matter associated with winter cooling, deep-mixing, and off-shelf canyon flows. In this context, both CR and PA seem to be suitable markers to describe the variability in the dark deep waters of the Mediterranean Sea. Despite the few data available on enzyme activities, the temporal decrease in their activity levels argues that hydrological changes could have affected negatively the deep-sea microbial decomposition processes. Future investigations are necessary to verify these hypotheses and describe the temporal evolution of these trends. The increases with depth of specific enzyme activities suggest comparable behaviour of the MED with open ocean tropical environments. On the contrary, respiratory rates and their variability indicate that the MED shows biogeochemical peculiarities different from other oceanic systems. Microbial activities in the deep layers of the MED provinces appear to be modulated by the deep circulatory patterns. Here, two pathways seemed to rule the MED biogeochemical arrangement: the EMT spreading across the entire basin mainly at deep levels, and the ‘classical’ LIW circulation route in the mesopelagic layer. In fact, notwithstanding the EMT climate event that temporarily changed the MED hydrological patterns, the intermediate waters maintain their unique and peculiar characteristics within the MED circulation.
Acknowledgments
We want to thank M. Dibitetto (CNR–DCSPI), M. Astraldi (CNR–ISMAR, La Spezia), L. Giuliano (CIESM), E. De Domenico (CONISMA–University of Messina) for allowing us to participate in MedGOOS, CIESM SUB 1/SUB 2, MedBio, FIRB-MIUR Cruises. The research was funded by: FIRB-MIUR Project ‘Biodiversity and organization of the communities in different environmental context’ and partially by University of Messina (PRA 2003 and 2004); by MedGOOS (Global Ocean Observing System for the Mediterranean), by VECTOR Project (VulnErabilità delle Coste e degli ecosistemi marini italiani ai cambiamenti climaTici e loro ruolO nei cicli del caRbonio mediterraneo) in the frame of the CARPEL–TRANSMED cruises. Again many thanks to the following colleagues of IAMC-UOS.ME: F. Soraci, F. Raffa and A. Cosenza for their valuable technical assistance and F. Genovese, P. De Francesco, M. Furnari for their prompt and friendly help in logistic support.
References
- Raven , JA and Falkowski , PG . 1999 . Ocean sink for atmospheric CO2 . Plant Cell Environ. , 22 : 741 – 755 .
- Packard , TT , Denis , M , Rodier , M and Garfield , P . 1988 . Deep-ocean metabolic CO2 production: calculations from ETS activity . Deep-Sea Res. , 35 : 371 – 382 .
- Arístegui , J , Gasol , GM , Duarte , CM and Herndl , GJ . 2009 . Microbial oceanography of the dark ocean's pelagic realm . Limnol. Oceanogr. , 54 ( 5 ) : 1501 – 1529 .
- Benitez-Nelson , CR . 2000 . The biogeochemical cycling of phosphorus in marine systems . Earth-Sci. Rev. , 51 : 109 – 135 .
- Morel , FMM and Price , NM . 2003 . The biogeochemical cycles of trace metals in the oceans . Science , 300 ( 5621 ) : 944 – 947 .
- Hansell , DA and Carlson , CA . 2002 . Biogeochemistry of Marine Dissolved Organic Matter , 1 – 774 . San Diego, CA : Academic Press .
- Hoppe , HG , Gocke , K , Koppe , R and Begler , C . 2002 . Bacterial growth and primary production along a north-south transect of the Atlantic Ocean . Nature , 416 : 168 – 171 .
- Andersson , JK , Wijsman , JWM , Herman , PMJ , Middelburg , JJ , Soetaert , K and Heip , C . 2004 . Respiration patterns in the deep ocean . Geoph. Res. Lett. , 31 : L03304 2004 doi: doi: 10.1029/2003GL018756
- La Ferla , R , Azzaro , M , Civitarese , G and Ribera D’Alcalà , M . 2003 . Distribution patterns of carbon oxidation in the Eastern Mediterranean Sea: evidence of changes in remineralization processes . J. Geophys. Res. , 108 ( C9 ) : 8111
- La Ferla , R , Azzaro , F , Azzaro , M , Caruso , G , Decembrini , F , Leonardi , M , Maimone , G , Monticelli , LS , Raffa , F , Santinelli , C , Zaccone , R and Ribera d’Alcalà , M . 2005 . Microbial processes contribution to carbon biogeochemistry in the Mediterranean sea: spatial and temporal scale variability of activities and biomass . J. Mar. Sys. , 57 : 146 – 166 .
- Zaccone , R , Monticelli , LS , Seritti , A , Santinelli , C , Azzaro , M , Boldrin , A , Ferla , RLa and d’Alcalà , MRibera . 2003 . Bacterial processes in the intermediate and deep layers of the Ionian Sea in winter 1999: vertical profiles and their relationship to the different water masses . J. Geophys. Res. , 108 (C9) : 8117 PBE 18 doi: doi: 10.1029/2002JC001625
- Baltar , F , Arístegui , J , Sintes , E , van Haken , HM , Gasol , JM and Herndl , GJ . 2009 . Prokariotic extracellular enzyme activity in relation to biomass production and respiration in the meso- and bathypelagic waters of the (sub)tropical Atlantic . Environ. Microbiol. , 11 ( 8 ) : 1998 – 2014 .
- del Giorgio , PA and Duarte , CM . 2002 . Respiration in the open ocean . Nature , 420 : 379 – 384 .
- Arístegui , J , Agustí , S , Middelburg , JJ and Duarte , CM . 2005 . “ Respiration in the mesopelagic and bathypelagic zones of the oceans ” . In Respiration in Aquatic Systems , Edited by: del Giorgio , PA and Williams , PJle B . 181 – 205 . New York : Ofxord University Press .
- Koppelmann , R and Weikert , H . 1992 . Full-depth zooplankton profiles over the deep bathyal of the NE Atlantic . Mar. Ecol. Prog. Ser. , 86 : 263 – 272 .
- Countway , PD , Gast , RJ , Denett , MR , Savai , P , Rose , JM and Caron , DA . 2007 . Distinct protistan assemblages characterize the euphotic zone and deep sea (2500 m) of the western North Atlantic (Sargasso Sea and Gulf Stream) . Environ. Microbiol. , 9 : 1219 – 1232 .
- Fukuda , H , Sohrin , R , Nagata , T and Koike , I . 2007 . Size distribution and biomass of nanoflagellates in meso- and bathypelagic layers of the subarctic Pacific . Aquat. Microb. Ecol. , 46 : 203 – 207 .
- Magagnini , M , Corinaldesi , C , Monticelli , LS , De Domenico , E and Danovaro , R . 2007 . Viral abundance and distribution in mesopelagic and bathypelagic waters of the Mediterranean Sea . Deep-Sea Res. I. , 54 : 1209 – 1220 .
- Pedros-Alió , C , Calderon-Paz , JI , Guixa-Boixereu , N , Estrada , M and Gasol , JM . 1999 . Bacterioplankton and phytoplankton biomass and production during summer stratification in the northwestern Mediterranean sea . Deep-Sea Res. I. , 46 : 985 – 1019 .
- Van Wambeke , F , Heussner , S , Diaz , F , Raimbault , P and Conan , P . 2002 . Small-scale variability in the coupling/uncoupling of bacteria, phytoplankton and organic carbon fluxes along the continental margin of the Gulf of Lions, Northwestern Mediterranean Sea . J. Mar. Syst. , 33–34 : 411 – 429 .
- Tanaka , T and Rassoulzadegan , F . 2002 . Full-depth profile (0–2000 m) of bacteria, heterotrophic nanoflagellates and ciliates in the NW Mediterranean Sea: vertical partitioning of microbial trophic structures . Deep-Sea Res. II. , 49 : 2093 – 2107 .
- Caruso , G , Monticelli , LS , Ferla , RLa , Maimone , G , Azzaro , M , Azzaro , F , Decembrini , F , Leonardi , M and Domenico , EDe . 2007 . “ Microbial distribution and activity within the epi-, meso- and bathypelagic layers of the southern Tyrrhenian Sea ” . In 3rd International Conference ‘Enzymes in the Environment: activity, ecology, applications’ Viterbo
- Danovaro , R , Dell’Anno , A , Corinaldesi , C , Magagnini , M , Noble , R , Tamburini , C and Weinbauer , M . 2008 . Major viral impact on the functioning of benthic deep-sea ecosystems . Nature. , 454 : 1084 – 1087 .
- Tamburini , C , Garel , M , Al Ali , B , Mérigot , B , Kriwy , P , Charrière , B and Budillon , G . 2008 . Distribution and activity of prokaryotes in the water column of the Tyrrhenian Sea . Deep-Sea Res. II. , 56 : 1533 – 1546 .
- Malanotte-Rizzoli , P , Manca , BB , Ribera d’Alcalà , M , Theocharis , A , Bergamasco , A , Bregant , D , Budillon , G , Civitarese , G , Georgopoulos , D , Korres , G , Lascaratos , A , Michelato , A , Sansone , E , Scarazzato , P and Souvermezoglou , E . 1997 . A synthesis of the Ionian Sea hydrography. Circulation and water mass pathways during POEM phase I . Progr. Oceanogr. , 39 ( 3 ) : 153 – 204 .
- Turley , CM . 1999 . The changing Mediterranean Sea–a sensitive ecosystem? . Progr. Oceanogr. , 44 : 387 – 400 .
- Robinson , AR , Leslie , WG , Theocharis , A and Lascaratos , A . 2001 . Mediterranean Sea circulation, , London : in Ocean Currents. Academic Press . doi: Doi:10.1006/rwos.2001.0376
- Briand F. (ed.), Climate warming and related changes in Mediterranean marine biota, in CIESM Workshop Monographs, Monaco, 35, (2008), 152 pp
- Schroeder , K , Ribotti , A , Borghini , M , Sorgente , R , Perilli , A and Gasparini , GP . 2008 . An extensive Western Mediterranean Deep Water Renewal between 2004 and 2006. Geophys. Res. Lett. , 35 : L18605 doi: doi: 10.1029/2008GL035146
- Manca , BB , Budillon , G , Scarazzato , P and Ursella , L . 2003 . Evolution of dynamics in the eastern Mediterranean affecting water mass structures and properties in the Ionian and Adriatic Sea . J. Geophys. Res. , 108 : 8102
- Roether , W , Klein , B , Manca , BB , Theocharis , A and Kioroglou , S . 2007 . Transient Eastern Mediterranean deep waters in response to the massive dense-water output of the Aegean Sea in the 1990s . Progr. Oceanogr. , 74 ( 4 ) : 540 – 571 .
- A. Seritti, B.B Manca., C. Santinelli, E. Murru, A. Boldrin, and L. Nannicini, Relationships between dissolved organic carbon (DOC) and water mass structure in the Ionian Sea (winter 1999). J. Geophys. Res. 108 (C9) (2003), 8112, PBE13.1–13
- Delfanti , R , Klein , B and Papucci , C . 2003 . Distribution of 137Cs and other radioactive tracers in the eastern Mediterranean: Relationship to the deepwater transient: Physical and Biochemical Evolution of the Eastern Mediterranean in the '90s (PBE) . J. Geoph. Res. , 108 ( C9 ) : PBE9.1 – PBE9.10 .
- Gasparini , GP , Ortona , A , Budillon , G , Astraldi , M and Sansone , E . 2005 . The effect of the Eastern Mediterranean Transient on the hydrographic characteristics in the Strait of Sicily and in the Tyrrhenian Sea . Deep-Sea Res. I. , 52 : 915 – 935 .
- Schroeder , K , Gasparini , GP , Borghini , M and Ribotti , A . 2009 . “ Experimental evidences of the recent abrupt changes in the deep Western Mediterranean Sea ” . In Dynamics of Mediterranean Deep Waters , CIESM Workshop Monographs Edited by: Briand , F . Vol. 38 , 51 – 56 . Monaco
- Santinelli , C , Gasparini , GP , Nannicini , L and Seritti , A . 2002 . Vertical distribution of dissolved organic carbon (DOC) in the Western Mediterranean Sea in relation to the hydrological characteristics . Deep-Sea Res. I. , 49 : 2203 – 2219 .
- Astraldi , M , Conversano , F , Civitarese , G , Gasparini , GP , Ribera d’Alcalà , M and Vetrano , A . 2002 . Water mass properties and chemical signatures in the central Mediterranean region . J. Mar. Sys. , 33–34 : 155 – 177 .
- La Ferla , R , Azzaro , M , Maimone , G and Ribera d’Alcalà , M . 2006 . Microbial biomass and respiration in the Western Mediterranean Sea , Napoli : XVII AIOL Congress .
- Monticelli , LS , Caruso , G , De Domenico , E , Azzaro , F , Azzaro , M , La Ferla , R , Leonardi , M , Maimone , G and Decembrini , F . 2008 . Attività e biomasse microbiche nelle acque superficiali del Mar Ionio . Proc. Italian Assoc Oceanol. Limnol. , 19 ( II ) : 349 – 359 .
- Ferla , RLa , Azzaro , M , Budillon , G , Caroppo , C , Decembrini , F and Maimone , G . Variability of microbial biomass and activities in the water masses of the South Tyrrhenian Sea (Mediterranean) . Aq. Microb. Ecol. , (submitted)
- Zaccone , R , Boldrin , G , Caruso , G , La Ferla , R , Maimone , G , Irrera , GP , Santinelli , C and Turchetto , M . 2009 . Attività sulla sostanza organica ed abbondanza microbica nel Mediterraneo durante la campagna Transmed , Venezia : XIX AIOL Congress .
- Hoppe , HG . 1983 . Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates . Mar. Ecol. Prog. Ser. , 11 : 299 – 308 .
- Hoppe , HG . 1993 . “ Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria ” . In Handbook of Methods in Aquatic Microbial Ecology , Edited by: Kemp , PF , Sherr , BF , Sherr , EB and Cole , JJ . 423 – 432 . Boca Raton, FL : Lewis Publisher .
- Smith , DC and Azam , F . 1992 . A simple, economical method for measuring bacterial protein synthesis rates in seawater using 3H leucine . Mar. Microb. Food Webs. , 6 : 107 – 114 .
- Kirchman , DL . 1993 . “ Leucine incorporation as a measure of biomass production by heterotrophic bacteria ” . In Current Methods in Aquatic Microbial Ecology , Edited by: Kemp , PF , Sherr , BF , Sherr , EB and Cole , JJ . 509 – 512 . Boca Raton, FL : Lewis Publisher .
- Packard , TT . 1971 . The measurement of respiratory electron transport activity in marine phytoplankton . J. Mar. Res. , 29 : 235 – 244 .
- Porter , KG and Feig , YS . 1980 . The use of DAPI for identifying and counting aquatic microflora . Limnol. Oceanogr. , 25 : 943 – 948 .
- La Ferla , R , Lo Giudice , A and Maimone , G . 2004 . Morphology and LPS content for the estimation of marine bacterioplankton biomass in the Ionian Sea . Sci. Mar. , 68 : 23 – 31 .
- Manca , BB , Ursella , L and Scarazzato , P . 2002 . New development of Eastern Mediterranean circulation from thermohaline properties and marine current measurements . P.S.Z.N. Mar. Ecol. , 23 ( 1 ) : 237 – 257 .
- Anderson , MJ . 2001 . A new method for non-parametric multivariate analysis of variance . Austral Ecol. , 26 : 32 – 46 .
- Christensen , JP , Packard , TT , Dortch , FQ , Minas , HJ , Gascard , JC , Richez , C and Garfield , PC . 1989 . Carbon oxidation in the deep Mediterranean Sea: evidence for dissolved organic carbon source . Global Biogeochem. Cycles. , 3 : 315 – 335 .
- Packard , TT and Christensen , JP . 2004 . Respiration and vertical carbon flux in the Gulf of Maine water column . J. Mar. Res. , 62 : 93 – 115 .
- Arístegui , J and Montero , MF . 1995 . The relationship between community respiration and ETS activity in the ocean . J. Plankton Res. , 17 ( 1 ) : 563 – 571 .
- del Giorgio , PA . 1992 . The relationship between ETS (electron transport system) activity and oxygen consumption in lake plankton: A cross-system calibration . J. Plankton Res. , 14 : 1723 – 1741 .
- Robinson , C and Williams , PJle B . 2005 . “ Respiration and its measurements in surface marine waters ” . In Respiration in Aquatic Systems , Edited by: del Giorgio , PA and Williams , PJle B . 147 – 180 . New York : Ofxord University Press .
- La Ferla , R , Azzaro , M and Chiodo , G . 1999 . Microplankton respiratory activity and CO2 production rates in the Otranto Strait (Mediterranean Sea) . Aquat. Ecol. , 33 : 157 – 165 .
- Koppelmenn , R , Weikert , H and Halsband-Lenk , C . 2004 . Mesozooplankton community respiration and its relation to particle flux in the oligotrophic eastern Mediterranean . Glob. Biogeochem. Cycles. , 18 : 1039
- Azzaro , M , La Ferla , R and Azzaro , F . 2006 . Microbial respiration in the aphotic zone of the Ross Sea (Antarctica) . Mar. Chem. , 99 ( 1–4 ) : 199 – 209 .
- Packard , TT and Codispoti , LA . 2007 . Respiration, mineralization, and biochemical properties of the particulate matter in the southern Nansen Basin water column in April 1981 . Deep Sea Res. I,. , 54 : 403 – 414 .
- Tholosan , O , Garcin , J and Bianchi , A . 1999 . Effects of hydrostatic pressure on microbial activity through a 2000 m deep water column in the NW Mediterranean Sea . Aquat. Microb. Ecol. , 32 : 209 – 218 .
- Tamburini , C , Garcin , J and Bianchi , A . 2002 . Role of deep-sea bacteria in organic matter mineralization and adaptation to hydrostatic pressure conditions in the NW Mediterranean Sea . Aquat. Microb. Ecol. , 32 : 209 – 218 .
- Misic , C , Castellano , M , Fabiano , M , Ruggieri , N , Saggiomo , V and Povero , P . 2006 . Ectoenzymatic activity in surface waters: A transect from the Mediterranean Sea across the Indian Ocean to Australia . Deep-Sea Res. I. , 53 : 1517 – 1532 .
- Van Wambeke , F , Christaki , U , Giannakourou , A , Moutin , T and Souvemerzoglou , K . 2002 . longitudinal and vertical trends of bacterial limitation by phosphorus and carbon in the Mediterranean Sea . Microbial Ecol. , 43 : 119 – 133 .
- La Ferla , R and Azzaro , M . 2001 . Microbial respiration in the Levantine Sea: evolution of the oxidative processes in relation to the main Mediterranean water masses . Deep-Sea Res. I. , 48 ( 10 ) : 2147 – 2159 .
- Packard , TT , Gòmez , M and Christensen , J . 2008 . “ Fueling Western Mediterranean deep metabolism by deep water formation and shelf-slope cascading; evidence from 1981 ” . In Dynamics of Mediterranean Deep Waters, CIESM Workshop Monographs , Edited by: Briand , F . 132 Monaco .
- Savenkoff , C , Lefèvre , D , Denis , M and Lambert , CE . 1993 . How do microbial communities keep living in the M editerranean outflow within northeast Atlantic Intermediate Waters? . Deep-Sea Res. II. , 40 ( 1–2 ) : 627 – 641 .