Abstract
The demographic transition of the 1930s–1960s dramatically improved life expectancy in some developing countries. Cohorts born during this time are increasingly characterized by their survivorship of poor early-life conditions, such as poor nutrition and infectious diseases. As a result, they are potentially more susceptible to the effects of these conditions at older ages. This study examines this conjecture by comparing obesity, diabetes, and hypertension in older adults born in the beginning portion of the 1930s–1960s across different mortality regimes using a subset of harmonized cross-national data from seven low- and middle-income countries (RELATE, n = 16,836). Using birthplace and height as indicators of early-life conditions, the results show (1) higher prevalence of obesity and diabetes and higher likelihood of obesity, diabetes, and hypertension in middle-income countries, but (2) no convincing evidence to indicate stronger effects of early-life conditions on health in these countries. However, shorter adults living in urban areas were more likely to be obese, indicating the overall importance of early-life conditions and the potential negative impact of urban exposures during adulthood. Obesity results may foreshadow the health of future cohorts born in the later portion of the 1930s–1960s as they reach older ages (60+).
Introduction
There has been solid evidence of associations between early-life conditions and older adult health (Almond and Currie Citation2010; Barker Citation1998; Crimmins and Finch Citation2006; Davey Smith et al. Citation1998; Elo and Preston Citation1992; Hayward and Gorman Citation2004; Wilkinson and Marmot Citation2003; van Ewijk, Painter, and Roseboom Citation2013; Lillard et al. Citation2015). Although there have been a few studies that examine the effects of macro-level events in early life on adult health (van den Berg, Doblhammer, and Christensen Citation2009), there have been almost no empirical studies regarding the degree to which important historical, demographic, and epidemiological changes in early life have influenced older adult health in the developing world. Reductions in infant and child mortality during the 1930s–1960s triggered by the medical and public health revolution led to dramatically increased life expectancy, but without parallel improvements in standards of living (Preston Citation1976). These early-life circumstances are predicted to affect elderly health in the developing world for at least the next 20–30 years (Palloni, Pinto-Aguirre, and Pelaez Citation2002) in the form of diseases such as obesity, diabetes, and heart disease, which are forecasted to increase in the developing world (WHO Citation2000; Kinsella and He Citation2009; Murray and Lopez Citation1996). Do the early-life circumstances of individuals born in the 1930s–1960s in any way help explain the increasing prevalence of these conditions among older adults in the developing world? The purpose of this study is to examine the evidence to answer this question by comparing the health of older adults in low- and middle-income countries born in the beginning decades of the period of the 1930s–1960s.
Public Health Interventions of the Early to Mid-Twentieth Century
Public health interventions in the developing world in the early to mid-twentieth century were focused primarily on reducing infectious disease by reducing infection through sanitary improvements, but not direct improvements to diet and nutrition (Farley Citation2003; Clark Citation1930), because the synergy between nutrition and infection was not fully recognized until later in the century (Scrimshaw Citation1968). This period saw stagnant economic growth for many developing countries (Maddison Citation2006), and, even though reducing infection indirectly improves nutrition (Floud et al. Citation2011), without economic improvements, many survivors continued to be exposed to poor nutrition and poor diet, resulting, for the most part, in a stunted population.
The public health interventions of the early to mid-twentieth century in developing countries initially benefited those living in urban areas because of limited coverage in rural areas. Although some smaller and poorer countries such as Costa Rica and Puerto Rico implemented country-wide interventions during the 1930s (Clark Citation1930; Rosero-Bixby Citation1990), public health interventions in other larger, present-day middle-income countries, such as Mexico, were mostly concentrated in urban areas during this period (Rodríguez de Romo and Rodríguez de Pérez Citation1998). Present-day low-income countries like China, Indonesia, Ghana, and India had less public health intervention coverage even in urban areas during the 1930s–1940s (Banister Citation1987; Ramasubban Citation2008; Dyson Citation1997; Patterson Citation1981; Patterson Citation1979; Caldwell Citation1967; Nitisastro Citation1970).
Most of the population in the developing world during this period lived in rural areas. In contrast to the developed world (Preston and Haines Citation1991), environmental conditions in rural areas of the developing world during the first part of the twentieth century were precarious and, for the most part, worse than those in urban areas in terms of exposure to poor diet and infectious diseases (Clark Citation1930; Rodríguez de Romo and Rodríguez de Pérez Citation1998)—circumstances still present in some rural areas (Sastry Citation1997).
As countries fully implemented public health interventions in both urban and rural areas, a larger population of infants and children survived. However, during the beginning period of the 1930s–1960s and prior to full country-wide implementation, increasing survivorship affected a smaller portion of the population that included mostly those living in urban areas in larger countries; in only a few exceptional cases did that portion include those living in both urban and rural areas in smaller countries.
Early-Life Mechanisms
While there are several early-life mechanisms that could explain older adult health (Barker Citation1998; Barker et al. Citation2010; Crimmins and Finch Citation2006; Davey Smith et al. Citation1998), the circumstances that produced unique cohorts increasingly characterized by their survival of poor early-life nutrition and infectious diseases during the historical period of the 1930s–1960s increase the chances of the manifestation of Barker-like effects at older ages. Low birth weight and stunted babies, a reflection of poor intrauterine and postgrowth growth, have an increased risk of obesity, diabetes, and hypertension at older ages (Barker Citation1998). Further, the mismatch between being born in a resource-scarce environment and then being exposed to a nutritionally richer environment later in life increases the risk of obesity, diabetes, and hypertension (Osmond and Barker Citation2000; Bateson et al. Citation2004), which in turn is associated with frailty and mortality (Kuh and Ben-Shlomo Citation2004).
Table 1 General framework for the nature of mortality decline across countries in the early to mid-twentieth century and expected health patterns in older adults
Some developing countries experienced significant improvements in economic conditions and increased urbanization later in the twentieth century (Maddison Citation2006; Henderson Citation2002), which might have increased access to a more enriched nutritional environment during adulthood for the survivors of poor early-life conditions from the 1930s–1960s, especially those living in urban areas. Diets increasingly higher in saturated fats are an important source of risk to health (Popkin Citation2006), and the combination of poor early nutritional environment and exposure to Western-style diets could be a lethal combination. Not all economic growth has benefited the entire population in the developing world (López-Alonso Citation2007). Economic improvements largely benefiting urban dwellers may have increased exposure to this type of nutrition in addition to other types of exposures for urban dwellers.
The macro-level changes of the 1930s–1960s may elucidate a clearer manifestation of the Barker-type hypothesis because cohorts are less affected by mortality-driven selection than preceding cohorts as a result of public health interventions and medical technology but are increasingly characterized by survivorship of poor early nutritional and infectious disease environments. However, the manifestation of these macro-level changes (i.e., rapid changes in mortality as a result of public health interventions and medical innovations) played out differently in various countries because of differences in the timing, pace, and reason for mortality decline between countries and mortality regimes. Thus, these differences may have produced cohorts with different mortality experiences that are now leading to different health patterns in later life (Palloni, Pinto-Aguirre, and Pelaez Citation2002).
At one extreme are developed, higher-income countries (denoted as type-A countries, ) that experienced an earlier and more graded mortality decline at a higher standard of living. At the other extreme are low-income countries (type-E countries, ) that experienced rapid mortality decline much later in the twentieth century primarily because of public health interventions and medical technology. In between are developing countries that experienced a mortality decline similar to that seen in the developed world (type-B countries) and countries that are present-day middle-income countries that experienced rapid improvement in life expectancy at some point during the 1930s–1960s (type-C and type-D countries). Differences in mortality decline among cohorts born during the 1930s–1960s in different mortality regimes partially depend on the timing of exposure to the country-wide implementation of public health interventions. Thus, moving from country type A to country type E as country-wide public health interventions are fully implemented in both urban and rural areas implies moving toward countries with cohorts increasingly characterized by their survivorship of poor early-life conditions and the effects of these conditions in older adulthood, including higher fragility, stronger effects of childhood conditions on health, sharper socioeconomic status (SES) inequalities, and increasing mortality risk.
Cohorts at the Beginning of the 1930s–1960s Period
While most of the mortality change during the period of the 1930s–1960s occurred after the mid-1940s in the developing world (Preston Citation1976), the cohorts of the 1940s–1960s have not all reached older ages (60+), and thus it is too early to fully examine those born in the mid-1940s through the 1960s. Nevertheless, recent evidence suggests that poor early-life conditions are, in part, associated with high levels of disease and disability and reduction in life expectancy at older ages in the unique cohorts from the beginning part of the 1930s–1960s (Palloni and Souza Citation2013). Cohorts from settings such as country types C and D (now predominantly middle-income countries) in some regions of the world are now experiencing an increasing prevalence of chronic conditions (Palloni et al. Citation2005), and evidence suggests the importance of early-life nutritional environments and diabetes prevalence among those countries undergoing demographic changes at the beginning of the period (McEniry Citation2014). The reported prevalence of diabetes in type-C and type-D countries is higher than what has appeared historically in developed countries (García-Palmieri et al. Citation1970; Gordon Citation1964; Hadden and Harris Citation1987; Harris et al. Citation1998; Wilkerson and Krall Citation1947).
Thus, examining the cohorts born in the late 1920s through the mid-1940s who are experiencing an increasing prevalence of chronic conditions at older ages is a relevant starting point to examine the conjecture regarding the demographic changes of the 1930s–1960s. It may be possible to reach conclusions regarding the long-term consequences of demographic change in some now-middle-income countries, especially among those most likely to have benefited from improving environmental conditions—those urban-born older adults exposed to poor nutrition and infectious diseases in large, now-middle-income countries or those rural-born older adults in those smaller countries with country-wide public health interventions coverage at the beginning of the 1930s–1960s.
This Study
In this study we examine the degree to which the prevalence and likelihood of chronic conditions are related to the demographic changes of the 1930s–1960s. We focus on adult obesity, diabetes, and hypertension—conditions known to originate in early life (Barker Citation1998)—and select two groups of older adults born in the beginning part of the 1930s–1960s period. The first group experienced increased survivorship of poor early-life conditions (individuals in now predominantly middle-income countries—Costa Rica, Mexico, South Africa, type-C and type-D countries), and the second group experienced very harsh environmental conditions in early life, with little decline in mortality (individuals in China, Ghana, India, Indonesia, type-E countries, low-income countries). Using a recently compiled cross-national dataset of older adults, we use two measures of early life common to many surveys of older adults, rural/urban birthplace and height, to examine the merit of the conjecture. Using the general framework of , we expect to observe a (1) higher prevalence and likelihood of older adult obesity, diabetes, and hypertension; and (2) stronger effects of early-life conditions (birthplace, low height) on these health conditions in older adults born in now-middle-income countries compared with those born in low-income countries. The stronger effects of early-life conditions should be more apparent for those most at risk—the most vulnerable population in now-middle-income countries who experienced increasing survivorship in the 1930s–1940s. In terms of birthplace, this population includes those born in rural areas in the smaller type-C countries where coverage of public health interventions extended into rural areas in the 1930s–1940s and those in urban areas for larger type-C and type-D countries where coverage of public health interventions had not yet reached full implementation in rural areas. In terms of height, this population is composed of shorter older adults.
Data and Methods
Data
The data to compare the health of older adults in low- and middle-income countries were drawn from a subset of the recently compiled Research on Early Life and Aging Trends and Effects (RELATE) database, which contains harmonized cross-sectional and panel data from major surveys of 147,278 older adults or households in 20 countries in Latin America, Asia, Africa, the United States, England, and The Netherlands (RELATE Citation2013). These studies are based on probability sampling and are representative of the older adult population either nationally or in major country provinces. All studies had good interviewer training, good questionnaire design, concern for data quality, and high response rates. The RELATE data were harmonized where possible to make cross-national comparisons across surveys possible (McEniry, Moen, and McDermott Citation2013).
The subset of RELATE data used for this study draws from seven surveys (n = 16,836) of low- and middle-income countries, including the Costa Rican Study of Longevity and Healthy Aging (CRELES); the Indonesian Family Life Survey (IFLS); and the WHO Study on Global Ageing and Adult Health Study (SAGE) from Mexico, China, Ghana, India, and South Africa. We included the SAGE survey because it has cross-national data on low- and middle-income countries, uses similar sample designs and questionnaire construction, and is representative at a country level in some instances.
Measures
Early-Life Conditions
Rural birthplace was used as an indicator of precarious environmental conditions in early life (exposure to poor nutrition and infectious diseases) and was defined according to questions asked of respondents regarding their birthplace and residence during childhood. Height was used as a marker of net nutritional status, reflecting the impact of childhood nutrition and disease (Floud et al. Citation2011), and we used the lowest quartile of adult height in the overall population to indicate stunting. For older adults born at the beginning of the 1930s–1960s period, the seven selected countries fell into two broad groups of mortality regimes at birth characterized by: (1) declining mortality and increased survivorship of poor early-life conditions as a result of public health interventions (Costa Rica, Mexico, South Africa) (Rosero-Bixby Citation1990; Rodríguez de Romo and Rodríguez de Pérez Citation1998; Beinart and Dubow Citation1995), and (2) little mortality decline and continued high infant and child mortality (China, Ghana, India, Indonesia) (Banister Citation1987; Patterson Citation1981; Patterson Citation1979; Caldwell Citation1967; Nitisastro Citation1970; Ramasubban Citation2008; Dyson Citation1997).
Adult Health
Obesity, diabetes, and hypertension were of particular interest because they are known to originate in early life as a result of poor nutrition and/or the synergy with poor nutrition and infectious diseases (Barker Citation1998). Obesity was calculated using measured height and weight. A body mass index of ≥ 30 identified obese individuals (1 = obese, 0 = not obese). Elderly diabetes was defined by dichotomous variables (1 = diabetic, 0 = not diabetic) from self-reports that were based on questions asked of the respondent about whether a doctor had ever diagnosed them with diabetes. Although self-reports for diabetes have shown validity in some settings (Banks et al. Citation2006; Brenes Citation2008; Goldman et al. Citation2003), there is a strong likelihood that diabetes is severely underreported in some developing countries, especially among those with limited access to quality health care. While biomarkers are preferable for the measurement of diabetes, biomarkers were not yet publicly available at the time of most of the selected studies. Hypertension was defined as a dichotomous variable (1 = hypertensive, 0 = not hypertensive) using criteria described in the literature (Yan et al. Citation2012). Hypertensive respondents showed systolic rates of ≥ 140 mmHg, diastolic rates of ≥ 90 mmHg, or reported that they were taking medication to control hypertension.
Other Variables
All statistical models controlled for age, gender, years of education, visits to a doctor, and current residence. Current residence was defined to be either rural (1) or urban (0). The visits to a doctor variable reflects, in part, preventive health care behaviour, and it was defined as a dichotomous variable to reflect at least one visit to a doctor within the last year.
Sample Selection
We selected older adults who were born in the late 1920s to the early 1940s and had participated in those studies that had measured blood pressure, obesity using measured height and weight, and self-reported diabetes in addition to childhood variables (rural birthplace, height). Imputation methods using Stata were used to address missing values (Raghunathan, Reiter, and Rubin Citation2003).
Analyses
Multivariate models using pooled country data for all seven countries and for SAGE countries only were estimated to examine the likelihood of older adult obesity, diabetes, or hypertension as a function of country and other predictor variables. Likelihood ratio tests were conducted comparing constrained and unconstrained models to determine the importance of including country dummy variables in models. Interaction terms between countries and birthplace and countries and low height were included in models to examine the likelihood of disease for those most at risk of manifesting the effects of poor early-life conditions between low- and middle-income countries. Predicted probabilities for pooled models were calculated and compared between countries using average responses on model variables.
Before pooling the data, country-specific models were estimated and associations between predictor variables and adult health compared to ensure comparability regarding the direction of associations across the different countries. We also compared country-specific models and pooled results using weighted and nonweighted models for only the SAGE countries because SAGE countries had similar sample designs and questionnaire construction. There were few differences noted between models with and without sample weights. Similarly, there were few differences noted between models using all seven countries versus only SAGE countries. Thus, reported results are based on nonweighted models.
Results
Sample Characteristics
Demographic and health characteristics of older adults born in the late 1920s to the early 1940s reveal a population of older adults who share some similarities but also exhibit differences in early-life conditions and who differ in terms of adult health patterns, health systems use, and changes in residence from rural to urban settings (). Across countries, there were lower levels of formal education achieved both for the respondent and for parents, with particularly low parental education seen in Ghana and China. Average height for both males and females was similar across countries and suggests a stunted population. Being rural-born was fairly disparate across countries in that, with the exception of Costa Rica, a higher percentage of respondents were born in rural areas in the low-income countries as compared with middle income countries. Comparing rural birthplace with rural residence suggests country differences in migration from rural to urban areas, particularly for Costa Rican adults, among whom 72 percent were born rural but only 38 percent currently resided in rural areas. A higher prevalence of obesity and diabetes was particularly notable in the middle-income countries; the highest prevalence of obesity but lowest prevalence of diabetes among middle-income countries occurred in South Africa. The prevalence of hypertension in the middle-income countries was slightly higher than in the low-income countries. The percentage of respondents who visited a medical doctor within the last year was highest in middle-income countries like Costa Rica and was lowest in Indonesia.
Table 2 Sample characteristics for cross-national data on aging populations born during the late 1920s–early 1940s in selected countries
Country Effects and Early-Life Effects
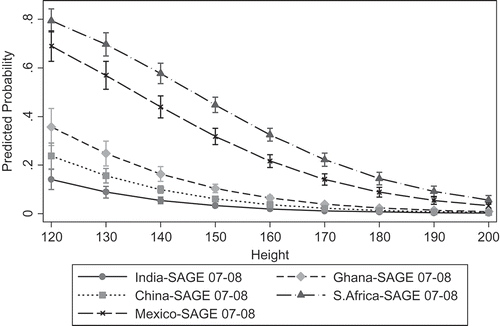
For obesity, a likelihood ratio test comparing models with and without country dummy variables indicated significant country differences in predicting obesity (χ2 (6) = 1894.02, p = 0.000). The likelihood of being obese was much higher in the middle-income countries such as Mexico (OR 5.29, 95% CI = 4.23–6.62) and South Africa (OR 8.14, 95% CI = 6.62–10.00) than in low-income countries such as China (OR 0.66, 95% CI = 0.54–0.82) and India (OR 0.39, 95% CI = 0.29–0.53), relative to Ghana. On average, across all SAGE countries, there was a higher likelihood of obesity for shorter individuals (OR 1.65, 95% CI = 1.45–1.88) and a reduced likelihood for those born in rural areas (OR 0.66, 95% CI = 0.57–0.76) (, Model 1). However, adding current residence (, Model 2) produced an attenuation of rural birthplace (OR 0.90, 95% CI = 0.74–1.10) but showed a significantly reduced odds for those currently living in rural areas (OR 0.64, 95% CI = 0.52–0.79). Similar results appeared for models using all seven countries (Appendix A). The odds of obesity increased slightly with increased level of education (OR 1.03, 95% CI = 1.02–1.05). There were no significant country interactions with height and an inconsistent pattern of country interactions for residence (results not shown). Predicted probabilities for obesity using only SAGE countries (Model 2) showed strong country differences between low- and middle-income countries. The predicted probability of being obese was 0.26 in Mexico and 0.37 in South Africa as compared with 0.04 in China, 0.07 in Ghana, and 0.03 in India (). Across countries there was a higher predicted probability of being obese for shorter individuals living in urban areas than for taller individuals in urban areas; however, there were higher probabilities of being obese and in the first quartile of height in Mexico and South Africa (). Re-estimating models using continuous height produced significant results for height (OR 0.95, 95% CI = 0.04–0.96; results not in table), and predicted probabilities across countries and height showed higher predicted probability of being obese for shorter individuals in Mexico and South Africa ().
Table 3 Models predicting obesity
Figure 1. Predicted probabilities for obesity by country.
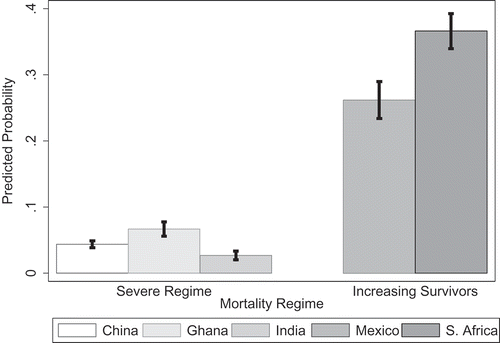
Figure 2. Predicted probabilities of being obese by country, height, and urban residence.
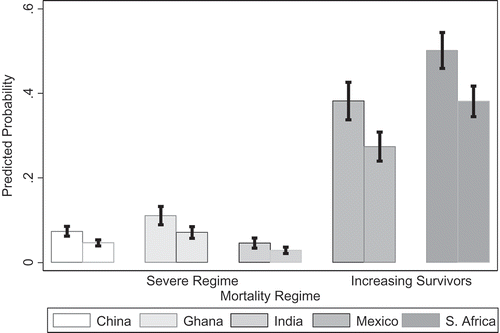
Figure 3. Predicted probabilities for obesity by country and height.
For diabetes, a likelihood ratio test comparing models with and without country dummy variables indicated significant country differences in predicting diabetes (χ2 (6) = 345.52, p = 0.000) although the contrasts between countries were not as sharp as with obesity. The odds of self-reporting diabetes was highest in Mexico (OR 5.55, 95% CI = 4.24–7.26), followed by India (OR 2.44, 95% CI = 1.88–3.18), South Africa (OR 2.36, 95% CI = 1.78–3.14), and China (OR 2.12, 95% CI = 1.67–2.70) (, Model 1). On average, across all low- and middle-income countries, being born in a rural area decreased the likelihood of diabetes (OR 0.49, 95% CI = 0.43–0.57) (, Model 1). Adding current residence made rural birthplace insignificant (OR 0.85, 95% CI = 0.70–1.03) but urban residence significant (OR 0.45, 95% CI = 0.36–0.55) (, Model 2). The odds of diabetes increased slightly with education (OR 1.05, 95% CI = 1.03–1.06). Similar to obesity, there were no significant interactions between countries and height and an inconsistent pattern of significant interactions between countries and residence (results not shown). Models using all countries produced similar results (Appendix A).
For hypertension, a likelihood ratio test comparing models with and without country dummy variables indicated significant country differences in predicting hypertension (χ2 (6) = 753.78, p = 0.000). SAGE-only models showed high likelihood of hypertension in South Africa (OR 2.54, 95% = CI 2.15–3.01), followed by Mexico (OR 1.64, 95% = CI 1.39–1.94) and China (OR 1.52, 95% CI = 1.36–1.70) (, Model 3). Initial effects of rural birthplace became insignificant when current residence was added, although current residence was also not significant (OR 0.89, 95% CI = 0.78–1.02) (, Model 4). The odds of hypertension increased slightly with education (OR 1.01, 95% CI = 1.00–1.02). Similar results were obtained for all seven countries (Appendix A).
Discussion
Demographic changes in the 1930s–1960s as a result of public health and medical interventions in the absence of parallel improvements in standards of living produced cohorts that may be more susceptible at older ages to the long-term consequences of poor early-life conditions. Expectations for a higher prevalence and likelihood of chronic conditions and stronger early-life effects on adult health among older adults born at the beginning of this period and in now-middle-income countries (country types C and D) were partially met for obesity and diabetes. We found a higher prevalence of obesity and diabetes in older adults born in middle-income countries but no large differences in hypertension (measured) and a higher likelihood of being obese, diabetic, and hypertensive in some selected middle-income countries. There was no strong evidence of stronger effects of early-life conditions in the middle-income countries, and urban residence was more predictive of disease than was urban birthplace. However, overall, being short and living in urban areas increased the likelihood of being obese, and shorter individuals from middle-income countries showed a higher probability of being obese. While these broad patterns in the data suggest the
Table 4 Models predicting diabetes and hypertension
overall importance of early-life conditions in predicting obesity in both low- and middle-income countries, there is insufficient evidence using available data to make a strong claim that the demographic changes of the 1930s–1960s and increasing survivorship of poor early-life conditions explain the notable higher prevalence of obesity and diabetes in middle-income countries in the cohort of older adults born in the beginning period of the 1930s–1960s.
A few additional results merit further discussion. First, the results show important differences between countries in disease patterns, especially concerning obesity. These differences suggest different determinants of older adult health, whether they be a result of macro-level events or stem from population differences. The higher prevalence of obesity and diabetes in middle-income countries is not surprising given that mortality resulting from diabetes has been increasing in some middle-income countries (Palloni et al. Citation2005). The higher prevalence of self-reported diabetes is higher than the prevalence of diabetes among present-day older adults and older adults several decades ago in the developed world (García-Palmieri et al. Citation1970; Gordon Citation1964; Hadden and Harris Citation1987; Harris et al. Citation1998; Wilkerson and Krall Citation1947). A high prevalence of conditions such as hypertension has been found in urban areas (Ibrahim and Damasceno Citation2012). Given that studies show increasing prevalence of these chronic conditions even in low-income countries (Hossain, Kawar, and El Nahas Citation2007; WHO Citation2000; Kinsella and He Citation2009; Murray and Lopez Citation1996; Lloyd-Sherlock et al. Citation2014; Gao et al. Citation2013; Méndez-Chacón, Santamaría-Ulloa, and Rosero-Bixby Citation2008), differences in the timing and development of disease as shown in this study remain an important consideration in understanding its determinants.
Second, the results indicate the overall importance of poor early-life conditions for adult obesity. Height is a marker of net nutritional status, and problems in intrauterine growth, resulting from either poor nutrition or infectious diseases, lead to low birth weight babies and stunting (Barker Citation1998; Crimmins and Finch Citation2006), with an increased risk of obesity and other conditions later in life. All seven countries in this study had very low caloric intake during the 1930s–1940s (FAO Citation1946), reflecting poor nutritional and infectious disease environments for most of the population. These types of conditions in early life affect infant and child health (Adair et al. Citation2013; Yajnik Citation2013; Yan et al. Citation2012), but they also increase the risk of poor adult health (Almond and Currie Citation2010; Crimmins and Finch Citation2006; Davey Smith et al. Citation1998; Elo, Martikainen, and Myrskylä Citation2010; Elo and Preston Citation1992; Hayward and Gorman Citation2004).
Third, the results indicating the importance of urban residence are not surprising given that trends in the developing world show a higher prevalence of chronic conditions in urban settings among older adults (e.g., Lloyd-Sherlock et al. Citation2014). However, that urban dwellers and shorter individuals are more at risk for obesity suggests the merit of early-life theories that stress the possible mismatch between being born in impoverished nutritional conditions and being exposed to a more nutritionally rich environment later in life (Barker Citation1998; Schmidhuber and Shetty Citation2005). All of the selected middle-income countries were very poor in the 1930s–1940s. Developing countries such as Costa Rica, China, and Indonesia experienced rapid economic growth during the adulthood of the cohort examined. Economic growth may have benefited urban dwellers more, potentially improving diet but also potentially increasing exposure to a more Western-style diet high in saturated fats. All developing countries in this study have seen an increase in caloric intake (FAO Citation1946; FAO Citation2010), but exposure to a more Western-style diet in adulthood may be more likely in urban areas. Exposure to this kind of diet may partially explain results (Popkin, Horton, and Kim Citation2001), and the combination of poor conditions during a critical early period with nutritional and lifestyle changes in later life might be a lethal combination for some older adults.
Fourth, the inconclusive result regarding the possible macro-level explanation for the noted differences in the cohorts of the 1930s–1940s—namely, the long-term consequences of demographic changes of the 1930s–1960s primarily as a result of country-wide public health interventions without parallel improvement in diet and nutrition—does not negate its importance for potentially explaining differences in disease. However, it may be too early to fully explore the merits of this explanation. Although smaller countries were experiencing country-wide public health interventions with consequent improvements in mortality in children and infants during the 1930s (Clark Citation1930; Rosero-Bixby Citation1990) and improvements were occurring in urban areas in the larger present-day middle-income countries (Rodríguez de Romo and Rodríguez de Pérez Citation1998), the full impact of these demographic changes may not have occurred until after 1945 with the introduction of antibiotics and with complete country-wide implementation of public health interventions in the larger countries. Fully implemented public health interventions would have resulted in increasing survivorship of the more vulnerable portion of the population, namely, those living in rural areas, resulting in sharp contrasts in adult health between vulnerable members of the population and everyone else.
The results showing that increased years of education was associated with higher likelihood for obesity and diabetes suggests a possible reversal of expected patterns of health as described in other studies (Monteiro et al. Citation2004). However, the effects of low height were much stronger for obesity. Given that obesity is a strong predictor of adult diabetes and hypertension, the results for obesity may be a precursor to what we will observe with diabetes and hypertension as countries undergo complete transitions. Thus, while the comparisons among older adults born in the 1930s–1940s provide a hazy glimpse at the impact of macro-level events, the question remains as to whether the results foreshadow in any way the health of cohorts of older adults born later in the period—the 1940s–1960s—in low-income countries.
There are a number of other possibilities that explain the weaker results for diabetes and hypertension. Although self-reports for diabetes show validity in some settings (Banks et al. Citation2006; Brenes Citation2008; Goldman et al. Citation2003), underestimation of diabetes using self-reports is undoubtedly problematic in the developing world, where access to good medical care and diagnostic tests is not widespread. Those who are shorter are more likely to also be poorer, with less access to these services. Additionally, there are undoubtedly large differences in health care infrastructure and urbanicity between low- and middle-income countries, with middle-income countries having a stronger health care infrastructure and a larger proportion of the population living in urban areas where older adults would be more likely to receive quality care and proper diagnosis of diabetes. In this study, low-income countries such as Ghana, India, and Indonesia indeed showed higher percentages of respondents living in rural areas. Even though we examined urban residents only, existing differences in health care infrastructure could have resulted in more severe underestimation in low-income countries. If we also consider that these differences are most likely to be correlated with mortality regimes and economic growth and development, we must conclude that this cross-national analysis between low- and middle-income countries using self-reported diabetes must be subject to cautious interpretation.
The weaker results for hypertension are surprising in that several studies have shown strong associations between low birth weight, infant mortality rate, poor SES, and adult hypertension (Barker Citation1998; Johnson and Schoeni Citation2011; Lawlor and Davey Smith Citation2005; McGovern Citation2012). However, similar results obtained for height and diabetes suggest that the pathway from stunting to diabetes and hypertension may operate mainly through obesity (Popkin, Horton, and Kim Citation2001). Thus, height may not be the best measure to sufficiently capture associations with adult diabetes and hypertension.
The study shows large differences among countries in terms of moving from rural to urban residences at some time during the life course. However, complete information about migration is limited in some surveys of older adults, making it harder to determine the effects of this migration. It was also not possible to observe on an individual level factors that may have mitigated the effects of poor early-life conditions, such as economic growth or factors that may have either compounded their effects or played a larger role in explaining adult health, such as diet. Rapid economic growth may help explain adult health for those exposed to poor early-life conditions (Steckel Citation2013), but speed of economic growth during adulthood might not be as important as who benefits from economic growth. Not all economic growth in the developing world has benefited the entire population (López-Alonso Citation2007). There is little information on individual diet during childhood or adulthood of older adult respondents in population-based studies, and diets differ tremendously between Latin America and Asia, making the interpretation of the results less clear in determining the relative importance of early life versus events such as the nutrition transition to a diet higher in saturated fat.
Additionally, the nature of the study prohibits the possibility of disentangling precise mechanisms in early life associated with older adult health because there is a complex synergy between nutrition and infection (Scrimshaw Citation1968). Birthplace is a broad measure that might also reflect epidemiological differences between rural and urban settings. Height could also be problematic given the imprecision of the lowest quartile of height as a measure of stunting and lost height at older ages. Some of the SAGE countries are representative of a particular province or region but not the country as a whole. Mexico and South Africa may be special cases, although there is evidence of increasing prevalence of these chronic conditions, especially in the Latin American region (Palloni, Pinto-Aguirre, and Pelaez Citation2002). The comparison by mortality regimes is based on analyses of historical mortality data from the early twentieth century (McEniry Citation2014), but these broadly defined mortality regimes may need refinement. Although we conducted analyses to show the similar direction of associations between predictors and health outcomes by country, the estimated models make an important assumption that countries can be pooled together.
In spite of the study limitations, the topic of early-life conditions and older adult health in the developing world remains important, and individual-level survey data of older adults are one of the better sources of data that we have to examine the topic. Even though the examination of trends in health in the developing world is complex, cross-national comparisons provide insight into health patterns and their determinants (National Research Council Citation2001). Rapid changes affecting developing countries and evidence from the middle-income countries pose relevant questions about the long-term consequences of macro-level events on health at older ages. Further examination of those born in the late 1940s–1960s will be essential to more fully examine if these questions have merit.
Acknowledgments
I am grateful for the feedback provided by Bob Schoeni, John Marcotte, and Sarah Moen. We are also very grateful for the helpful comments made by the editors and anonymous reviewers.
Funding
This research was supported by a grant awarded from the Population Studies Center, Institute for Social Research at the University of Michigan from the Ronald and Deborah Freedman Fund for International Population Activities. Research work for University of Michigan researchers is supported by a core NICHD grant (R24 HD041028) to the Population Studies Center at the University of Michigan. ICPSR at the Institute for Social Research also supports research work for its University of Michigan researchers. Data for the study are stored with the University of Wisconsin–Madison Social Science Computing Cooperative.
Additional information
Funding
References
- Adair, L. S., C. H. D. Fall, C. Osmond, A. D. Stein, R. Martorell, M. Ramirez-Zea, H. S. Sachdev, et al. 2013. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: Findings from five birth cohort studies. Lancet 382 (9891): 525–34. doi:10.1016/S0140-6736(13)60103-8.
- Ahmad, O. B., C. Boschi-Pinto, A. D. Lopez, C. J. L. Murray, R. Lozano, and M. Inoue. 2001. Age standardization of rates: A new WHO standard. GPE Discussion Paper Series No. 31. Geneva, Switzerland: World Health Organization.
- Almond, D., and J. Currie. 2010. Human capital development before age five. Working Paper No. 15827. Cambridge, MA: National Bureau of Economic Research.
- Banister, J. 1987. China’s changing population. Stanford, CA: Stanford University Press.
- Banks, J., M. Marmot, Z. Oldfield, and J. P. Smith. 2006. Disease and disadvantage in the United States and in England. Journal of the American Medical Association 295 (17): 2037–45.
- Barker, D. J. P. 1998. Mothers, babies and health in later life. 2nd ed. Edinburgh: Churchill Livingstone.
- Barker, D. J. P., K. L. Thornburg, C. Osmond, E. Kajantie, and J. G. Eriksson. 2010. Beyond birthweight: The maternal and placental origins of chronic disease. Journal of Developmental Origins of Health and Disease 1 (6): 360–64. doi:10.1017/S2040174410000280.
- Bateson, P., D. Barker, T. Clutton-Brock, D. Deb, B. D’Udine, R. A. Foley, P. Gluckman, et al. 2004. Developmental plasticity and human health. Nature 430:419–21. doi:10.1038/nature02725.
- Beinart, W., and S. Dubow, eds. 1995. Segregation and apartheid in twentieth century South Africa. London: Routledge.
- Brenes, G. 2008. The effect of early life events on the burden of diabetes mellitus among Costa Rican elderly: Estimates and projections. Ph.D. diss., University of Wisconsin—Madison.
- Caldwell, J. C. 1967. Population change. In A study of contemporary Ghana, ed. W. Birmingham, I. Neustadt, and E. N. Omaboe, 78–110. London: George Allen & Unwin Ltd.
- Clark, V. S. 1930. Porto Rico and its problems. Washington, D.C.: Brookings Institution.
- Crimmins, E. M., and C. E. Finch. 2006. Infection, inflammation, height and longevity. Proceedings of the National Academy of Sciences of the United States of America 103 (2): 498–503. doi:10.1073/pnas.0501470103.
- Davey Smith, G., C. Hart, D. Blane, and D. Hole. 1998. Adverse socioeconomic conditions in childhood and cause specific adult mortality: Prospective observational study. British Medical Journal 316:1631–35. doi:10.1136/bmj.316.7145.1631.
- Dyson, T. 1997. Infant and child moratlity in the Indian subcontinent, 1881–1947. In Infant and child mortality in the past, ed. A. Bideau, B. Desjardina, and H. P. Brignoli, 109–34. Oxford, UK: Clarendon.
- Elo, I. T., P. Martikainen, and M. Myrskylä. 2010. Early life conditions and cause-specific mortality in Finland. Working paper 10-04, University of Pennsylvania Population Aging Research Center.
- Elo, I. T., and S. H. Preston. 1992. Effects of early-life conditions on adult mortality: A review. Population Index 58 (2): 186–212.
- Farley, J. 2003. To cast out disease: A history of the International Health Division of the Rockefeller Foundation (1913–1951). Oxford, UK: Oxford University Press.
- Floud, R., R. W. Fogel, B. Harris, and S. C. Hong. 2011. The changing body: Health, nutrition, and human development in the Western world since 1700. Cambridge, UK: Cambridge University Press.
- Food and Agriculture Organization of the United Nations (FAO). 1946. World food survey. Washington, D.C.: United Nations.
- Food and Agriculture Organization of the United Nations (FAO). 2010. Statistics Division. Food Balance Sheets. http://faostat.fao.org/site/368/default.aspx#ancor ( accessed January, 2012).
- Gao, Y., G. Chen, H. Tian, L. Lin, L. Juming, J. Weng, W. Jia, et al. 2013. Prevalence of hypertension in China: A cross-sectional study. PLOS One 8 (6): e65938. doi:10.1371/journal.pone.0065938.
- García-Palmieri, M. R., R. Costas, M. Cruz-Vidal, M. Cortés-Alicea, A. A. Colón, M. Feliberti, A. M. Ayala, et al. 1970. Risk factors and prevalence of coronary heart disease in Puerto Rico. Circulation 42:541–49. doi:10.1161/01.CIR.42.3.541.
- Goldman, N., I.-F. Lin, M. Weinstein, and Y.-H. Lin. 2003. Evaluating the quality of self-reports of hypertension and diabetes. Journal of Clinical Epidemiology 56:148–54. doi:10.1016/S0895-4356(02)00580-2.
- Gordon, T. 1964. Glucose tolerance of adults: United States—1960–1962. Public Health Service Publication No. 1000—Series 11, No. 2, U.S. Government Printing Office, Washington, D.C.
- Hadden, W. C., and M. I. Harris. 1987. Prevalence of diagnosed diabetes, undiagnosed diabetes, and impaired glucose tolerance in adults 20–74 years of age. Data from the National Health Survey Series 11, No. 237, U.S. Department of Health and Human Services, Hyattsville, MD.
- Harris, M. I., K. M. Flegal, C. C. Cowie, M. S. Eberhardt, D. E. Goldstein, R. R. Little, H.-M. Wiedmeyer, and D. D. Byrd-Hold. 1998. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21 (4): 518–24. doi:10.2337/diacare.21.4.518.
- Hayward, M. D., and B. K. Gorman. 2004. The long arm of childhood: The influence of early-life social conditions on men’s mortality. Demography 41 (1): 87–107. doi:10.1353/dem.2004.0005.
- Henderson, V. 2002. Urbanization in developing countries. World Bank Research Observer 17 (1): 89–112. doi:10.1093/wbro/17.1.89.
- Hossain, P., B. Kawar, and M. El Nahas. 2007. Obesity and diabetes in the developing world—A growing challenge. New England Journal of Medicine 356:213–15. doi:10.1056/NEJMp068177.
- Ibrahim, M. M., and A. Damasceno. 2012. Hypertension in developing countries. Lancet 380 (9841): 611–19. doi:10.1016/S0140-6736(12)60861-7.
- Johnson, R. C., and R. F. Schoeni. 2011. Early-life origins of adult disease: National longitudinal population-based study of the United States. American Journal of Public Health 101:2317–24. doi:10.2105/AJPH.2011.300252.
- Kinsella, K., and W. He. 2009. An aging world: 2008. U.S. Census Bureau, International Population Reports, P95/09-1. U.S. Government Printing Office, Washington, D.C.
- Kuh, D., and Y. Ben-Shlomo, eds. 2004. A life course approach to chronic disease epidemiology. Oxford, UK: Oxford University Press.
- Lawlor, D. A., and G. Davey Smith. 2005. Early life determinants of adult blood pressure. Current Opinions in Nephrology and Hypertension 14 (3): 259–64. doi:10.1097/01.mnh.0000165893.13620.2b. PMID 15821420.
- Lillard, D. R., R. V. Burkhauser, M. H. Hahn, and R. Wilkins. 2015. Does early-life income inequality predict self-reported health in later life? Evidence from the United States. Social Science and Medicine 128:347–55. doi:10.1016/j.socscimed.2014.12.026.
- Lloyd-Sherlock, P., J. Beard, N. Minicuci, S. Ebrahim, and S. Chatterji. 2014. Hypertension among older adults in low- and middle-income countries: Prevalence, awareness and control. International Journal of Epidemiology 43 (1): 116–28. doi:10.1093/ije/dyt215.
- López-Alonso, M. 2007. Growth with inequality: Living standards in Mexico, 1850–1950. Journal of Latin American Studies 39:81–105.
- Maddison, A. 2006. The world economy. Paris: OECD Publishing.
- McEniry, M. 2014. Early life conditions and rapid demographic changes in the developing world: Consequences for older adult health. Dordrecht, Netherlands: Springer Science and Business Media.
- McEniry, M., S. Moen, and J. McDermott. 2013. Methods report on the compilation of the RELATE cross-national data on older adults from 20 low, middle and high income countries. Ann Arbor: University of Michigan.
- McGovern, M. E. 2012. Don’t stress: Early life conditions, hypertension, and selection into associated risk factors. Geary Institute Discussion Paper WP2012/23, Cambridge, MA.
- Méndez-Chacón, E., C. Santamaría-Ulloa, and L. Rosero-Bixby. 2008. Factors associated with hypertension prevalence, unawareness and treatment among Costa Rican elderly. BMC Public Health 8:275. doi:10.1186/1471-2458-8-275.
- Monteiro, C. A., W. L. Conde, B. Lu, and B. M. Popkin. 2004. Obesity and inequities in health in the developing world. International Journal of Obesity 28:1181–86. doi:10.1038/sj.ijo.0802716.
- Murray, C. J. L., and A. D. Lopez, eds. 1996. Global health statistics: Global burden of disease and injury series. 2nd vol. Boston: Harvard School of Public Health.
- National Research Council. 2001. Preparing for an aging world: The case for cross-national research. Panel on a Research Agenda and New Data for an Aging World, Committee on Population and Committee on National Statistics, Division of Behavioral and Social Sciences and Education. Washington, D.C.: National Academy Press.
- Nitisastro, W. 1970. Population trends in Indonesia. Ithaca, NY: Cornell University Press.
- Osmond, C., and D. J. P. Barker. 2000. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environmental Health Perspectives 108 (suppl 3): S545–S53. doi:10.1289/ehp.00108s3545. PubMed: 10852853.
- Palloni, A., M. McEniry, A. L. Dávila, and A. G. Gurucharri. 2005. The influence of early conditions on health status among elderly Puerto Ricans. Social Biology 52 (3/4): 132–64.
- Palloni, A., G. Pinto-Aguirre, and M. Pelaez. 2002. Demographic and health conditions of ageing in Latin America and the Caribbean. International Journal of Epidemiology 31:762–71. doi:10.1093/ije/31.4.762.
- Palloni, A., and L. Souza. 2013. The fragility of the future and the tug of the past: Longevity in Latin America and the Caribbean. Demographic Research 29 (21): 543–78. doi:10.4054/DemRes.2013.29.21.
- Patterson, K. D. 1979. Health in urban Ghana: The case of Accra 1900–1940. Social Science and Medicine 138:251–68.
- Patterson, K. D. 1981. Health in colonial Ghana: Disease, medicine and socio-economic change 1900–1955. Waltham, MA: Crossroads Press.
- Popkin, B. M. 2006. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases (NCDs). American Journal of Clinical Nutrition 84:289–98.
- Popkin, B. M., S. Horton, and S. Kim. 2001. The nutritional transition and diet-related chronic diseases in Asia: Implications for prevention. Food Consumption and Nutrition Division Discussion Paper No. 105. Washington, DC: International Food Policy Research Institute.
- Preston, S. H. 1976. Mortality patterns in national populations with special reference to recorded causes of death. New York: Academic Press.
- Preston, S. H., and M. R. Haines. 1991. Fatal years: Child mortality in late nineteenth-century America. Princeton, NJ: Princeton University Press.
- Raghunathan, T. E., J. P. Reiter, and D. B. Rubin. 2003. Multiple imputation for disclosure limitation. Journal of Official Statistics 19:1–16.
- Ramasubban, R. 2008. History of public health in modern India: 1857–2005. In Public health in Asia and the Pacific: Routledge advances in Asia-Pacific studies, ed. M. J. Lewis and K. L. MacPherson, 87–105. New York: Routledge Taylor & Francis Group.
- RELATE (Research on Early Life and Aging: Trends and Effects): A cross national study. Principal Investigator: Mary C. McEniry. ICPSR34241-v1. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2013 -06-12. doi:10.3886/ICPSR34241.v1.
- Rodríguez de Romo, A. C., and M. E. Rodríguez de Pérez. 1998. Historia de la salud pública en México: Siglos XIX y XX [History of public health in Mexico: 19th and 20th century]. História, Ciências, Saúde-Manguinhos 5 (2): 293–310. doi:10.1590/S0104-59701998000200002.
- Rosero-Bixby, L. 1990. Socioeconomic development, health interventions and mortality decline in Costa Rica. Scandinavian Journal of Social Medicine 46:33–42.
- Sastry, N. 1997. What explains rural-urban differentials in child mortality in Brazil? Social Science and Medicine 44 (7): 989–1002. doi:10.1016/S0277-9536(96)00224-9.
- Schmidhuber, J., and P. Shetty. 2005. The nutrition transition to 2030: Why developing countries are likely to bear the major burden. Plenary paper presented at the 97th Seminar of the European Association of Agricultural Economists, University of Reading, England, April 21–22. doi:10.1080/16507540500534812.
- Scrimshaw, N. S. 1968. Interaction of nutrition and infection. New York: World Health Organization.
- Steckel, R. H. 2013. The hidden cost of moving up: Type 2 diabetes and the escape from persistent poverty in the American South. American Journal of Human Biology 25 (4): 508–15. doi:10.1002/ajhb.22399.
- van den Berg, G. J., G. Doblhammer, and K. Christensen. 2009. Exogenous determinants of early-life conditions, and mortality later in life. Social Science and Medicine 68:1591–98. doi:10.1016/j.socscimed.2009.02.007.
- van Ewijk, R. J. G., R. C. Painter, and T. J. Roseboom. 2013. Associations of prenatal exposure to Ramadan with small stature and thinness in adulthood: Results from a large Indonesian population-based study. American Journal of Epidemiology 177 (8): 729–36. doi:10.1093/aje/kwt023.
- Wilkerson, H. L. C., and L. P. Krall. 1947. Diabetes in a New England town; study of 3,516 persons in Oxford, Mass. JAMA 135:209–16.
- Wilkinson, R., and M. Marmot, eds. 2003. Social determinants of health: The solid facts. 2nd ed. Copenhagen: World Health Organization.
- World Health Organization (WHO). 2000. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. Geneva: World Health Organization.
- Yajnik, C. S. 2013. Commentary: Thrifty phenotype: 20 years later. International Journal of Epidemiology 42 (5): 1227–29. doi:10.1093/ije/dyt132.
- Yan, S., J. Li, S. Li, B. Zhang, S. Du, P. Gordon-Larsen, L. Adair, and B. Popkin. 2012. The expanding burden of cardiometabolic risk in China: The China Health and Nutrition Survey. Obesity Reviews 13 (9): 810–21. doi:10.1111/obr.2012.13.issue-9.
Appendix A
Table A1 Models using all seven countries predicting health outcomes
