Abstract
The gut of the human neonate is colonized rapidly after birth from an early sparse and highly distinct microbiota to a more adult-like and convergent state, within 1 to 3 years. The progression of colonizing bacterial species is non-random. During the first months of life several shifts commonly occur in the species prevalent in our guts. Although the sequential progression of these species is remarkably consistent across individuals and geographies, there is inter-individual variation in the rate of progression. Our study and others suggest that the rate is influenced by environmental factors, and influences our future health. In this article, we review our recent contribution to cataloging the developing infant gut microbiota alongside other important recent studies. We suggest testable hypotheses that arise from this synthesis.
The fetus is almost sterile during the gestational period,Citation1 and birth starts a process of colonization by microorganisms. How the gut is colonized is of interest for at least three reasons. Firstly, the make-up of the gut microbiota has been causally linked with metabolic health and disease.Citation2-4 Secondly, microbiota may be a mechanism for developmental programming;Citation5 while the infant gut microbial composition does not necessarily predict the make-up of the adult gut microbiota,Citation6 it does affect the infant's developing immune system and metabolism.Citation7,8 Finally, the gut microbiota can be sampled non-invasively, albeit with some loss of accuracy and complexity, by studying fecal matter.Citation9
Yatsunenko et al,Citation10 among others found that the fecal microbiome varies strongly but predictably with age over the first few years of life. They also found that this progression with age was essentially consistent across Venezuela, Malawi and USA, although there were some strong differences in microbiota content across geographies. In a fascinating study of premature infants in a neonatal intensive care ward designed to minimize sources of bacterial exposure, La Rosa et al,Citation11 found that the progression of microbial species found in fecal samples followed a similar pattern to that described before and included discrete stages where particular phyla dominated. They found that extrinsic factors “influenced the pace, but not the sequence, of progression.” These studies might suggest that intrinsic factors such as gut maturity are driving the progression. External environmental factors such as delivery mode, antibiotic exposure and feeding style strongly affect the infant fecal microbiota,Citation12 but maybe only the rate of its establishment.
In our recent publication,Citation13 we found that 75 newborn babies from the Singaporean GUSTO birth cohort went through a progression of gut microbiota acquisition, similar to the studies previously mentioned and others. We used 16S rRNA pyrosequencing of stool samples collected at ages 3 days, 3 weeks, 3 months and 6 months and classified the fecal microbiota by non-supervised clustering; over this period the majority of infants progressed from a “cluster-2” fecal microbial profile (high in Enterobacteriaceae and Klebsiella) to a “cluster-3” fecal microbial profile (high in Bifidobacteria and Collinsella). In our study, 88% of the babies reached a cluster-3 classified profile by age 6 months, but there was substantial inter-individual variation in the rate at which it was reached. At day 3 26% of the neonates already had fecal microbiota which classified as cluster-3, while 1% of the babies only reached cluster-3 by month 6; the remaining reached there at week 3 (35%) or month 3 (26%). Most studies of infants that studied inter-individual variation have reported differences between individuals of the same age. However, we postulated that the rate of microbiota acquisition is an important source of inter-individual variation.
Subsequent to the publication of our article, Bäckhed et al,Citation14 published a comprehensive study of the fecal microbiota of 98 Swedish infants sampled at birth, month 4 and month 12 along with fecal samples from their mothers. Crucially, they employed metagenomic sequencing, a much more quantitative and comprehensive methodology, which allows identification of bacterial species and genes, and so can describe functional capacity. Both Bäckhed et al,Citation14 and our own study,Citation13 found that fecal samples were more similar within time-points than within individuals.
Bäckhed et al,Citation14 also described a shift in the fecal microbiota over time. Their showed microbiota within a week of birth very similar to our cluster 2 microbiota, high in Enterobacteriaceae species such as Escherichia/Shigella and in Streptococcus. Species characteristic of cluster 3 in our study,Citation13 such as Bifidobacterium and Collinsella, peaked in relative abundance at month 4 in Bäckhed's data. The Swedish data showed a further large transition between the month 4 and month 12 samples. Our data did not extend past month 6 and so did not include this shift to the Bacteroidetes and Firmicutes species documented by Bäckhed et al.
presents a simplified schematic of what is known about the progression of species in the infant fecal microbiota over time. The Bacilli class dominated stage A is found chiefly in premature infants, as documented by La Rosa et al.,Citation11 and rarely in other infants. Stage B dominated by Proteobacteria and termed Cluster 2 in our study, seems to exist from birth or soon after in term infants; Bäckhed et al.,Citation14 also observed this profile in samples at birth, La Rosa et al.,Citation11 observed it at post-conceptional ages 32–34 weeks, and many other studies have also reported a similar profile.Citation12 Progression from Stage B to Stage C (Actinobacteria dominated, called Cluster 3 in our study) occurred between birth and month 6 in our study.Citation13 The species characteristic of Cluster 3, e.g. Bifidobacterium, peaked in relative abundance in the samples at month 4 in Bäckhed et al.Citation14 It was also the most frequent profile observed in fecal samples of 24 Canadian infants at 4 months of age.Citation15 Interestingly it was absent from the study of premature infants by La Rosa et al.Citation11 Another dramatic shift from Actinobacteria to Firmicutes and Bacteroidetes occurs between month 4 and years 1-3 in term infants,Citation14,16 and perhaps earlier in premature infants.Citation11 Stage D represents a more adult-like microbiota.Citation10,12,14
Figure 1. (a) schematic diagram representing a simplified view of the progression of the infant fecal microbiota across the first year of life, incorporating Stages (A–D). (b–e) present putative modifications to the progression caused by intrinsic and extrinsic factors.
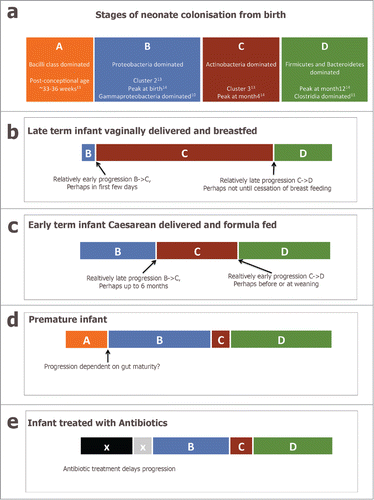
Our thesis is that population shifts in the core microbiota are developmental-stage specific. Our study and others suggest that extrinsic factors primarily contribute to inter-individual diversity in rate of progression. Furthermore, the rate of progression influences health later in life.
In both our study,Citation13 and Bäckhed et al.Citation14 there was a profound effect of delivery mode on the infant gut microbiota, which was most marked at early time-points and decreased at later ages. In Bäckhed et al (2015) the infants born by Caesarean section had much sparser early fecal microbiota, which tended to contain species usually found on adult skin, while the fecal microbiota of the vaginally delivered infants closely resembled the fecal microbiota of their mothers. Even at the 12-month time-point the Bacteroidetes phylum was less abundant in infants born by Cesarean section. We also found that Bacteroidetes was absent from infants born at Caesarean section at all time-points, although we note that it was variably present in the vaginally born infants. These findings echo those of Jakobsson et al.Citation17 Bäckhed et al.,Citation14 found strong evidence for vertical transmission of Bacteroides fragilis and Bacteroides thetiaot-aomicron among other species in vaginally delivered infants.
So, delivery mode affects the infant microbiota in cross-sectional comparisons, i.e. when comparing infants at the same age. However, this apparent difference in microbiota composition could be explained by differences in rate of progression through the same stages. In our study those infants who were vaginally delivered tended to reach the Actinobacteria dominated Cluster-3/ Stage C earlier. It is difficult to determine if the rate of progression from Stage B to Stage C is affected by delivery mode in the data of Bäckhed et al.,Citation14 but it is possible to observe in their that many of the vaginally delivered babies already had high levels of Bifidobacterium and Collinsella as neonates.
Bäckhed et al.Citation14 used the random forest approach, which was developed by Subramanian et al.Citation18 to describe delay in the normal development of the gut microbiota in malnourished children. They found that babies born by Cesarean section (or fed formula) had fecal microbiota that were characterized as older than their chronological age at 4 months. At first appearance this is in contrast to our conclusion,Citation13 which proposed babies born by Cesarean tended to reach Cluster 3 (Stage C) later than vaginally delivered infants. However, Cluster 3 represents a month 6 pre-weaning microbiota and not the Stage D weaned microbiota that represents maturity (and on which the random forests algorithm was trained) in the Bäckhed et al. data. Perhaps the Caesarean delivered infants spend a relatively compressed time at Stage C but instead convert relatively rapidly from Stage B to Stage D (Fig. 1c).
Premature infants have repeatedly been shown to have a markedly different gut microbiota to term infants,Citation19 with a bacilli dominated phase,Citation11 delayed or missed acquisition of Bifidobacteria,Citation11,20,21 and earlier acquisition of Firmicutes,Citation11 (). Our study was unusual in showing a difference in the acquisition of gut microbiota across the normal range of gestational ages. All the infants in our study were born at term but those born at 38 weeks tended to reach Cluster 3 later than those born at 39 or more weeks' gestation. Indeed, the median gestation for those who reached Cluster 3 at day 3 was 39.2 weeks as compared to 38.5 weeks for those who reached Cluster 3 at month 6. Overall, a difference in gestational duration of about a week was associated with a lag in gut microbiota acquisition measured in months.
Others have suggested that host physiology drives the differences in gut microbiota between premature and term infants.Citation16,22 In particular a defective mucin barrier especially MUC2 may lead to unbalanced microbiota.Citation23,24 Perhaps subtle immaturity of the gut in the early term infants is enough to delay microbiota acquisition. However cause and effect are difficult to unpick: Athalye-Jape et al. showed that administering probiotics can speed up gut maturation in premature infants,Citation25 suggesting that the gut microbiome can influence physiology and vice-versa. Administering IGF1 in utero may speed development of the guts of premature infants,Citation26 and it would be telling if this also affected the gut microbiota.
The most dramatic shift in the Bäckhed et al.Citation14 data is between the months 4 and 12, Stage C to Stage D. The month 12 microbiota of the infants is more similar to the adult profiles of their mothers than to either the birth or month 4 profiles and led the authors to speculate that infants could reach a “steady-state” profile at about one year old, similar but a bit earlier to the 1-3 y time-point proposed by Yatsunenko et al.Citation10 The introduction of solid food (weaning) usually within 4 and 12 months of life has been previously implicated in this shift,Citation16 but Bäckhed et al.Citation14 emphasize the role of breast-feeding. Infants that were still breastfed at age 12 months tended to have fecal microbiota containing the Actinobacterial species seen at 4 months of age and characteristic of Stage C. Also, as mentioned above the machine learning approach employed by Bäckhed et al.Citation14 determined that formula fed infants tended to have a profile that was characterized as “older” than that of breast fed infants at the birth and 4-month time-points. Therefore, breastfeeding seems to delay the progression to Stage D, even after introduction of solid food.
In summary, the timing of the transition between Stages B and C is affected by delivery mode and gestational duration. Similarly, the timing of the transition between Stage C and D seems to also be influenced by mode of delivery and more importantly the intake of breast milk and solid food. An obvious following question is: does the timing matter?
Cox et al.Citation27 showed in mice that disruption of the early microbiota (by a low dose of penicillin limited to gestation and infancy) could enhance the effect of diet in inducing obesity in adulthood. In humans too, early treatment with antibiotics is associated with later adiposity.Citation28,29 Taking antibiotics delays the progression of the gut microbiota,Citation30,31 (). Antibiotic usage at the end of gestation and during early infancy correlates with increased body size in childhood,Citation32 and risk of being overweight.Citation33
Babies born prematurely show a lag in the proper colonization of the gut,Citation31 and less time at Bifidobacteria dominated Stage C;Citation20 they are also at higher risk for obesity later in life.Citation34,35 Similarly individuals born by Caesarean section have a slower rate of progression to Stage C,Citation13,14 and are also at higher risk for obesity.Citation36,37 However, longer duration of breastfeeding is associated with longer at Stage C,14 and is protective of obesity.Citation36,38 In our study,Citation13 those infants which reached Cluster 3 (aka Stage C) later were more likely to be of median adiposity at 18 months old, while those who got there later were more likely to be significantly underweight at age 18 months. Those who had high levels of the Firmicutes genera Streptococcus at the month 6 time-point (characteristic of Stage D and so presumably these infants paused at Stage C for a shorter time), had a higher than average adiposity gain between birth and 18 months of age. Previous cross-sectional studies have found lower Bifidobacterium levels in fecal samples from children younger than 6 months correlates with future obesity at 7 and 10 y of age, compared to normal weight controls.Citation39,40
Many researchers are asking if changing the early microbiota by pre- or probiotics, could affect later phenotype and perhaps prevent obesity.Citation41 In agreement with the hypothesis presented here, others have suggested that infants born at earlier gestations,Citation42 or by Caesarean section,Citation43 would benefit from intervention to get them to Stage C faster and keep them there longer.Citation44,45
Our present understanding of the progression of the infant gut microbiota is crude. represents a testable hypothesis but is oversimplified and most likely incorrect in detail. More studies sampling infants at more frequent time-points are needed to build a better and more complete picture. Employing the type of metagenomic approach exemplified by Bäckhed et al,Citation14 is not only more quantitative but starts to provide insights into the functional capacity of the microbiota. Even more informative would be metatranscriptomic approaches that provide information of which genes are expressed in the microbiome.
The following is a short-listing of some of the most pressing questions:
There are almost certainly more stages we have yet been able to capture - what are they?
Are the stages discrete or are they more gradual than our sparse data suggests at present?
Do all infants progress through all stages or do some infants miss some stages completely?
What are the intrinsic and extrinsic factors that influence speed of progression at different points? How do they interact?
Several studies have documented the influence of the maternal microbiome and vertical transmission,Citation46 is this how factors such as delivery mode affect rate of progression?
Is it beneficial to progress through some stages fast and others more slowly? For instance, is it beneficial for future metabolic health to progress through Stage C slowly?
It is interesting to note that the recovery of the childhood and adult gut microbiota after diarrhea has also been observed to be orderly and reproducible.Citation47,48 If as suggested by the authors, intrinsic factors govern the progression: are there similarities within individuals between the original process of colonization and the re-colonization that occurs after diarrhea?
Disclosure of Potential Conflicts of Interest
OS, CNB, WB, BB and HB are employees of the Nestlé Research Center, a commercial entity that aims to enhance the quality of consumers' lives through nutrition, health and wellness. Nestle is active in research into prebiotics and probiotics and produces infant formula. KMG and YSC have received reimbursement for speaking at conferences sponsored by companies selling nutritional products, and are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors have no potential conflicts of interest. A patent in a related field has been submitted (No. 14181275.0).
References
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014; 6:237ra65; PMID:24848255
- Gao X, Jia R, Xie L, Kuang L, Feng L, Wan C. Obesity in school-aged children and its correlation with Gut E.coli and Bifidobacteria: a case-control study. BMC Pediatr 2015; 15:64; PMID:26024884; http://dx.doi.org/10.1186/s12887-015-0384-x
- Karl JP, Fu X, Wang X, Zhao Y, Shen J, Zhang C, Wolfe BE, Saltzman E, Zhao L, Booth SL. Fecal menaquinone profiles of overweight adults are associated with gut microbiota composition during a gut microbiota-targeted dietary intervention. Am J Clin Nutr 2015; 102(1):84-93; PMID:26016865
- Zhong CY, Sun WW, Ma Y, Zhu H, Yang P, Wei H, Zeng BH, Zhang Q, Liu Y, Li WX, et al. Microbiota prevents cholesterol loss from the body by regulating host gene expression in mice. Sci Rep 2015; 5:10512; PMID:26015368; http://dx.doi.org/10.1038/srep10512
- Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr Res 2014; 76:2-10; PMID:24732106; http://dx.doi.org/10.1038/pr.2014.49
- Knights D, Ward TL, McKinlay CE, Miller H, Gonzalez A, McDonald D, Knight R. Rethinking “enterotypes.” Cell Host Microbe 2014; 16:433-7; PMID:25299329; http://dx.doi.org/10.1016/j.chom.2014.09.013
- Collado MC, Cernada M, Bauerl C, Vento M, Perez-Martinez G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 2012; 3:352-65; PMID:22743759; http://dx.doi.org/10.4161/gmic.21215
- Kerr CA, Grice DM, Tran CD, Bauer DC, Li D, Hendry P, Hannan GN. Early life events influence whole-of-life metabolic health via gut microflora and gut permeability. Crit Rev Microbiol 2014; 41(3):326-40; PMID:24645635
- Romano-Keeler J, Moore DJ, Wang C, Brucker RM, Fonnesbeck C, Slaughter JC, Li H, Curran DP, Meng S, Correa H, et al. Early life establishment of site-specific microbial communities in the gut. Gut Microbes 2014; 5:192-201; PMID:24637795; http://dx.doi.org/10.4161/gmic.28442
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486:222-7; PMID:22699611
- La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, Stevens HJ, Bennett WE, Jr., Shaikh N, Linneman LA, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci USA 2014; 111:12522-7; PMID:25114261; http://dx.doi.org/10.1073/pnas.1409497111
- Matamoros S, Gras-Leguen C, Le Vacon F, Potel G, de La Cochetiere MF. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol 2013; 21:167-73; PMID:23332725; http://dx.doi.org/10.1016/j.tim.2012.12.001
- Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Bruck WM, Berger B, Brussow H, Lee YS, Yap F, Chong YS, et al. Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. MBio 2015; 6:e02419-14; PMID:25650398
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015; 17:690-703; PMID:25974306; http://dx.doi.org/10.1016/j.chom.2015.04.004
- Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 2013; 185:385-94; PMID:23401405
- Gritz EC, Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr 2015; 3:17; PMID:25798435
- Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Bjorksten B, Engstrand L, Andersson AF. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014; 63:559-66; PMID:23926244; http://dx.doi.org/10.1136/gutjnl-2012-303249
- Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014; 510:417-21; PMID:24896187
- Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, Gilbert JA. Development of the preterm infant gut microbiome: a research priority. Microbiome 2014; 2:38; PMID:25332768; http://dx.doi.org/10.1186/2049-2618-2-38
- Butel MJ, Suau A, Campeotto F, Magne F, Aires J, Ferraris L, Kalach N, Leroux B, Dupont C. Conditions of bifidobacterial colonization in preterm infants: a prospective analysis. J Pediatr Gastroenterol Nutr 2007; 44:577-82; PMID:17460489; http://dx.doi.org/10.1097/MPG.0b013e3180406b20
- Collado MC, Cernada M, Neu J, Perez-Martinez G, Gormaz M, Vento M. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res 2015; 77:726-31; PMID:25760550; http://dx.doi.org/10.1038/pr.2015.54
- Stockinger S, Hornef MW, Chassin C. Establishment of intestinal homeostasis during the neonatal period. Cell Mol Life Sci 2011; 68:3699-712; PMID:21952827; http://dx.doi.org/10.1007/s00018-011-0831-2
- Elson CO, Alexander KL. Host-microbiota interactions in the intestine. Dig Dis 2015; 33:131-6; PMID:25925913; http://dx.doi.org/10.1159/000369534
- Cobo ER, Kissoon-Singh V, Moreau F, Chadee K. Colonic MUC2 mucin regulates the expression and antimicrobial activity of beta-defensin 2. Mucosal Immunol 2015; PMID:25921338; http://dx.doi.org/10.1038/mi.2015.27
- Athalye-Jape G, Deshpande G, Rao S, Patole S. Benefits of probiotics on enteral nutrition in preterm neonates: a systematic review. Am J Clin Nutr 2014; 100:1508-19; http://dx.doi.org/10.3945/ajcn.114.092551
- Kimble RM, Breier BH, Gluckman PD, Harding JE. Enteral IGF-I enhances fetal growth and gastrointestinal development in oesophageal ligated fetal sheep. J Endocrinol 1999; 162:227-35; PMID:10425460; http://dx.doi.org/10.1677/joe.0.1620227
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158:705-21; PMID:25126780; http://dx.doi.org/10.1016/j.cell.2014.05.052
- Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes 2013; 37:16-23; PMID:22907693; http://dx.doi.org/10.1038/ijo.2012.132
- Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes 2014; 38:1290-8; http://dx.doi.org/10.1038/ijo.2014.119
- Dardas M, Gill SR, Grier A, Pryhuber GS, Gill AL, Lee YH, Guillet R. The impact of postnatal antibiotics on the preterm intestinal microbiome. Pediatr Res 2014; 76:150-8; PMID:24819377; http://dx.doi.org/10.1038/pr.2014.69
- Arboleya S, Sanchez B, Milani C, Duranti S, Solis G, Fernandez N, de los Reyes-Gavilan CG, Ventura M, Margolles A, Gueimonde M. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr 2015; 166:538-44; http://dx.doi.org/10.1016/j.jpeds.2014.09.041
- Gough EK, Moodie EE, Prendergast AJ, Johnson SM, Humphrey JH, Stoltzfus RJ, Walker AS, Trehan I, Gibb DM, Goto R, et al. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. Bmj 2014; 348:g2267; PMID:24735883; http://dx.doi.org/10.1136/bmj.g2267
- Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obesity 2011; 35:522-9; http://dx.doi.org/10.1038/ijo.2011.27
- Lane RH. Fetal programming, epigenetics, and adult onset disease. Clin Perinatol 2014; 41:815-31; PMID:25459776; http://dx.doi.org/10.1016/j.clp.2014.08.006
- Bayman E, Drake AJ, Piyasena C. Prematurity and programming of cardiovascular disease risk: a future challenge for public health? Arch Dis Child Fetal Neonatal Ed 2014; 99:F510-4; PMID:25135955; http://dx.doi.org/10.1136/archdischild-2014-306742
- Portela DS, Vieira TO, Matos SM, de Oliveira NF, Vieira GO. Maternal obesity, environmental factors, cesarean delivery and breastfeeding as determinants of overweight and obesity in children: results from a cohort. BMC Pregnancy Childbirth 2015; 15:94; PMID:25884808; http://dx.doi.org/10.1186/s12884-015-0518-z
- Kuhle S, Tong OS, Woolcott CG. Association between caesarean section and childhood obesity: a systematic review and meta-analysis. Obes Rev 2015; 16:295-303; PMID:25752886; http://dx.doi.org/10.1111/obr.12267
- Zhu Y, Hernandez LM, Dong Y, Himes JH, Hirschfeld S, Forman MR. Longer breastfeeding duration reduces the positive relationships among gestational weight gain, birth weight and childhood anthropometrics. J Epidemiol Community Health 2015; 69(7):632-8; PMID:25680365
- Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clinical Nutr 2008; 87:534-8
- Luoto R, Kalliomaki M, Laitinen K, Delzenne NM, Cani PD, Salminen S, Isolauri E. Initial dietary and microbiological environments deviate in normal-weight compared to overweight children at 10 years of age. J Pediatr Gastroenterol Nutri 2011; 52:90-5; http://dx.doi.org/10.1097/MPG.0b013e3181f3457f
- Subramanian S, Blanton LV, Frese SA, Charbonneau M, Mills DA, Gordon JI. Cultivating Healthy Growth and Nutrition through the Gut Microbiota. Cell 2015; 161:36-48; PMID:25815983; http://dx.doi.org/10.1016/j.cell.2015.03.013
- Dutta S, Ray P, Narang A. Comparison of Stool Colonization in Premature Infants by Three Dose Regimes of a Probiotic Combination: A Randomized Controlled Trial. Am J Perinatol 2014; 32(8):733-40; PMID:25519197
- Miniello VL, Colasanto A, Cristofori F, Diaferio L, Ficele L, Lieggi MS, Santoiemma V, Francavilla R. Gut microbiota biomodulators, when the stork comes by the scalpel. Clin Chim Acta 2015; PMID:25668229; http://dx.doi.org/10.1016/j.cca.2015.01.022
- Pacheco AR, Barile D, Underwood MA, Mills DA. The impact of the milk glycobiome on the neonate gut microbiota. Annu Rev Anim Biosci 2015; 3:419-45; PMID:25387230; http://dx.doi.org/10.1146/annurev-animal-022114-111112
- Arboleya S, Bahrami B, Macfarlane S, Gueimonde M, Macfarlane GT, de Los Reyes-Gavilan CG. Production of immune response mediators by HT-29 intestinal cell-lines in the presence of Bifidobacterium-treated infant microbiota. Benef Microbes 2015; 6(4):1-10; PMID:25691102
- Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med 2015; 21:109-17; PMID:25578246; http://dx.doi.org/10.1016/j.molmed.2014.12.002
- David LA, Weil A, Ryan ET, Calderwood SB, Harris JB, Chowdhury F, Begum Y, Qadri F, LaRocque RC, Turnbaugh PJ. Gut microbial succession follows acute secretory diarrhea in humans. mBio 2015; 6:e00381-15
- Hsiao A, Ahmed AM, Subramanian S, Griffin NW, Drewry LL, Petri WA, Jr., Haque R, Ahmed T, Gordon JI. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nat 2014; 515:423-6; PMID:25231861; http://dx.doi.org/10.1038/nature13738

