ABSTRACT
Emerging evidence strongly suggest that the human “microbiome” plays an important role in both health and disease. Bile acids function both as detergents molecules promoting nutrient absorption in the intestines and as hormones regulating nutrient metabolism. Bile acids regulate metabolism via activation of specific nuclear receptors (NR) and G-protein coupled receptors (GPCRs). The circulating bile acid pool composition consists of primary bile acids produced from cholesterol in the liver, and secondary bile acids formed by specific gut bacteria. The various biotransformation of bile acids carried out by gut bacteria appear to regulate the structure of the gut microbiome and host physiology. Increased levels of secondary bile acids are associated with specific diseases of the GI system. Elucidating methods to control the gut microbiome and bile acid pool composition in humans may lead to a reduction in some of the major diseases of the liver, gall bladder and colon.
Introduction
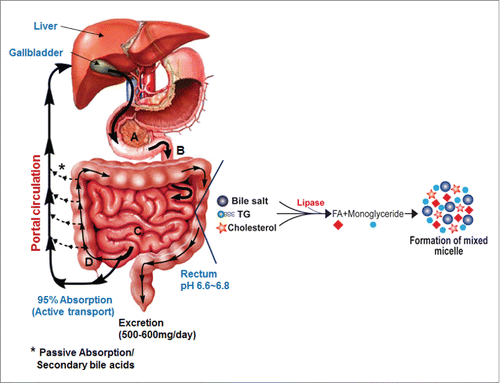
Bile acids are synthesized from cholesterol in the liver hepatocytes. In humans, the liver synthesizes 2 primary bile acids, cholic acid (CA) and chenodeoxycholic acid; (CDCA); whereas, in rodents the primary bile acids include CA, CDCA and muricholic acids (MCA) which are 6-hydroxylated derivatives of CDCA. Before active secretion from the liver, via the canalicular membrane of hepatocytes, bile acids are conjugated to either taurine or glycine at the C-24 carboxyl group. In the human liver, unlike rodents, the ratio of taurine to glycine conjugation is regulated by diet.Citation1 Following secretion, conjugated bile acids (CBA) are stored in the gall bladder along with phosphatidylcholine and cholesterol. CBAs function to help keep cholesterol in solution in the gall bladder by forming mixed micelles. Following a meal, the gall bladder is stimulated to contract releasing its contents into the upper small intestines. Here, CBAs activate pancreatic lipase, and form mixed micelles with monoglyceride, cholesterol, partially ionized fatty acids and fat soluble vitamins (A, D, K and E) promoting their absorption by enterocytes (). Unconjugated and some glycine-conjugated bile acids are reabsorbed via passive diffusion throughout the small intestine. Active transport of bile acids occurs in the ileum and passive absorption of hydrophobic secondary bile acids occurs in the colon.Citation2 The composition of serum bile acids returning from the gut to the liver is a mixture of free and CBA, secondary, and oxo and β-hydroxyl bile acids. The enterohepatic circulation of bile acids occurs several times each day and there is an increased secretion of fecal bile acids in individuals on a Western diet.
Figure 1. The gastrointestinal system and the role of bile salts in nutrient absorption. Bile salts activate pancreatic lipase producing monoglycerides and free fatty acids. Bile salts aid in the solubilization and absorption of lipids, cholesterol and fat-soluble vitamins by forming mixed micelles. The capital letters A-D indicate duodenum, jejunum, ileum and colon, respectively.
Starting in 1999, it became clear that bile acids also serve endocrine functions in the body largely through their binding and activation of the nuclear receptor, Farnesoid X Receptor (FXR),Citation3,4 and the plasma membrane-bile acid receptor TGR5.Citation5 Through activation of these 2 receptors, bile acids regulate their own synthesis, conjugation, transport and detoxification, as well as lipid, glucose, and energy homeostasis.Citation6 Furthermore, bile acids play an important role in maintaining intestinal barrier function, as antimicrobial agents that help determine the gut microbiome structure, and as inducers of genes encoding anti-microbial peptides and lectins via FXR.Citation7
Bile acid biotransformation of gut bacteria
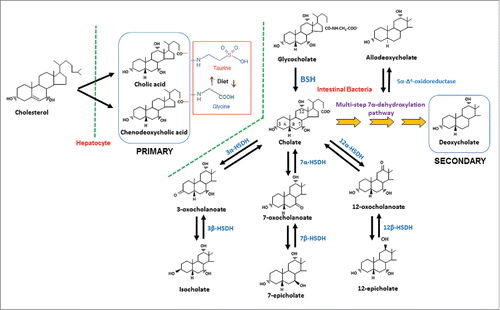
Gut bacteria are capable of carrying out numerous biotransformations of bile salts during their enterohepatic circulation (). The major biotransformations include: hydrolysis of conjugated bile acids to free bile acids and glycine or taurine by bile salt hydrolase (BSH); 7α-dehydroxylation of CA and CDCA yielding DCA and LCA, respectively; bile acid 7β-dehydroxylation of UDCA yielding LCA. The bile acid 7α/7β-dehydroxylations involve a multistep biochemical pathway found only in anaerobic gut bacteria.Citation8 In addition, the gut microbiota are capable of the oxidation and epimerization of hydroxy groups at the C3, C7 and C12 position of bile acids yielding isobile (β-hydroxy) acids.
Figure 2. Synthesis of primary bile acids in the liver and biotransformations of bile acids carried out by gut bacteria. In humans, the liver synthesizes cholic acid and chenodeoxycholic acid from cholesterol. The major quantitative and irreversible reactions carried out by gut bacteria include the hydrolysis of conjugated bile acids by BSH and 7α-dehydroxylation of cholic acid and chenodeoxycholic acid. More than 20 bile acid metabolites can be produced from primary bile acids by the gut microbiota. Abb. BSH, bile salt hydrolase; 3α-HSDH, 3α-hydroxysteroid dehydrogenase; 3β-HSDH, 3β-hydroxysteroid dehydrogenase; 7α-HSDH, 7α-hydroxysteroid dehydrogenase; 7β-HSDH, 7β-hydroxysteroid dehydrogenase; 12α-HSDH, 12α-hydroxysteroid dehydrogenase; 12β-HSDH, 12β-hydroxysteroid dehydrogenase.
Bile salt hydrolase
Bile salts in the gallbladder of vertebrates are conjugated to either taurine or glycine in varying proportions due to a combination of nature and nurture. The murine bile acid pool is almost entirely taurine conjugated,Citation9 irrespective of diet. Typically, the glycine:taurine ratio in humans is 3:1.Citation10 However, taurine-conjugation (but not glycine-conjugation) of bile acids in humans is diet-dependent, with “Western-diets” high in animal protein favoring taurine-conjugation; whereas, vegetarian diets tend to shift the biliary pool toward glycine conjugation.Citation1,11
The composition of bile salts in the small intestine more closely resembles the biliary pool; whereas, the fecal bile acid profile is almost entirely unconjugated and secondary bile acids owing to the action of bile salt hydrolases (BSH) and 7α-dehydroxylation.Citation8 BSH activity is widespread in commensal bacteria inhabiting both the small intestine and large bowel. Gram-positive gut bacteria have the most diverse distribution of BSH including; Clostridium,Citation12,13 Enterococcus,Citation14 Bifidobacterium,Citation15,16 and Lactobacillus,Citation17,18 while the distribution of BSH in Gram-negatives is, so far, detected only in members of the genus Bacteroides.Citation19 Species of human intestinal archaea such as Methanobrevibacter smithii and Methanosphera stadmanae were both shown to encode BSH capable of hydrolyzing both taurine- and glycine-conjugates.Citation20 Metagenomic “mining” of environmental bacteria for BSH and characterization of cloned homologues revealed penicillin V amidase activity, but not BSH activity, suggesting BSH is a gut-specific function.Citation20
BSH hydrolyze the C-24 N-acyl bond which links the bile acid to the amino acid conjugate. BSH enzymes are classified as N-terminal nucleophilic hydrolases (Ntn), sharing this classification with penicillin amidases both of which have a catalytic N-terminal cysteine residue.Citation16 Indeed mutation of Cys-1 to serine or threonine (OH rather than SH) abolishes activity in the BSH of Bifidobacterium bifidum.Citation21 Chemical agents that oxidize thiol groups inhibit BSH activity. Sequence alignment of BSH suggests C1, D20, Y82, N175, and R228 are highly conserved among BSH; and structural characterization and sequence alignment of multiple BSH has provides further support for these amino acids in catalysis.Citation16
In general, BSH are insensitive to oxygen, have pH optima between 5 and 6, and are located intracellularly. Substrate specificity is typically in relation to the amino acid moiety with higher affinity for glycine-conjugated bile acids.Citation16,21-23
Bile acids regulate the gut microbiome structure
Resistance to bile salts is a major selective pressure modulating microbiome structure in the gut.Citation24 Gut bacteria are resistant to bile salts through a number of mechanisms including; efflux pumps,Citation25,26 alteration of membrane lipidCitation27,28 and proteinCitation29,30 composition. A major mechanism by which bile acids act as antimicrobial agents in the gut is through membrane-damage. The detergent nature of bile acids suggests that concentration and bile acid hydrophobicity play an important role in their antimicrobial action. Unconjugated bile acids are generally more hydrophobic than the corresponding conjugated forms. Indeed, increasing concentrations of bile acids have been shown to solubilize membranes and cause the dissociation of integral membrane proteins,Citation31,32 resulting in leakage of intracellular content.Citation33,34 Even at sub-micellar concentrations, bile salts can alter membrane lipid composition.Citation35,36
The conversion of primary bile acids to secondary bile acids is correlated with increased hydrophobicity and enhanced binding to membrane lipids.Citation37,38 Unconjugated dihydroxy bile acids passively flip-flop across phospholipid bilayers more rapidly than trihydroxy bile acids.Citation39 Cell membrane architecture has been observed to alter bile tolerance; for instance, growth in the presence of sodium monooleate resulted in altered membrane lipid profile and enhanced tolerance to bile salts.Citation28 B. longum BBMN68 expresses a hemolysin-like protein that provides greater tolerance to taurine-conjugated bile acids but not glycine-conjugated bile acids.Citation29 Once inside the bacterial cell, bile acids have been shown to alter macromolecular structure/function through detergent effects, damage DNA, and activate DNA repair enzymes.
Gram-negative bacteria are thought to have a higher bile acid tolerance than Gram-positives due to their lesser degree of sensitivity to oxgall, used in enrichment of MacConkey agar medium. Indeed, Salmonella typhimurium and Campylobacter are often isolated from bile or from the gallbladder.Citation40 Interestingly, studies involving rodents fed bile acids or high fat diets (regardless of obesity phenotype) appear to select against the Bacteroidetes (Gram-negative) allowing expansion of the Firmicutes (Gram-positive).Citation41
A microbial view of bile salt hydrolysis
The physiological role of BSH is a subject of controversy and appears to vary between bacterial isolates; however, several hypotheses have been proposed. Free bile acids are more damaging to cellular membranes than conjugated bile acids. Floch et al.Citation42 presented data on 2 unidentified Lactobacillus isolates in addition to strains of Enterococcus, Bacteroides and Clostridium perfringens,Citation42 that suggests the gene encoding BSH evolved to generate free bile acids whose toxicity is greater than conjugated bile salts. BSH activity was active against glycine-conjugates of hydrophobic BAs CDCA and DCA, but not the hydrophilic BA CA. GCA was tolerated at 10 mM; whereas, GDCA and GCDCA were inhibitory (defined as a 2-log10 reduction in growth) between 1–5 mM, values similar to DCA and CDCA.Citation42
Mechanisms by which free BA inhibit lactobacilli and bifidobacteria suggest a combination of membrane-perturbation and intracellular accumulation of protonated free BA. Accumulation of free BA was shown to reduce intracellular pH (ΔpH) similar to other hydrophobic weak organic acids, such as short chain fatty acids (SCFA). However, at the minimum inhibitory concentration, free BA dissipated ΔpH and, unlike SCFA, also collapsed transmembrane electrical potential (ΔΨ) due to membrane perturbations. Again, secondary bile acids were more toxic i.e. DCA has a 10-fold lower minimum inhibitory concentration (MIC) than CA.Citation43 Thus, liberation of free bile acids results in toxicity for specific Lactobacillus species.
In contrast, studies comparing wild type and mutant BSH positive isolates suggest conjugated bile acids are more toxic to many gut microbes and that BSH plays a detoxification role. BSH mutant strains of Lactobacillus amylovorus,Citation44 and Listeria monocytogenesCitation45,46 were shown to be significantly more sensitive to conjugated bile salts than their wild-type counterparts. Indeed, glycine-conjugated BA was found to be significantly more inhibitory than taurine-conjugates, particularly at low environmental pH.Citation22,47 The explanation proposed is that because the pKa of conjugated BA is significantly lower than free bile acids the conjugate acts as a weak acid reducing intracellular pH. By hydrolyzing the conjugate, the weaker carboxyl group of DCA allows recapture and export of DCA (and thus protons) thus reestablishing ΔpH. Additional experiments with bsh mutant isogenic strains will be important in clarifying the relative toxicity of free and conjugated bile acids for particular bacterial strains.
Bile salt hydrolysis provides a source of amino acids
BSH may function in some bacterial strains as a means to acquire a source of energy and building blocks for biosynthesis. Glycine is metabolized to ammonia and carbon dioxide by some bacteria. Taurine is catabolized to ammonia and carbon dioxide with the additional release of sulfite. It has been observed that some Clostridium spp. with BSH activity are able to utilize taurine in Stickland fermentation reactions resulting in improved growth.Citation48 Furthermore, taurine metabolism from TCA by a strain of Bacteroides was shown to stimulate cholic acid 7α-dehydroxylation via release of sulfite suggesting end-product metabolism may further enhance bile salt degradation.Citation49 Consumption of taurine and TCA has been shown recently to cause a bloom in the sulfidogenic bacterium, Bilophila wadsworthia, resulting in colitis in the IL10-/- mouse model.Citation50
Consequences of bile salt hydrolysis for the mammalian host
Cardiovascular disease is responsible for 16.7 million deaths worldwide and is the leading cause of mortality and morbidity worldwide.Citation51 There is a clear link between serum cholesterol and cardiovascular disease, particularly coronary artery disease (CAD).Citation52 A strong correlation exists between low-density lipoprotein cholesterol (LDL-C) and risk for CAD.Citation53 The major focus of BSH research is aimed at treating/preventing CAD caused by hypercholesterolemia (high LDL-C) in humans.Citation54 LDL-C may be decreased by therapeutic oral intake of strains of probiotic bacteria with high BSH activity. Not surprisingly, therefore, much literature in probiotics is devoted to screening potential probiotic bacteria for BSH activity and specificity.Citation55
A major question that is the topic of much research focus is the mechanism by which certain probiotic bacteria reduce serum cholesterol. In the small intestine, conjugated bile salts form small mixed micelles over a narrow concentration range (critical micellar concentration) with cholesterol, fatty acids and monoglycerides (). Bile salts are amphipathic molecules with a hydrophobic side characterized by the steroid ring moiety, and a hydrophilic face characterized by one to 3 hydroxy groups and the amide carbonyl. Bile acids aggregate to form micelles, in which their hydrophilic face is exposed to the aqueous medium while their hydrophobic face is sequestered from the polar aqueous environment. The micelle thus provides a solvent vehicle for water insoluble compounds, allowing their absorption along the small intestine.
Increased BSH activity in the small intestine disrupts micelle formation and absorption of cholesterol and lipids. This is hypothesized to lower serum LDL-C and triglycerides as cholesterol output is increased due to lower uptake. Probiotics can also increase the output of bile acids in feces possibly by binding or uptake of bile acids. Increased fecal secretion of bile acids requires increased hepatic catabolism of cholesterol into bile acid synthesis. Elucidation of mechanisms involved in lowering of serum cholesterol by probiotic bacteria should focus on comparison of BSH expressing strains vs. isogenic mutants as controls in which the only variable is BSH. This should allow better comparisons within studies and between studies using different strains of bacteria. Lipidomics/metabolomics profiles at all points along the EHC along with key genes regulating cholesterol and bile acid synthesis and transport should further clarify the mode of action. There is also a balance in levels in BSH activity that needs to be determined between healthy cholesterol lowering and prevention of malabsorption/steatorrhea in patients.
Bile Acid 7α/7β-dehydroxylation by intestinal bacteria
The term “secondary bile acid” typically denotes the removal of the 7α-hydroxy or 7β-hydroxy group from primary bile acids produced in the liver of humans and other vertebrates. Germ-free animals have been shown to lack secondary bile acids.Citation56,57 Bacteria capable of converting primary to secondary bile acids have been isolated from humans.Citation58–60 The rodent isolate required unidentified Bacteroides spp. in co-culture due to an unidentified syntrophy.Citation61 This is in contrast to human isolates that are readily isolated in pure culture using anaerobic media. Rodent,Citation61 but not human bile acid 7α-dehydroxylating bacteria are capable of 7α-dehydroxylating muricholic acids (6α, 7β-hydroxy; 6β, 7β-hydroxy).Citation62
Characterization of the genetics and molecular biology of bile acid 7α/β-dehydroxylation has been performed mainly in strains of Clostridium scindens,Citation8,63 particularly C. scindens VPI 12708 (formerly Eubacterium sp. strain VPI 12708).Citation64-67 The type strain, C. scindens ATCC 35704 (formerly Clostridium strain 19) was characterized as having steroid-17,20-desmolase (SD) activity,Citation68 which cleaves the side chain from glucocorticoids (C21) converting them to androgens (C19).Citation69,70 Interestingly, there are important differences in the “sterolbiome” of C. scindens ATCC 35704 and C. scindens VPI 12708 with respect to sterol metabolism.Citation71 Strain 35705 encodes the desABCD operon which contain the gene for 20α-HSDH and the putative SD, while strain 12708 lacks this operon. Interestingly, C. scindens VPI 12708 metabolizes the 11β-hydroxyandrostenedione (11β-OHAD) product, reducing the 17-keto group to a 17α-hydroxy.Citation70 Previously, we reported that testosterone enhances 7α-dehydroxylating activity in C. hylemonae TN27172Citation72; however, it is unknown what effect 11β-OHAD, and its subsequent gut microbial metabolites has on bile acid 7α-dehydroxylation.
While genetic knockouts of genes in the 7α-dehydroxylation pathway have yet to be reported, our laboratory has built a model for the multi-step bile acid 7α-dehydroxylating pathway in C. scindens based on characterization of native or recombinant enzymes encoded by the bai operon ().Citation8 In this pathway, primary unconjugated bile acids are transported into the cell by a proton-dependent bile acids transporter encoded by the baiG gene,Citation73 ATP-dependent CoA ligation is catalyzed by the baiB gene,Citation64 while ATP-independent CoA transfer from secondary to primary bile acids is catalyzed by the baiF and baiK gene products.63 () Oxidation of the 3-hydroxy group is catalyzed by the baiA gene, which has specificity for CoA conjugates.Citation74 A C4˭C5 bond is introduced in 7α-hydroxy, 3-dehydro-bile acids by an NADH:flavin-dependent oxidoreductase encoded by the baiCD gene while a homologous enzyme encoded by the baiH gene recognizes 7β-hydroxy, 3-dehydro-bile acids.Citation67 The rate-limiting step in this pathway is bile acid 7α-dehydration.Citation65 The baiE gene encodes the bile acid 7α-dehydratase,Citation65 and it is hypothesized that the baiI gene encodes the bile acid 7β-dehydratase.Citation71 The genes involved in reduction of the 3-dehydro-4,6-deoxycholyl-CoA in the case of CA metabolism or 3-dehydro-4,6-lithocholyl-CoA in the case of CDCA or UDCA metabolism have yet to be identified.
Figure 3. Proposed bile acid 7α-dehydroxylation pathway of cholic acid in Clostridium scindens. The multistep 7α-dehydroxylation pathway converts the primary bile acids cholic acid and chenodeoxycholic acid into the secondary bile acids deoxycholic acid and lithocholic acid, respectively. Abb. baiG, primary bile acid transporter; baiB, bile acid-coenzyme A ligase; baiA, 3α-hydroxysteroid dehydrogenase; baiCD, 7α-hydroxy-3-oxo-Δ4-cholenoic acid oxidoreductase; baiF, bile acid coenzyme A transferase/hydrolase; baiE, bile acid 7α-dehydratase.
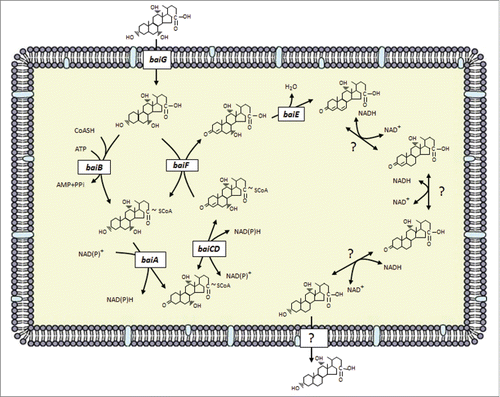
Figure 4. Arrangement of genes in the bile acid inducible (bai) operon in various species of bile acid 7α/β-dehydroxylating gut bacteria. The genes encoding enzymes carrying out bile acid metabolism in gut bacteria capable of producing secondary bile acids. Biochemical pathway leading to secondary bile acid formation is shown in . Amino acid accession numbers are as follows: C. scindens ATCC 35704 baiABCDEAFGHI (EDS05761-EDS05768), baiA2 (EDS06021), 7α-HSDH (EDS05904); C. scindens VPI 12708 baiBCDEA2FGHI (AAC45410-AAC45418), baiJKL (ACF20978-ACF20980), baiA1 (P07914), 7α-HSDH(AAB61151); C. hiranonis TO-931 (DSM 13275) baiBCDEA2FG (AAF22844-AAF22849), baiH (ABQ12723), baiJ (WP_006439754) 7α-HSDH (WP_006440226; C. sordellii VPI 9048 baiCD, baiA2 (EPZ56913, EPZ56915), baiH, baiE (EPZ56916, EPZ56912), 7α-HSDH (EPZ54454); C. hylemonae TN271 baiBCDEA2FGHI (ACF20984-ACF20990), baiJKL (ACF20981-ACF20983) baiA1 (WP_006441384), 7α-HSDH (WP_040435027).
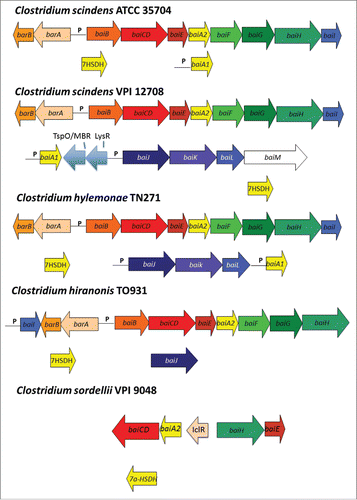
In a ground-breaking paper demonstrating a causal-link between deoxycholic acid and liver cancer, it was shown that a single OTU in Clostridium Cluster XI closely related to C. sordellii constituted 12% of fecal bacteria fed the high-fat diet, but was not found in HFD+vancomycin.Citation75 It was also found that the baiJ gene was in high abundance, consistent with C. sordellii levels.Citation73 We reported the baiJKL operon in C. hylemonae and C. scindens which encodes a bile acid CoA transferase (baiK) with similar function and to the baiF gene, a homolog on the baiBCDEAFGHI operon ().Citation63,72 The function of the baiJ gene is unknown, though it is predicted to encode a flavoprotein possibly involved in C-C double bond oxidation/reduction. However, it is doubtful it is involved in the “reductive” arm of the pathway toward secondary bile acids because it is absent in the genomes of several isolates capable of secondary bile acid production (). A BLASTP search of the NCBI nr database, as well as Genome-BLAST against the C. sordellii VPI 9048 genome suggests the baiJ gene is absent. It may be that some C. sordellii strains may have acquired/lost the baiJKL operon (). In the same study, sequences most closely related to C. hylemonae, which encodes the baiJ gene were found to make up only 0.5% of OTU on the HFD.Citation75 In any case, this data is consistent with several studies that have shown increased levels of bile acid 7α-dehydroxylating bacteria on a high-fat dietCitation76–78 or bile acid feeding.Citation79
Genome-sequencing projects of individual gut isolates and metagenomic libraries have provides additional information about potential bai genes in other gut bacteria providing new questions that can begin to be answered; however, a further discussion of these hypothetical genes is beyond the scope of this review.
Structure-function characteristics of enzymes in the bile acid 7α-dehydroxylation pathway
To date, crystal structures of 3 key enzymes of the bile acid 7α-dehydroxylation pathway, namely those encoded by the baiA2, baiB and baiE genes, have been solved.Citation80,81 The structures along with functional characterization provide important insights into the mechanisms of key biochemical steps of this pathway. Structural information is essential for designing small molecule inhibitors of key enzymes in this pathway.
The baiB gene encodes a bile acid Co-enzyme A ligase that catalyzes the thioesterification of bile acids to Coenzyme A (CoA) following uptake of primary bile acids into the bacterial cell.Citation64 The crystal structure of baiB gene product from Clostridium scindens VPI 12708 revealed a monomeric assembly confirmed by analytical size exclusion chromatography (unpublished data). The overall fold of the protein is similar to members of the AMP binding family consisting of separate binding pockets for CoA, bile acid and ATP. As previously described, thioesterification of bile acid to CoA is a 2-step ATP-dependent process, which involves the ATP-dependent adenylation of the bile acid followed by CoA exchange with release of AMP.Citation64
The crystal structure of bile acid 3α-hydroxysteroid dehydrogenase (3α-HSDH), encoded by the baiA gene product, revealed a tetrameric assembly commensurate with analytical size exclusion chromatography.Citation80 The constituting monomers are all a single domain protein bearing the characteristic Rossmann fold observed in other members of the short-chain dehydrogenase/reductases (SDR) family with distinct substrate and co-factor binding sites. Catalytically conserved amino acid residues are located at the interface of substrate and co-factor binding sites. The substrate binding site continues into the co-factor binding site suggesting binding of the co-factor in its respective site is essential to orient the functional group of the substrate to the active site residues. There is evident from steady-state enzyme kinetic studies that indicate substrate turnover occurs in the presence of co-factor. Steady-state kinetic characterization of baiA1 and baiA2 also revealed NAD+ as the preferred co-factor. The specificity constant (kcat/KM) of NADP+ is at least an order of magnitude lower than NAD+. The NAD+-bound crystal structure of baiA2 provides the basis of such a preference to restrictive conformational flexibility of residue Glu42 in the co-factor binding site that participates in a salt bridge interaction with Arg16. Mutagenesis studies where Glu42 is replaced with Ala improved specificity toward NADP+ by fold10- compared to the wild-type enzyme. Steady-state kinetic characterization studies also established that bile acid-CoA esters are the preferred substrates suggesting a possible interaction of the CoA moiety with the enzyme. However, the crystal structures did not reveal a separate binding pocket for CoA. It is likely that an induced pocket is generated upon binding of the bile acid-CoA ester.
Bile acid 7α-dehydratase (BA 7α-DeOH), encoded by the baiE gene, catalyzes the rate limiting and irreversible step in the bile acid 7α-dehydroxylation pathway.Citation65 The recently solved crystal structure of this enzyme revealed a trimeric assembly for all 3 key homologs whereas the monomers exhibit the canonical twisted α+β barrel fold characteristic of the Nuclear Transport Factor 2 (NTF2) family.Citation81 The trimers appear to be bonded together by a divalent metal cation, probably Zn2+. The binding of bile acid substrate to BA 7α-DeOH is shown in Site-directed mutagenesis studies established the role of 3 conserved residues in the active site namely, Tyr30, Asp35 and His83, as important for catalysis (). In addition, 3 other non-active site residues, Tyr54, Asp106 and Arg146, are found to be essential for substrate binding and turnover. This correlates with the co-crystal structure of baiE from Clostridium hiranonis DSM13275 with the partially bound product 3-oxo-Δ4,6-lithocholyl-CoA (3-oxo-Δ4,6-LC-CoA), where the loop constituting of residues 48–63 forming the roof of the active site that reveals a distinct conformation for all the apo-structures. Conformational flexibility of this loop may be essential for substrate binding. Site-directed mutagenesis study of Tyr54 detected impaired substrate turnover indicating the importance of this loop in catalysis. In all, the apo-structures of baiE residues Asp106 and Arg146 in the substrate binding pocket interact via a salt bridge.Citation81 Severance of the salt bridge by replacing with residues Asn and Gln at position 106 and 146, respectively, abolished catalysis. Furthermore, steady-state kinetic characterization studies revealed that unlike baiA1 and baiA2 all baiE homologs are able to turnover 3-oxo-Δ4-bile acid and 3-oxo-Δ4-bile acid-CoA thioester substrates with comparable efficiency. This suggests that the CoA moiety maybe removed from the bile acid either before or after the elimination of C7-hydroxy group. The apo- crystal structures of baiE, like the crystal structure of baiA2, did not reveal a separate binding pocket for the CoA moiety. However, the 3-oxo-Δ4,6-LC-CoA bound crystal structure of baiE from Clostridium hiranonis DSM13275 revealed an extended binding pocket generated by substrate binding sites of 2 protomers that originate from 2 different trimer assemblies. In that structure, the loop formed by residues 48-63 participates in forming the extended substrate-binding pocket.
Figure 5. Binding of substrate to bile acid 7α-dehydratase from Clostridium scindens. (A) Predicted binding mode of 3-oxo-Δ4-chenodeoxylcholyl-CoA (3-oxo-Δ4-CDCA-CoA) in the crystal structure of this enzyme. (B) Observed binding of the product, 3-oxo-Δ4,6-lithocholyl-CoA (3-oxo-Δ4,6-LCA-CoA), in product bound crystal structure of bile acid 7α-dehydratase.Citation81
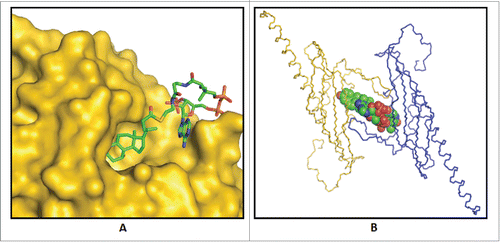
Figure 6. Predicted enzyme substrate interactions in the active site of bile acid 7α-dehydratase from Clostridium scindens. (A) Probable binding mode of 3-oxo-Δ4-chenodeoxylcholyl-CoA (3-oxo-Δ4-CDCA-CoA). Blue dashed lines and adjacent numbers are predicted interaction of His83-Nϵ2 atom with C7-OH and C6 atoms and Y30-OH group with C3-oxo atom of 3-oxo-Δ4-CDCA-CoA. The 6α-H closest to H83-Nϵ2 atom colored magenta and 6β-H away from H83-Nϵ2 atom colored brown. (B) Predicted stacking interaction involving the adenine group of the Coenzyme (CoA) moiety of oxo-Δ4-chenodeoxycholyl CoA (3-oxo-Δ4-CDC-CoA) with Y115. Carbon atoms of protein residues and product molecule are colored gold and green, respectively. H, O, N, P and S atoms are colored gray, red, blue, orange and olive, respectively.Citation81
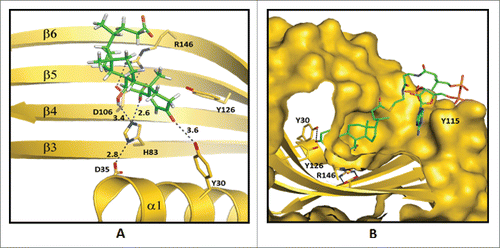
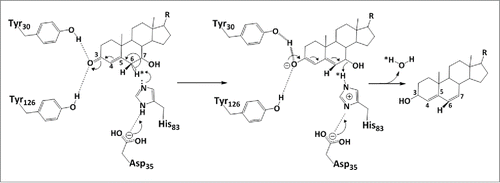
Figure 7. Proposed catalytic mechanism of bile acid 7α-dehydratase from Clostridium scindens. The role of catalytically important amino acid residues in elimination of the 7α-hydroxy group through release of a water molecule are illustrated. Tyr 30 acts as a general acid (assisted by Tyr 126) to withdraw electrons from the 3-oxo group. This allows an electron shift and destabilization of the bond holding the 6α-hydrogen. In this model, H83 accepts the 6α-hydrogen and may either protonate the 7α-hydroxy group or activate D106 or both.
Esterification of fecal bile acids in the gut
Over the past 50 y there have been a number of reports of esterified bile acids in fecal samples from rodents and humans.Citation82 A recent study by Kakiyama G. et al.Citation83 showed that treatment of different human fecal samples with 0.1 N NaOH at 60°C for 2 hours significantly increased the yield (28% to 48%) of total bile acids as compared to those samples without base hydrolysis. In this regard, there have been reports of ethyl-esters and long-chain fatty acid esters of LCA,Citation84 as well as polyesters of DCA.Citation85 It has been estimated that esterified bile acids may constitute more than 25% of total fecal bile acids. This could be important as esterification of bile acids make these molecules more hydrophobic, less soluble, and should decrease the concentration of bile acids in fecal water. Unfortunately, little is known about the role of gut bacteria in carrying out esterification of bile acids.
Pathophysiological relevance of secondary bile acids to host
In recent years it has become clear that bile acids serve endocrine functions largely through their activation of nuclear receptors including: Farnesoid X Receptor (FXR), Vitamin D receptor, and the Pregnane-activated Receptor (PXR) as well as the G-protein coupled plasma membrane receptors (GPCR) including; TGR5, muscarinic receptors (M2,3) and the sphingosine-1-phosphate receptor 2 (S1PR2).Citation86 Both nuclear receptors and GPCRs have varying affinities for different bile acids (). In this regard, by altering the bile acid pol composition, gut bacteria have the potential to alter cellular metabolism and host physiology and metabolism of cells exposed to bile acids.
Table 1. Secondary bile acids differentially activate nuclear Receptors and G-protein coupled receptors as compared to primary bile acids.
Recent studies also illustrate the important role gut microbes play in the levels and profiles of bile acids in various tissues of the body including liver, kidney, plasma, and heart.Citation87 The global physiological significance of these alterations in bile acid profiles is unknown. However, decades of research strongly suggest that secondary bile acids (DCA and LCA), a product of bacterial metabolism of primary bile acids, are involved in disease processes including cancers of the colon,Citation76 liver,Citation75 and cholesterol gallstone disease in some patients.Citation88 The level of DCA in bile of male VA patients in Richmond, Virginia, varied from <1% to >60% with a mean of 35%.Citation8 The average DCA level of the normal population is ~20%.Citation10 The level of DCA in bile is believed to be controlled by 2 major factors: A) levels and activities of bile acid 7α-dehydroxylating gut bacteria, and B) colonic transit time.Citation88,89 Both the levels of bile acid 7α-dehydroxylating gut bacteria and colonic transit time can be strongly influenced by dietary habits. Finding ways to decrease the percentage of secondary bile acids in blood, bile and feces may result in a decrease in the incidence of some GI cancers.
Oxidation and epimerization of bile acid hydroxy groups by gut microbiota
Members of the intestinal microbiota have genes that encode a variety of pyridine nucleotide-dependent hydroxysteroid dehydrogenases (HSDH). HSDHs are widely distributed in various members of the gut microbiota.Citation8 The gut microbiota is capable of the oxidation/reduction of hydroxy groups at the 3-, 7-, and 12- carbons of bile acids (). The epimerization of bile acid hydroxy groups (α ↔ β) requires 2 position-specific bile acid α and β HSDHs which generates a stable oxo-bile acid intermediate i.e., 7α-hydroxy ↔ 7-oxo ↔ 7β-hydroxy. The extent of epimerization and accumulation of the oxo group intermediate appears to be influenced by the oxidation/reduction potential of the cellular environment. For example, the formation of oxo-bile acids may be more favorable in bacteria near the mucosal surface of the gut where there is a potential for a higher redox potential than in the lumen of the intestines. Bacterial bile acids HSDHs differ in their pH optima, pyridine nucleotide specificity NAD(H) or NADP(H) or both, subunit molecular weight, and gene regulation.Citation8 Amino acid sequence analysis suggests that most bacterial HSDHs in the gut microbiota belong to the short-chain alcohol/polyol dehydrogenase gene family.Citation8
What is the physiological significance of bile acid hydroxy group biotransformations to gut bacteria and host? In the highly evolutionary competitive environment found in the human gut microbiome, the persistence of genes usually indicates they increase the organism’s ability to survive. The epimerization of bile acid 7α-hydroxy group decreases the toxicity of chenodeoxycholic acid (CDCA) for gut microbiota. CDCA is a relatively hydrophobic primary bile acid. In contrast, ursodeoxycholic acid (UDCA), the 7β-epimer of CDCA, is a much more hydrophilic, and therefore less toxic to gut bacteria.Citation90,91 Clostridium absonum, which has both 7α and 7β-bile acid HSDHs, converts CDCA into UDCA. It follows that any bacterium able to biotransform CDCA into UDCA may have a survival advantage in the highly competitive environment found in the lumen of our gastrointestinal system. Bacterial HSDHs have the ability to epimerize other hydroxyl groups on bile acids in addition to the 7-oxo group, including the 3- and 12-oxo groups.Citation8 A recent study that elucidated genes responsible for the production of enzymes that epimerize DCA to 3-oxo-DCA and subsequently to 3-iso-DCA in Ruminococcus gnavus reported that these bile acids have reduced toxicity.Citation92 Given these findings, a reasonable hypothesis is that in response to these membrane-disrupting primary and secondary bile acids, gut microbes utilize HSDHs as a means of detoxifying bile acids.
Bile acid 7α-HSDH could also be an important enzyme regulating substrate availability of bile acid 7α-dehydroxylation pathway by converting primary bile acids into 7-oxo-bile acids that are not substrates for 7α-dehydroxylation. Other bacteria may use bile acid HSDHs to co-opt primary bile acids into signaling molecules for their own autocrine or paracrine uses, though this area has yet to be thoroughly investigated.
The formation of 7-oxo-bile acid by gut microbiota may also have effects on host glucocorticoid metabolism. Recent studies reported that 7-oxo-lithocholic acid (7-oxo-LCA), a bacterial metabolite of CDCA, acts as a competitive inhibitor of human hepatic 11β-hydroxysteroid dehydrogenase-1 (11β-HSDH1).Citation93 Hepatic 11β-HSDH1 recognizes 7-oxo-LCA as a substrate and reduces this bacterial metabolite back to CDCA.Citation94 However, in the process, 7-oxo-LCA inhibits cortisone reduction back to active cortisol. In this instance of possible inter-kingdom signaling, a bacterial bile acid metabolite has the ability to modulate host endogenous active steroid hormone levels. Steroid hormones can be effective at very low concentrations and enzymes such as 11β-HSDH1 are responsible for modulating the levels of active vs. inactive steroid hormones.Citation94 Any alteration in the levels of steroid hormones by gut microbes could be potentially significant to host physiology, though the extent of these affects in vivo are currently unknown.
Not only do primary and secondary bile acids alter human steroid hormone levels, but they are also implicated in a host of other signaling pathways. Antimicrobial peptides, such as cathelicidin produced in the colon, are an important component of our innate immune system.Citation95 Their production by the colonic epithelium is increased by activation of FXR.Citation96 CDCA is a high affinity agonist for FXR,Citation4 whereas 7-oxo-LCA and UDCA, bacterial metabolites of CDCA, are intermediate and non-active agonists, respectively.Citation97,98 Because cathelicidin has broad-spectrum antimicrobial activityCitation99 and its expression is controlled by FXR, it follows that by lessening the affinity of bile acids for FXR, a microbe could increase its fitness in the lumen of the large intestine. The extent to which these alterations in bile acid levels by gut flora affect both host FXR activation and downstream antimicrobial peptide production has yet to be fully explored.
Inactivation of ursodeoxycholic acid by 7β-dehydroxylation
UDCA is synthesized chemically on the order of 1,000 tons each year for pharmaceutical use or in some countries as an over-the-counter supplemen.Citation100 UDCA is prescribed therapeutically for the dissolution of gallstones, recurrent pancreatitis, primary biliary cirrhosis, and primary sclerosing cholangitis (PSC).Citation101,102 Rodent models even suggest UDCA may be therapeutic in fructose-induced metabolic syndrome.Citation103 Recently, trials have been underway to determine the efficacy of UDCA to prevent colorectal adenoma recurrence, and results have been mixed.Citation104-106 Animal models have suggested that UDCA can prevent azomethane-induced colon carcinogenesis.Citation107-109 UDCA treatment dilutes the concentration of DCA, and is thought to modulate the membrane and inflammatory effects of secondary bile acids on the colonic mucosa by increasing the hydrophilicity of the colonic bile acid milieu, and suppressing cell-signaling pathways activated by DCA.Citation110,111 Problematically, bile acid 7α-dehydroxylating bacteria, including Clostridium scindens and C. hiranonas are capable of converting UDCA to LCA through bile acid 7β-dehydroxylation.Citation8 Studies have shown that LCA is toxic, and causes DNA damage in bacteriaCitation112 and colonocytes.Citation113,114Thus, one might hypothesize that inhibition of bile acid 7β-dehydroxylation would improve the therapeutic potential of UDCA.
Bile acids regulate Clostridium difficile colonization and growth in the GI tract
C. difficile, the cause of antibiotic-associated diarrhea and colitis, is a growing health threat for patients taking broad spectrum antibiotics.Citation115 It has been estimated that C. difficile may be responsible for almost a half a million infections and 29,000 deaths in the US per year.Citation116 Moreover, as the world population ages, a higher percentage of the elderly are colonized by C. difficile and are more often treated with antibiotics. Treatment of patients with broad-spectrum antibiotics markedly reduces the level of the normally protective gut microbiota and allows proliferation of C. difficile normally found in low levels in some individuals. Patients treated with antibiotics, especially in hospitals, are also at risk from colonization by C. difficile spores, which germinate in the GI tract producing vegetative cells that secrete toxin A and B causing diarrhea and severe colitis in some cases.Citation117,118 Patients with antibiotics associated diarrhea and/or colitis are initially treated with either metronidazole or vancomycin to kill C. difficile colonizing the gut; however, between 10 to 40% of patients successfully treated with these antibiotics will relapse following cessation of antibiotic therapy. Fecal transplants, using gut microbiota from healthy donors, have been very successful in treating relapsing patient.Citation119 Recent studies have been undertaken to determine which members of the gut microbiota are responsible for resistance to C. difficile infection.Citation120 In this regard, it was recently reported that Clostridium scindens, a human gut bacterium that converts primary bile acids CA to DCA, is strongly associated with inhibition of Clostridium difficile colonization and colitis in animal models and in human patients.
What are the links between C. difficile infection of the GI tract and bile acids? More than 30 y ago, Wilson K.H. et al. reported that the addition of taurocholate, CA or DCA to specialized culture media stimulated C. difficile spore germination.Citation121-123 Later studies by Sorg J.A. et al. demonstrated that taurocholate, cholate and deoxycholate, but not chenodeoxycholate, stimulated the germination of C. difficile spores in vitro.Citation124 Moreover, a combination of taurocholate plus glycine proved to be a more potent combination for C. difficile spore germination.Citation123 Additional studies by Sorg J. A. et al. showed that chenodeoxycholate and muricholic acids were competitive inhibitors of taurocholate stimulated germination of C. difficile spores.Citation125 These studies suggest that a bile acid with a 12α-hydroxy group is necessary for binding to a putative germinant receptor on C. difficile spores. In 2013, the Sorg Laboratory reported the identification of a unique germinant receptor (CspC) recognized by 12α-hydroxylated bile acids.Citation126 Binding of bile acids to the CspC gene product is believed to lead to the release of Ca2+-dipicolinic acid (DPA) from the spore cortex in exchange for water. Hydration of the cortex then allows cell wall hydrolysis and outgrowth of a vegetative cell. Some studies report that DCA is an inhibitor of C. difficile growth in vitro and in the intestinal tract explaining why C. scindens may be a crucial member of the gut microbiota regulating levels of C. difficile. However, the molecular mechanism(s) of how DCA may inhibit C. difficile colonization and growth are currently unclear.
Summary and future directions
It is becoming clear that our view of the human body is changing. We now recognize the human body as a complex ecosystem of interacting prokaryotic and eukaryotic cells. Diet is a regulator of both our microbiome composition as well as mammalian cell physiology and metabolism. As a result of the new “omics” technologies and systems biology approaches, we are beginning to unravel the complex interactions between the liver, gastrointestinal tract, gut microbiome and bile acids. Specific members of the gut microbiome, in conjunction with diet, determine the composition of the circulating bile acid pool in humans. In this regard, individual bile acids are differentially recognized by specific nuclear receptors and G-protein receptors (GPCRs) of the host controlling the extent of activation of different cell signaling pathways and gene expression patterns in host cells exposed to bile acids. This trait allows bile acids to function as both detergents promoting nutrient absorption in the gut and as hormones regulating nutrient metabolism during the feed/fast cycle. Future studies should be aimed at efforts to control the composition and metabolic activities of our gut microbiome as well as the bile acid pool composition in order to decrease pathophysiological effects on the host.
Abbreviations
| BA | = | bile acid |
| BA7 | = | bile acid 7α/β-dehydroxylation |
| BSH | = | bile salt hydrolase |
| CA | = | cholic acid |
| CBA | = | conjugated bile acid |
| CDCA | = | chenodeoxycholic acid |
| DCA | = | deoxycholic acid |
| FXR | = | Farnesoid X Receptor |
| GCA | = | glycocholic acid |
| GCDCA | = | glycochenodeoxycholic acid |
| GDCA | = | glycodeoxycholic acid |
| GPCR | = | G-protein coupled receptor |
| HSDH | = | hydroxysteroid dehydrogenase |
| LCA | = | lithocholic acid |
| MCA | = | muricholic acid |
| SCFA | = | short chain fatty acid |
| SD | = | steroid-17.20-desmolase |
| TCA | = | taurocholic acid |
| UDCA | = | ursodeoxycholic acid |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Supported by VA Merit Grant; BX001328 (to PBH).
References
- Hardison WG. Hepatic taurine concentration and dietary taurine as regulators of bile acid conjugation with taurine. Gastroenterology 1978; 75(1):71–5; PMID:401099
- Dawaon PA, Karpen SJ. J Lipid Res 2015; 56(6):1085–99; PMID:25210150; http://dx.doi.org/10.1194/jlr.R054114
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig, KD, Mangelsdorf, DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 1999; 284:1362–5; PMID:10334992; http://dx.doi.org/10.1126/science.284.5418.1362
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Wilson TM, Zavacki AM, Moore DD, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 1999; 284:1365-8.
- Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y et al. A G-protein-coupled receptor responsive to bile acids. J Biol Chem 2003; 278:9435–40
- Li T, Chiang JY. Bile acids as metabolic reglators. Curr Opin Gastroenterol 2015; 31(2):159–65; PMID:25584736; http://dx.doi.org/10.1097/MOG.0000000000-000156
- D'Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, Wendum D, Firrincieli D, Coilly A, Fouassier L, Corpechot C, Poupon R, Housset C, et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology 2009; 136(4):1435–43; PMID:19245866; http://dx.doi.org/10.1053/j.gastro.2008.12.040
- Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006; 47(2):241–59; PMID:16299351; http://dx.doi.org/10.1194/jlr.R500013-JLR200
- Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 873(2):209–17; PMID:18801708; http://dx.doi.org/10.1016/j.jchromb.2008.08.018
- Vlahcevic ZR, Heuman DM, Hylemon PB. Physiology and pathophysiology of enterohepatic circulation of bile acids. In Hepatology: A Textbook of Liver Disease. 3rd edition. Vol. 1. D. Zakim and T. Boyer, editors. Saunders, Philadelphia, PA. 1996. 376–417.
- Sjövall J. Dietary glycine and taurine on bile acid conjugation in man:bile acids and steroids 75. Proc Soc Exp Biol Med 1959; 100:676–78; PMID:13645682; http://dx.doi.org/10.3181/00379727-100-24741
- Gopal-Srivastava R, Hylemon PB. Purification and characterization of bile salt hydrolase from Clostridium perfringens. J Lipid Res 1988; 29(8):1079–85; PMID:2903208
- Masuda N. Deconjugation of bile salts by Bacteroides and Clostridium. Microbiol Immunol 1981; 25(1):1–11; PMID:6265737; http://dx.doi.org/10.1111/j.1348-0421.1981.tb00001.x
- Wijaya A, Hermann A, Abriouel H, Specht I, Yousif NM, Holzapfel WH, Franz CM. Cloning of the bile salt hydrolase (bsh) gene from Enterococcus faecium FAIR-E 345 and chromosomal location of bsh genes from food enterococci. J Food Prot 2004; 67(12):2772–8; PMID:15633685
- Jarocki P, Targoński Z. Genetic diversity of bile salt hydrolases among human intestinal bifidobacteria. Curr Microbiol 2013; 67(3):286–92; PMID:23591474; http://dx.doi.org/10.1007/s00284-013-0362-1
- Tanaka H, Hashiba H, Kok J, Mierau I. Bile salt hydrolase of Bifidobacterium longum biochemical and genetic characterization. Appl Environ Microbiol 2000; 66(6):2502–12; PMID:10831430; http://dx.doi.org/10.1128/AEM.66.6.2502-2512.2000
- Oh HK, Lee JY, Lim SJ, Kim MJ, Kim JH, Hong SK, Kang DK. Molecular cloning and characterization of a bile salt hydrolase from Lactobacillus acidophilus PF01. J Microbiol Biotechnol 2008; 18(3):449-56.1; PMID:18388461
- De Smet I, Van Hoorde L, Vande Woestyne M, Christiaens H, Verstraete W. Significance of bile salt hydrolytic activities of lactobacilli. J Appl Bacteriol 1995; 79(3):292–301; PMID:7592123; http://dx.doi.org/10.1111/j.1365-2672.1995.tb03140.x
- Stellwag EJ, Hylemon PB. Purification and characterization of bile salt hydrolase from Bacteroides fragilis subsp. fragilis. Biochim Biophys Acta 1976; 452(1):165–76; PMID:10993; http://dx.doi.org/10.1016/0005-2744(76)90068-1
- Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA 2008; 105(36):13580–5; PMID:18757757; http://dx.doi.org/10.1073/pnas.0804437105
- Kim GB, Miyamoto CM, Meighen EA, Lee BH. Cloning and characterization of the bile salt hydrolase genes (bsh) from Bifidobacterium bifidum strains. Appl Environ Microbiol 2004; 70(9):5603–12; PMID:15345449; http://dx.doi.org/10.1128/AEM.70.9.5603-5612.2004
- Kumar RS, Brannigan JA, Prabhune AA, Pundle AV, Dodson GG, Dodson EJ, Suresh CG. Structural and functional analysis of a conjugated bile salt hydrolase from Bifidobacterium longum reveals an evolutionary relationship with penicillin V acylase. J Biol Chem 2006 Oct 27; 281(43):32516–25; http://dx.doi.org/10.1074/jbc.M6041-72200
- Coleman JP, Hudson LL. Cloning and characterization of a conjugated bile acid hydrolase gene from Clostridium perfringens. Appl Environ Microbiol 1995; 61(7):2514–20; PMID:7618863
- Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 2014; 30(3):332–8; PMID:24625896; http://dx.doi.org/10.1097/MOG.0000000000000057
- Lin J, Sahin O, Michel LO, Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun 2003; 71(8):4250–59; PMID:12874300; http://dx.doi.org/10.1128/IAI.71.8.4250-4259.2003
- Yokota A, Veenstra M, Kurdi P, van Veen HW, Konings WN. Cholate resistance in Lactococcus lactis is mediated by an ATP-dependent multispecific organic anion transporter. J Bacteriol 2000; 182(18):5196–201; PMID:10960105; http://dx.doi.org/10.1128/JB.182.18.5196-5201.2000
- Fernandez Murga ML, Bernick D, de Valdez GF, Disalvo AE. Permeability and stability properties of membranes formed by lipids extracted from Lactobacillus acidophilus grown at different temperatures. Arch Biochem Biophys 1999; 364:115–121; PMID:10087172; http://dx.doi.org/10.1006/abbi.1998.1093
- Kimoto H, Ohmomo S, Okamoto T. Enhancement of bile tolerance in Lactococci by Tween 80. J Appl Micro 2002; 92:41–46; http://dx.doi.org/10.1046/j.1365-2672.2002.01505.x
- Liu Y, An H, Zhang J, Zhou J, Zhou H, Ren F, Hao Y. Functional role of tlyC1 encoding a hemolysin-like protein from Bifidobacterium longum BBMN68 in bile tolerance. FEMS Microbiol Lett 2014; 360:167–73; PMID:25227940; http://dx.doi.org/10.1111/1574-6968.12601
- Ruiz L, Couté Y, Sánchez B, de los Reyes-Gavilán CG, Sanchez J, Margolles A. The cell-envelope proteome of Bifidobacterium longum in an in vivo bile environment. Microbiology 2009; 155:957–67; PMID:19246766; http://dx.doi.org/10.1099/mic.0.024273-0
- Coleman R, Lowe PJ, Billington D. Membrane lipid composition and susceptibility to bile salt damage. Biochim Biophys Acta 1980; 599(1):294–300; PMID:7397151; http://dx.doi.org/10.1016/0005-2736(80)90075-9
- Heuman DM, Bajaj RS, Lin Q. Adsorption of mixtures of bile salt taurine conjugates to lecithin-cholesterol membranes: implications for bile salt toxicity and cytoprotection. J Lipid Res 1996; 37(3):562–73; PMID:8728319
- Fujisawa T, Mori M. Influence of bile salts on beta-glucuronidase activity of intestinal bacteria. Lett Appl Microbiol 1996; 22(4):271–74; PMID:8934784; http://dx.doi.org/10.1111/j.1472-765X.1996.tb01159.x
- Noh DO, Gilliland SE. Influence of bile on cellular integrity and beta-galactosidase activity of Lactobacillus acidophilus. J Dairy Sci 1993; 76(5):1253–59; PMID:8505417; http://dx.doi.org/10.3168/jds.S0022-0302(93)77454-8
- Gómez Zavagilia A, Kociubinski G, Pérez P, Disalvo E, De Antoni G. Effect of bile on the lipid composition and surface properties of bifidobacteria. J Appl Microbiol 2002; 93(5):794–99; http://dx.doi.org/10.1046/j.1365-2672.2002.01747.x
- Taranto MP, Perez-Martinez G, Font de Valdez G. Effect of bile acid on the cell membrane functionality of lactic acid bacteria for oral administration. Res Microbiol 2006; 157(8):720–25; PMID:16730163; http://dx.doi.org/10.1016/j.resmic.2006.04.002
- Hofmann M, Schumann C, Zimmer G, Henzel K, Locher U, Leuschner U. LUV’s lipid composition modulates diffusion of bile acids. Chem Phys Lipid 2001; 110(2):165–71; http://dx.doi.org/10.1016/S0009-3084(01)00131-1
- Schubert R, Jaroni H, Schoelmerich J, Schmidt KH. Studies on the mechanism of bile salt-induced liposomal membrane damage. Digestion 1983; 28(3):181–90; PMID:6365666; http://dx.doi.org/10.1159/000198984
- Cabral DJ, Small DM, Lilly HS, Hamilton JA. Transbilayer movement of bile acids in model membranes. Biochemistry 1987; 26(7):1801–4; PMID:3593691; http://dx.doi.org/10.1021/bi00381a002
- Prouty AM, Schwesinger WH, Gunn JS. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immunol 2002; 70:2640–49; http://dx.doi.org/10.1128/IAI.70.5.2640-2649.2002
- Hildebrandt MA, Hoffman C, SHerill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009; 137(5):1716–24; PMID:19706296; http://dx.doi.org/10.1053/j.gastro.2009.08.042
- Floch MH, Binder HJ, Filburn B, Gershengorn W. The effect of bile acids on intestinal microflora. Am J Clin Nutr 1972; (12):1418–26; PMID:4344803
- Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol 2006; 188(5):1979–86; PMID:16484210; http://dx.doi.org/10.1128/JB.188.5.1979-1986.2006
- Grill JP, Cayuela C, Antoine JM, Schneider F. Isolation and characterization of a Lactobacillus amylovorus mutant depleted in conjugated bile salt hydrolase activity: relation between activity and bile salt resistance. J Appl Microbiol 2000; 89:553–563; PMID:11054157; http://dx.doi.org/10.1046/j.1365-2672.2000.01147.x
- Dussurget O, Cabanes D, Dehoux P, Lecuit M, Buchrieser C, Glaser P, Cossart P, European Listeria Genome Consortoum. Mol Microbiol 2002; 10(5):238–45
- Dussurget O, Cabanes D, Dehoux P, Lecuit M, Buchrieser C, Glaser P, Cossart P; European Listeria Genome Consortium. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol Microbiol 2002 Aug; 45(4):1095–106; http://dx.doi.org/10.1046/j.1365-2958.2002.03080.x
- Dunne C, O’Mahony L, Murphy L, Thornton G, Morrissey D, O’Halloran S, Feeney M, Flynn S, Fitzgerald G, Daly C, et al. In vitro selection criteria for probiotic bacteria of human origin: correlations with in vivo findings. Am J Clin Nutr 2001; 73(2 Suppl):386S–92S; PMID:11157346
- Huijghebaert SM, Eyssen HJ. Specificity of bile salt sulfatase activity from Clostridium sp. strains S1. Appl Environ Microbiol 1982; 44(5):1030–1034; PMID:7181500
- Van Eldere J, Celis P, De Pauw G, Lesaffre E, Eyssen H. Tauroconjugation of cholic acid stimulates 7 alpha-dehydroxylation by fecal bacteria. Appl Environ Microbiol 1996; 62(2):656–661; PMID:8593067
- Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012; 487(7405):104–8; PMID:22722865
- Guilbert JJ. The world health report 2002-reducing risks, promoting healthy life. Educ Health 2003; 16(2):230; http://dx.doi.org/10.1080/1357628031000116808
- Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ 1990; 301:309–14; PMID:2144195; http://dx.doi.org/10.1136/bmj.301.6747.309
- Evans M, Roberts S, Davies S, Rees A. Medical lipid-regulating therapy: current evidence, ongoing trails and future developments. Drugs 2004; 64(11):1181–96; PMID:15161326; http://dx.doi.org/10.2165/00003495-200464110-00003
- Jones ML, Tomaro-Duchesneau C, Martoni CJ, Prakash S. Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin Biol Ther 2013; 13(5):631–642; PMID:23350815; http://dx.doi.org/10.1517/14712598.2013.758706
- Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 2006; 72(3):1729–38; PMID:16517616; http://dx.doi.org/10.1128/AEM.72.3.1729-1738.2006
- Gustafsson BE, Midvedt T, Norman A. Metabolism of cholic acid in germfree animals after the establishment in the intestinal tract of deconjugating and 7alpha-dehydroxylating bacteria. Acta Pathol Microbiol Scand 1968; 72(3):433–43; PMID:4297296; http://dx.doi.org/10.1111/j.1699-0463.1968.tb00457.x
- Narushima S, Itoha K, Miyamoto Y, Park SH, Nagata K, Kuruma K, Uchida K. Deoxycholic acid formation in gnotobiotic mice associated with human intestinal bacteria. Lipids 2006; 41(9):835–43; PMID:17152920; http://dx.doi.org/10.1007/s11745-006-5038-1
- Wells JE, Berr F, Thomas LA, Dowling RH, Hylemon PB. Isolation and characterization of cholic acid 7alpha-dehydroxylating fecal bacteria from cholesterol gallstone patients. J Hepatol 2000; 32(1):4–10; PMID:10673060; http://dx.doi.org/10.1016/S0168-8278(00)80183-X
- Takamine F, Imamura T. Isolation and characterization of bile acid 7-dehydroxylating bacteria from human feces. Microbiol Immunol 1995; 39(1):11–8; PMID:7783673; http://dx.doi.org/10.1111/j.1348-0421.1995.tb02162.x
- Hirano S, Nakama R, Tamaki M, Masuda N, Oda H. Isolation and characterization of thirteen intestinal microorganisms capable of 7alpha-dehydroxylating bile acids. Appl Environ Microbiol 1981; 41(3):737–45; PMID:7224633
- Eyssen HJ, De Pauw G, Van Eldere J. Formation of hyodeoxycholic acid from muricholic acid and hyocholic acid by an unidentified gram-positive rod termed HDCA-1 isolated from rat intestinal microflora. Appl Environ Microbiol 1999; 65(7):3158–63; PMID:10388717
- Sacquet EC, Gadelle DP, Riottot MJ, Raibaud PM. Absence of transformation of beta-muricholic acid by human microflora implanted in the digestive tracts of germfree male rats. Appl Environ Microbiol 1984; 47(5):1167–68; PMID:6742831
- Ridlon JM, Hylemon PB. Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7alpha-dehydroxylating bacterium. J Lipid Res 2012; 53(1):66–76; PMID:22021638; http://dx.doi.org/10.1194/jlr.M020313
- Mallonee DH, Adams JL, Hylemon PB. The bile acid-inducible baiB gene from Eubacterium sp. strain VPI 12708 encodes a bile acid-coenzyme A ligase. 1992; 174(7):2065–71; PMID:1551828
- Dawson JA, Mallonee DH, Björkhem I, Hylemon PB. Expression and characterization of a C24 bile acid 7 alpha-dehydratase from Eubacterium sp. strain VPI 12708 in Escherichia coli. J Lipid Res 1996; 37(6):1258–67; PMID:8808760
- Coleman JP, White WB, Hylemon PB. Molecular cloning of bile acid 7-dehydroxylase from Eubacterium sp. strain VPI 12708. J Bacteriol 1987; 169(4):1516–21; PMID:3549693
- Kang DJ, Ridlon JM, Moore DR 2nd, Barnes S, Hylemon PB. Clostridium scindens baiCD and baiH genes encode stereo-specific 7alpha/7beta-hydroxy-3-oxo-delta-4-cholenoic acid oxidoreductases. Biochim Biophys Acta 2008; 1781(1-2):16–25; PMID:18047844; http://dx.doi.org/10.1016/j.bbalip.2007.10.008
- Winter J, Morris GN, O’Rourke-Locascio S, Bokkenheuser VD, Mosbach EH, Cohen BI, Hylemon PB. Mode of action of steroid desmolase and reductases synthesized by Clostridium “scindens” (formerly Clostridium strain 19). J Lipid Res 1984; 25(10):1124–31; PMID:6512418
- Bokkenheuser VD, Morris GN, Ritchie AE, Holdeman LV, Winter J. Biosynthesis of androgen from cortisol by a species of Clostridium recovered from human fecal flora. J Infect Dis 1984; 149(4):489–94; PMID:6725987; http://dx.doi.org/10.1093/infdis/149.4.489
- Ridlon JM, Ikegawa S, Alves JM, Zhou B, Kobayaski A, Iida T, Mitamura K, Tanabe G, Serrano M, De Guzman A, et al. Clostridium scindens: a human gut microbe with high potential to convert glucocorticoids into androgens. J Lipid Res 2013; 54(9):2437–49; PMID:23772041; http://dx.doi.org/10.1194/jlr.M038869
- Ridlon JM, Bajaj JS. The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics. Acta Pharmaceutica Sinica B 2015; 5(2):99–105; PMID:26579434; http://dx.doi.org/10.1016/j.apsb.2015.01.006
- Ridlon JM, Kang DJ, Hylemon PB. Isolation and characterization of a bile acid inducible 7alpha-dehydroxylating operon in Clostridium hylemonae TN271. Anaerobe 2010; 16(2):137–46; PMID:19464381; http://dx.doi.org/10.1016/j.anaerobe.2009.05.004
- Mallonee DH, Hylemon PB. Sequencing and expression of a gene encoding a bile acid transporter from Eubacterium sp. strain VPI 12708. J Bacteriol 1996; 178(24):7053–58; PMID:8955384
- Mallonee DH, Lijewski MA, Hylemon PB. Expression in Escherichia coli and characterization of a bile acid-inducible 3 alpha-hydroxysteroid dehydrogenase from Eubacterium sp. strain VPI 12708. Curr Microbiol 1995; 30(5):259–63; PMID:7766153; http://dx.doi.org/10.1007/BF00295498
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013; 499(7456):97–101; PMID:23803760
- Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, Gaskins HR, O’Keefe SJ. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr 2013; 98(1):111–20; PMID:23719549; http://dx.doi.org/10.3945/ajcn.112.056689
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505(7484):559–63; PMID:24336217; http://dx.doi.org/10.1038/nature12820
- O’Keefe DJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun 2015; 6:6342; http://dx.doi.org/10.1038/ncomms7342
- Islam KB, Fukiya S, Hagio M, Fujii N, Ischizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011; 141(5):1773–81; PMID:21839040; http://dx.doi.org/10.1053/j.gastro.2011.07.046
- Bhowmik S, Jones DH, Chiu HP, Park IH, Chiu HJ, Axelrod HL, Farr CL, Tien HJ, Agarwalla S, Lesley SA. Structural and functional characterization of BaiA, an enzyme involved in secondary bile acid synthesis in human gut microbe. Proteins 2014; 82(2):216–29; PMID:23836456; http://dx.doi.org/10.1002/prot.24353
- Bhowmik B, Chiu H-P, Jones DH, Chiu H-J, Miller MD, Xu Q, Farr CL, Ridlon JM, Wells JE, Elsliger M-A et al. Structure and functional characterization of a Bile Acid 7α dehydratase BaiE in secondary bile acid synthesis. Proteins 2015; in press; PMID:26650892; http://dx.doi.org/10.1002/prot.2471 after 2015
- Korpela JT, Fotsis T, Adlercreutz H. Multicomponent analysis of bile acids in faeces by anion exchange and capillary column gas-liquid chromatography: application in oxytetracycline treated subjects. J Steroid Biochem 1986; 25(2):277-84.7; PMID:3747527; http://dx.doi.org/10.1016/0022-4731(86)90429-2
- Kakiyama G, Muto A, Takei H, Nittono H, Murai T, Kurosawa T, Hofmann AF, Pandak WM, Bajaj JS. A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: validation by GC-MS and LC-MS. J Lipid Res 2014; 55(5):978–90; PMID:24627129; http://dx.doi.org/10.1194/jlr.D047506
- Kelsey MI, Molina JE, Huang SK, Hwang KK. The identification of microbial metabolites of sulfolithocholic acid. J Lipid Res 1980; 21(6)751–9.6; PMID:7419986
- Benson GM, Haskins NJ, Eckers C, Moore PJ, Reid DG, Mitchell RC, Waghmare S, Suckling KE. Polydeoxycholate in human and hamster feces: a major product of cholate metabolism. J Lipid Res 1993; 34(12):2121–34.1; PMID:8301231
- Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, Pandak WM, Dent P, Spiegel S, Shi R, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 2012 Jan; 55(1):267–76; http://dx.doi.org/10.1002/hep.24681
- Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A 2009; 108 Suppl 1:4523–30
- Berr F, Kullak-Ublick GA, Paumgartner G, Münzing W, Hylemon PB. 7α-dehydroxylating bacteria enhance deoxycholic acid input and cholesterol saturation of bile in patients with gallstones. Gastroenterology 1996; 111(6):1611–201; PMID:8942741; http://dx.doi.org/10.1016/S0016-5085(96)70024-0
- Thomas LA, Veysey MJ, Murphy GM, Russell-Jones D, French GL, Wass JA, Dowling RH. Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly Gut 2005; 54(5):630–5; PMID:15831907
- Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res 1989; 30(5):719–30; PMID:2760545
- Matsuoka K, Moroi Y. Micelle formation of sodium deoxycholate and sodium ursodeoxycholate (part 1). Biochim Biophys Acta. 2002; 1580(2-3):189–99; PMID:11880243; http://dx.doi.org/10.1016/S1388-1981(01)00203-7
- Devlin AS, Fischbach MA. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat Chem Biol 2015; 11(9):685–90; PMID:26192599; http://dx.doi.org/10.1038/nchembio.1864
- Odermatt A, Da Cunha T, Penno CA, Chandsawangbhuwana C, Reichert C, Wolf A, Dong M, Baker ME. Hepatic reduction of the secondary bile acid 7-oxolithocholic acid is mediated by 11beta-hydroxysteroid dehydrogenase 1. Biochem J 2011; 436(3):621–9; PMID:21453287; http://dx.doi.org/10.1042/BJ20110022
- Odermatt A, Klusonova P. 11beta-Hydroxysteroid dehydrogenase 1: Regeneration of active glucocorticoids is only part of the story. J Steroid Biochem Mol Biol 2015; 151:85–92; PMID:25151952; http://dx.doi.org/10.1016/j.jsbmb.2014.08.011
- Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun 2002; 70(2):953–63; PMID:11796631; http://dx.doi.org/10.1128/IAI.70.2.953-963.2002
- Campbell Y, Fantacone ML, Gombart AF. Regulation of antimicrobial peptide gene expression by nutrients and by-products of microbial metabolism. Eur J Nutr 2012; 51(8):899–907; PMID:22797470; http://dx.doi.org/10.1007/s00394-012-0415-4
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 1999; 3(5):543–53; PMID:10360171; http://dx.doi.org/10.1016/S1097-2765(00)80348-2
- Fujino T, Une M, Imanaka T, Inoue K, Nishimaki-Mogami T. Structure-activity relationship of bile acids and bile acid analogs in regard to FXR activation. J Lipid Res 2004; 45(1):132–8; PMID:13130122; http://dx.doi.org/10.1194/jlr.M300215-JLR200
- Hase K, Murakami M, Iimura M, Cole SP, Horibe Y, Ohtake T, et al. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 2003; 125(6):1613–25; PMID:14724813; http://dx.doi.org/10.1053/j.gastro.2003.08.028
- Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res 2014; 55(8):1553–1595; http://dx.doi.org/10.1194/jlr.R049437
- Guarino MP, Cocca S, Altomare A, Emerenziani S, Cicala M. Ursodeoxycholic acid therapy in gallbladder disease, a story not yet completed. World J. Gastroenterol 2013; 19(31):5029–5034
- Czul F, Peyton A, Levy C. Primary biliary cirrhosis: therapeutic advances. Clin Liver Dis 2013.17(2):229–242; PMID:23540499; http://dx.doi.org/10.1016/j.cld.2012.12.003
- Mahmoud MF, Elshazly SM. Ursodeoxycholic acid ameliorates fructose-induced metabolic syndrome in rats. PLoS One 2014; 9(9):e106993; PMID:25202970; http://dx.doi.org/10.1371/journal.pone.0106993
- ALberts DS, Martinez ME, Hess LM, Einspahr JG, Green SB, Bhattacharyya AK, Guillen J, Krutzsch M, Batta AK, Salen G, et al, Phoenix and Tucson Gastroenterologist Networks. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst 2005; 97(11):846–53; PMID:15928305; http://dx.doi.org/10.1093/jnci/dji144
- Serfaty L. Chemoprevention of colorectal cancer with ursodeoxycholic acid: pro. Clin Res Hepatol Gastroenterol 2012; 36 Suppl 1:S53–S60; PMID:23141895; http://dx.doi.org/10.1016/S2210-7401(12)70022-0
- Carey EJ, Lindor KD. Chemoprevention of colorectal cancer with ursodeoxycholic acid: cons. Clin Res Hepatol Gastroenterol 2012; 36 Suppl 1:S61–S64
- Earnest DL, Holubec H, Wali RK, Jolley CS, Bissonette M, Bhattacharyya AK, Roy H, Khare S, Brasitus TA. Chemoprevention of azomethane-induced colonic carcinogenesis by supplemental dietary ursodeoxycholic acid. Cancer Res 1994 Oct 1; 54(19):5071–4
- Brasitus TA. Primary chemoprevention strategies for colorectal cancer: ursodeoxycholic acid and other agents. Gastroenterology 1995; 109(6):2036–38; PMID:7498674; http://dx.doi.org/10.1016/0016-5085(95)90776-9
- Wali RK, Frawley BP Jr, Hartmann S, Roy HK, Khare S, Scaglione-Sewell BA, Earnest DL, Sitrin MD, Brasitus TA, Bissonnette M. Mechanism of action of chemopreventative ursodeoxycholate in the azomethane model of rat colonic carcinogenesis: potential roles of protein kinase C-alpha, -beta II, and –zeta. Cancer Res 1995 Nov 15; 55(22):5257–64
- Im E, Martinez JD. Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/Raf-1/ERK signaling in human colon cancer cells. J Nutr 2004; 134(2):483–86; PMID:14747693
- Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med 1998 Mar; 4(3):165–78
- Kulkarni MS, Heidepriem PM, Yielding KL. Production by lithocholic acid of DNA strand breaks in L1210 cells. Cancer Res 1980; 40:2666–2669; PMID:7388816
- Booth LA, Gilmore IT, Bilton RF. Secondary bile acid induced DNA damage in HT29 cells: are free radicals involved? Free Radic Res 1997; 26(2):135–44; PMID:9257125; http://dx.doi.org/10.3109/10715769709-097792
- Kulkarni MS, Cox BA, Yielding KL. Requirements for induction of DNA strand breaks by lithocholic acid. Cancer Res 1982; 42(7):2792–95; PMID:7083168
- Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature Rev Microbiol 2009; 7:526–536; http://dx.doi.org/10.1038/nrmicro2164
- Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 26; 372(9):825–34; http://dx.doi.org/10.1056/NEJMoa1408913
- Wilson KH, Perini F. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect Immun 1988; 56:2610–14; PMID:3417352
- Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature 2010; 467(7316):711–3; PMID:20844489; http://dx.doi.org/10.1038/nature09397
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al. Duodenal infusion of feces for recurrent Clostridium difficile. N Engl J Med 2013; 368(22):407–415; PMID:23323867; http://dx.doi.org/10.1056/NEJMoa1205037
- Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015; 517(7533):205–8; PMID:25337874; http://dx.doi.org/10.1038/nature13828
- Wilson KH. Eficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J Clin Microbiol 1983; (4):1017–9
- Wilson KH, Kennedy MJ, Fekety FR. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile J Clin Microbiol 1982; (3):443–6
- Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 2008; 190(7):2505–12; PMID:18245298; http://dx.doi.org/10.1128/JB.01765-07
- Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol 2010; 192(19):4983–90; PMID:20675492; http://dx.doi.org/10.1128/JB.00610-10
- Francis MB, Allen CA, Sorg JA. Muricholic acids inhibit Clostridium difficile spore germination and growth. PLoS One 2013; 8(9):e73653; PMID:24040011; http://dx.doi.org/10.1371/journal.pone.0073653
- Francis MB, Allen CA, Shrestha R, Sorg JA. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog 2013; 9(5):e1003356; PMID:23675301; http://dx.doi.org/10.1371/journal.ppat.1003356
